Manipulation of Dietary Intake on Changes in Circulating Testosterone Concentrations
Abstract
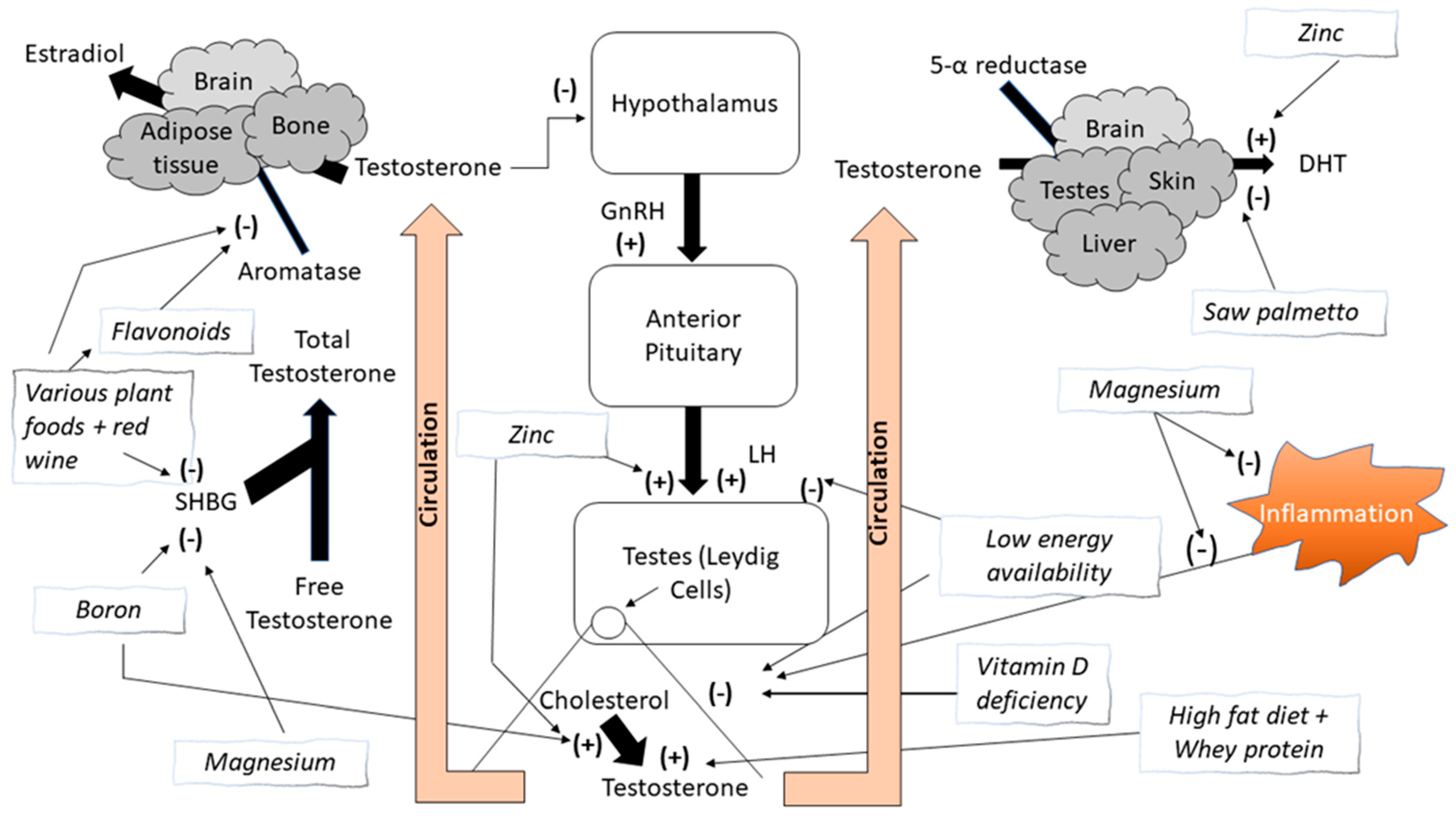
1. Introduction
1.1. Natural Product Extracts and Aromatase Inhibition
1.2. Flavonoids
1.3. Other Nutrients
2. Macronutrient Effects on Changes in Testosterone Concentrations
2.1. Low Energy Availability and Calorie Intake
| Source | Participants | Duration | Intervention | Key Findings |
|---|---|---|---|---|
| [78] | n = 14 men Elite bodybuilders | 11 weeks |
|
|
| [76] | n = 34 Healthy adults (men and women) | 31 days |
|
|
| [79] | n = 24 males Elite distance runners | 7 days |
|
|
2.2. High-Fat Diets and Dietary Fats
2.3. Dietary Protein and Protein Supplements
| Source | Participants | Duration | Intervention | Key Findings |
|---|---|---|---|---|
| [89] | n = 48 Premenopausal healthy women | 14 weeks |
|
|
| [108] | n = 10 Healthy resistance-trained men (crossover design) | 10 weeks |
|
|
| [109] | n = 47 Healthy college-aged men performing a resistance training program | 12 weeks |
|
|
| [86] | n = 25 Resistance-trained men | 11 weeks |
|
|
| [111] | n = 18 Experienced, resistance-trained, middle-aged men | 8 weeks |
|
|
3. Micronutrient Effects on Testosterone Concentrations
3.1. Vitamin D
3.2. Zinc
3.3. Magnesium
| Source | Participants | Duration | Intervention | Key Findings |
|---|---|---|---|---|
| [159] | n = 10 Healthy college-aged men | 4 weeks |
|
|
| [132] | n = 54 Healthy overweight men | 12 months |
|
|
| [160] | n = 32 Male cyclists | 4 weeks |
|
|
| [171] | n = 30 Healthy college-aged men participating in an aerobic -training program | 4 weeks |
|
|
4. Summary
Author Contributions
Funding
Conflicts of Interest
References
- Hackett, G.; Kirby, M.; Edwards, D.; Jones, T.H.; Wylie, K.; Ossei-Gerning, N.; David, J.; Muneer, A. British Society for Sexual Medicine Guidelines on Adult Testosterone Deficiency, with Statements for UK Practice. J. Sex. Med. 2017, 14, 1504–1523. [Google Scholar] [CrossRef]
- Bhasin, S.; Hatfield, D.L.; Hoffman, J.R.; Kraemer, W.J.; Labotz, M.; Phillips, S.M.; Ratamess, N.A. Anabolic-Androgenic Steroid Use in Sports, Health, and Society. Med. Sci. Sports Exerc. 2021, 53, 1778–1794. [Google Scholar] [CrossRef]
- Kraemer, W.J.; Ratamess, N.A.; Nindl, B.C. Recovery responses of testosterone, growth hormone, and IGF-1 after resistance exercise. J. Appl. Physiol. 2017, 122, 549–558. [Google Scholar] [CrossRef]
- A Hiipakka, R.; Liao, S. Molecular Mechanism of Androgen Action. Trends Endocrinol. Metab. 1998, 9, 317–324. [Google Scholar] [CrossRef]
- Ratnasabapathy, R.; Dhillo, W.S. The effects of kisspeptin in human reproductive function—Therapeutic implications. Curr. Drug Targets 2013, 14, 365–371. [Google Scholar] [PubMed]
- Hoffman, J.R.; Kraemer, W.J.; Bhasin, S.; Storer, T.; Ratamess, N.A.; Haff, G.G.; Willoughby, D.S.; Rogol, A.D. Position stand on androgen and human growth hormone use. J. Strength Cond. Res. 2009, 23, S1–S59. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, L.; Stanton, P.; de Kretser, D.M. Endocrinology of the Male Reproductive System and Spermatogenesis; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., de Herder, W.W., Dhatariya, K., Dungan, K., Grossman, A., Hershman, J.M., Hofland, J., et al., Eds.; Endotext [Internet]; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Dunn, J.F.; Nisula, B.C.; Rodbard, D. Transport of Steroid Hormones: Binding of 21 Endogenous Steroids to Both Testosterone-Binding Globulin and Corticosteroid-Binding Globulin in Human Plasma. J. Clin. Endocrinol. Metab. 1981, 53, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J.R. Testosterone: A review of exercise responses and physiological effects. NSCA Journal. 1992, 14, 10–17. [Google Scholar]
- Breiner, M.; Romalo, G.; Schweikert, H.-U. Inhibition of androgen receptor binding by natural and synthetic steroids in cultured human genital skin fibroblasts. J. Mol. Med. 1986, 64, 732–737. [Google Scholar] [CrossRef]
- Kraemer, W.J.; Ratamess, N.A.; Hymer, W.C.; Nindl, B.C.; Fragala, M.S. Growth Hormone(s), Testosterone, Insulin-Like Growth Factors, and Cortisol: Roles and Integration for Cellular Development and Growth with Exercise. Front. Endocrinol. 2020, 11, 33. [Google Scholar] [CrossRef]
- Bennett, N.C.; Gardiner, R.A.; Hooper, J.; Johnson, D.; Gobe, G.C. Molecular cell biology of androgen receptor signalling. Int. J. Biochem. Cell Biol. 2010, 42, 813–827. [Google Scholar] [CrossRef]
- Hooper, D.R.; Kraemer, W.J.; Saenz, C.; Schill, K.E.; Focht, B.C.; Volek, J.S.; Maresh, C.M. The presence of symptoms of testosterone deficiency in the exercise-hypogonadal male condition and the role of nutrition. Eur. J. Appl. Physiol. 2017, 117, 1349–1357. [Google Scholar] [CrossRef]
- Wrzosek, M.; Woźniak, J.; Włodarek, D. The causes of adverse changes of testosterone levels in men. Expert Rev. Endocrinol. Metab. 2020, 15, 355–362. [Google Scholar] [CrossRef]
- Fantus, R.J.; Halpern, J.A.; Chang, C.; Keeter, M.K.; Bennett, N.E.; Helfand, B.; Brannigan, R.E. The Association between Popular Diets and Serum Testosterone among Men in the United States. J. Urol. 2020, 203, 398–404. [Google Scholar] [CrossRef]
- Baltaci, A.K.; Mogulkoc, R.; Baltaci, S.B. Review: The role of zinc in the endocrine system. Pak. J. Pharm. Sci. 2019, 32, 231–239. [Google Scholar] [PubMed]
- Van Kiem, P.; Dat, N.T.; Van Minh, C.; Lee, J.J.; Kim, Y.H. Lupane-triterpenes from the leaves of Brassaiopsis glomerulata. Arch. Pharmacal Res. 2003, 26, 594–596. [Google Scholar] [CrossRef] [PubMed]
- Balunas, M.J.; Su, B.; Riswan, S.; Fong, H.H.; Brueggemeier, R.W.; Pezzuto, J.M.; Kinghorn, A.D. Isolation and characterization of aromatase inhibitors from Brassaiopsis glomerulata (Araliaceae). Phytochem. Lett. 2009, 2, 29–33. [Google Scholar] [CrossRef]
- Kowalska, M.T.; Itzhak, Y.; Puett, D. Presence of aromatase inhibitors in cycads. J. Ethnopharmacol. 1995, 47, 113–116. [Google Scholar] [CrossRef]
- Hargrove, J.L.; Greenspan, P.; Hartle, D.K.; Dowd, C. Inhibition of Aromatase and α-Amylase by Flavonoids and Proanthocyanidins from Sorghum bicolor Bran Extracts. J. Med. Food 2011, 14, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Whang, W.K.; Kim, I.H. Constituents of the herb ofIsodon excisus var.coreanus. Arch. Pharmacal Res. 1997, 20, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.-J.; Chang, L.C.; Kim, H.-K.; Kim, I.-H.; Kinghorn, A.D.; Pezzuto, J.M. Aromatase inhibitors fromIsodon excisus var.coreanus. Arch. Pharmacal Res. 2000, 23, 243–245. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Kim, D.; Han, J.; Kim, C. Inhibitory Effects of Scutellaria Barbata D. Don. and Euonymus Alatus Sieb. on Aromatase Activity of Human Leiomyomal Cells. Immunopharmacol. Immunotoxicol. 2004, 26, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Golan, R.; Gepner, Y.; Shai, I. Wine and Health-New Evidence. Eur. J. Clin. Nutr. 2019, 72, 55–59. [Google Scholar] [CrossRef]
- Eng, E.T.; Ye, J.; Williams, D.; Phung, S.; Moore, R.; Young, M.K.; Gruntmanis, U.; Braunstein, G.; Chen, S. Suppression of estrogen biosynthesis by procyanidin dimers in red wine and grape seeds. Cancer Res. 2003, 63, 8516–8522. [Google Scholar]
- Eng, E.T.; Williams, D.; Mandava, U.; Kirma, N.; Tekmal, R.R.; Chen, S. Anti-aromatase chemicals in red wine. Ann. N. Y. Acad. Sci. 2006, 963, 239–246. [Google Scholar] [CrossRef]
- Eng, E.T.; Williams, D.; Mandava, U.; Kirma, N.; Tekmal, R.R.; Chen, S. Suppression of aromatase (estrogen synthetase) by red wine phytochemicals. Breast Cancer Res. Treat. 2001, 67, 133–146. [Google Scholar] [CrossRef]
- Kijima, I.; Phung, S.; Hur, G.; Kwok, S.-L.; Chen, S. Grape Seed Extract Is an Aromatase Inhibitor and a Suppressor of Aromatase Expression. Cancer Res. 2006, 66, 5960–5967. [Google Scholar] [CrossRef]
- Shufelt, C.; Merz, C.N.B.; Yang, Y.; Kirschner, J.; Polk, D.; Stanczyk, F.; Paul-Labrador, M.; Braunstein, G.D. Red Versus White Wine as a Nutritional Aromatase Inhibitor in Premenopausal Women: A Pilot Study. J. Women’s Health 2012, 21, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Guo, Z.; Meydani, S.N.; Wu, D. White Button Mushroom Enhances Maturation of Bone Marrow-Derived Dendritic Cells and Their Antigen Presenting Function in Mice. J. Nutr. 2008, 138, 544–550. [Google Scholar] [CrossRef]
- Rop, O.; Mlcek, J.; Juríková, T. Beta-glucans in higher fungi and their health effects. Nutr. Rev. 2009, 67, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Grube, B.J.; Eng, E.T.; Kao, Y.-C.; Kwon, A.; Chen, S. White button mushroom phytochemicals inhibit aromatase activity and breast cancer cell proliferation. J. Nutr. 2001, 131, 3288–3293. [Google Scholar] [CrossRef]
- Loing, E.; Lachance, R.; Ollier, V.; Hocquaux, M. A new strategy to modulate alopecia using a combination of two specific and unique ingredients. J. Cosmet. Sci. 2013, 64, 45–58. [Google Scholar]
- Lipovac, M.; Chedraui, P.; Gruenhut, C.; Gocan, A.; Kurz, C.; Neuber, B.; Imhof, M. Effect of Red Clover Isoflavones over Skin, Appendages, and Mucosal Status in Postmenopausal Women. Obstet. Gynecol. Int. 2011, 2011, 1–6. [Google Scholar] [CrossRef]
- Almstrup, K.; Fernandez, M.F.; Petersen, J.H.; Olea, N.; Skakkebaek, N.E.; Leffers, H. Dual effects of phytoestrogens result in u-shaped dose-response curves. Environ. Health Perspect. 2002, 110, 743–748. [Google Scholar] [CrossRef]
- Balunas, M.; Su, B.; Brueggemeier, R.W.; Kinghorn, A.D. Xanthones from the Botanical Dietary Supplement Mangosteen (Garcinia mangostana) with Aromatase Inhibitory Activity. J. Nat. Prod. 2008, 71, 1161–1166. [Google Scholar] [CrossRef] [PubMed]
- Konda, M.R.; Alluri, K.V.; Janardhanan, P.K.; Trimurtulu, G.; Sengupta, K. Combined extracts of Garcinia mangostana fruit rind and Cinnamomum tamala leaf supplementation enhances muscle strength and endurance in resistance trained males. J. Int. Soc. Sports Nutr. 2018, 15, 50. [Google Scholar] [CrossRef]
- Sultan, C.; Terraza, A.; Devillier, C.; Carilla, E.; Briley, M.; Loire, C.; Descomps, B. Inhibition of androgen metabolism and binding by a liposterolic extract of “serenoa repens B” in human foreskin fibroblasts. J. Steroid Biochem. 1984, 20, 515–519. [Google Scholar] [CrossRef]
- Sudeep, H.V.; Thomas, J.V.; Shyamprasad, K. A double blind, placebo-controlled randomized comparative study on the efficacy of phytosterol-enriched and conventional saw palmetto oil in mitigating benign prostate hyperplasia and androgen deficiency. BMC Urol. 2020, 20, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Angwafor, F.; Anderson, M.L. An open label, dose response study to determine the effect of a dietary supplement on dihydrotestosterone, testosterone and estradiol levels in healthy males. J. Int. Soc. Sports Nutr. 2008, 5, 12. [Google Scholar] [CrossRef]
- Osawa, Y.; Tochigi, B.; Tochigi, M.; Ohnishi, S.; Watanabe, Y.; Bullion, K.; Osawa, G.; Nakabayashi, Y.; Yarborough, C. Aromatase Inhibitors in Cigarette Smoke, Tobacco Leaves and Other Plants. J. Enzym. Inhib. 1990, 4, 187–200. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Hatti, K.S.; Diwakar, L.; Rao, G.V.; Kush, A.; Reddy, G.C. Abyssinones and related flavonoids as potential steroidogenesis modulators. Bioinformation 2009, 3, 399–402. [Google Scholar] [CrossRef][Green Version]
- Bors, W.; Michel, C.; Stettmaier, K. Antioxidant effects of flavonoids. BioFactors 1997, 6, 399–402. [Google Scholar] [CrossRef]
- Arango, D.; Morohashi, K.; Yilmaz, A.; Kuramochi, K.; Parihar, A.; Brahimaj, B.; Grotewold, E.; Doseff, A.I. Molecular basis for the action of a dietary flavonoid revealed by the comprehensive identification of apigenin human targets. Proc. Natl. Acad. Sci. USA 2013, 110, E2153–E2162. [Google Scholar] [CrossRef]
- Monteiro, R.; Becker, H.; Azevedo, I.; Calhau, C. Effect of Hop (Humulus lupulus L.) Flavonoids on Aromatase (Estrogen Synthase) Activity. J. Agric. Food Chem. 2006, 54, 2938–2943. [Google Scholar] [CrossRef]
- Tomas-Barberán, F.A.; Cienfuegos-Jovellanos, E.; Marín, A.; Muguerza, B.; Gil-Izquierdo, A.; Cerdá, B.; Zafrilla, P.; Morillas, J.; Mulero, J.; Ibarra, A.; et al. A New Process to Develop a Cocoa Powder with Higher Flavonoid Monomer Content and Enhanced Bioavailability in Healthy Humans. J. Agric. Food Chem. 2007, 55, 3926–3935. [Google Scholar] [CrossRef] [PubMed]
- Chou, H.-C.; Šuklje, K.; Antalick, G.; Schmidtke, L.M.; Blackman, J.W. Late-Season Shiraz Berry Dehydration That Alters Composition and Sensory Traits of Wine. J. Agric. Food Chem. 2018, 66, 7750–7757. [Google Scholar] [CrossRef] [PubMed]
- Pacheco-Palencia, L.A.; Mertens-Talcott, S.; Talcott, S.T. Chemical composition, antioxidant properties, and thermal stability of a phytochemical enriched oil from Acai (Euterpe oleracea Mart.). J. Agric. Food Chem. 2008, 56, 4631–4636. [Google Scholar] [CrossRef] [PubMed]
- Neves, M.A.C.; Dinis, T.C.P.; Colombo, G.; e Melo, M.L.S. Combining Computational and Biochemical Studies for a Rationale on the Anti-Aromatase Activity of Natural Polyphenols. ChemMedChem 2007, 2, 1750–1762. [Google Scholar] [CrossRef] [PubMed]
- Kao, Y.C.; Zhou, C.; Sherman, M.; Laughton, C.A.; Chen, S. Molecular basis of the inhibition of human aromatase (estrogen synthetase) by flavone and isoflavone phytoestrogens: A site-directed mutagenesis study. Environ. Health Perspect 1998, 106, 85–92. [Google Scholar] [CrossRef]
- Kellis, J.; Vickery, L. Inhibition of human estrogen synthetase (aromatase) by flavones. Science 1984, 225, 1032–1034. [Google Scholar] [CrossRef]
- Gambelunghe, C.; Rossi, R.; Sommavilla, M.; Ferranti, C.; Rossi, R.; Ciculi, C.; Gizzi, S.; Micheletti, A.; Rufini, S. Effects of Chrysin on Urinary Testosterone Levels in Human Males. J. Med. Food 2003, 6, 387–390. [Google Scholar] [CrossRef]
- Brown, G.A.; Vukovich, M.D.; Martini, E.R.; Kohut, M.L.; Franke, W.D.; Jackson, D.A.; King, D.S. Effects of Androstenedione-Herbal Supplementation on Serum Sex Hormone Concentrations in 30- to 59-Year-old Men. Int. J. Vitam. Nutr. Res. 2001, 71, 293–301. [Google Scholar] [CrossRef]
- Nielsen, F.H.; Hunt, C.D.; Mullen, L.M.; Hunt, J.R. Effect of dietary boron on mineral, estrogen, and testosterone metabolism in postmenopausal women 1. FASEB J. 1987, 1, 394–397. [Google Scholar] [CrossRef] [PubMed]
- Naghii, M.R.; Samman, S. The effect of boron supplementation on its urinary excretion and selected cardiovascular risk factors in healthy male subjects. Biol. Trace Elem. Res. 1997, 56, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Green, N.R.; Ferrando, A.A. Plasma boron and the effects of boron supplementation in males. Environ. Health Perspect. 1994, 102 (Suppl. 7), 73–77. [Google Scholar] [PubMed]
- Naghii, M.; Samman, S. The effect of boron on plasma testosterone and plasma lipids in rats. Nutr. Res. 1997, 17, 523–531. [Google Scholar] [CrossRef]
- Naghii, M.R.; Mofid, M.; Asgari, A.R.; Hedayati, M.; Daneshpour, M.A. Comparative effects of daily and weekly boron supplementation on plasma steroid hormones and proinflammatory cytokines. J. Trace Elem. Med. Biol. 2011, 25, 54–58. [Google Scholar] [CrossRef]
- Rainey, C.J.; A Nyquist, L.; E Christensen, R.; Strong, P.L.; Culver, B.D.; Coughlin, J.R. Daily Boron Intake from the American Diet. J. Am. Diet. Assoc. 1999, 99, 335–340. [Google Scholar] [CrossRef]
- Starks, M.A.; Starks, S.L.; Kingsley, M.; Purpura, M.; Jäger, R. The effects of phosphatidylserine on endocrine response to moderate intensity exercise. J. Int. Soc. Sports Nutr. 2008, 5, 11. [Google Scholar] [CrossRef]
- Hoffman, J.R.; Stout, J.R.; Williams, D.R.; Wells, A.J.; Fragala, M.S.; Mangine, G.T.; Gonzalez, A.M.; Emerson, N.S.; McCormack, W.P.; Scanlon, T.C.; et al. Efficacy of phosphatidic acid ingestion on lean body mass, muscle thickness and strength gains in resistance-trained men. J. Int. Soc. Sports Nutr. 2012, 9, 47. [Google Scholar] [CrossRef]
- Balasubramanian, A.; Thirumavalavan, N.; Srivatsav, A.; Yu, J.; Lipshultz, L.I.; Pastuszak, A.W. Testosterone Imposters: An Analysis of Popular Online Testosterone Boosting Supplements. J. Sex. Med. 2019, 16, 203–212. [Google Scholar] [CrossRef]
- Clemesha, C.G.; Thaker, H.; Samplaski, M.K. ‘Testosterone Boosting’ Supplements Composition and Claims Are not Supported by the Academic Literature. World J. Men’s Health 2020, 38, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Kavvoura, A.; Zaras, N.; Stasinaki, A.-N.; Arnaoutis, G.; Methenitis, S.; Terzis, G. The Importance of Lean Body Mass for the Rate of Force Development in Taekwondo Athletes and Track and Field Throwers. J. Funct. Morphol. Kinesiol. 2018, 3, 43. [Google Scholar] [CrossRef] [PubMed]
- Zaras, N.; Stasinaki, A.-N.; Spiliopoulou, P.; Hadjicharalambous, M.; Terzis, G. Lean Body Mass, Muscle Architecture, and Performance in Well-Trained Female Weightlifters. Sports 2020, 8, 67. [Google Scholar] [CrossRef] [PubMed]
- Sundgot-Borgen, J.; Torstveit, M.K. Aspects of disordered eating continuum in elite high-intensity sports. Scand. J. Med. Sci. Sports 2010, 20, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Wasserfurth, P.; Palmowski, J.; Hahn, A.; Krüger, K. Reasons for and Consequences of Low Energy Availability in Female and Male Athletes: Social Environment, Adaptations, and Prevention. Sports Med. Open 2020, 6, 1–14. [Google Scholar] [CrossRef]
- Mountjoy, M.; Sundgot-Borgen, J.K.; Burke, L.M.; Ackerman, K.E.; Blauwet, C.; Constantini, N.; Lebrun, C.; Lundy, B.; Melin, A.K.; Meyer, N.L.; et al. IOC consensus statement on relative energy deficiency in sport (RED-S): 2018 update. Br. J. Sports Med. 2018, 52, 687–697. [Google Scholar] [CrossRef]
- Melin, A.K.; Heikura, I.; Tenforde, A.; Mountjoy, M. Energy Availability in Athletics: Health, Performance, and Physique. Int. J. Sport Nutr. Exerc. Metab. 2019, 29, 152–164. [Google Scholar] [CrossRef]
- Egger, T.; Flueck, J.L. Energy Availability in Male and Female Elite Wheelchair Athletes over Seven Consecutive Training Days. Nutr. 2020, 12, 3262. [Google Scholar] [CrossRef]
- Loucks, A.B.; Heath, E.M. Dietary restriction reduces luteinizing hormone (LH) pulse frequency during waking hours and increases LH pulse amplitude during sleep in young menstruating women. J. Clin. Endocrinol. Metab. 1994, 78, 910–915. [Google Scholar] [CrossRef] [PubMed]
- Loucks, A.B.; Verdun, M. Slow restoration of LH pulsatility by refeeding in energetically disrupted women. Am. J. Physiol. Integr. Comp. Physiol. 1998, 275, R1218–R1226. [Google Scholar] [CrossRef] [PubMed]
- Loucks, A.B.; Thuma, J.R. Luteinizing Hormone Pulsatility Is Disrupted at a Threshold of Energy Availability in Regularly Menstruating Women. J. Clin. Endocrinol. Metab. 2003, 88, 297–311. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.-Y.; Chen, Y.C.; Lin, P.; Shih, C.-K.; Bai, C.-H.; Yuan, K.-C.; Lee, S.-Y.; Chang, J.-S. Testosterone-Associated Dietary Pattern Predicts Low Testosterone Levels and Hypogonadism. Nutrients 2018, 10, 1786. [Google Scholar] [CrossRef]
- Henning, P.C.; Margolis, L.M.; McClung, J.P.; Young, A.J.; Pasiakos, S.M. High protein diets do not attenuate decrements in testosterone and IGF-I during energy deficit. Metabolism 2014, 63, 628–632. [Google Scholar] [CrossRef]
- Hooper, D.R.; Tenforde, A.S.; Hackney, A.C. Treating exercise-associated low testosterone and its related symptoms. Physician Sportsmed. 2018, 46, 427–434. [Google Scholar] [CrossRef]
- Mäestu, J.; Eliakim, A.; Jürimäe, J.; Valter, I.; Jürimäe, T. Anabolic and Catabolic Hormones and Energy Balance of the Male Bodybuilders During the Preparation for the Competition. J. Strength Cond. Res. 2010, 24, 1074–1081. [Google Scholar] [CrossRef]
- Heikura, I.A.; Uusitalo, A.L.T.; Stellingwerff, T.; Bergland, D.; Mero, A.A.; Burke, L.M. Low Energy Availability Is Difficult to Assess but Outcomes Have Large Impact on Bone Injury Rates in Elite Distance Athletes. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 403–411. [Google Scholar] [CrossRef]
- Torstveit, M.K.; Fahrenholtz, I.; Stenqvist, T.B.; Sylta, Ø.; Melin, A. Within-Day Energy Deficiency and Metabolic Perturbation in Male Endurance Athletes. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 419–427. [Google Scholar] [CrossRef]
- Lichtenstein, A.H.; Kennedy, E.; Barrier, P.; Danford, D.; Ernst, N.D.; Grundy, S.M.; Leveille, G.A.; Horn, L.; Williams, C.L.; Booth, S.L. Dietary Fat Consumption and Health. Nutr. Rev. 2009, 56, 3–19. [Google Scholar] [CrossRef]
- Trumbo, P.; Schlicker, S.; Yates, A.A.; Poos, M. Food and Nutrition Board of the Institute of Medicine TeNA: Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. J. Am. Diet. Assoc. 2002, 102, 1621–1630. [Google Scholar] [CrossRef]
- Mota, J.A.; Nuckols, G.; Smith-Ryan, A.E. Nutritional Periodization: Applications for the Strength Athlete. Strength Cond. J. 2019, 41, 69–78. [Google Scholar] [CrossRef]
- Aranceta, J.; Pérez-Rodrigo, C. Recommended dietary reference intakes, nutritional goals and dietary guidelines for fat and fatty acids: A systematic review. Br. J. Nutr. 2012, 107, S8–S22. [Google Scholar] [CrossRef]
- Whittaker, J.; Wu, K. Low-fat diets and testosterone in men: Systematic review and meta-analysis of intervention studies. J. Steroid Biochem. Mol. Biol. 2021, 210, 105878. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.M.; Lowery, R.P.; Roberts, M.D.; Sharp, M.H.; Joy, J.M.; Shields, K.A.; Partl, J.; Volek, J.S.; D’Agostino, D. The Effects of Ketogenic Dieting on Body Composition, Strength, Power, and Hormonal Profiles in Resistance Training Males. J. Strength Cond. Res. 2020, 34, 3463–3474. [Google Scholar] [CrossRef]
- Volek, J.S.; Kraemer, W.J.; Bush, J.A.; Incledon, T.; Boetes, M. Testosterone and cortisol in relationship to dietary nutrients and resistance exercise. J. Appl. Physiol. 1997, 82, 49–54. [Google Scholar] [CrossRef]
- Santos, H.O. Ketogenic diet and testosterone increase: Is the increased cholesterol intake responsible? To what extent and under what circumstances can there be benefits? Hormones 2017, 16, 266–270. [Google Scholar] [CrossRef]
- Goldin, B.R.; Woods, M.N.; Spiegelman, D.L.; Longcope, C.; Morrill-LaBrode, A.; Dwyer, J.; Gualtieri, L.J.; Hertzmark, E.; Gorbach, S.L. The effect of dietary fat and fiber on serum estrogen concentrations in premenopausal women under controlled dietary conditions. Cancer 1994, 74, 1125–1131. [Google Scholar] [CrossRef]
- Helge, J.W. A high carbohydrate diet remains the evidence based choice for elite athletes to optimise performance. J. Physiol. 2017, 595, 2775. [Google Scholar] [CrossRef] [PubMed]
- Vigh-Larsen, J.F.; Ørtenblad, N.; Spriet, L.L.; Overgaard, K.; Mohr, M. Muscle Glycogen Metabolism and High-Intensity Exercise Performance: A Narrative Review. Sports Med. 2021, 51, 1855–1874. [Google Scholar] [CrossRef] [PubMed]
- Burke, L.M. Re-Examining High-Fat Diets for Sports Performance: Did We Call the ‘Nail in the Coffin’ Too Soon? Sports Med. 2015, 45, 33–49. [Google Scholar] [CrossRef]
- Taber, C.B.; Vigotsky, A.; Nuckols, G.; Haun, C.T. Exercise-Induced Myofibrillar Hypertrophy is a Contributory Cause of Gains in Muscle Strength. Sports Med. 2019, 49, 993–997. [Google Scholar] [CrossRef]
- Slater, G.J.; Dieter, B.P.; Marsh, D.J.; Helms, E.R.; Shaw, G.; Iraki, J. Is an Energy Surplus Required to Maximize Skeletal Muscle Hypertrophy Associated With Resistance Training. Front. Nutr. 2019, 6, 131. [Google Scholar] [CrossRef]
- Morton, R.W.; McGlory, C.; Phillips, S.M. Nutritional interventions to augment resistance training-induced skeletal muscle hypertrophy. Front. Physiol. 2015, 6, 245. [Google Scholar] [CrossRef]
- Jäger, R.; Kerksick, C.M.; Campbell, B.I.; Cribb, P.J.; Wells, S.D.; Skwiat, T.M.; Purpura, M.; Ziegenfuss, T.N.; Ferrando, A.A.; Arent, S.M.; et al. International Society of Sports Nutrition Position Stand: Protein and exercise. J. Int. Soc. Sports Nutr. 2017, 14, 20. [Google Scholar] [CrossRef]
- Stokes, T.; Hector, A.J.; Morton, R.W.; McGlory, C.; Phillips, S.M. Recent Perspectives Regarding the Role of Dietary Protein for the Promotion of Muscle Hypertrophy with Resistance Exercise Training. Nutrients 2018, 10, 180. [Google Scholar] [CrossRef]
- 98. Morton, R.W.; Murphy, K.T.; McKellar, S.R.; Schoenfeld, B.J.; Henselmans, M.; Helms, E.; Aragon, A.A.; Devries, M.C.; Banfield, L.; Krieger, J.W.; et al. A systematic review, meta-analysis and meta-regression of the effect of protein supplementation on resistance training-induced gains in muscle mass and strength in healthy adults. Br. J. Sports Med. 2018, 52, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Candow, D.G.; Burke, N.C.; Smith-Palmer, T.; Burke, D.G. Effect of whey and soy protein supplementation combined with resistance training in young adults. Int. J. Sport Nutr. Exerc. Metab. 2006, 16, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Messina, M.; Nagata, C.; Wu, A.H. Estimated Asian Adult Soy Protein and Isoflavone Intakes. Nutr. Cancer 2006, 55, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kuiper, G.G.; Carlsson, B.; Grandien, K.; Enmark, E.; Häggblad, J.; Nilsson, S.; Gustafsson, J.A. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology 1997, 138, 863–870. [Google Scholar] [CrossRef] [PubMed]
- Kuiper, G.G.; Lemmen, J.G.; Carlsson, B.; Corton, J.C.; Safe, S.H.; van der Saag, P.T.; van der Burg, B.; Gustafsson, J.A. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology 1998, 139, 4252–4263. [Google Scholar] [CrossRef] [PubMed]
- Weber, K.; Setchell, K.; Stocco, D.; Lephart, E.; Weber, S. Dietary soy-phytoestrogens decrease testosterone levels and prostate weight without altering LH, prostate 5alpha-reductase or testicular steroidogenic acute regulatory peptide levels in adult male Sprague-Dawley rats. J. Endocrinol. 2001, 170, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Strauss, L.; Mäkelä, S.; Joshi, S.; Huhtaniemi, I.; Santti, R. Genistein exerts estrogen-like effects in male mouse reproductive tract. Mol. Cell. Endocrinol. 1998, 144, 83–93. [Google Scholar] [CrossRef]
- Kwon, S.M.; Kim, S.I.; Chun, D.C.; Cho, N.H.; Chung, B.C.; Park, B.W.; Hong, S.J. Development of Rat Prostatitis Model by Oral Administration of Isoflavone and Its Characteristics. Yonsei Med. J. 2001, 42, 395–404. [Google Scholar] [CrossRef]
- Roberts, D.; Veeramachaneni, D.N.R.; Schlaff, W.D.; Awoniyi, C.A. Effects of Chronic Dietary Exposure to Genistein, a Phytoestrogen, During Various Stages of Development on Reproductive Hormones and Spermatogenesis in Rats. Endocrine 2000, 13, 281–286. [Google Scholar] [CrossRef]
- Hamilton-Reeves, J.M.; Vazquez, G.; Duval, S.J.; Phipps, W.R.; Kurzer, M.S.; Messina, M.J. Clinical studies show no effects of soy protein or isoflavones on reproductive hormones in men: Results of a meta-analysis. Fertil. Steril. 2010, 94, 997–1007. [Google Scholar] [CrossRef]
- Kraemer, W.J.; Solomon-Hill, G.; Volk, B.M.; Kupchak, B.R.; Looney, D.P.; Dunn-Lewis, C.; Comstock, B.A.; Szivak, T.K.; Hooper, D.R.; Flanagan, S.D.; et al. The effects of soy and whey protein supplementation on acute hormonal reponses to resistance exercise in men. J. Am. Coll. Nutr. 2013, 32, 66–74. [Google Scholar] [CrossRef]
- Haun, C.T.; Mobley, C.B.; Vann, C.G.; Romero, M.A.; Roberson, P.A.; Mumford, P.W.; Kephart, W.C.; Healy, J.C.; Patel, R.K.; Osburn, S.C.; et al. Soy protein supplementation is not androgenic or estrogenic in college-aged men when combined with resistance exercise training. Sci. Rep. 2018, 8, 11151. [Google Scholar] [CrossRef]
- Kalman, D.; Feldman, S.; Martinez, M.; Krieger, D.R.; Tallon, M.J. Effect of protein source and resistance training on body composition and sex hormones. J. Int. Soc. Sports Nutr. 2007, 4, 4. [Google Scholar] [CrossRef]
- Vidić, V.; Ilić, V.; Toskić, L.; Janković, N.; Ugarković, D. Effects of calorie restricted low carbohydrate high fat ketogenic vs. non-ketogenic diet on strength, body-composition, hormonal and lipid profile in trained middle-aged men. Clin. Nutr. 2021, 40, 1495–1502. [Google Scholar] [CrossRef]
- Bendik, I.; Friedel, A.; Roos, F.F.; Weber, P.; Eggersdorfer, M. Vitamin D: A critical and essential micronutrient for human health. Front. Physiol. 2014, 5, 248. [Google Scholar] [CrossRef]
- Zhang, R.; Naughton, D.P. Vitamin D in health and disease: Current perspectives. Nutr. J. 2010, 9, 65. [Google Scholar] [CrossRef]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Guidelines for Preventing and Treating Vitamin D Deficiency and Insufficiency Revisited. J. Clin. Endocrinol. Metab. 2012, 97, 1153–1158. [Google Scholar] [CrossRef]
- Bikle, D.D. Vitamin D Metabolism, Mechanism of Action, and Clinical Applications. Chem. Biol. 2014, 21, 319–329. [Google Scholar] [CrossRef] [PubMed]
- de la Puente Yagüe, M.; Collado Yurrita, L.; Cuadrado Cenzual, M.A. Faculty Opinions recommendation of Role of vitamin D in athletes and their performance: Current concepts and new trends. Fac. Opin. Post-Publ. Peer Rev. Biomed. Lit. 2020, 12, 579. [Google Scholar] [CrossRef]
- Lips, P. Vitamin D physiology. Prog. Biophys. Mol. Biol. 2006, 92, 4–8. [Google Scholar] [CrossRef]
- Tripkovic, L.; Lambert, H.; Hart, K.; Smith, C.P.; Bucca, G.; Penson, S.; Chope, G.; Hypponen, E.; Berry, J.; Vieth, R.; et al. Comparison of vitamin D2 and vitamin D3 supplementation in raising serum 25-hydroxyvitamin D status: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2012, 95, 1357–1364. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.C.; Manson, J.E.; Abrams, S.A.; Aloia, J.F.; Brannon, P.M.; Clinton, S.K.; Durazo-Arvizu, R.A.; Gallagher, J.C.; Gallo, R.L.; Jones, G.; et al. The 2011 Report on Dietary Reference Intakes for Calcium and Vitamin D from the Institute of Medicine: What Clinicians Need to Know. J. Clin. Endocrinol. Metab. 2011, 96, 53–58. [Google Scholar] [CrossRef]
- Jung, H.C.; Seo, M.W.; Lee, S.; Jung, S.W.; Song, J.K. Correcting Vitamin D Insufficiency Improves Some But Not All Aspects of Physical Performance During Winter Training in Taekwondo Athletes. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 635–643. [Google Scholar] [CrossRef]
- Farrokhyar, F.; Tabasinejad, R.; Dao, D.; Peterson, D.; Ayeni, O.R.; Hadioonzadeh, R.; Bhandari, M. Prevalence of Vitamin D Inadequacy in Athletes: A Systematic-Review and Meta-Analysis. Sports Med. 2015, 45, 365–378. [Google Scholar] [CrossRef]
- Habib, F.; Maddy, S.; Gelly, K. Characterisation of receptors for 1,25-dihydroxyvitamin D3 in the human testis. J. Steroid Biochem. 1990, 35, 195–199. [Google Scholar] [CrossRef]
- Johnson, J.A.; Grande, J.P.; Roche, P.C.; Kumar, R. Immunohistochemical detection and distribution of the 1,25-dihydroxyvitamin D3 receptor in rat reproductive tissues. Histochem. Cell Biol. 1996, 105, 7–15. [Google Scholar] [CrossRef]
- Aquila, S.; Guido, C.; Perrotta, I.; Tripepi, S.; Nastro, A.; Andò, S. Human sperm anatomy: Ultrastructural localization of 1α,25-dihydroxyvitamin D3 receptor and its possible role in the human male gamete. J. Anat. 2008, 213, 555–564. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, J.; DeLuca, H.F. Where is the vitamin D receptor? Arch. Biochem. Biophys. 2012, 523, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Wehr, E.; Pilz, S.; Boehm, B.O.; MãRz, W.; Obermayer-Pietsch, B. Association of vitamin D status with serum androgen levels in men. Clin. Endocrinol. 2009, 73, 243–248. [Google Scholar] [CrossRef]
- Lee, D.M.; Tajar, A.; Pye, S.R.; Boonen, S.; Vanderschueren, D.; Bouillon, R.; O’Neill, T.W.; Bartfai, G.; Casanueva, F.F.; Finn, J.D.; et al. Association of hypogonadism with vitamin D status: The European Male Ageing Study. Eur. J. Endocrinol. 2012, 166, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Nimptsch, K.; Platz, E.A.; Willett, W.C.; Giovannucci, E. Association between plasma 25-OH vitamin D and testosterone levels in men. Clin. Endocrinol. 2012, 77, 106–112. [Google Scholar] [CrossRef]
- Close, G.; Russell, J.; Cobley, J.; Owens, D.; Wilson, G.; Gregson, W.; Fraser, W.; Morton, J. Assessment of vitamin D concentration in non-supplemented professional athletes and healthy adults during the winter months in the UK: Implications for skeletal muscle function. J. Sports Sci. 2013, 31, 344–353. [Google Scholar] [CrossRef]
- Farrokhyar, F.; Sivakumar, G.K.; Savage, K.; Koziarz, A.; Jamshidi, S.; Ayeni, O.R.; Peterson, D.; Bhandari, M. Effects of Vitamin D Supplementation on Serum 25-Hydroxyvitamin D Concentrations and Physical Performance in Athletes: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Sports Med. 2017, 47, 2323–2339. [Google Scholar] [CrossRef] [PubMed]
- Dubnov-Raz, G.; Livne, N.; Raz, R.; Cohen, A.H.; Constantini, N.W. Vitamin D Supplementation and Physical Performance in Adolescent Swimmers. Int. J. Sport Nutr. Exerc. Metab. 2015, 25, 317–325. [Google Scholar] [CrossRef]
- Pilz, S.; Frisch, S.; Koertke, H.; Kuhn, J.; Dreier, J.; Obermayer-Pietsch, B.; Wehr, E.; Zittermann, A. Effect of Vitamin D Supplementation on Testosterone Levels in Men. Horm. Metab. Res. 2010, 43, 223–225. [Google Scholar] [CrossRef]
- Dahlquist, D.T.; Dieter, B.P.; Koehle, M.S. Plausible ergogenic effects of vitamin D on athletic performance and recovery. J. Int. Soc. Sports Nutr. 2015, 12, 1–12. [Google Scholar] [CrossRef]
- He, C.-S.; Fraser, W.D.; Tang, J.; Brown, K.; Renwick, S.; Rudland-Thomas, J.; Teah, J.; Tanqueray, E.; Gleeson, M. The effect of 14 weeks of vitamin D 3 supplementation on antimicrobial peptides and proteins in athletes. J. Sports Sci. 2015, 34, 1–8. [Google Scholar] [CrossRef]
- 135. Micheletti, A.; Rossi, R.; Rufini, S. Zinc status in athletes: Relation to diet and exercise. Sports Med. 2001, 31, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Miranda, E.R.; Dey, C.S. Effect of Chromium and Zinc on Insulin Signaling in Skeletal Muscle Cells. Biol. Trace Elem. Res. 2004, 101, 19–36. [Google Scholar] [CrossRef]
- Foster, M.; Samman, S. Zinc and Regulation of Inflammatory Cytokines: Implications for Cardiometabolic Disease. Nutrients 2012, 4, 676–694. [Google Scholar] [CrossRef] [PubMed]
- König, D.; Weinstock, C.; Keul, J.; Northoff, H.; Berg, A. Zinc, iron, and magnesium status in athletes--influence on the regulation of exercise-induced stress and immune function. Exerc. Immunol. Rev. 1998, 4, 2–21. [Google Scholar] [PubMed]
- Freeland-Graves, J.H.; Ebangit, M.L.; Bodzy, P.W. Zinc and copper content of foods used in vegetarian diets. J. Am. Diet. Assoc. 1980, 77, 648–654. [Google Scholar]
- Foster, M.; Chu, A.; Petocz, P.; Samman, S. Effect of vegetarian diets on zinc status: A systematic review and meta-analysis of studies in humans. J. Sci. Food Agric. 2013, 93, 2362–2371. [Google Scholar] [CrossRef]
- Hunt, J.R.; Matthys, L.A.; Johnson, L.K. Zinc absorption, mineral balance, and blood lipids in women consuming controlled lactoovovegetarian and omnivorous diets for 8 wk. Am. J. Clin. Nutr 1998, 67, 421–430. [Google Scholar] [CrossRef]
- Maret, W.; Sandstead, H.H. Zinc requirements and the risks and benefits of zinc supplementation. J. Trace Elem. Med. Biol. 2006, 20, 3–18. [Google Scholar] [CrossRef]
- 143. Trumbo, P.; Yates, A.A.; Schlicker, S.; Poos, M. Dietary reference intakes: Vitamin A, Vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. J. Am. Diet. Assoc. 2001, 101, 294–301. [Google Scholar] [CrossRef]
- Om, A.-S.; Chung, K.-W. Dietary Zinc Deficiency Alters 5α-Reduction and Aromatization of Testosterone and Androgen and Estrogen Receptors in Rat Liver. J. Nutr. 1996, 126, 842–848. [Google Scholar] [CrossRef] [PubMed]
- Swerdloff, R.S.; Dudley, R.E.; Page, S.T.; Wang, C.; Salameh, W. Dihydrotestosterone: Biochemistry, Physiology, and Clinical Implications of Elevated Blood Levels. Endocr. Rev. 2017, 38, 220–254. [Google Scholar] [CrossRef] [PubMed]
- Grino, P.B.; Griffin, J.E.; Wilson, J.D. Testosterone at High Concentrations Interacts with the Human Androgen Receptor Similarly to Dihydrotestosterone*. Endocrinol. 1990, 126, 1165–1172. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Bohl, C.E.; Dalton, J.T. Chemistry and Structural Biology of Androgen Receptor. Chem. Rev. 2005, 105, 3352–3370. [Google Scholar] [CrossRef] [PubMed]
- Wilson, E.M.; French, F.S. Binding properties of androgen receptors. Evidence for identical receptors in rat testis, epididymis, and prostate. J. Biol. Chem. 1976, 251, 5620–5629. [Google Scholar] [CrossRef]
- Marchetti, P.M.; Barth, J.H. Clinical biochemistry of dihydrotestosterone. Ann. Clin. Biochem. 2013, 50, 95–107. [Google Scholar] [CrossRef]
- Bernstein, K.E.; Khan, Z.; Giani, J.F.; Cao, D.-Y.; Bernstein, E.A.; Shen, X.Z. Angiotensin-converting enzyme in innate and adaptive immunity. Nat. Rev. Nephrol. 2018, 14, 325–336. [Google Scholar] [CrossRef]
- Towler, P.; Staker, B.; Prasad, S.G.; Menon, S.; Tang, J.; Parsons, T.; Ryan, D.; Fisher, M.; Williams, D.; Dales, N.A.; et al. ACE2 X-Ray Structures Reveal a Large Hinge-bending Motion Important for Inhibitor Binding and Catalysis. J. Biol. Chem. 2004, 279, 17996–18007. [Google Scholar] [CrossRef]
- Kwok, T.; Ohlsson, C.; Vandenput, L.; Tang, N.; Zhang, Y.; Tomlinson, B.; Leung, P. ACE inhibitor use was associated with lower serum dehydroepiandrosterone concentrations in older men. Clin. Chim. Acta 2010, 411, 1122–1125. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Prasad, A.S.; Mantzoros, C.S.; Beck, F.W.; Hess, J.W.; Brewer, G.J. Zinc status and serum testosterone levels of healthy adults. Nutrition 1996, 12, 344–348. [Google Scholar] [CrossRef]
- Jalali, G.R.; Roozbeh, J.; Mohammadzadeh, A.; Sharifian, M.; Sagheb, M.M.; Jahromi, A.H.; Shabani, S.; Ghaffarpasand, F.; Afshariani, R. Impact of oral zinc therapy on the level of sex hormones in male patients on hemodialysis. Ren. Fail. 2010, 32, 417–419. [Google Scholar] [CrossRef] [PubMed]
- Santos, H.O.; Teixeira, F. Use of medicinal doses of zinc as a safe and efficient coadjutant in the treatment of male hypogonadism. Aging Male 2020, 23, 669–678. [Google Scholar] [CrossRef]
- McClung, J.P. Iron, Zinc, and Physical Performance. Biol. Trace Elem. Res. 2018, 188, 135–139. [Google Scholar] [CrossRef]
- Chu, A.; Holdaway, C.; Varma, T.; Petocz, P.; Samman, S. Lower Serum Zinc Concentration Despite Higher Dietary Zinc Intake in Athletes: A Systematic Review and Meta-analysis. Sports Med. 2017, 48, 327–336. [Google Scholar] [CrossRef]
- Koehler, K.; Parr, M.K.; Geyer, H.; Mester, J.; Schänzer, W. Serum testosterone and urinary excretion of steroid hormone metabolites after administration of a high-dose zinc supplement. Eur. J. Clin. Nutr. 2007, 63, 65–70. [Google Scholar] [CrossRef]
- Kilic, M. Effect of fatiguing bicycle exercise on thyroid hormone and testosterone levels in sedentary males supplemented with oral zinc. Neuro Endocrinol. Lett. 2007, 28, 681–685. [Google Scholar]
- Shafiei Neek, L.; Gaeini, A.A.; Choobineh, S. Effect of zinc and selenium supplementation on serum testosterone and plasma lactate in cyclist after an exhaustive exercise bout. Biol. Trace Elem. Res. 2011, 144, 454–462. [Google Scholar] [CrossRef]
- Volpe, S.L. Magnesium in Disease Prevention and Overall Health. Adv. Nutr. 2013, 4, 378S–383S. [Google Scholar] [CrossRef]
- Volpe, S.L. Magnesium and the Athlete. Curr. Sports Med. Rep. 2015, 14, 279–283. [Google Scholar] [CrossRef]
- Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride. National Academies Press: Washington, DC, USA, 1997. [Google Scholar]
- de Sousa, E.F.; Da Costa, T.H.; Nogueira, J.A.; Vivaldi, L.J. Assessment of nutrient and water intake among adolescents from sports federations in the Federal District, Brazil. Br. J. Nutr. 2008, 99, 1275–1283. [Google Scholar] [CrossRef] [PubMed]
- Juzwiak, C.R.; Amancio, O.M.S.; Vitalle, M.S.S.; Pinheiro, M.M.; Szejnfeld, V.L. Body composition and nutritional profile of male adolescent tennis players. J. Sports Sci. 2008, 26, 1209–1217. [Google Scholar] [CrossRef] [PubMed]
- Heaney, S.; O’Connor, H.; Gifford, J.; Naughton, G. Comparison of strategies for assessing nutritional adequacy in elite female athletes’ dietary intake. Int. J. Sport Nutr. Exerc. Metab. 2010, 20, 245–256. [Google Scholar] [CrossRef]
- Silva, M.R.; Paiva, T. Low energy availability and low body fat of female gymnasts before an international competition. Eur. J. Sport Sci. 2015, 15, 591–599. [Google Scholar] [CrossRef]
- Maggio, M.; De Vita, F.; Lauretani, F.; Nouvenne, A.; Meschi, T.; Ticinesi, A.; Dominguez, L.J.; Barbagallo, M.; Dall’Aglio, E.; Ceda, G.P. The Interplay between Magnesium and Testosterone in Modulating Physical Function in Men. Int. J. Endocrinol. 2014, 2014, 1–9. [Google Scholar] [CrossRef]
- Maggio, M.; Ceda, G.P.; Lauretani, F.; Cattabiani, C.; Avantaggiato, E.; Morganti, S.; Ablondi, F.; Bandinelli, S.; Dominguez, L.J.; Barbagallo, M.; et al. Magnesium and anabolic hormones in older men. Int. J. Androl. 2011, 34, e594–e600. [Google Scholar] [CrossRef] [PubMed]
- Rotter, I.; Kosik-Bogacka, D.; Dołęgowska, B.; Safranow, K.; Karakiewicz, B.; Laszczyńska, M. Relationship between serum magnesium concentration and metabolic and hormonal disorders in middle-aged and older men. Magnes. Res. 2015, 28, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Cinar, V.; Polat, Y.; Baltaci, A.K.; Mogulkoc, R. Effects of magnesium supplementation on testosterone levels of athletes and sedentary subjects at rest and after exhaustion. Biol. Trace Elem. Res. 2011, 140, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Barbagallo, M.; Dominguez, L.J. Magnesium and Aging. Curr. Pharm. Des. 2010, 16, 832–839. [Google Scholar] [CrossRef]
- Dickens, B.; Weglicki, W.; Li, Y.-S.; Mak, I. Magnesium deficiency in vitro enhances free radical-induced intracellular oxidation and cytotoxicity in endothelial cells. FEBS Lett. 1992, 311, 187–191. [Google Scholar] [CrossRef]
- Freedman, A.M.; Mak, I.T.; Stafford, R.E.; Dickens, B.F.; Cassidy, M.M.; Muesing, R.A.; Weglicki, W.B. Erythrocytes from magnesium-deficient hamsters display an enhanced susceptibility to oxidative stress. Am. J. Physiol. Physiol. 1992, 262, C1371–C1375. [Google Scholar] [CrossRef] [PubMed]
- Demirbag, R.; Yilmaz, R.; Erel, O. The association of total antioxidant capacity with sex hormones. Scand. Cardiovasc. J. 2005, 39, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Blache, D.; Devaux, S.; Joubert, O.; Loreau, N.; Schneider, M.; Durand, P.; Prost, M.; Gaume, V.; Adrian, M.; Laurant, P.; et al. Long-term moderate magnesium-deficient diet shows relationships between blood pressure, inflammation and oxidant stress defense in aging rats. Free. Radic. Biol. Med. 2006, 41, 277–284. [Google Scholar] [CrossRef]
- Hans, C.P.; Chaudhary, D.P.; Bansal, D.D. Effect of magnesium supplementation on oxidative stress in alloxanic diabetic rats. Magnes. Res. 2003, 16, 13–19. [Google Scholar] [PubMed]
- Yang, Y.; Wu, Z.; Chen, Y.; Qiao, J.; Gao, M.; Yuan, J.; Nie, W.; Guo, Y. Magnesium Deficiency Enhances Hydrogen Peroxide Production and Oxidative Damage in Chick Embryo Hepatocyte In Vitro. BioMetals 2006, 19, 71–81. [Google Scholar] [CrossRef]
- Afanas’Ev, I.B.; Suslova, T.B.; Cheremisina, Z.P.; Abramova, N.E.; Korkina, L.G. Study of antioxidant properties of metal aspartates. Analyst 1995, 120, 859–862. [Google Scholar] [CrossRef]
- Nielsen, F.H.; Johnson, L.K.; Zeng, H. Magnesium supplementation improves indicators of low magnesium status and inflammatory stress in adults older than 51 years with poor quality sleep. Magnes. Res. 2011, 23, 158–168. [Google Scholar] [CrossRef]
- Weglicki, W.B.; Dickens, B.F.; Wagner, T.L.; Chmielinska, J.J.; Phillips, T.M. Immunoregulation by neuropeptides in magnesium deficiency: Ex vivo effect of enhanced substance P production on circulating T lymphocytes from magnesium-deficient mice. Magnes. Res. 1996, 9, 3–11. [Google Scholar]
- Nielsen, F.H.; Lukaski, H.C. Update on the relationship between magnesium and exercise. Magnes. Res. 2006, 19, 180–189. [Google Scholar]
- I Spratt, D.; Orav, J.; Moloney, J.; Bigos, T.; Cox, P. Reproductive axis suppression in acute illness is related to disease severity. J. Clin. Endocrinol. Metab. 1993, 76, 1548–1554. [Google Scholar] [CrossRef]
- Hong, C.Y.; Park, J.H.; Ahn, R.S.; Im, S.Y.; Choi, H.-S.; Soh, J.; Mellon, S.H.; Lee, K. Molecular Mechanism of Suppression of Testicular Steroidogenesis by Proinflammatory Cytokine Tumor Necrosis Factor Alpha. Mol. Cell. Biol. 2004, 24, 2593–2604. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, T. The nuclear factor NF-kappaB pathway in inflammation. Cold. Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef] [PubMed]
- Rochelson, B.; Dowling, O.; Schwartz, N.; Metz, C.N. Magnesium sulfate suppresses inflammatory responses by human umbilical vein endothelial cells (HuVECs) through the NFkappaB pathway. J. Reprod. Immunol. 2007, 73, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Steward, C.J.; Zhou, Y.; Keane, G.; Cook, M.D.; Liu, Y.; Cullen, T. One week of magnesium supplementation lowers IL-6, muscle soreness and increases post-exercise blood glucose in response to downhill running. Graefe’s Arch. Clin. Exp. Ophthalmol. 2019, 119, 2617–2627. [Google Scholar] [CrossRef] [PubMed]
- Excoffon, L.; Guillaume, Y.; Woronoff-Lemsi, M.; André, C. Magnesium effect on testosterone–SHBG association studied by a novel molecular chromatography approach. J. Pharm. Biomed. Anal. 2009, 49, 175–180. [Google Scholar] [CrossRef]
- Emadi-Konjin, P.; Bain, J.; Bromberg, I.L. Evaluation of an algorithm for calculation of serum “bioavailable” testosterone (BAT). Clin. Biochem. 2003, 36, 591–596. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zamir, A.; Ben-Zeev, T.; Hoffman, J.R. Manipulation of Dietary Intake on Changes in Circulating Testosterone Concentrations. Nutrients 2021, 13, 3375. https://doi.org/10.3390/nu13103375
Zamir A, Ben-Zeev T, Hoffman JR. Manipulation of Dietary Intake on Changes in Circulating Testosterone Concentrations. Nutrients. 2021; 13(10):3375. https://doi.org/10.3390/nu13103375
Chicago/Turabian StyleZamir, Amit, Tavor Ben-Zeev, and Jay R. Hoffman. 2021. "Manipulation of Dietary Intake on Changes in Circulating Testosterone Concentrations" Nutrients 13, no. 10: 3375. https://doi.org/10.3390/nu13103375
APA StyleZamir, A., Ben-Zeev, T., & Hoffman, J. R. (2021). Manipulation of Dietary Intake on Changes in Circulating Testosterone Concentrations. Nutrients, 13(10), 3375. https://doi.org/10.3390/nu13103375






