Meal Timing and Glycemic Control during Pregnancy—Is There a Link?
Abstract
1. Introduction
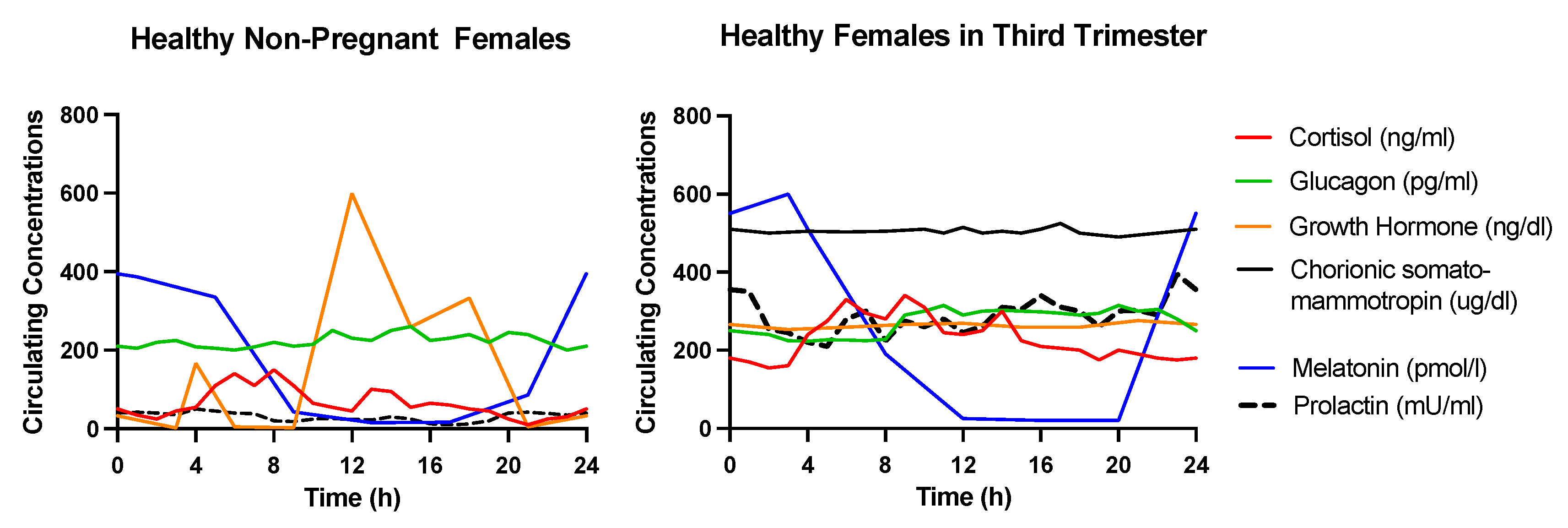
2. Endocrine Mechanisms Underlying the Alterations in Glucose Metabolism during Pregnancy
2.1. Growth Hormone (GH)
2.2. Human Chorionic Somatomammotropin (hCS)
2.3. Prolactin
2.4. Cortisol
2.5. Glucagon
2.6. Melatonin
3. Fasting Duration and Glycemic Control during Gestation
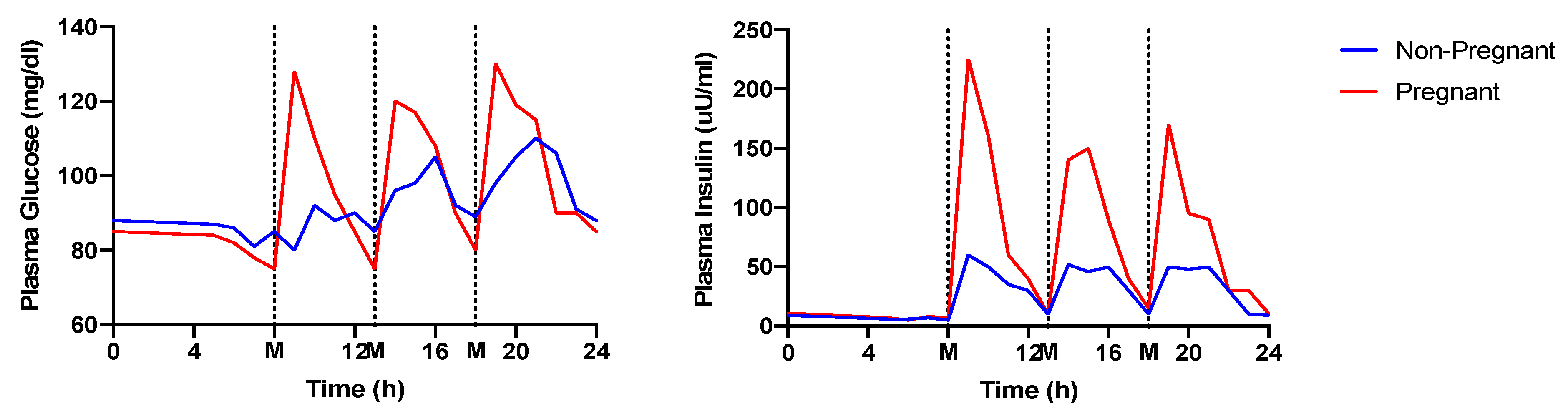
4. Meal Timing and Glycemic Control during Gestation
5. Twenty-Four-Hour Energy and Carbohydrate Distribution and Glycemic Control during Gestation
6. Eating Frequency and Glycemic Control during Gestation
7. Future Research
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Diabetes Federation Atlas; International Diabetes Federation: Brussels, Belgium, 2017; Volume 147.
- Zhou, T.; Sun, D.; Li, X.; Heianza, Y.; Nisa, H.; Hu, G.; Pei, X.; Shang, X.; Qi, L. Prevalence and Trends in Gestational Diabetes Mellitus among Women in the United States, 2006–2016; The American Diabetes Association: Arlington, VA, USA, 2018. [Google Scholar]
- American Diabetes Association. 2. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes—2021. Diabetes Care 2021, 44, S15–S33. [Google Scholar] [CrossRef]
- Bryson, C.L.; Ioannou, G.N.; Rulyak, S.J.; Critchlow, C. Association between gestational diabetes and pregnancy-induced hypertension. Am. J. Epidemiol. 2003, 158, 1148–1153. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, M.W. Gestational diabetes, pregnancy hypertension, and late vascular disease. Diabetes Care 2007, 30 (Suppl. 2), S246–S250. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Yuan, S.; Ma, Y.; An, Y.X.; Yang, Y.X.; Yang, J.K. Associations of maternal hyperglycemia in the second and third trimesters of pregnancy with prematurity. Medicine 2020, 99, e19663. [Google Scholar] [CrossRef]
- Goldenberg, R.L.; Culhane, J.F.; Iams, J.D.; Romero, R. Epidemiology and causes of preterm birth. Lancet 2008, 371, 75–84. [Google Scholar] [CrossRef]
- Ehrenberg, H.M.; Mercer, B.M.; Catalano, P.M. The influence of obesity and diabetes on the prevalence of macrosomia. Am. J. Obstet. Gynecol. 2004, 191, 964–968. [Google Scholar] [CrossRef]
- Hill, M.G.; Cohen, W.R. Shoulder dystocia: Prediction and management. Womens Health 2016, 12, 251–261. [Google Scholar] [CrossRef]
- Voormolen, D.N.; de Wit, L.; van Rijn, B.B.; DeVries, J.H.; Heringa, M.P.; Franx, A.; Groenendaal, F.; Lamain-de Ruiter, M. Neonatal hypoglycemia following diet-controlled and insulin-treated gestational diabetes mellitus. Diabetes Care 2018, 41, 1385–1390. [Google Scholar] [CrossRef] [PubMed]
- Desoye, G.; Nolan, C.J. The fetal glucose steal: An underappreciated phenomenon in diabetic pregnancy. Diabetologia 2016, 59, 1089–1094. [Google Scholar] [CrossRef]
- Daly, B.; Toulis, K.A.; Thomas, N.; Gokhale, K.; Martin, J.; Webber, J.; Keerthy, D.; Jolly, K.; Saravanan, P.; Nirantharakumar, K. Increased risk of ischemic heart disease, hypertension, and type 2 diabetes in women with previous gestational diabetes mellitus, a target group in general practice for preventive interventions: A population-based cohort study. PLoS Med. 2018, 15, e1002488. [Google Scholar] [CrossRef]
- Tobias, D.K.; Hu, F.B.; Forman, J.P.; Chavarro, J.; Zhang, C. Increased risk of hypertension after gestational diabetes mellitus: Findings from a large prospective cohort study. Diabetes Care 2011, 34, 1582–1584. [Google Scholar] [CrossRef]
- Kramer, C.K.; Campbell, S.; Retnakaran, R. Gestational diabetes and the risk of cardiovascular disease in women: A systematic review and meta-analysis. Diabetologia 2019, 62, 905–914. [Google Scholar] [CrossRef]
- Kim, S.Y.; Sharma, A.J.; Callaghan, W.M. Gestational diabetes and childhood obesity: What is the link? Curr. Opin. Obstet. Gynecol. 2012, 24, 376–381. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. 13. Management of Diabetes in Pregnancy. Diabetes Care 2017, 40, S114–S119. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Diabetes and Digestive and Kidney Diseases. Managing & Treating Gestational Diabetes. Available online: https://www.niddk.nih.gov/health-information/diabetes/overview/what-is-diabetes/gestational/management-treatment (accessed on 24 May 2021).
- Hui, A.L.; Sevenhuysen, G.; Harvey, D.; Salamon, E. Barriers and coping strategies of women with gestational diabetes to follow dietary advice. Women Birth 2014, 27, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Hatori, M.; Vollmers, C.; Zarrinpar, A.; DiTacchio, L.; Bushong, E.A.; Gill, S.; Leblanc, M.; Chaix, A.; Joens, M.; Fitzpatrick, J.A.; et al. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 2012, 15, 848–860. [Google Scholar] [CrossRef] [PubMed]
- Chaix, A.; Zarrinpar, A.; Miu, P.; Panda, S. Time-restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges. Cell Metab. 2014, 20, 991–1005. [Google Scholar] [CrossRef] [PubMed]
- Chow, L.S.; Manoogian, E.N.; Alvear, A.; Fleischer, J.G.; Thor, H.; Dietsche, K.; Wang, Q.; Hodges, J.S.; Esch, N.; Malaeb, S. Time-restricted eating effects on body composition and metabolic measures in humans who are overweight: A feasibility study. Obesity 2020, 28, 860–869. [Google Scholar] [CrossRef]
- Cienfuegos, S.; Gabel, K.; Kalam, F.; Ezpeleta, M.; Wiseman, E.; Pavlou, V.; Lin, S.; Oliveira, M.L.; Varady, K.A. Effects of 4- and 6-h time-restricted feeding on weight and cardiometabolic health: A randomized controlled trial in adults with obesity. Cell Metab. 2020, 32, 366–378.e3. [Google Scholar] [CrossRef]
- Sutton, E.F.; Beyl, R.; Early, K.S.; Cefalu, W.T.; Ravussin, E.; Peterson, C.M. Early time-restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes. Cell Metab. 2018, 27, 1212–1221.e3. [Google Scholar] [CrossRef]
- Hutchison, A.T.; Regmi, P.; Manoogian, E.N.; Fleischer, J.G.; Wittert, G.A.; Panda, S.; Heilbronn, L.K. Time-restricted feeding improves glucose tolerance in men at risk for type 2 diabetes: A randomized crossover trial. Obesity 2019, 27, 724–732. [Google Scholar] [CrossRef]
- Zhu, S.; Surampudi, P.; Rosharavan, B.; Chondronikola, M. Intermittent fasting as a nutrition approach against obesity and metabolic disease. Curr. Opin. Clin. Nutr. Metab. Care 2020, 23, 387–394. [Google Scholar] [CrossRef]
- Kessler, K.; Hornemann, S.; Petzke, K.J.; Kemper, M.; Kramer, A.; Pfeiffer, A.F.; Pivovarova, O.; Rudovich, N. The effect of diurnal distribution of carbohydrates and fat on glycaemic control in humans: A randomized controlled trial. Sci. Rep. 2017, 7, 44170. [Google Scholar] [CrossRef] [PubMed]
- Pearce, K.L.; Noakes, M.; Keogh, J.; Clifton, P.M. Effect of carbohydrate distribution on postprandial glucose peaks with the use of continuous glucose monitoring in type 2 diabetes. Am. J. Clin. Nutr. 2008, 87, 638–644. [Google Scholar] [CrossRef] [PubMed]
- Kanaley, J.A.; Heden, T.D.; Liu, Y.; Fairchild, T.J. Alteration of postprandial glucose and insulin concentrations with meal frequency and composition. Br. J. Nutr. 2014, 112, 1484–1493. [Google Scholar] [CrossRef] [PubMed]
- Lammi-Keefe, C.J.; Couch, S.C.; Kirwan, J.P. Handbook of Nutrition and Pregnancy; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Phelps, R.L.; Metzger, B.E.; Freinkel, N. Carbohydrate metabolism in pregnancy. XVII. Diurnal profiles of plasma glucose, insulin, free fatty acids, triglycerides, cholesterol, and individual amino acids in late normal pregnancy. Am. J. Obstet. Gynecol. 1981, 140, 730–736. [Google Scholar] [CrossRef]
- Plows, J.F.; Stanley, J.L.; Baker, P.N.; Reynolds, C.M.; Vickers, M.H. The Pathophysiology of Gestational Diabetes Mellitus. Int. J. Mol. Sci. 2018, 19, 3342. [Google Scholar] [CrossRef]
- Hollingsworth, D.R. Alterations of maternal metabolism in normal and diabetic pregnancies: Differences in insulin-dependent, non-insulin-dependent, and gestational diabetes. Am. J. Obstet. Gynecol. 1983, 146, 417–429. [Google Scholar] [CrossRef]
- Eriksson, L. Growth hormone in human pregnancy. Maternal 24-hour serum profiles and experimental effects of continuous GH secretion. Acta Obstet. Gynecol. Scand. 1989, 147, 1–38. [Google Scholar] [CrossRef]
- Gillmer, M.D.; Oakley, N.W.; Beard, R.W.; Brooke, F.M.; Brudenell, M.; Chard, T. Plasma human placental lactogen profiles over 24 hours in normal and diabetic pregnancy. Br. J. Obstet. Gynaecol. 1977, 84, 197–204. [Google Scholar] [CrossRef]
- Kivela, A. Serum melatonin during human pregnancy. Acta Endocrinol. 1991, 124, 233–237. [Google Scholar] [CrossRef]
- Gunn, P.J.; Middleton, B.; Davies, S.K.; Revell, V.L.; Skene, D.J. Sex differences in the circadian profiles of melatonin and cortisol in plasma and urine matrices under constant routine conditions. Chronobiol. Int. 2016, 33, 39–50. [Google Scholar] [CrossRef]
- Handwerger, S.; Freemark, M. The roles of placental growth hormone and placental lactogen in the regulation of human fetal growth and development. J. Pediatric Endocrinol. Metab. 2000, 13, 343–356. [Google Scholar] [CrossRef]
- Eriksson, L.; Eden, S.; Frohlander, N.; Bengtsson, B.A.; von Schoultz, B. Continuous 24-hour secretion of growth hormone during late pregnancy. A regulator of maternal metabolic adjustment? Acta Obstet. Gynecol. Scand. 1988, 67, 543–547. [Google Scholar] [CrossRef]
- Vijayakumar, A.; Novosyadlyy, R.; Wu, Y.; Yakar, S.; LeRoith, D. Biological effects of growth hormone on carbohydrate and lipid metabolism. Growth Horm. IGF Res. 2010, 20, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Grumbach, M.M.; Kaplan, S.L.; Sciarra, J.; Burr, I.M. Chorionic growth hormone-prolactin (CGP): Secretion, disposition, biologic activity in man, and postulated function as the “growth hormone” of the second half of pregnancy. Ann. N. Y. Acad. Sci. 1968, 148, 501–531. [Google Scholar] [CrossRef]
- Baeyens, L.; Hindi, S.; Sorenson, R.L.; German, M.S. β-Cell adaptation in pregnancy. Diabetes Obes. Metab. 2016, 18, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Maltepe, E.; Penn, A.A. Development, Function, and Pathology of the Placenta. In Avery’s Diseases of the Newborn; Elsevier: Amsterdam, The Netherlands, 2018; pp. 40–60.e8. [Google Scholar]
- Berga, S.L.; Nitsche, J.F.; Braunstein, G.D. Endocrine changes in pregnancy. In Williams Textbook of Endocrinology; Elsevier: Amsterdam, The Netherlands, 2016; pp. 831–848. [Google Scholar]
- Tenorio, F.; Simoes Mde, J.; Teixeira, V.W.; Teixeira, A.A. Effects of melatonin and prolactin in reproduction: Review of literature. Rev. Assoc. Med. Bras. 2015, 61, 269–274. [Google Scholar] [CrossRef]
- Daimon, M.; Kamba, A.; Murakami, H.; Mizushiri, S.; Osonoi, S.; Yamaichi, M.; Matsuki, K.; Sato, E.; Tanabe, J.; Takayasu, S.; et al. Association between serum prolactin levels and insulin resistance in non-diabetic men. PLoS ONE 2017, 12, e0175204. [Google Scholar] [CrossRef] [PubMed]
- Auriemma, R.S.; De Alcubierre, D.; Pirchio, R.; Pivonello, R.; Colao, A. Glucose Abnormalities Associated to Prolactin Secreting Pituitary Adenomas. Front. Endocrinol. 2019, 10, 327. [Google Scholar] [CrossRef] [PubMed]
- Entringer, S.; Buss, C.; Shirtcliff, E.A.; Cammack, A.L.; Yim, I.S.; Chicz-DeMet, A.; Sandman, C.A.; Wadhwa, P.D. Attenuation of maternal psychophysiological stress responses and the maternal cortisol awakening response over the course of human pregnancy. Stress 2010, 13, 258–268. [Google Scholar] [CrossRef]
- Khani, S.; Tayek, J.A. Cortisol increases gluconeogenesis in humans: Its role in the metabolic syndrome. Clin. Sci. 2001, 101, 739–747. [Google Scholar] [CrossRef]
- Zinker, B.; Mika, A.; Nguyen, P.; Wilcox, D.; Öhman, L.; von Geldern, T.W.; Opgenorth, T.; Jacobson, P. Liver-selective glucocorticoid receptor antagonism decreases glucose production and increases glucose disposal, ameliorating insulin resistance. Metabolism 2007, 56, 380–387. [Google Scholar] [CrossRef]
- Weinstein, S.P.; Wilson, C.M.; Pritsker, A.; Cushman, S.W. Dexamethasone inhibits insulin-stimulated recruitment of GLUT4 to the cell surface in rat skeletal muscle. Metabolism 1998, 47, 3–6. [Google Scholar] [CrossRef]
- Weinstein, S.P.; Paquin, T.; Pritsker, A.; Haber, R.S. Glucocorticoid-induced insulin resistance: Dexamethasone inhibits the activation of glucose transport in rat skeletal muscle by both insulin-and non-insulin-related stimuli. Diabetes 1995, 44, 441–445. [Google Scholar] [CrossRef]
- Lundgren, M.; Burén, J.; Ruge, T.; Myrnäs, T.; Eriksson, J.W. Glucocorticoids down-regulate glucose uptake capacity and insulin-signaling proteins in omental but not subcutaneous human adipocytes. J. Clin. Endocrinol. Metab. 2004, 89, 2989–2997. [Google Scholar] [CrossRef] [PubMed]
- Plat, L.; Byrne, M.M.; Sturis, J.; Polonsky, K.S.; Mockel, J.; Féry, F.; Van Cauter, E. Effects of morning cortisol elevation on insulin secretion and glucose regulation in humans. Am. J. Physiol. 1996, 270, E36–E42. [Google Scholar] [CrossRef]
- Fine, N.H.F.; Doig, C.L.; Elhassan, Y.S.; Vierra, N.C.; Marchetti, P.; Bugliani, M.; Nano, R.; Piemonti, L.; Rutter, G.A.; Jacobson, D.A.; et al. Glucocorticoids reprogram β-cell signaling to preserve insulin secretion. Diabetes 2018, 67, 278–290. [Google Scholar] [CrossRef] [PubMed]
- Rix, I.; Nexøe-Larsen, C.; Bergmann, N.C.; Lund, A.; Knop, F.K. Glucagon Physiology; MDText.com, Inc.: South Dartmouth, MA, USA, 2015. [Google Scholar]
- Müller, T.D.; Finan, B.; Clemmensen, C.; DiMarchi, R.D.; Tschöp, M.H. The New Biology and Pharmacology of Glucagon. Physiol. Rev. 2017, 97, 721–766. [Google Scholar] [CrossRef] [PubMed]
- Lind, T. Metabolic changes in pregnancy relevant to diabetes mellitus. Postgrad. Med. J. 1979, 55, 353–357. [Google Scholar] [CrossRef][Green Version]
- Cipolla-Neto, J.; Amaral, F.G.; Afeche, S.C.; Tan, D.X.; Reiter, R.J. Melatonin, energy metabolism, and obesity: A review. J. Pineal Res. 2014, 56, 371–381. [Google Scholar] [CrossRef]
- Nakamura, Y.; Tamura, H.; Kashida, S.; Takayama, H.; Yamagata, Y.; Karube, A.; Sugino, N.; Kato, H. Changes of serum melatonin level and its relationship to feto-placental unit during pregnancy. J. Pineal Res. 2001, 30, 29–33. [Google Scholar] [CrossRef]
- de Cabo, R.; Mattson, M.P. Effects of Intermittent Fasting on Health, Aging, and Disease. N. Engl. J. Med. 2019, 381, 2541–2551. [Google Scholar] [CrossRef]
- Di Francesco, A.; Di Germanio, C.; Bernier, M.; de Cabo, R. A time to fast. Science 2018, 362, 770–775. [Google Scholar] [CrossRef]
- Safari, K.; Piro, T.J.; Ahmad, H.M. Perspectives and pregnancy outcomes of maternal Ramadan fasting in the second trimester of pregnancy. BMC Pregnancy Childbirth 2019, 19, 128. [Google Scholar] [CrossRef]
- Loy, S.L.; Chan, J.K.; Wee, P.H.; Colega, M.T.; Cheung, Y.B.; Godfrey, K.M.; Kwek, K.; Saw, S.M.; Chong, Y.S.; Natarajan, P.; et al. Maternal circadian eating time and frequency are associated with blood glucose concentrations during pregnancy. J. Nutr. 2017, 147, 70–77. [Google Scholar] [CrossRef]
- Metzger, B.E.; Freinkel, N. Accelerated starvation in pregnancy: Implications for dietary treatment of obesity and gestational diabetes mellitus. Biol. Neonate 1987, 51, 78–85. [Google Scholar] [CrossRef]
- Sacks, D.A.; Chen, W.; Wolde-Tsadik, G.; Buchanan, T.A. When is fasting really fasting? The influence of time of day, interval after a meal, and maternal body mass on maternal glycemia in gestational diabetes. Am. J. Obstet. Gynecol. 1999, 181, 904–911. [Google Scholar] [CrossRef]
- Peterson, C.M.; Jovanovic-Peterson, L. Percentage of carbohydrate and glycemic response to breakfast, lunch, and dinner in women with gestational diabetes. Diabetes 1991, 40, 172–174. [Google Scholar] [CrossRef]
- Deniz, C.D.; Ozler, S.; Sayin, F.K.; Eryilmaz, M.A. Associations between night eating syndrome and metabolic parameters in pregnant women. Turk. J. Obstet. Gynecol. 2019, 16, 107–111. [Google Scholar] [CrossRef]
- O’Reardon, J.P.; Peshek, A.; Allison, K.C. Night Eating Syndrome. CNS Drugs 2005, 19, 997–1008. [Google Scholar] [CrossRef] [PubMed]
- Van Cauter, E.; Shapiro, E.T.; Tillil, H.; Polonsky, K.S. Circadian modulation of glucose and insulin responses to meals: Relationship to cortisol rhythm. Am. J. Physiol. 1992, 262, E467–E475. [Google Scholar] [CrossRef]
- Jakubowicz, D.; Barnea, M.; Wainstein, J.; Froy, O. Effects of caloric intake timing on insulin resistance and hyperandrogenism in lean women with polycystic ovary syndrome. Clin. Sci. 2013, 125, 423–432. [Google Scholar] [CrossRef]
- Jakubowicz, D.; Barnea, M.; Wainstein, J.; Froy, O. High caloric intake at breakfast vs. dinner differentially influences weight loss of overweight and obese women. Obesity 2013, 21, 2504–2512. [Google Scholar] [CrossRef]
- Chandler-Laney, P.C.; Schneider, C.R.; Gower, B.A.; Granger, W.M.; Mancuso, M.S.; Biggio, J.R. Association of late-night carbohydrate intake with glucose tolerance among pregnant African American women. Matern. Child Nutr. 2016, 12, 688–698. [Google Scholar] [CrossRef]
- Loy, S.L.; Cheng, T.S.; Colega, M.T.; Cheung, Y.B.; Godfrey, K.M.; Gluckman, P.D.; Kwek, K.; Saw, S.M.; Chong, Y.S.; Padmapriya, N.; et al. Predominantly night-time feeding and maternal glycaemic levels during pregnancy. Br. J. Nutr. 2016, 115, 1563–1570. [Google Scholar] [CrossRef]
- Rasmussen, L.; Christensen, M.L.; Poulsen, C.W.; Rud, C.; Christensen, A.S.; Andersen, J.R.; Kampmann, U.; Ovesen, P.G. Effect of high versus low carbohydrate intake in the morning on glycemic variability and glycemic control measured by continuous blood glucose monitoring in women with gestational diabetes mellitus-A randomized crossover study. Nutrients 2020, 12, 475. [Google Scholar] [CrossRef]
- Aljuraiban, G.S.; Chan, Q.; Oude Griep, L.M.; Brown, I.J.; Daviglus, M.L.; Stamler, J.; Van Horn, L.; Elliott, P.; Frost, G.S.; Group, I.R. The impact of eating frequency and time of intake on nutrient quality and Body Mass Index: The INTERMAP Study, a Population-Based Study. J. Acad. Nutr. Diet. 2015, 115, 528–536.e521. [Google Scholar] [CrossRef]
- Ruidavets, J.B.; Bongard, V.; Bataille, V.; Gourdy, P.; Ferrieres, J. Eating frequency and body fatness in middle-aged men. Int. J. Obes. Relat. Metab. Disord. 2002, 26, 1476–1483. [Google Scholar] [CrossRef]
- Titan, S.M.; Bingham, S.; Welch, A.; Luben, R.; Oakes, S.; Day, N.; Khaw, K.T. Frequency of eating and concentrations of serum cholesterol in the Norfolk population of the European prospective investigation into cancer (EPIC-Norfolk): Cross sectional study. BMJ 2001, 323, 1286–1288. [Google Scholar] [CrossRef]
- Drummond, S.E.; Crombie, N.E.; Cursiter, M.C.; Kirk, T.R. Evidence that eating frequency is inversely related to body weight status in male, but not female, non-obese adults reporting valid dietary intakes. Int. J. Obes. Relat. Metab. Disord. 1998, 22, 105–112. [Google Scholar] [CrossRef]
- Arnold, L.M.; Ball, M.J.; Duncan, A.W.; Mann, J. Effect of isoenergetic intake of three or nine meals on plasma lipoproteins and glucose metabolism. Am. J. Clin. Nutr. 1993, 57, 446–451. [Google Scholar] [CrossRef]
- Solomon, T.P.; Chambers, E.S.; Jeukendrup, A.E.; Toogood, A.A.; Blannin, A.K. The effect of feeding frequency on insulin and ghrelin responses in human subjects. Br. J. Nutr. 2008, 100, 810–819. [Google Scholar] [CrossRef]
- Finkelstein, B.; Fryer, B.A. Meal frequency and weight reduction of young women. Am. J. Clin. Nutr. 1971, 24, 465–468. [Google Scholar] [CrossRef]
| Author, Year, Study Design | Population | Methods | CHO and EI Distribution, and Appetite | Metabolic Outcomes | Other Outcomes |
|---|---|---|---|---|---|
| Peterson et al., 1991 [66] Cross-sectional study | Females with OW and GDM in 3rd trimester (n = 14) | Dietary advice to consume 24 kcal/kg/d. Self-prepared 3 meals and 3 snacks per day. Energy distribution: B: 12.5%, L: 28%, D: 28%, two S: 10.5% each. | NA | ↑ correlation between CHO and 1 h pp glucose during dinner | NA |
| Sacks et al., 1999 [65] 1-Day randomized crossover trial | Females in 3rd trimester singleton with GDM (n = 16 OB, 14 NW; n = 23, 75% Hispanic) | 9 h mixed meal test after ≥5 h fasting AM meal test (07:00): 564 kcal, 51% CHO, 17% protein, 32% fat PM meal test (21:00): 567 kcal, 44% CHO, 26% protein, 30% fat | NA | AM vs. PM meal test ↑ 1 h pp glucose and insulin ↑ glucose decline rate Ø 2–9 h pp insulin, pp insulin/glucose ratio ↓ FFA 2–3 h and ↑ 5–9 h | NA |
| Chandler-Laney et al., 2016 [72] Cross-sectional | Healthy African American females in 3rd trimester singleton pregnancy (n = 20 NW, n = 20 OB) | 3-day food record verified in-person interview OGTT after >12 h overnight fast Daytime intake: 06:00–19:59 Nighttime intake: 20:00–05:59 | OB vs. NW Ø earliest and latest EI time Ø EI after 20:00 ↑ daily EI (640 kcal) trend for ↑ nighttime CHO% intake Ø total daily macronutrient composition | Both groups
OB group:
| NA |
| Loy et al., 2016 [73] Cross-sectional | Asian females in late 2nd trimester pregnancy (n = 534 NW, n = 451 OW) | Nutrition habits obtained using 24 h food recall. OGTT after overnight fast AM eater (n = 83): >50% EI 07:00–18:59 PM eater (n = 147): >50% EI 19:00–06:59 | PM vs. AM eating NW: ↓ total EI OW: ↓ %CHO trend for ↑ %fat | NW: PM eating associated with ↑ fasting glucose (2.5 mg/dL), but not 2 h OGTT glucose OW: PM eating not associated with fasting or 2 h OGTT glucose | Ø PA Ø Sleep duration |
| Loy et al., 2017 [63] Cross-sectional | Asian females in late 2nd trimester singleton pregnancy (n = 1061) | Nutrition habits obtained using 24 h food recall and 3-day food record. OGTT after 8–10 h overnight fast Night fasting: hours with no EI 19:00–06:59 EO: EI ≥50 kcal with ≥15 min apart | Participants with ↑ night fasting had: ↓ daily EI and EO
| Night fasting associated with: ↓ fasting glucose (9.1–10.9 vs. 11–12 h/day) Ø 2 h OGTT glucose Daily EO associated with: Ø fasting glucose ↑ 2 h pp glucose (4 vs. 5–10 EO/day) | Participants with ↑ night fasting:
|
| Deniz et al., 2019 [67] Cross-sectional study | OW females in 3rd trimester healthy singleton pregnancy (n = 17 NES, n = 131 non-NES) | NES score assessed with validated questionnaire Hunger assessed via interview Infant information obtained by medical record. | NES vs. Non-NES ↑ morning hunger, Ø night hunger ↑ B skipping | NES vs. Non-NES ↑ fasting insulin, HOMA-IR index, and HDL-c trend for ↑ fasting glucose and total cholesterol Ø BMI, LDL-c, HbA1c NES score correlated with fasting insulin, HOMA-IR, HbA1c, HDL-c | NES vs. Non-NES trend for ↑ fetal weight |
| Safari et al., 2019 [62] Prospective observational study | Females in 2nd trimester healthy pregnancy | All data collected via questionnaires and hospital records | NA | Ramadan Fasting vs. No fasting ↓ GDM prevalence | Ramadan Fasting vs. No fasting ↓ GWG (~0.5 kg) Ø pre-eclampsia, preterm labor, fetal birth weight or other fetal outcomes |
| Rasmussen et al., 2020 [74] 4-Day randomized crossover trial | NW females in 3rd trimester with GDM (n = 12) | Eucaloric meal intervention (46% CHO, 20% protein, 34% fat). Self-prepared 3 meals and 2 snacks per day High CHO and EI AM
| High CHO and EI AM vs. PM Ø daily EI, CHO and protein intake ↓ fat intake (9 g) | High CHO and EI AM vs. PM ↓ 4-day mean glucose (5.4 mg/dL) ↓ day 4 fasting glucose (9.0 mg/dL) ↑ mean glucose excursion (10.8 mg/dL) ↑ variation coefficient (5.2%) Ø change in insulin sensitivity, total, LDL-c, HDL-c, TG | NA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, S.; Surampudi, P.; Field, N.T.; Chondronikola, M. Meal Timing and Glycemic Control during Pregnancy—Is There a Link? Nutrients 2021, 13, 3379. https://doi.org/10.3390/nu13103379
Zhu S, Surampudi P, Field NT, Chondronikola M. Meal Timing and Glycemic Control during Pregnancy—Is There a Link? Nutrients. 2021; 13(10):3379. https://doi.org/10.3390/nu13103379
Chicago/Turabian StyleZhu, Shengjie, Prasanth Surampudi, Nancy T. Field, and Maria Chondronikola. 2021. "Meal Timing and Glycemic Control during Pregnancy—Is There a Link?" Nutrients 13, no. 10: 3379. https://doi.org/10.3390/nu13103379
APA StyleZhu, S., Surampudi, P., Field, N. T., & Chondronikola, M. (2021). Meal Timing and Glycemic Control during Pregnancy—Is There a Link? Nutrients, 13(10), 3379. https://doi.org/10.3390/nu13103379






