Targeting Probiotics in Rheumatoid Arthritis
Abstract
:1. Introduction
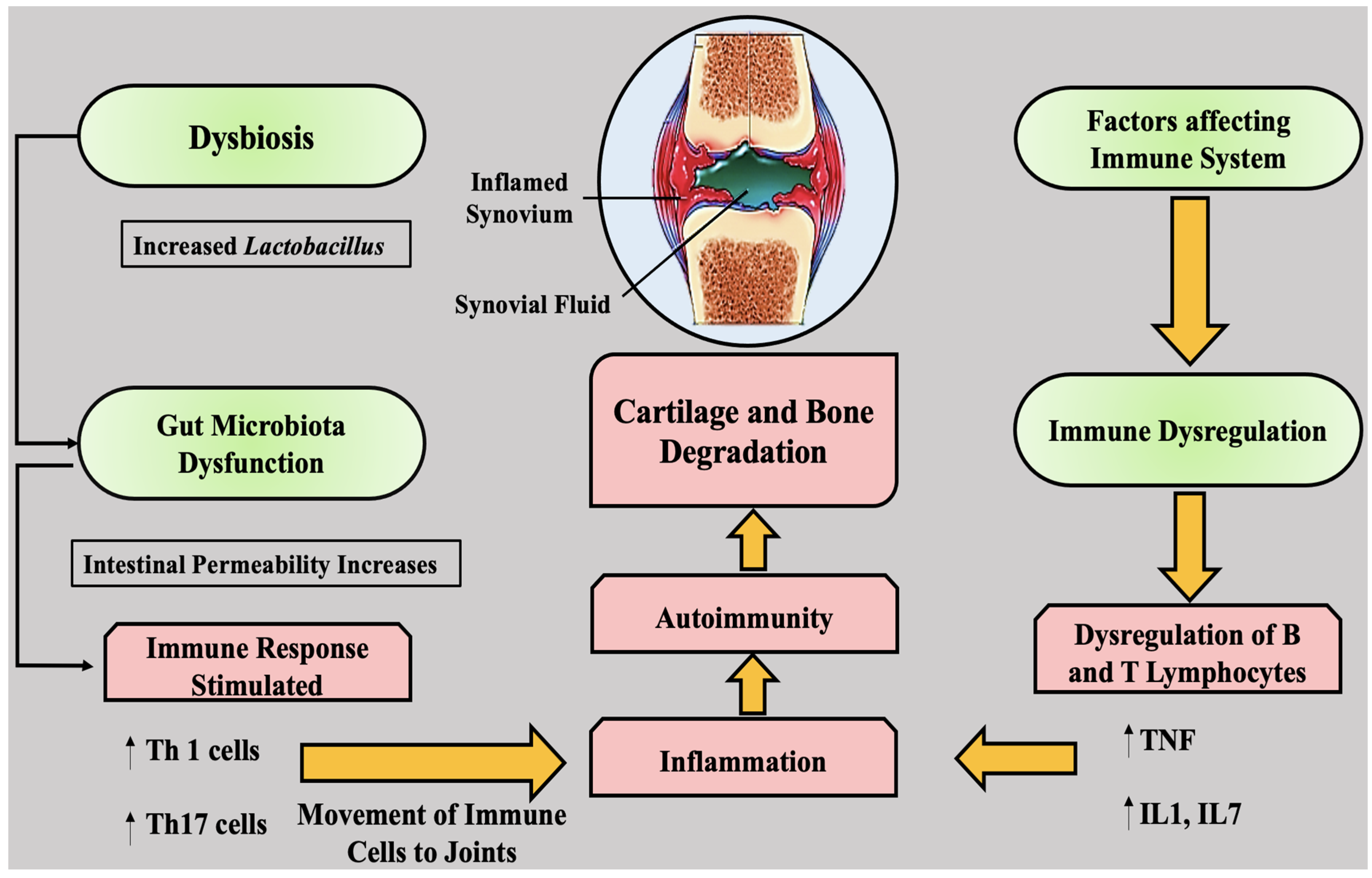
2. An Inflammatory Pathway in Rheumatoid Arthritis
3. Probiotics and Their Interventions in RA
- Keeping an equilibrium of “beneficial” and “harmful” bacteria in the body;
- Diminishing harmful bacteria that can cause allergies and ailments;
- Renewing beneficial bacteria that have been lost post sickness.
- Accelerating the treatment of some intestinal infections;
- Assisting in the reduction of gas and bloating;
- Preventing or minimizing the severity of colds and flu;
- Improving blood pressure;
- Relieving symptoms of inflammatory bowel illnesses such as Crohn’s disease and ulcerative colitis.
3.1. Probiotics Selection
3.2. Role of Diet in RA
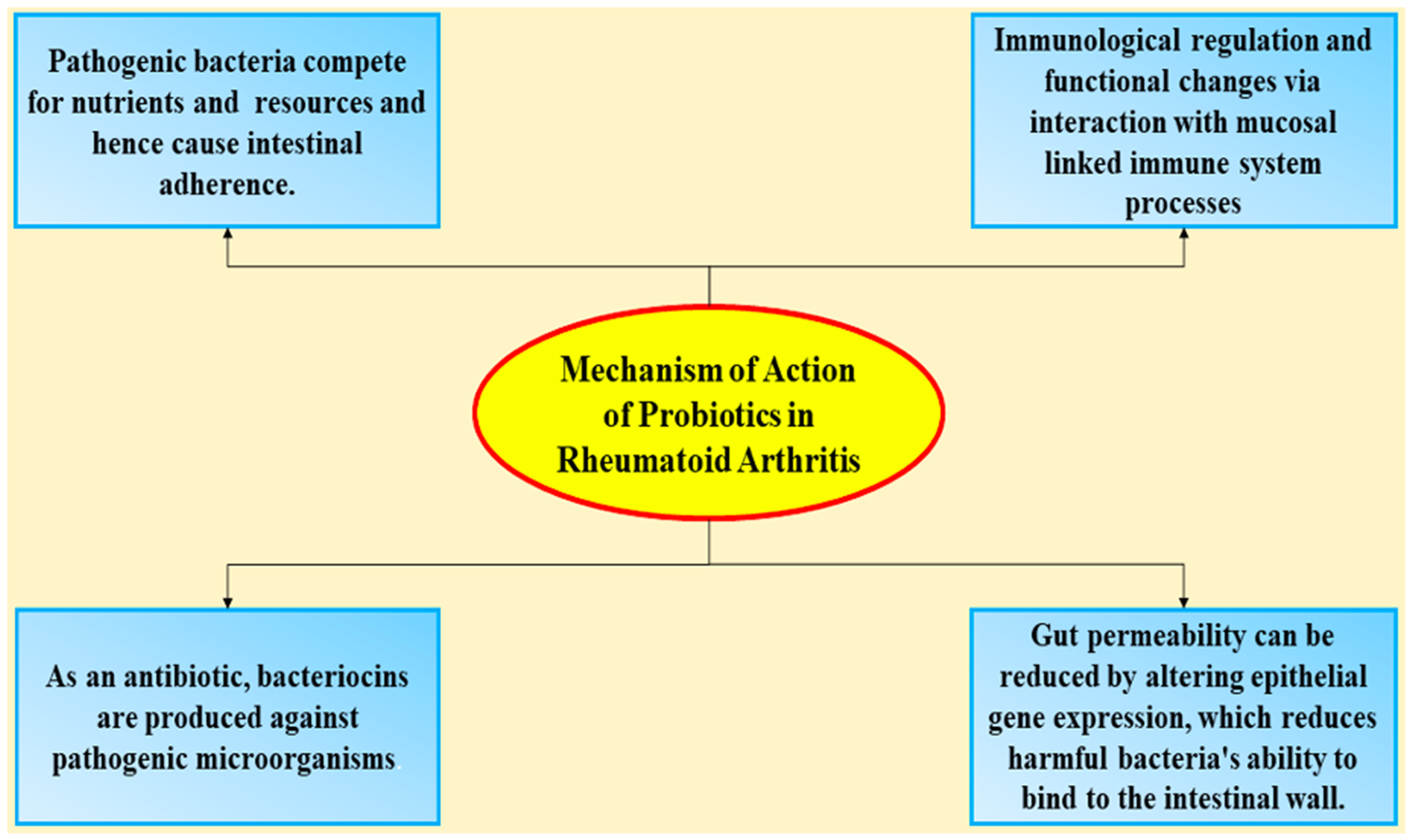
4. Mechanism of Action of Probiotics in Rheumatoid Arthritis
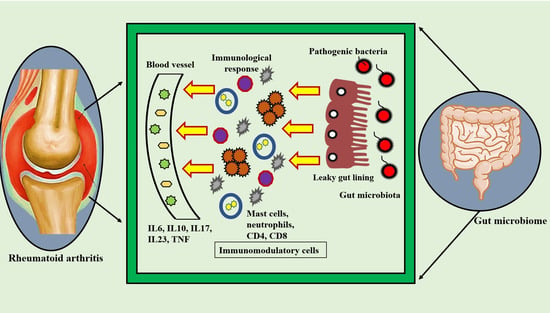
5. Rheumatoid Arthritis and Gut Microbiota
5.1. Bacillus coagulans in Rheumatoid Arthritis
5.2. Preclinical Studies on the Effects of Lactobacillus Probiotics on Arthritis
5.3. Clinic Applications of Lactobacillus Probiotics in the Treatment of Rheumatoid Arthritis
6. Probiotics’ Efficacy in the Treatment of Rheumatoid Arthritis
7. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Wang, W.; Zhou, H.; Liu, L. Side effects of methotrexate therapy for rheumatoid arthritis: A systematic review. Eur. J. Med. Chem. 2018, 158, 502–516. [Google Scholar] [CrossRef]
- Tilstra, J.S.; Lienesch, D.W. Rheumatoid nodules. Dermatol. Clin. 2015, 33, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Sepriano, A.; Kerschbaumer, A.; Smolen, J.S.; Van Der Heijde, D.; Dougados, M.; Van Vollenhoven, R.; McInnes, I.B.; Bijlsma, J.W.; Burmester, G.R.; De Wit, M. Safety of synthetic and biological DMARDs: A systematic literature review informing the 2019 update of the EULAR recommendations for the management of rheumatoid arthritis. Ann. Rheum. Dis. 2020, 79, 760–770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schrezenmeier, E.; Dörner, T. Mechanisms of action of hydroxychloroquine and chloroquine: Implications for rheumatology. Nat. Rev. Rheumatol. 2020, 16, 155–166. [Google Scholar] [CrossRef]
- Reis, E.T.d.; Kakehasi, A.M.; Pinheiro, M.d.M.; Ferreira, G.A.; Marques, C.D.L.; Mota, L.M.H.d.; Paiva, E.d.S.; Pileggi, G.C.S.; Sato, E.I.; Reis, A.P.M.G. Revisiting hydroxychloroquine and chloroquine for patients with chronic immunity-mediated inflammatory rheumatic diseases. Adv. Rheumatol. 2020, 60, 32. [Google Scholar] [CrossRef]
- Jorge, A.; Ung, C.; Young, L.H.; Melles, R.B.; Choi, H.K. Hydroxychloroquine retinopathy—implications of research advances for rheumatology care. Nat. Rev. Rheumatol. 2018, 14, 693–703. [Google Scholar] [CrossRef]
- Stokkermans, T.J.; Goyal, A.; Bansal, P.; Trichonas, G. Chloroquine and Hydroxychloroquine Toxicity; StatPearls Publishing: Treasure Island, FL, USA, 2019. [Google Scholar]
- Gerriets, V.; Bansal, P.; Goyal, A.; Khaddour, K. Tumor Necrosis Factor Inhibitors; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Zelová, H.; Hošek, J. TNF-α signalling and inflammation: Interactions between old acquaintances. Inflamm. Res. 2013, 62, 641–651. [Google Scholar] [CrossRef]
- Emery, P.; Vlahos, B.; Szczypa, P.; Thakur, M.; Jones, H.E.; Woolcott, J.; Estrella, P.V.S.; Rolland, C.; Gibofsky, A.; Citera, G. Longterm drug survival of tumor necrosis factor inhibitors in patients with rheumatoid arthritis. J. Rheumatol. 2020, 47, 493–501. [Google Scholar] [CrossRef]
- Reed, G.W.; Gerber, R.A.; Shan, Y.; Takiya, L.; Dandreo, K.J.; Gruben, D.; Kremer, J.; Wallenstein, G. Real-world comparative effectiveness of Tofacitinib and tumor necrosis factor inhibitors as monotherapy and combination therapy for treatment of rheumatoid arthritis. Rheumatol. Ther. 2019, 6, 573–586. [Google Scholar] [CrossRef] [Green Version]
- Furst, D.E. The risk of infections with biologic therapies for rheumatoid arthritis. Semin. Arthritis Rheum. 2010, 39, 327–346. [Google Scholar] [CrossRef]
- Pérez-Sola, M.J.; Torre-Cisneros, J.; Perez-Zafrilla, B.; Carmona, L.; Descalzo, M.A.; Gómez-Reino, J.J.; Group, B.S. Infections in patients treated with tumor necrosis factor antagonists: Incidence, etiology and mortality in the BIOBADASER registry. Med. Clin. 2011, 137, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Lindhaus, C.; Tittelbach, J.; Elsner, P. Cutaneous side effects of TNF-alpha inhibitors. JDDG J. Der Dtsch. Dermatol. Ges. 2017, 15, 281–288. [Google Scholar] [CrossRef]
- Cencic, A.; Chingwaru, W. The Role of Functional Foods, Nutraceuticals, and Food Supplements in Intestinal Health. Nutrients 2010, 2, 611–625. [Google Scholar] [CrossRef] [PubMed]
- Krathen, M.S.; Gottlieb, A.B.; Mease, P.J. Pharmacologic immunomodulation and cutaneous malignancy in rheumatoid arthritis, psoriasis, and psoriatic arthritis. J. Rheumatol. 2010, 37, 2205–2215. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Friedman, M.; Liu, G.; Deodhar, A.; Chu, C.-Q. Do tumor necrosis factor inhibitors increase cancer risk in patients with chronic immune-mediated inflammatory disorders? Cytokine 2018, 101, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Raaschou, P.; Soderling, J.; Turesson, C.; Askling, J.; ARTIS Study Group. Tumor necrosis factor inhibitors and cancer recurrence in Swedish patients with rheumatoid arthritis: A nationwide population-based cohort study. Ann. Intern. Med. 2018, 169, 9. [Google Scholar] [CrossRef]
- McFarland, L.V. From yaks to yogurt: The history, development, and current use of probiotics. Clin. Infect. Dis. 2015, 60, S85–S90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gómez-Llorente, C.; Muñoz, S.; Gil, A. Role of Toll-like receptors in the development of immunotolerance mediated by probiotics. Proc. Nutr. Soc. 2010, 69, 381–389. [Google Scholar] [CrossRef]
- Malysheva, O.; Baerwald, C.G. Low-dose corticosteroids and disease-modifying drugs in patients with rheumatoid arthritis. Clin. Exp. Rheumatol.-Incl Suppl. 2011, 29, S113. [Google Scholar]
- Petta, I.; Peene, I.; Elewaut, D.; Vereecke, L.; De Bosscher, K. Risks and benefits of corticosteroids in arthritic diseases in the clinic. Biochem. Pharmacol. 2019, 165, 112–125. [Google Scholar] [CrossRef]
- Zamora, N.V.; Christensen, R.; Goel, N.; Klokker, L.; Lopez-Olivo, M.A.; Kristensen, L.E.; Carmona, L.; Strand, V.; Curtis, J.R.; Suarez-Almazor, M.E. Critical Outcomes in Longitudinal Observational Studies and Registries in Patients with Rheumatoid Arthritis: An OMERACT Special Interest Group Report. J. Rheumatol. 2017, 44, 1894–1898. [Google Scholar] [CrossRef] [PubMed]
- Oliviero, F.; Spinella, P.; Fiocco, U.; Ramonda, R.; Sfriso, P.; Punzi, L. How the Mediterranean diet and some of its components modulate inflammatory pathways in arthritis. Swiss Med. Wkly. 2015, 145, w14190. [Google Scholar] [CrossRef]
- Draper, K.; Ley, C.; Parsonnet, J. A survey of probiotic use practices among patients at a tertiary medical centre. Benef Microbes. 2017, 8, 345–351. [Google Scholar] [CrossRef]
- Aletaha, D.; Smolen, J.S. Diagnosis and management of rheumatoid arthritis: A review. JAMA 2018, 320, 1360–1372. [Google Scholar] [CrossRef]
- Cruz-Topete, D.; Cidlowski, J.A. One hormone, two actions: Anti-and pro-inflammatory effects of glucocorticoids. Neuroimmunomodulation 2015, 22, 20–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandel, D.R.; Eichas, K.; Holmes, J. Bacillus coagulans: A viable adjunct therapy for relieving symptoms of rheumatoid arthritis according to a randomized, controlled trial. BMC Complement. Altern. Med. 2010, 10, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de los Angeles Pineda, M.; Thompson, S.F.; Summers, K.; de Leon, F.; Pope, J.; Reid, G. A randomized, double-blinded, placebo-controlled pilot study of probiotics in active rheumatoid arthritis. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2011, 17, CR347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strowski, M.Z.; Wiedenmann, B. Probiotic carbohydrates reduce intestinal permeability and inflammation in metabolic diseases. Gut 2009, 58, 1044–1045. [Google Scholar] [CrossRef]
- Keller, D.; Farmer, S.; McCartney, A.; Gibson, G. Bacillus coagulansas a probiotic. Food Sci. Technol. Bull. 2011, 7, 103–109. [Google Scholar] [CrossRef]
- Needle, C. Gut Instinct: AIDS healthcare foundation trains a research lens on probiotics. A&U Magazine, 6 November 2010. [Google Scholar]
- Wang, P.; Tao, J.-H.; Pan, H.-F. Probiotic bacteria: A viable adjuvant therapy for relieving symptoms of rheumatoid arthritis. Inflammopharmacology 2016, 24, 189–196. [Google Scholar] [CrossRef]
- Hagen, K.B.; Byfuglien, M.G.; Falzon, L.; Olsen, S.U.; Smedslund, G. Dietary interventions for rheumatoid arthritis. Cochrane Database Syst Rev. 2009, 1, CD006400. [Google Scholar] [CrossRef]
- Alipour, B.; Homayouni-Rad, A.; Vaghef-Mehrabany, E.; Sharif, S.K.; Vaghef-Mehrabany, L.; Asghari-Jafarabadi, M.; Nakhjavani, M.R.; Mohtadi-Nia, J. Effects of L actobacillus casei supplementation on disease activity and inflammatory cytokines in rheumatoid arthritis patients: A randomized double-blind clinical trial. Int. J. Rheum. Dis. 2014, 17, 519–527. [Google Scholar] [PubMed]
- Schorpion, A.; Kolasinski, S.L. Can probiotic supplements improve outcomes in rheumatoid arthritis? Curr. Rheumatol. Rep. 2017, 19, 1–7. [Google Scholar] [CrossRef]
- Vaghef-Mehrabany, E.; Alipour, B.; Homayouni-Rad, A.; Sharif, S.-K.; Asghari-Jafarabadi, M.; Zavvari, S. Probiotic supplementation improves inflammatory status in patients with rheumatoid arthritis. Nutrition 2014, 30, 430–435. [Google Scholar] [CrossRef]
- Vaghef-Mehrabany, E.; Homayouni-Rad, A.; Alipour, B.; Sharif, S.-K.; Vaghef-Mehrabany, L.; Alipour-Ajiry, S. Effects of probiotic supplementation on oxidative stress indices in women with rheumatoid arthritis: A randomized double-blind clinical trial. J. Am. Coll. Nutr. 2016, 35, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A.T.; Khattab, M.; Ahmed, A.M.; Turk, T.; Sakr, N.; Khalil, A.M.; Abdelhalim, M.; Sawaf, B.; Hirayama, K.; Huy, N.T. The therapeutic effect of probiotics on rheumatoid arthritis: A systematic review and meta-analysis of randomized control trials. Clin. Rheumatol. 2017, 36, 2697–2707. [Google Scholar] [CrossRef]
- Bedaiwi, M.K.; Inman, R.D. Microbiome and probiotics: Link to arthritis. Curr. Opin. Rheumatol. 2014, 26, 410–415. [Google Scholar] [CrossRef]
- Abdollahi-Roodsaz, S.; Abramson, S.B.; Scher, J.U. The metabolic role of the gut microbiota in health and rheumatic disease: Mechanisms and interventions. Nat. Rev. Rheumatol. 2016, 12, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-E.; Chae, C.S.; Kim, G.-C.; Hwang, W.; Hwang, J.-s.; Hwang, S.-M.; Kim, Y.; Ahn, Y.-T.; Park, S.-G.; Jun, C.-D.; et al. Lactobacillus helveticus suppresses experimental rheumatoid arthritis by reducing inflammatory T cell responses. J. Funct. Foods 2015, 13, 350–362. [Google Scholar] [CrossRef]
- Marietta, E.V.; Murray, J.A.; Luckey, D.H.; Jeraldo, P.R.; Lamba, A.; Patel, R.; Luthra, H.S.; Mangalam, A.; Taneja, V. Suppression of inflammatory arthritis by human gut-derived Prevotella histicola in humanized mice. Arthritis Rheumatol. 2016, 68, 2878–2888. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Feng, X.; Tan, W.; Gu, W.; Guo, D.; Zhang, M.; Wang, F. IL-29 enhances Toll-like receptor-mediated IL-6 and IL-8 production by the synovial fibroblasts from rheumatoid arthritis patients. Arthritis Res. Ther. 2013, 15, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Thompson, C.; Davies, R.; Choy, E. Anti cytokine therapy in chronic inflammatory arthritis. Cytokine 2016, 86, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, T. Discovery of IL-6 and development of anti-IL-6R antibody. Keio J. Med. 2019, 68, 96. [Google Scholar] [CrossRef]
- Alam, J.; Jantan, I.; Bukhari, S.N.A. Rheumatoid arthritis: Recent advances on its etiology, role of cytokines and pharmacotherapy. Biomed. Pharmacother. 2017, 92, 615–633. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, A.; Bagchi, A.; De, S.; Mitra, S.; Mukherjee, S.; Ghosh, P.; Ghosh, A.; Chatterjee, M. Role of redox imbalance and cytokines in mediating oxidative damage and disease progression of patients with rheumatoid arthritis. Free Radic. Res. 2019, 53, 768–779. [Google Scholar] [CrossRef] [PubMed]
- González-Rodríguez, I.; Sánchez, B.; Ruiz, L.; Turroni, F.; Ventura, M.; Ruas-Madiedo, P.; Gueimonde, M.; Margolles, A. Role of extracellular transaldolase from Bifidobacterium bifidum in mucin adhesion and aggregation. Appl. Environ. Microbiol. 2012, 78, 3992–3998. [Google Scholar] [CrossRef] [Green Version]
- Villena, J.; Barbieri, N.; Salva, S.; Herrera, M.; Alvarez, S. Enhanced immune response to pneumococcal infection in malnourished mice nasally treated with heat-killed Lactobacillus casei. Microbiol. Immunol. 2009, 53, 636–646. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, H.; Taniguchi, A.; Tsuboi, H.; Kano, H.; Asami, Y. Hypouricaemic effects of yoghurt containing Lactobacillus gasseri PA-3 in patients with hyperuricaemia and/or gout: A randomised, double-blind, placebo-controlled study. Mod. Rheumatol. 2019, 29, 146–150. [Google Scholar] [CrossRef]
- de Oliveira, G.L.V.; Leite, A.Z.; Higuchi, B.S.; Gonzaga, M.I.; Mariano, V.S. Intestinal dysbiosis and probiotic applications in autoimmune diseases. Immunology. 2017, 152, 1–12. [Google Scholar] [CrossRef]
- Chen, J.; Wright, K.; Davis, J.M.; Jeraldo, P.; Marietta, E.V.; Murray, J.; Nelson, H.; Matteson, E.L.; Taneja, V. An expansion of rare lineage intestinal microbes characterizes rheumatoid arthritis. Genome Med. 2016, 8, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Amdekar, S.; Singh, V.; Singh, R.; Sharma, P.; Keshav, P.; Kumar, A. Lactobacillus casei reduces the inflammatory joint damage associated with collagen-induced arthritis (CIA) by reducing the pro-inflammatory cytokines: Lactobacillus casei: COX-2 inhibitor. J. Clin. Immunol. 2011, 31, 147–154. [Google Scholar] [CrossRef]
- Azad, M.A.K.; Sarker, M.; Wan, D. Immunomodulatory Effects of Probiotics on Cytokine Profiles. Biomed. Res. Int. 2018, 2018, 8063647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Achi, S.C.; Talahalli, R.R.; Halami, P.M. Prophylactic effects of probiotic Bifidobacterium spp. in the resolution of inflammation in arthritic rats. Appl. Microbiol. Biotechnol. 2019, 103, 6287–6296. [Google Scholar] [CrossRef]
- Derrien, M.; Veiga, P. Rethinking Diet to Aid Human-Microbe Symbiosis. Trends Microbiol. 2017, 25, 100–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deehan, E.C.; Walter, J. The Fiber Gap and the Disappearing Gut Microbiome: Implications for Human Nutrition. Trends Endocrinol. Metab. 2016, 27, 239–242. [Google Scholar] [CrossRef]
- Hu, Y.; Sparks, J.A.; Malspeis, S.; Costenbader, K.H.; Hu, F.B.; Karlson, E.W.; Lu, B. Long-term dietary quality and risk of developing rheumatoid arthritis in women. Ann. Rheum. Dis. 2017, 76, 1357–1364. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Lee, N.K.; Lee, S.Y. Current understanding of RANK signaling in osteoclast differentiation and maturation. Mol. Cells 2017, 40, 706. [Google Scholar] [PubMed] [Green Version]
- Hatano, Y.; Matsuoka, H.; Lam, L.; Currow, D.C. Side effects of corticosteroids in patients with advanced cancer: A systematic review. Support. Care Cancer 2018, 26, 3979–3983. [Google Scholar] [CrossRef]
- Mateen, S.; Zafar, A.; Moin, S.; Khan, A.Q.; Zubair, S. Understanding the role of cytokines in the pathogenesis of rheumatoid arthritis. Clin. Chim. Acta 2016, 455, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Goh, Y.J.; Klaenhammer, T.R. Functional roles of aggregation-promoting-like factor in stress tolerance and adherence of Lactobacillus acidophilus NCFM. Appl. Environ. Microbiol. 2010, 76, 5005–5012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez, B.; González-Tejedo, C.; Ruas-Madiedo, P.; Urdaci, M.C.; Margolles, A. Lactobacillus plantarum extracellular chitin-binding protein and its role in the interaction between chitin, Caco-2 cells, and mucin. Appl. Environ. Microbiol. 2011, 77, 1123–1126. [Google Scholar] [CrossRef] [Green Version]
- von Ossowski, I.; Reunanen, J.; Satokari, R.; Vesterlund, S.; Kankainen, M.; Huhtinen, H.; Tynkkynen, S.; Salminen, S.; de Vos, W.M.; Palva, A. Mucosal adhesion properties of the probiotic Lactobacillus rhamnosus GG SpaCBA and SpaFED pilin subunits. Appl. Environ. Microbiol. 2010, 76, 2049–2057. [Google Scholar] [CrossRef] [Green Version]
- von Ossowski, I.; Satokari, R.; Reunanen, J.; Lebeer, S.; De Keersmaecker, S.C.; Vanderleyden, J.; de Vos, W.M.; Palva, A. Functional characterization of a mucus-specific LPXTG surface adhesin from probiotic Lactobacillus rhamnosus GG. Appl. Environ. Microbiol. 2011, 77, 4465–4472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Candela, M.; Centanni, M.; Fiori, J.; Biagi, E.; Turroni, S.; Orrico, C.; Bergmann, S.; Hammerschmidt, S.; Brigidi, P. DnaK from Bifidobacterium animalis subsp. lactis is a surface-exposed human plasminogen receptor upregulated in response to bile salts. Microbiology 2010, 156, 1609–1618. [Google Scholar] [CrossRef] [Green Version]
- La Fata, G.; Weber, P.; Mohajeri, M.H. Probiotics and the Gut Immune System: Indirect Regulation. Probiotics Antimicrob. Proteins 2018, 10, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Kalinkovich, A.; Livshits, G. A cross talk between dysbiosis and gut-associated immune system governs the development of inflammatory arthropathies. Semin. Arthritis Rheum. 2019, 49, 474–484. [Google Scholar] [CrossRef]
- Plaza-Diaz, J.; Ruiz-Ojeda, F.J.; Gil-Campos, M.; Gil, A. Mechanisms of Action of Probiotics. Adv. Nutr. 2019, 10, S49–S66. [Google Scholar] [CrossRef] [Green Version]
- Van Spaendonk, H.; Ceuleers, H.; Witters, L.; Patteet, E.; Joossens, J.; Augustyns, K.; Lambeir, A.M.; De Meester, I.; De Man, J.G.; De Winter, B.Y. Regulation of intestinal permeability: The role of proteases. World J. Gastroenterol. 2017, 23, 2106–2123. [Google Scholar] [CrossRef] [PubMed]
- Volkov, M.; van Schie, K.A.; van der Woude, D. Autoantibodies and B Cells: The ABC of rheumatoid arthritis pathophysiology. Immunol. Rev. 2020, 294, 148–163. [Google Scholar] [CrossRef] [Green Version]
- Amdekar, S.; Singh, V.; Kumar, A.; Sharma, P.; Singh, R. Lactobacillus casei and Lactobacillus acidophilus regulate inflammatory pathway and improve antioxidant status in collagen-induced arthritic rats. J. Interferon Cytokine Res. 2013, 33, 1–8. [Google Scholar] [CrossRef] [PubMed]
- van Hamburg, J.P.; Tas, S.W. Molecular mechanisms underpinning T helper 17 cell heterogeneity and functions in rheumatoid arthritis. J. Autoimmun. 2018, 87, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-Q.; Szodoray, P.; Zeher, M. Toll-like receptor pathways in autoimmune diseases. Clin. Rev. Allergy Immunol. 2016, 50, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piccinini, A.M.; Williams, L.; McCann, F.E.; Midwood, K.S. Investigating the role of Toll-like receptors in models of arthritis. In Toll-Like Receptors; Springer: Berlin/Heidelberg, Germany, 2016; pp. 351–381. [Google Scholar]
- Elshabrawy, H.A.; Essani, A.E.; Szekanecz, Z.; Fox, D.A.; Shahrara, S. TLRs, future potential therapeutic targets for RA. Autoimmun. Rev. 2017, 16, 103–113. [Google Scholar] [CrossRef] [Green Version]
- McGarry, T.; Veale, D.J.; Gao, W.; Orr, C.; Fearon, U.; Connolly, M. Toll-like receptor 2 (TLR2) induces migration and invasive mechanisms in rheumatoid arthritis. Arthritis Res. Ther. 2015, 17, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Ramezani, A.; Raj, D.S. The gut microbiome, kidney disease, and targeted interventions. J. Am. Soc. Nephrol. 2014, 25, 657–670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dias Bastos, P.A.; Lara Santos, L.; Pinheiro Vitorino, R.M. How are the expression patterns of gut antimicrobial peptides modulated by human gastrointestinal diseases? A bridge between infectious, inflammatory, and malignant diseases. J. Pept. Sci. 2018, 24, e3071. [Google Scholar] [CrossRef]
- Boulangé, C.L.; Neves, A.L.; Chilloux, J.; Nicholson, J.K.; Dumas, M.-E. Impact of the gut microbiota on inflammation, obesity, and metabolic disease. Genome Med. 2016, 8, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Kau, A.L.; Ahern, P.P.; Griffin, N.W.; Goodman, A.L.; Gordon, J.I. Human nutrition, the gut microbiome and the immune system. Nature 2011, 474, 327–336. [Google Scholar] [CrossRef] [Green Version]
- Haber, A.L.; Biton, M.; Rogel, N.; Herbst, R.H.; Shekhar, K.; Smillie, C.; Burgin, G.; Delorey, T.M.; Howitt, M.R.; Katz, Y. A single-cell survey of the small intestinal epithelium. Nature 2017, 551, 333–339. [Google Scholar] [CrossRef]
- Bodkhe, R.; Balakrishnan, B.; Taneja, V. The role of microbiome in rheumatoid arthritis treatment. Ther. Adv. Musculoskelet. Dis. 2019, 11, 1759720X19844632. [Google Scholar] [CrossRef]
- Manasson, J.; Blank, R.B.; Scher, J.U. The microbiome in rheumatology: Where are we and where should we go? Ann. Rheum. Dis. 2020, 79, 727–733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rouhani, S.; Griffin, N.W.; Yori, P.P.; Gehrig, J.L.; Olortegui, M.P.; Salas, M.S.; Trigoso, D.R.; Moulton, L.H.; Houpt, E.R.; Barratt, M.J. Diarrhea as a potential cause and consequence of reduced gut microbial diversity among undernourished children in Peru. Clin. Infect. Dis. 2020, 71, 989–999. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.K.; Paul, A.; Jahan, R.; Jannat, K.; Bondhon, T.A.; Hasan, A.; Nissapatorn, V.; Pereira, M.L.; Wilairatana, P.; Rahmatullah, M. Probiotics and Amelioration of Rheumatoid Arthritis: Significant Roles of Lactobacillus casei and Lactobacillus acidophilus. Microorganisms 2021, 9, 1070. [Google Scholar] [CrossRef]
- Huang, Z.; Stabler, T.; Pei, F.; Kraus, V.B. Both systemic and local lipopolysaccharide (LPS) burden are associated with knee OA severity and inflammation. Osteoarthr. Cartil. 2016, 24, 1769–1775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pretorius, E.; Akeredolu, O.-O.; Soma, P.; Kell, D.B. Major involvement of bacterial components in rheumatoid arthritis and its accompanying oxidative stress, systemic inflammation and hypercoagulability. Exp. Biol. Med. 2017, 242, 355–373. [Google Scholar] [CrossRef]
- Alpizar-Rodriguez, D.; Lesker, T.R.; Gronow, A.; Gilbert, B.; Raemy, E.; Lamacchia, C.; Gabay, C.; Finckh, A.; Strowig, T. Prevotella copri in individuals at risk for rheumatoid arthritis. Ann. Rheum. Dis. 2019, 78, 590–593. [Google Scholar] [CrossRef]
- Scher, J.U.; Sczesnak, A.; Longman, R.S.; Segata, N.; Ubeda, C.; Bielski, C.; Rostron, T.; Cerundolo, V.; Pamer, E.G.; Abramson, S.B. Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. eLife 2013, 2, e01202. [Google Scholar] [CrossRef]
- Maeda, Y.; Kurakawa, T.; Umemoto, E.; Motooka, D.; Ito, Y.; Gotoh, K.; Hirota, K.; Matsushita, M.; Furuta, Y.; Narazaki, M. Dysbiosis contributes to arthritis development via activation of autoreactive T cells in the intestine. Arthritis Rheumatol. 2016, 68, 2646–2661. [Google Scholar] [CrossRef]
- Wells, P.M.; Adebayo, A.S.; Bowyer, R.C.; Freidin, M.B.; Finckh, A.; Strowig, T.; Lesker, T.R.; Alpizar-Rodriguez, D.; Gilbert, B.; Kirkham, B. Associations between gut microbiota and genetic risk for rheumatoid arthritis in the absence of disease: A cross-sectional study. Lancet Rheumatol. 2020, 2, e418–e427. [Google Scholar] [CrossRef]
- Pianta, A.; Arvikar, S.; Strle, K.; Drouin, E.E.; Wang, Q.; Costello, C.E.; Steere, A.C. Evidence of the immune relevance of Prevotella copri, a gut microbe, in patients with rheumatoid arthritis. Arthritis Rheumatol. 2017, 69, 964–975. [Google Scholar] [CrossRef] [Green Version]
- Larsen, J.M. The immune response to Prevotella bacteria in chronic inflammatory disease. Immunology 2017, 151, 363–374. [Google Scholar] [CrossRef] [Green Version]
- Balakrishnan, B.; Luckey, D.; Taneja, V. Autoimmunity-associated gut commensals modulate gut permeability and immunity in humanized mice. Mil. Med. 2019, 184, 529–536. [Google Scholar] [CrossRef] [Green Version]
- Ferreira-Halder, C.V.; de Sousa Faria, A.V.; Andrade, S.S. Action and function of Faecalibacterium prausnitzii in health and disease. Best Pract. Res. Clin. Gastroenterol. 2017, 31, 643–648. [Google Scholar] [CrossRef]
- Chu, X.-J.; Cao, N.-W.; Zhou, H.-Y.; Meng, X.; Guo, B.; Zhang, H.-Y.; Li, B.-Z. The oral and gut microbiome in rheumatoid arthritis patients: A systematic review. Rheumatology 2021, 60, 1054–1066. [Google Scholar] [CrossRef]
- Möller, B.; Kollert, F.; Sculean, A.; Villiger, P.M. Infectious triggers in periodontitis and the gut in rheumatoid arthritis (RA): A complex story about association and causality. Front. Immunol. 2020, 11, 1108. [Google Scholar] [CrossRef] [PubMed]
- van Vollenhoven, R.F. Sex differences in rheumatoid arthritis: More than meets the eye. BMC Med. 2009, 7, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Sapir-Koren, R.; Livshits, G. Postmenopausal osteoporosis in rheumatoid arthritis: The estrogen deficiency-immune mechanisms link. Bone 2017, 103, 102–115. [Google Scholar] [CrossRef] [PubMed]
- Tit, D.M.; Bungau, S.; Iovan, C.; Cseppento, D.C.N.; Endres, L.; Sava, C.; Sabau, A.M.; Furau, G.; Furau, C. Effects of the Hormone Replacement Therapy and of Soy Isoflavones on Bone Resorption in Postmenopause. J. Clin. Med. 2018, 7, 297. [Google Scholar] [CrossRef] [Green Version]
- Tit, D.M.; Pallag, A.; Iovan, C.; Furau, G.; Furau, C.; Bungau, S. Somatic-vegetative Symptoms Evolution in Postmenopausal Women Treated with Phytoestrogens and Hormone Replacement Therapy. Iran. J. Public Health 2017, 46, 1528–1534. [Google Scholar] [PubMed]
- Favalli, E.G.; Biggioggero, M.; Crotti, C.; Becciolini, A.; Raimondo, M.G.; Meroni, P.L. Sex and management of rheumatoid arthritis. Clin. Rev. Allergy Immunol. 2019, 56, 333–345. [Google Scholar] [CrossRef] [PubMed]
- Islander, U.; Jochems, C.; Lagerquist, M.K.; Forsblad-d’Elia, H.; Carlsten, H. Estrogens in rheumatoid arthritis; the immune system and bone. Mol. Cell. Endocrinol. 2011, 335, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Fert-Bober, J.; Darrah, E.; Andrade, F. Insights into the study and origin of the citrullinome in rheumatoid arthritis. Immunol. Rev. 2020, 294, 133–147. [Google Scholar] [CrossRef] [PubMed]
- Aletaha, D.; Martinez-Avila, J.; Kvien, T.K.; Smolen, J.S. Definition of treatment response in rheumatoid arthritis based on the simplified and the clinical disease activity index. Ann. Rheum Dis. 2012, 71, 1190–1196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, S.W.; Kim, J.H.; Bae, H.J.; Ham, J.S.; Yoo, J.G.; Chung, K.S.; Oh, M.H. Selection and characterization of broad-spectrum antibacterial substance-producing Lactobacillus curvatus PA 40 as a potential probiotic for feed additives. Anim. Sci. J. 2018, 89, 1459–1467. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bungau, S.G.; Behl, T.; Singh, A.; Sehgal, A.; Singh, S.; Chigurupati, S.; Vijayabalan, S.; Das, S.; Palanimuthu, V.R. Targeting Probiotics in Rheumatoid Arthritis. Nutrients 2021, 13, 3376. https://doi.org/10.3390/nu13103376
Bungau SG, Behl T, Singh A, Sehgal A, Singh S, Chigurupati S, Vijayabalan S, Das S, Palanimuthu VR. Targeting Probiotics in Rheumatoid Arthritis. Nutrients. 2021; 13(10):3376. https://doi.org/10.3390/nu13103376
Chicago/Turabian StyleBungau, Simona Gabriela, Tapan Behl, Anuja Singh, Aayush Sehgal, Sukhbir Singh, Sridevi Chigurupati, Shantini Vijayabalan, Suprava Das, and Vasanth Raj Palanimuthu. 2021. "Targeting Probiotics in Rheumatoid Arthritis" Nutrients 13, no. 10: 3376. https://doi.org/10.3390/nu13103376
APA StyleBungau, S. G., Behl, T., Singh, A., Sehgal, A., Singh, S., Chigurupati, S., Vijayabalan, S., Das, S., & Palanimuthu, V. R. (2021). Targeting Probiotics in Rheumatoid Arthritis. Nutrients, 13(10), 3376. https://doi.org/10.3390/nu13103376