The Renaissance of Plant Mucilage in Health Promotion and Industrial Applications: A Review
Abstract
:1. Introduction
2. Materials and Methods
3. General Aspects of Plant-Derived Mucilages
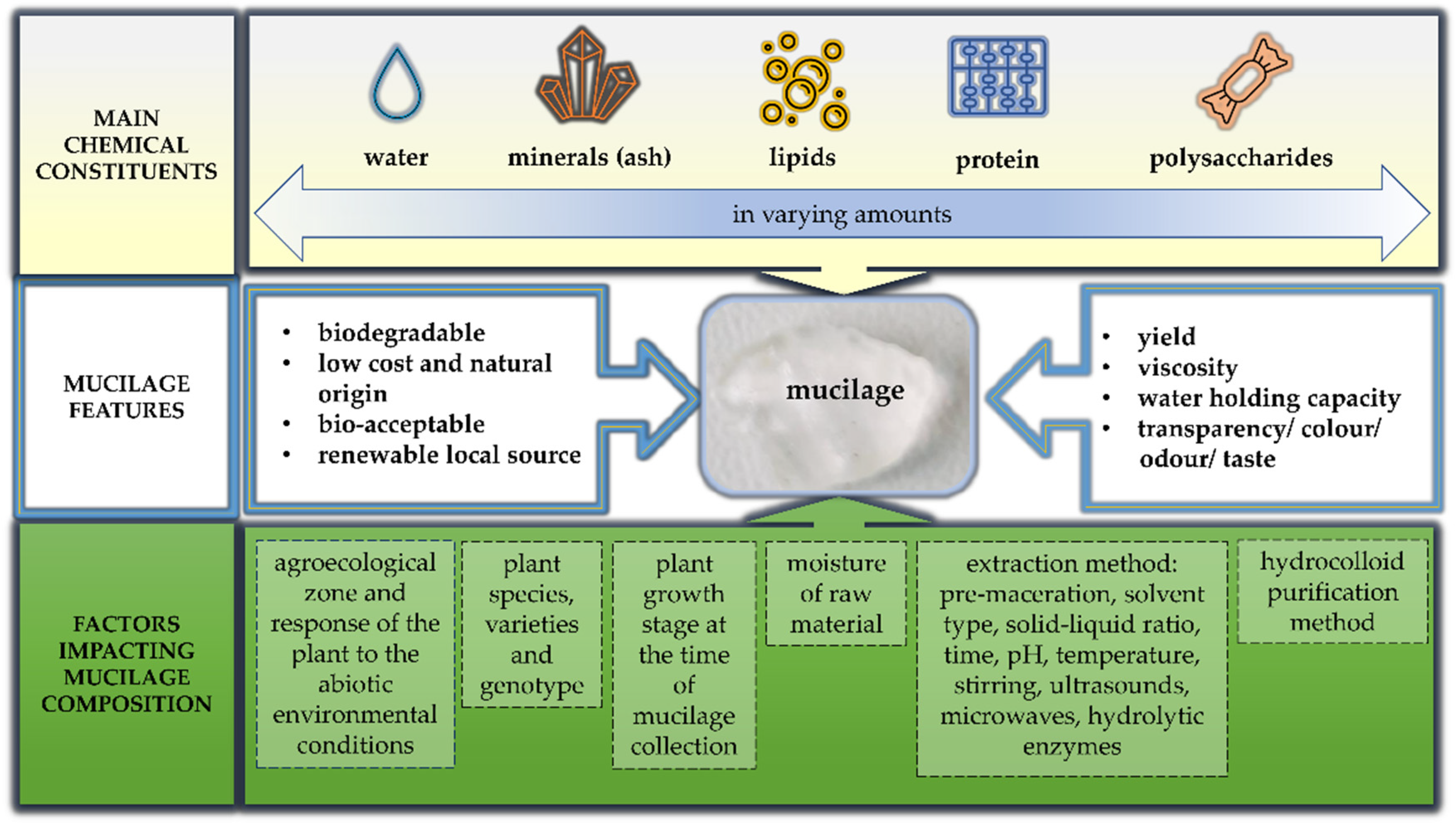
3.1. Chemical Characterization of Mucilages
3.2. Function of Mucilages in Plants
3.3. Application of Plant-Derived Mucilage
4. Selected Plants as a Mucilage Origin in the Light of Recent Research
4.1. Salvia
4.2. Ocimum basilium
4.3. Hyptis suaveolens
4.4. Plantago
4.5. Trigonella foenum-graecum
4.6. Cassia
4.7. Basella alba
4.8. Spinacia oleracea
4.9. Talinum triangulare
4.10. Opuntia
4.11. Linum usitatissimum
4.12. Aloe vera
4.13. Solanum betaceum
4.14. Cydonia oblonga
5. Summary and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Liu, Y.; Liu, Z.; Zhu, X.; Hu, X.; Zhang, H.; Guo, Q.; Yada, R.Y.; Cui, S.W. Seed Coat Mucilages: Structural, Functional/Bioactive Properties, and Genetic Information. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2534–2559. [Google Scholar] [CrossRef]
- Tosif, M.M.; Najda, A.; Bains, A.; Kaushik, R.; Dhull, S.B.; Chawla, P.; Walasek-Janusz, M. A Comprehensive Review on Plant-Derived Mucilage: Characterization, Functional Properties, Applications, and Its Utilization for Nanocarrier Fabrication. Polymers 2021, 13, 1066. [Google Scholar] [CrossRef]
- Soukoulis, C.; Gaiani, C.; Hoffmann, L. Plant Seed Mucilage as Emerging Biopolymer in Food Industry Applications. Curr. Opin. Food Sci. 2018, 22, 28–42. [Google Scholar] [CrossRef]
- Muñoz, L.A.; Cobos, A.; Diaz, O.; Aguilera, J.M. Chia Seed (Salvia Hispanica): An Ancient Grain and a New Functional Food. Food Rev. Int. 2013, 29, 394–408. [Google Scholar] [CrossRef]
- Shahrajabian, M.H.; Sun, W.; Cheng, Q. Chemical Components and Pharmacological Benefits of Basil (Ocimum Basilicum): A Review. Int. J. Food Prop. 2020, 23, 1961–1970. [Google Scholar] [CrossRef]
- Nazir, S.; Wani, I.A. Functional Characterization of Basil (Ocimum Basilicum L.) Seed Mucilage. Bioact. Carbohydr. Diet. Fibre 2021, 25, 100261. [Google Scholar] [CrossRef]
- Li, R.; Tang, G.; Liu, X.; Li, J.; Wang, D.; Ji, S. An Ethnopharmacological Review of Hyptis Suaveolens (L.) Poit. Trop. J. Pharm. Res. 2020, 19, 1541–1550. [Google Scholar] [CrossRef]
- Mueller, M.; Čavarkapa, A.; Unger, F.M.; Viernstein, H.; Praznik, W. Prebiotic Potential of Neutral Oligo- and Polysaccharides from Seed Mucilage of Hyptis Suaveolens. Food Chem. 2017, 221, 508–514. [Google Scholar] [CrossRef]
- Madgulkar, A.R.; Rao, M.R.P.; Warrier, D. Characterization of Psyllium (Plantago ovata) Polysaccharide and Its Uses. In Polysaccharides; Springer: Cham, Switzerland, 2014; pp. 1–17. [Google Scholar]
- Mahmood, D. Plantago Ovata: A Comprehensive Review On Cultivation, Biochemical, Pharmaceutical And Pharmacological Aspects. Acta Pol. Pharm. 2018, 74, 739–746. [Google Scholar]
- Turker, A.U.; Gurel, E. Common Mullein (Verbascum Thapsus L.): Recent Advances in Research. Phyther. Res. 2005, 19, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Jamshidi-Kia, F.; Lorigooini, Z.; Asgari, S.; Saeidi, K. Iranian Species of Verbascum: A Review of Botany, Phytochemistry, and Pharmacological Effects. Toxin Rev. 2019, 38, 255–262. [Google Scholar] [CrossRef]
- Pastorino, G.; Cornara, L.; Soares, S.; Rodrigues, F.; Oliveira, M.B.P.P. Liquorice (Glycyrrhiza Glabra): A Phytochemical and Pharmacological Review. Phyther. Res. 2018, 32, 2323–2339. [Google Scholar] [CrossRef] [PubMed]
- Shi, B.; Zhang, W.; Jiang, H.; Zhu, Y. A New Anthraquinone from Seed of Cassia Obtusifolia. Nat. Prod. Res. 2016, 30, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Mudgil, D.; Barak, S.; Khatkar, B.S. Guar Gum: Processing, Properties and Food Applications—A Review. J. Food Sci. Technol. 2014, 51, 409–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khole, S.; Chatterjee, S.; Variyar, P.; Sharma, A.; Devasagayam, T.P.A.; Ghaskadbi, S. Bioactive Constituents of Germinated Fenugreek Seeds with Strong Antioxidant Potential. J. Funct. Foods 2014, 6, 270–279. [Google Scholar] [CrossRef]
- Chaudhari, N.B.; Patil, V.R. Isolation and Evaluation of Cassia Fistula Seed Gum as Film Coating Material. Int. J. PharmTech Res. 2011, 3, 1478–1481. [Google Scholar]
- Deore, U.V.; Mahajan, H.S.; Surana, S.J.; Wagh, R.D. Thiolated and Carboxymethylated Cassia obtusifolia Seed Mucilage as Novel Excipient for Drug Delivery: Development and Characterisation. Mater. Technol. 2020, 1–11. [Google Scholar] [CrossRef]
- Deore, U.V.; Mahajan, H.S. Isolation and Structural Characterization of Mucilaginous Polysaccharides Obtained from the Seeds of Cassia uniflora for Industrial Application. Food Chem. 2021, 351, 129262. [Google Scholar] [CrossRef]
- Gupta, S.; Jain, R.; Kachhwaha, S.; Kothari, S.L. Nutritional and Medicinal Applications of Moringa oleifera Lam.—Review of Current Status and Future Possibilities. J. Herb. Med. 2018, 11, 1–11. [Google Scholar] [CrossRef]
- Badwaik, H.R.; Al Hoque, A.; Kumari, L.; Sakure, K.; Baghel, M.; Giri, T.K. Moringa Gum and Its Modified Form as a Potential Green Polymer Used in Biomedical Field. Carbohydr. Polym. 2020, 249, 116893. [Google Scholar] [CrossRef]
- Ajayi, O.O.; Held, M.A.; Showalter, A.M. Two β-Glucuronosyltransferases Involved in the Biosynthesis of Type II Arabinogalactans Function in Mucilage Polysaccharide Matrix Organization in Arabidopsis thaliana. BMC Plant. Biol. 2021, 21, 245. [Google Scholar] [CrossRef]
- Wu, Y.; Hui, D.; Eskin, N.A.M.; Cui, S.W. Water-Soluble Yellow Mustard Mucilage: A Novel Ingredient with Potent Antioxidant Properties. Int. J. Biol. Macromol. 2016, 91, 710–715. [Google Scholar] [CrossRef] [PubMed]
- Koocheki, A.; Razavi, S.M.A.; Hesarinejad, M.A. Effect of Extraction Procedures on Functional Properties of Eruca sativa Seed Mucilage. Food Biophys. 2012, 7, 84–92. [Google Scholar] [CrossRef]
- Kutlu, G.; Akcicek, A.; Bozkurt, F.; Karasu, S.; Tekin-Cakmak, Z.H. Rocket Seed (Eruca sativa Mill) Gum: Physicochemical and Comprehensive Rheological Characterization. Food Sci. Technol. 2021. [Google Scholar] [CrossRef]
- Shah, S.M.A.; Akhtar, N.; Akram, M.; Shah, P.A.; Saeed, T.; Ahmed, K.; Asif, H.M. Pharmacological Activity of Althaea officinalis L. J. Med. Plants Res. 2011, 5, 5662–5666. [Google Scholar]
- Al-Snafi, A.E. The Pharmaceutical Importance of Althaea officinalis and Althaea rosea: A Review. Int. J. Pharm Tech. Res. 2013, 5, 1385–1387. [Google Scholar]
- Lee, H.-B.; Son, S.-U.; Lee, J.-E.; Lee, S.-H.; Kang, C.-H.; Kim, Y.-S.; Shin, K.-S.; Park, H.-Y. Characterization, Prebiotic and Immune-Enhancing Activities of Rhamnogalacturonan-I-Rich Polysaccharide Fraction from Molokhia Leaves. Int. J. Biol. Macromol. 2021, 175, 443–450. [Google Scholar] [CrossRef]
- Pal, A.P.; Chakraborty, P. Investigation of Corchorus olitorius Mucilage as a Potential Mucoadhesive Agent in Developing in Situ Mucoadhesive Nasal Gel. J. Appl. Pharm. Sci. 2020, 10, 90–98. [Google Scholar] [CrossRef]
- Ahmed, F. Nutraceutical Potential of Molokhia (Corchorus olitorius L.): A Versatile Green Leafy Vegetable. Pharmacognosy Res. 2021, 13, 1–12. [Google Scholar] [CrossRef]
- Jani, G.K.; Shah, D.P. Evaluation of Mucilage of Hibiscus rosasinensis Linn as Rate Controlling Matrix for Sustained Release of Diclofenac. Drug Dev. Ind. Pharm. 2008, 34, 807–816. [Google Scholar] [CrossRef]
- Somya, G.; Nayyar, P.; Sharma, P.K. Extraction and Characterization of Hibiscus rosasinensis Mucilage as Pharmaceutical Adjuvant. World Appl. Sci. J. 2015, 33, 136–141. [Google Scholar]
- Gemede, H.F.; Ratta, N.; Haki, G.D.; Woldegiorgis, A.Z.; Beyene, F. Nutritional Quality and Health Benefits of Okra (Abelmoschus esculentus): A Review. J. Food Process. Technol 2015, 6, 2. [Google Scholar] [CrossRef]
- Zhu, X.; Xu, R.; Wang, H.; Chen, J.; Tu, Z. Structural Properties, Bioactivities, and Applications of Polysaccharides from Okra [Abelmoschus esculentus (L.) Moench]: A Review. J. Agric. Food Chem. 2020, 68, 14091–14103. [Google Scholar] [CrossRef]
- Deshmukh, S.A.; Gaikwad, D.K. A Review of the Taxonomy, Ethnobotany, Phytochemistry and Pharmacology of Basella alba (Basellaceae). J. Appl. Pharm. Sci. 2014, 4, 153–165. [Google Scholar] [CrossRef]
- Pal, D.; Pany, D.; Mohanty, B.; Nayak, A. Evaluation of Spinacia oleracea L. Leaves Mucilage as an Innovative Suspending Agent. J. Adv. Pharm. Technol. Res. 2010, 1, 338. [Google Scholar] [CrossRef] [Green Version]
- Mzoughi, Z.; Souid, G.; Timoumi, R.; Le Cerf, D.; Majdoub, H. Partial Characterization of the Edible Spinacia oleracea Polysaccharides: Cytoprotective and Antioxidant Potentials against Cd Induced Toxicity in HCT116 and HEK293 Cells. Int. J. Biol. Macromol. 2019, 136, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Adetuyi, F.O.; Dada, I.B.O. Nutritional, Phytoconstituent and Antioxidant Potential of Mucilage Extract of Okra (Abelmoschus esculentus), Water Leaf (Talinum triangulare) and Jews Mallow (Corchorus olitorius). Int. Food Res. J. 2014, 21, 2345. [Google Scholar]
- Yeh, S.-H.; Hsu, W.-K.; Chang, Z.-Q.; Wang, S.-H.; Hsieh, C.-W.; Liou, G.-G.; Lee, H.-B.; Jiang, B.-H.; Tsou, H.-K.; Tsai, M.-S. Purification and Characterization of Fractions Containing Polysaccharides from Talinum triangulare and Their Immunomodulatory Effects. Processes 2021, 9, 709. [Google Scholar] [CrossRef]
- Monrroy, M.; García, E.; Ríos, K.; García, J.R. Extraction and Physicochemical Characterization of Mucilage from Opuntia cochenillifera (L.) Miller. J. Chem. 2017, 2017, 430190. [Google Scholar] [CrossRef] [Green Version]
- Salehi, E.; Emam-Djomeh, Z.; Fathi, M.; Askari, G. Opuntia ficus-indica Mucilage. In Emerging Natural Hydrocolloids; John Wiley & Sons: Chichester, UK, 2019; pp. 425–449. [Google Scholar]
- Jamkhande, P.G.; Barde, S.R.; Patwekar, S.L.; Tidke, P.S. Plant Profile, Phytochemistry and Pharmacology of Cordia dichotoma (Indian Cherry): A Review. Asian Pac. J. Trop. Biomed. 2013, 3, 1009–1012. [Google Scholar] [CrossRef] [Green Version]
- El-Newary, S.A.; Sulieman, A.M.; El-Attar, S.R.; Sitohy, M.Z. Hypolipidemic and Antioxidant Activity of the Aqueous Extract from the Uneaten Pulp of the Fruit from Cordia dichotoma in Healthy and Hyperlipidemic Wistar Albino Rats. J. Nat. Med. 2016, 70, 539–553. [Google Scholar] [CrossRef]
- Singh, S.; Bothara, S.B. Physico-Chemical and Structural Characterization of Mucilage Isolated from Seeds of Diospyros melonoxylon Roxb. Braz. J. Pharm. Sci. 2014, 50, 713–725. [Google Scholar] [CrossRef]
- Metia, P.K.; Bandyopadhyay, A.K. In Vitro Evaluation of Novel Mucoadhesive Buccal Tablet of Oxytocin Prepared with Diospyros peregrina Fruits Mucilages. Yakugaku Zasshi 2008, 128, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Kajla, P.; Sharma, A.; Sood, D.R. Flaxseed—A Potential Functional Food Source. J. Food Sci. Technol. 2015, 52, 1857–1871. [Google Scholar] [CrossRef] [PubMed]
- Dzuvor, C.; Taylor, J.; Acquah, C.; Pan, S.; Agyei, D. Bioprocessing of Functional Ingredients from Flaxseed. Molecules 2018, 23, 2444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, S.; Chung, M.-H. A Review on the Relationship between Aloe vera Components and Their Biologic Effects. Semin. Integr. Med. 2003, 1, 53–62. [Google Scholar] [CrossRef]
- do Nascimento, G.E.; Iacomini, M.; Cordeiro, L.M.C. A Comparative Study of Mucilage and Pulp Polysaccharides from Tamarillo Fruit (Solanum betaceum Cav.). Plant. Physiol. Biochem. 2016, 104, 278–283. [Google Scholar] [CrossRef]
- Epping, J.; Laibach, N. An Underutilized Orphan Tuber Crop—Chinese Yam: A Review. Planta 2020, 252, 58. [Google Scholar] [CrossRef]
- Patel, N.C.; Shah, V.N.; Mahajan, A.N.; Shah, D.A. Isolation of Mucilage from Cydonia vulgaris Pers. Seeds and Its Evaluation as Superdisintegrant. J. Appl. Pharm. Sci. 2011, 1, 11. [Google Scholar]
- Guzelgulgen, M.; Ozkendir-Inanc, D.; Yildiz, U.H.; Arslan-Yildiz, A. Glucuronoxylan-Based Quince Seed Hydrogel: A Promising Scaffold for Tissue Engineering Applications. Int. J. Biol. Macromol. 2021, 180, 729–738. [Google Scholar] [CrossRef]
- Kassem, I.A.A.; Joshua Ashaolu, T.; Kamel, R.; Elkasabgy, N.A.; Afifi, S.M.; Farag, M.A. Mucilage as a Functional Food Hydrocolloid: Ongoing and Potential Applications in Prebiotics and Nutraceuticals. Food Funct. 2021, 12, 4738–4748. [Google Scholar] [CrossRef]
- Nazari, M.; Riebeling, S.; Banfield, C.C.; Akale, A.; Crosta, M.; Mason-Jones, K.; Dippold, M.A.; Ahmed, M.A. Mucilage Polysaccharide Composition and Exudation in Maize From Contrasting Climatic Regions. Front. Plant. Sci. 2020, 11, 1968. [Google Scholar] [CrossRef]
- Teixeira, A.; Iannetta, P.; Binnie, K.; Valentine, T.A.; Toorop, P. Myxospermous Seed-Mucilage Quantity Correlates with Environmental Gradients Indicative of Water-Deficit Stress: Plantago Species as a Model. Plant. Soil 2020, 446, 343–356. [Google Scholar] [CrossRef] [Green Version]
- Izydorczyk, M.; Cui, S.W.; Wang, Q. Polysaccharide Gums: Structures, Functional Properties, and Applications. Food Carbohydr. Chem. Phys. Prop. Appl. 2005, 293, 299. [Google Scholar]
- Amiri, M.S.; Mohammadzadeh, V.; Yazdi, M.E.T.; Barani, M.; Rahdar, A.; Kyzas, G.Z. Plant-Based Gums and Mucilages Applications in Pharmacology and Nanomedicine: A Review. Molecules 2021, 26, 1770. [Google Scholar] [CrossRef] [PubMed]
- Galloway, A.F.; Knox, P.; Krause, K. Sticky Mucilages and Exudates of Plants: Putative Microenvironmental Design Elements with Biotechnological Value. New Phytol. 2020, 225, 1461–1469. [Google Scholar] [CrossRef] [Green Version]
- Rashid, F.; Ahmed, Z.; Hussain, S.; Huang, J.-Y.; Ahmad, A. Linum Usitatissimum L. Seeds: Flax Gum Extraction, Physicochemical and Functional Characterization. Carbohydr. Polym. 2019, 215, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Viudes, S.; Burlat, V.; Dunand, C. Seed Mucilage Evolution: Diverse Molecular Mechanisms Generate Versatile Ecological Functions for Particular Environments. Plant. Cell Environ. 2020, 43, 2857–2870. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Baskin, J.M.; Baskin, C.C.; Huang, Z. More than just a coating: Ecological importance, taxonomic occurrence and phylogenetic relationships of seed coat mucilage. Perspect. Plant Ecol. Evol. Syst. 2012, 14, 434–442. [Google Scholar] [CrossRef]
- Huang, D.; Wang, C.; Yuan, J.; Cao, J.; Lan, H. Differentiation of the Seed Coat and Composition of the Mucilage of Lepidium perfoliatum L.: A Desert Annual with Typical Myxospermy. Acta Biochim. Biophys. Sin. 2015, 47, 775–787. [Google Scholar] [CrossRef] [Green Version]
- Kreitschitz, A.; Haase, E.; Gorb, S.N. The Role of Mucilage Envelope in the Endozoochory of Selected Plant Taxa. Sci. Nat. 2021, 108, 2. [Google Scholar] [CrossRef]
- Añibarro-Ortega, M.; Pinela, J.; Barros, L.; Ćirić, A.; Silva, S.P.; Coelho, E.; Mocan, A.; Calhelha, R.C.; Soković, M.; Coimbra, M.A.; et al. Compositional Features and Bioactive Properties of Aloe vera Leaf (Fillet, Mucilage, and Rind) and Flower. Antioxidants 2019, 8, 444. [Google Scholar] [CrossRef] [Green Version]
- Ventura-Aguilar, R.I.; Bosquez-Molina, E.; Bautista-Baños, S.; Rivera-Cabrera, F. Cactus Stem (Opuntia ficus-indica Mill): Anatomy, Physiology and Chemical Composition with Emphasis on Its Biofunctional Properties. J. Sci. Food Agric. 2017, 97, 5065–5073. [Google Scholar] [CrossRef]
- Hadi, H.; Razali, S.N.S.; Awadh, A.I. A Comprehensive Review of the Cosmeceutical Benefits of Vanda Species (Orchidaceae). Nat. Prod. Commun. 2015, 10, 1483–1488. [Google Scholar] [CrossRef] [Green Version]
- Cassola, F.; Nunes, C.E.P.; Lusa, M.G.; Garcia, V.L.; Mayer, J.L.S. Deep in the Jelly: Histochemical and Functional Aspects of Mucilage-Secreting Floral Colleters in the Orchids Elleanthus Brasiliensis and E. Crinipes. Front. Plant. Sci. 2019, 10, 518. [Google Scholar] [CrossRef]
- Knee, E.M.; Gong, F.-C.; Gao, M.; Teplitski, M.; Jones, A.R.; Foxworthy, A.; Mort, A.J.; Bauer, W.D. Root Mucilage from Pea and Its Utilization by Rhizosphere Bacteria as a Sole Carbon Source. Mol. Plant. Microbe Interact. 2001, 14, 775–784. [Google Scholar] [CrossRef] [Green Version]
- Korus, J.; Witczak, T.; Ziobro, R.; Juszczak, L. Linseed (Linum usitatissimum L.) Mucilage as a Novel Structure Forming Agent in Gluten-Free Bread. LWT Food Sci. Technol. 2015, 62, 257–264. [Google Scholar] [CrossRef]
- Knez Hrnčič, M.; Ivanovski, M.; Cör, D.; Knez, Ž. Chia Seeds (Salvia Hispanica L.): An Overview—Phytochemical Profile, Isolation Methods, and Application. Molecules 2020, 25, 11. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, S.S.; de las Mercedes Salas-Mellado, M. Addition of Chia Seed Mucilage for Reduction of Fat Content in Bread and Cakes. Food Chem. 2017, 227, 237–244. [Google Scholar] [CrossRef]
- Ribes, S.; Peña, N.; Fuentes, A.; Talens, P.; Barat, J.M. Chia (Salvia hispanica L.) Seed Mucilage as a Fat Replacer in Yogurts: Effect on Their Nutritional, Technological, and Sensory Properties. J. Dairy Sci. 2021, 104, 2822–2833. [Google Scholar] [CrossRef]
- Kozlu, A.; Elmacı, Y. Quince Seed Mucilage as Edible Coating for Mandarin Fruit Determination of the Quality Characteristics during Storage. J. Food Process. Preserv. 2020, 44, e14854. [Google Scholar] [CrossRef]
- Choudhary, P.D.; Pawar, H.A. Recently Investigated Natural Gums and Mucilages as Pharmaceutical Excipients: An Overview. J. Pharm. 2014, 2014, 204849. [Google Scholar] [CrossRef] [Green Version]
- Kawahara, T.; Tsutsui, K.; Nakanishi, E.; Inoue, T.; Hamauzu, Y. Effect of the Topical Application of an Ethanol Extract of Quince Seeds on the Development of Atopic Dermatitis-like Symptoms in NC/Nga Mice. BMC Complement. Altern. Med. 2017, 17, 80. [Google Scholar] [CrossRef] [Green Version]
- Hamdani, A.M.; Wani, I.A.; Bhat, N.A. Sources, Structure, Properties and Health Benefits of Plant Gums: A Review. Int. J. Biol. Macromol. 2019, 135, 46–61. [Google Scholar] [CrossRef]
- Quintal-Bojórquez, N.D.C.; Carrillo-Cocom, L.M.; Hernández-Álvarez, A.J.; Segura-Campos, M.R. Anticancer Activity of Protein Fractions from Chia (Salvia hispanica L.). J. Food Sci. 2021, 86, 2861–2871. [Google Scholar] [CrossRef]
- Tavares, L.S.; Junqueira, L.A.; de Oliveira Guimarães, Í.C.; de Resende, J.V. Cold Extraction Method of Chia Seed Mucilage (Salvia Hispanica L.): Effect on Yield and Rheological Behavior. J. Food Sci. Technol. 2018, 55, 457–466. [Google Scholar] [CrossRef]
- Brütsch, L.; Stringer, F.J.; Kuster, S.; Windhab, E.J.; Fischer, P. Chia Seed Mucilage—A Vegan Thickener: Isolation, Tailoring Viscoelasticity and Rehydration. Food Funct. 2019, 10, 4854–4860. [Google Scholar] [CrossRef] [Green Version]
- Capitani, M.I.; Ixtaina, V.Y.; Nolasco, S.M.; Tomás, M.C. Microstructure, Chemical Composition and Mucilage Exudation of Chia (Salvia hispanica L.) Nutlets from Argentina. J. Sci. Food Agric. 2013, 93, 3856–3862. [Google Scholar] [CrossRef]
- Muñoz, L.A.; Cobos, A.; Diaz, O.; Aguilera, J.M. Chia Seeds: Microstructure, Mucilage Extraction and Hydration. J. Food Eng. 2012, 108, 216–224. [Google Scholar] [CrossRef]
- Sacco, P.; Lipari, S.; Cok, M.; Colella, M.; Marsich, E.; Lopez, F.; Donati, I. Insights into Mechanical Behavior and Biological Properties of Chia Seed Mucilage Hydrogels. Gels 2021, 7, 47. [Google Scholar] [CrossRef]
- Rocha, M.C.; da Penha Píccolo, M.; de Abreu, W.C.; Maradini Filho, A.M.; Barcelos, M.D.F.P. Physicochemical Properties And Use Of Chia Mucilage (Salvia hispanica L.) In The Reduction Of Fat In Cookies. Braz. J. Dev. 2020, 6, 69019–69034. [Google Scholar] [CrossRef]
- Chiang, J.H.; Ong, D.S.M.; Ng, F.S.K.; Hua, X.Y.; Tay, W.L.W.; Henry, C.J. Application of Chia (Salvia hispanica) Mucilage as an Ingredient Replacer in Foods. Trends Food Sci. Technol. 2021, 115, 105–116. [Google Scholar] [CrossRef]
- Xing, X.; Hsieh, Y.S.Y.; Yap, K.; Ang, M.E.; Lahnstein, J.; Tucker, M.R.; Burton, R.A.; Bulone, V. Isolation and Structural Elucidation by 2D NMR of Planteose, a Major Oligosaccharide in the Mucilage of Chia (Salvia hispanica L.) Seeds. Carbohydr. Polym. 2017, 175, 231–240. [Google Scholar] [CrossRef]
- García-Salcedo, Á.J.; Torres-Vargas, O.L.; del Real, A.; Contreras-Jiménez, B.; Rodriguez-Garcia, M.E. Pasting, Viscoelastic, and Physicochemical Properties of Chia (Salvia hispanica L.) Flour and Mucilage. Food Struct. 2018, 16, 59–66. [Google Scholar] [CrossRef]
- Câmara, A.K.F.I.; Okuro, P.K.; Santos, M.; de Souza Paglarini, C.; da Cunha, R.L.; Ruiz-Capillas, C.; Herrero, A.M.; Pollonio, M.A.R. Understanding the Role of Chia (Salvia hispanica L.) Mucilage on Olive Oil-Based Emulsion Gels as a New Fat Substitute in Emulsified Meat Products. Eur. Food Res. Technol. 2020, 246, 909–922. [Google Scholar] [CrossRef]
- Atik, D.S.; Demirci, T.; Öztürk, H.İ.; Demirci, S.; Sert, D.; Akın, N. Chia Seed Mucilage Versus Guar Gum: Effects on Microstructural, Textural, and Antioxidative Properties of Set-Type Yoghurts. Braz. Arch. Biol. Technol. 2020, 63. [Google Scholar] [CrossRef]
- Morales-Tovar, M.E.; Ramos-Ramírez, E.G.; Salazar-Montoya, J.A. Modeling and Optimization of the Parameters Affecting Extraction of the Chan Seed Mucilage (Hyptis suaveolens (L.) Poit) by Mechanical Agitation (MA) and Ultrasound-Assisted Extraction (UAE) in a Multiple Variables System. Food Bioprod. Process. 2020, 120, 166–178. [Google Scholar] [CrossRef]
- Pintado, T.; Ruiz-Capillas, C.; Jiménez-Colmenero, F.; Herrero, A.M. Impact of Culinary Procedures on Nutritional and Technological Properties of Reduced-Fat Longanizas Formulated with Chia (Salvia hispanica L.) or Oat (Avena Sativa L.) Emulsion Gel. Foods 2020, 9, 1847. [Google Scholar] [CrossRef]
- Niknia, S.; Razavi, S.M.A.; Koocheki, A.; Nayebzadeh, A. The Influence of Application of Basil Seed and Sage Seed Gums on the Sensory Properties and Stability of Mayonnaise. Q. Electron. J. Food Process. Preserv. 2011, 2, 61–79. [Google Scholar]
- Gallo, L.R.D.R.; Assunção Botelho, R.B.; Ginani, V.C.; de Lacerda de Oliveira, L.; Riquette, R.F.R.; Leandro, E.D.S. Chia (Salvia hispanica L.) Gel as Egg Replacer in Chocolate Cakes: Applicability and Microbial and Sensory Qualities After Storage. J. Culin. Sci. Technol. 2020, 18, 29–39. [Google Scholar] [CrossRef]
- Felisberto, M.H.F.; Wahanik, A.L.; Gomes-Ruffi, C.R.; Clerici, M.T.P.S.; Chang, Y.K.; Steel, C.J. Use of Chia (Salvia hispanica L.) Mucilage Gel to Reduce Fat in Pound Cakes. LWT Food Sci. Technol. 2015, 63, 1049–1055. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez-Luna, K.; Ansorena, D.; Astiasarán, I. Flax and Hempseed Oil Functional Ingredient Stabilized by Inulin and Chia Mucilage as a Butter Replacer in Muffin Formulations. J. Food Sci. 2020, 85, 3072–3080. [Google Scholar] [CrossRef] [PubMed]
- Guiotto, E.N.; Tomas, M.C.; Haros, C.M. Development of Highly Nutritional Breads with By-Products of Chia (Salvia hispanica L.) Seeds. Foods 2020, 9, 819. [Google Scholar] [CrossRef]
- Salgado-Cruz, M.D.L.P.; Ramírez-Miranda, M.; Díaz-Ramírez, M.; Alamilla-Beltran, L.; Calderón-Domínguez, G. Microstructural Characterisation and Glycemic Index Evaluation of Pita Bread Enriched with Chia Mucilage. Food Hydrocoll. 2017, 69, 141–149. [Google Scholar] [CrossRef]
- Ziemichód, A.; Wójcik, M.; Różyło, R. Ocimum tenuiflorum Seeds and Salvia hispanica Seeds: Mineral and Amino Acid Composition, Physical Properties, and Use in Gluten-Free Bread. CyTA J. Food 2019, 17, 804–813. [Google Scholar] [CrossRef] [Green Version]
- Menga, V.; Amato, M.; Phillips, T.D.; Angelino, D.; Morreale, F.; Fares, C. Gluten-Free Pasta Incorporating Chia (Salvia hispanica L.) as Thickening Agent: An Approach to Naturally Improve the Nutritional Profile and the in Vitro Carbohydrate Digestibility. Food Chem. 2017, 221, 1954–1961. [Google Scholar] [CrossRef]
- Rentería-Ortega, M.; Salgado-Cruz, M.D.L.P.; Morales-Sánchez, E.; Alamilla-Beltrán, L.; Valdespino-León, M.; Calderón-Domínguez, G. Glucose Oxidase Release of Stressed Chia Mucilage-sodium Alginate Capsules Prepared by Electrospraying. J. Food Process. Preserv. 2021, 45, e15484. [Google Scholar] [CrossRef]
- Song, K.Y.; Joung, K.Y.; Shin, S.Y.; Kim, Y.S. Effects of Basil (Ocimum basilicum L.) Seed Mucilage Substituted for Fat Source in Sponge Cake: Physicochemical, Structural, and Retrogradation Properties. Ital. J. Food Sci. 2017, 29, 681–696. [Google Scholar] [CrossRef]
- de Campo, C.; dos Santos, P.P.; Costa, T.M.H.; Paese, K.; Guterres, S.S.; Rios, A.D.O.; Flôres, S.H. Nanoencapsulation of Chia Seed Oil with Chia Mucilage (Salvia hispanica L.) as Wall Material: Characterization and Stability Evaluation. Food Chem. 2017, 234, 1–9. [Google Scholar] [CrossRef]
- Bustamante, M.; Oomah, B.D.; Rubilar, M.; Shene, C. Effective Lactobacillus plantarum and Bifidobacterium infantis Encapsulation with Chia Seed (Salvia hispanica L.) and Flaxseed (Linum usitatissimum L.) Mucilage and Soluble Protein by Spray Drying. Food Chem. 2017, 216, 97–105. [Google Scholar] [CrossRef]
- Dehghani, S.; Noshad, M.; Rastegarzadeh, S.; Hojjati, M.; Fazlara, A. Electrospun Chia Seed Mucilage/PVA Encapsulated with Green Cardamonmum Essential Oils: Antioxidant and Antibacterial Property. Int. J. Biol. Macromol. 2020, 161, 1–9. [Google Scholar] [CrossRef]
- Muñoz-Tebar, N.; Molina, A.; Carmona, M.; Berruga, M.I. Use of Chia By-Products Obtained from the Extraction of Seeds Oil for the Development of New Biodegradable Films for the Agri-Food Industry. Foods 2021, 10, 620. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, L.A.; Aguilera, J.M.; Rodriguez-Turienzo, L.; Cobos, A.; Diaz, O. Characterization and Microstructure of Films Made from Mucilage of Salvia hispanica and Whey Protein Concentrate. J. Food Eng. 2012, 111, 511–518. [Google Scholar] [CrossRef]
- Ruiz-Ruiz, J.C.; Segura-Campos, M.R. Films Based on Salvia hispanica and Clove Oil: Physical, Optical and Mechanical Properties. Emerg. Mater. Res. 2021, 10, 2–6. [Google Scholar] [CrossRef]
- Luo, M.; Cao, Y.; Wang, W.; Chen, X.; Cai, J.; Wang, L.; Xiao, J. Sustained-Release Antimicrobial Gelatin Film: Effect of Chia Mucilage on Physicochemical and Antimicrobial Properties. Food Hydrocoll. 2019, 87, 783–791. [Google Scholar] [CrossRef]
- Mujtaba, M.; Akyuz, L.; Koc, B.; Kaya, M.; Ilk, S.; Cansaran-Duman, D.; Martinez, A.S.; Cakmak, Y.S.; Labidi, J.; Boufi, S. Novel, Multifunctional Mucilage Composite Films Incorporated with Cellulose Nanofibers. Food Hydrocoll. 2019, 89, 20–28. [Google Scholar] [CrossRef]
- Madaan, R.; Bala, R.; Zandu, S.K.; Singh, I. Formulation and Characterization of Fast Dissolving Tablets Using Salvia hispanica (Chia Seed) Mucilage as Superdisintegrant. ACTA Pharm. Sci. 2020, 58, 69. [Google Scholar] [CrossRef]
- Velázquez-Gutiérrez, S.K.; Figueira, A.C.; Rodríguez-Huezo, M.E.; Román-Guerrero, A.; Carrillo-Navas, H.; Pérez-Alonso, C. Sorption Isotherms, Thermodynamic Properties and Glass Transition Temperature of Mucilage Extracted from Chia Seeds (Salvia hispanica L.). Carbohydr. Polym. 2015, 121, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Castejón, N.; Luna, P.; Señoráns, F.J. Ultrasonic Removal of Mucilage for Pressurized Liquid Extraction of Omega-3 Rich Oil from Chia Seeds (Salvia hispanica L.). J. Agric. Food Chem. 2017, 65, 2572–2579. [Google Scholar] [CrossRef]
- Cuomo, F.; Iacovino, S.; Messia, M.C.; Sacco, P.; Lopez, F. Protective Action of Lemongrass Essential Oil on Mucilage from Chia (Salvia hispanica) Seeds. Food Hydrocoll. 2020, 105, 105860. [Google Scholar] [CrossRef]
- Cuomo, F.; Iacovino, S.; Cinelli, G.; Messia, M.C.; Marconi, E.; Lopez, F. Effect of Additives on Chia Mucilage Suspensions: A Rheological Approach. Food Hydrocoll. 2020, 109, 106118. [Google Scholar] [CrossRef]
- Naji-Tabasi, S.; Niazmand, R.; Modiri-Dovom, A. Application of Mucilaginous Seeds (Alyssum homolocarpum and Salvia macrosiphon Boiss) and Wheat Bran in Improving Technological and Nutritional Properties of Pasta. J. Food Sci. 2021, 86, 2288–2299. [Google Scholar] [CrossRef]
- Nasiri, H.; Golestan, L.; Shahidi, S.-A.; Darjani, P. Encapsulation of Lactobacillus casei in Sodium Alginate Microcapsules: Improvement of the Bacterial Viability under Simulated Gastrointestinal Conditions Using Wild Sage Seed Mucilage. J. Food Meas. Charact. 2021, 15, 4726–4734. [Google Scholar] [CrossRef]
- Davachi, S.M.; Shekarabi, A.S. Preparation and Characterization of Antibacterial, Eco-Friendly Edible Nanocomposite Films Containing Salvia macrosiphon and Nanoclay. Int. J. Biol. Macromol. 2018, 113, 66–72. [Google Scholar] [CrossRef]
- Davoodi, S.; Davachi, S.M.; Ghorbani Golkhajeh, A.; Shekarabi, A.S.; Abbaspourrad, A. Development and Characterization of Salvia Macrosiphon/Chitosan Edible Films. ACS Sustain. Chem. Eng. 2020, 8, 1487–1496. [Google Scholar] [CrossRef]
- Bostan, A.; Razavi, S.M.A.; Farhoosh, R. Optimization of Hydrocolloid Extraction From Wild Sage Seed (Salvia macrosiphon) Using Response Surface. Int. J. Food Prop. 2010, 13, 1380–1392. [Google Scholar] [CrossRef] [Green Version]
- Razavi, S.M.A.; Mortazavi, S.A.; Matia-Merino, L.; Hosseini-Parvar, S.H.; Motamedzadegan, A.; Khanipour, E. Optimisation Study of Gum Extraction from Basil Seeds (Ocimum basilicum L.). Int. J. Food Sci. Technol. 2009, 44, 1755–1762. [Google Scholar] [CrossRef]
- Naji-Tabasi, S.; Razavi, S.M.A.; Mohebbi, M.; Malaekeh-Nikouei, B. New Studies on Basil (Ocimum bacilicum L.) Seed Gum: Part I—Fractionation, Physicochemical and Surface Activity Characterization. Food Hydrocoll. 2016, 52, 350–358. [Google Scholar] [CrossRef]
- Naji-Tabasi, S.; Razavi, S.M.A. Functional Properties and Applications of Basil Seed Gum: An Overview. Food Hydrocoll. 2017, 73, 313–325. [Google Scholar] [CrossRef]
- Mohammad Amini, A.; Razavi, S.M.A.; Zahedi, Y. The Influence of Different Plasticisers and Fatty Acids on Functional Properties of Basil Seed Gum Edible Film. Int. J. Food Sci. Technol. 2015, 50, 1137–1143. [Google Scholar] [CrossRef]
- Wongputtisin, P.; Khanongnuch, C. Prebiotic Properties of Crude Oligosaccharide Prepared from Enzymatic Hydrolysis of Basil Seed Gum. Food Sci. Biotechnol. 2015, 24, 1767–1773. [Google Scholar] [CrossRef]
- Ghasempour, Z.; Javanmard, N.; Mojaddar Langroodi, A.; Alizadeh-Sani, M.; Ehsani, A.; Moghaddas Kia, E. Development of Probiotic Yogurt Containing Red Beet Extract and Basil Seed Gum; Techno-Functional, Microbial and Sensorial Characterization. Biocatal. Agric. Biotechnol. 2020, 29, 101785. [Google Scholar] [CrossRef]
- Imam, H.; Lian, S.; Kasimu, R.; Rakhmanberdyeva, R.K.; Aisa, H.A. Extraction of an Antidiabetic Polysaccharide from Seeds of Ocimum basilicum and Determination of the Monosaccharide Composition by Precolumn High-Efficiency Capillary Electrophoresis A. Chem. Nat. Compd. 2012, 48, 653–654. [Google Scholar] [CrossRef]
- Feng, B.; Zhu, Y.; Sun, C.; Su, Z.; Tang, L.; Li, C.; Zheng, G. Basil Polysaccharide Inhibits Hypoxia-Induced Hepatocellular Carcinoma Metastasis and Progression through Suppression of HIF-1α-Mediated Epithelial-Mesenchymal Transition. Int. J. Biol. Macromol. 2019, 137, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Gajendiran, A.; Thangaraman, V.; Thangamani, S.; Ravi, D.; Abraham, J. Antimicrobial, Antioxidant and Anticancer Screening of Ocimum basilicum Seeds. Bull. Pharm. Res. 2016, 6, 114–119. [Google Scholar]
- Praznik, W.; Čavarkapa, A.; Unger, F.M.; Loeppert, R.; Holzer, W.; Viernstein, H.; Mueller, M. Molecular Dimensions and Structural Features of Neutral Polysaccharides from the Seed Mucilage of Hyptis suaveolens L. Food Chem. 2017, 221, 1997–2004. [Google Scholar] [CrossRef] [PubMed]
- Ngozi, L. The Efficacy of Hyptis suaveolens: A Review of Its Nutritional and Medicinal Applications. European J. Med. Plants 2014, 4, 661–674. [Google Scholar] [CrossRef]
- Aspinall, G.O.; Capek, P.; Carpenter, R.C.; Gowda, D.C.; Szafranek, J. A Novel L-Fuco-4-O-Methyl-d-Glucurono-d-Xylan from Hyptis Suaveolens. Carbohydr. Res. 1991, 214, 107–113. [Google Scholar] [CrossRef]
- Rodriguez, A.A.; Alfaro, J.; Vargas, R.; Pacheco, J.; Araya, J.J. Evaluation of Mucilages Isolated from Seeds of Hyptis suaveolens, Salvia hispanica and Linum usitatissimum as Pharmaceutical Excipients in Solid Dose and Liquid Formulations. J. Excip. Food Chem. 2018, 9, 67–79. [Google Scholar]
- Gonçalves, S.; Romano, A. The Medicinal Potential of Plants from the Genus Plantago (Plantaginaceae). Ind. Crops Prod. 2016, 83, 213–226. [Google Scholar] [CrossRef]
- Ramawat, K.G.; Mérillon, J.M. Polysaccharides: Bioactivity and Biotechnology; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1–2241. [Google Scholar] [CrossRef]
- Benaoun, F.; Delattre, C.; Boual, Z.; Ursu, A.V.; Vial, C.; Gardarin, C.; Wadouachi, A.; Le Cerf, D.; Varacavoudin, T.; Ould El-Hadj, M.D.; et al. Structural Characterization and Rheological Behavior of a Heteroxylan Extracted from Plantago Notata Lagasca (Plantaginaceae) Seeds. Carbohydr. Polym. 2017, 175, 96–104. [Google Scholar] [CrossRef]
- Patel, M.K.; Tanna, B.; Gupta, H.; Mishra, A.; Jha, B. Physicochemical, Scavenging and Anti-Proliferative Analyses of Polysaccharides Extracted from Psyllium (Plantago ovata Forssk) Husk and Seeds. Int. J. Biol. Macromol. 2019, 133, 190–201. [Google Scholar] [CrossRef]
- Cheng, J.; Tennilä, J.; Stenman, L.; Ibarra, A.; Kumar, M.; Gupta, K.K.; Sharma, S.S.; Sen, D.; Garg, S.; Penurkar, M. Influence of Lactitol and Psyllium on Bowel Function in Constipated Indian Volunteers: A Randomized, Controlled Trial. Nutrients 2019, 11, 1130. [Google Scholar] [CrossRef] [Green Version]
- Hasheminasab, F.S.; Hashemi, S.M.; Dehghan, A.; Sharififar, F.; Setayesh, M.; Sasanpour, P.; Tasbandi, M.; Raeiszadeh, M. Effects of a Plantago ovata-Based Herbal Compound in Prevention and Treatment of Oral Mucositis in Patients with Breast Cancer Receiving Chemotherapy: A Double-Blind, Randomized, Controlled Crossover Trial. J. Integr. Med. 2020, 18, 214–221. [Google Scholar] [CrossRef]
- Draksiene, G.; Kopustinskiene, D.M.; Lazauskas, R.; Bernatoniene, J. Psyllium (Plantago ovata Forsk) Husk Powder as a Natural Superdisintegrant for Orodispersible Formulations: A Study on Meloxicam Tablets. Molecules 2019, 24, 3255. [Google Scholar] [CrossRef] [Green Version]
- Yu, L.; Yakubov, G.E.; Zeng, W.; Xing, X.; Stenson, J.; Bulone, V.; Stokes, J.R. Multi-Layer Mucilage of Plantago ovata Seeds: Rheological Differences Arise from Variations in Arabinoxylan Side Chains. Carbohydr. Polym. 2017, 165, 132–141. [Google Scholar] [CrossRef] [Green Version]
- Yin, J.-Y.; Chen, H.-H.; Lin, H.-X.; Xie, M.-Y.; Nie, S.-P. Structural Features of Alkaline Extracted Polysaccharide from the Seeds of Plantago asiatica L. and Its Rheological Properties. Molecules 2016, 21, 1181. [Google Scholar] [CrossRef] [Green Version]
- Kreitschitz, A.; Kovalev, A.; Gorb, S.N. Plant Seed Mucilage as a Glue: Adhesive Properties of Hydrated and Dried-in-Contact Seed Mucilage of Five Plant Species. Int. J. Mol. Sci. 2021, 22, 1443. [Google Scholar] [CrossRef]
- Cowley, J.M.; O’Donovan, L.A.; Burton, R.A. The Composition of Australian Plantago Seeds Highlights Their Potential as Nutritionally-Rich Functional Food Ingredients. Sci. Rep. 2021, 11, 12692. [Google Scholar] [CrossRef] [PubMed]
- Kreitschitz, A.; Kovalev, A.; Gorb, S.N. “Sticky Invasion”—The Physical Properties of Plantago lanceolata L. Seed Mucilage. Beilstein J. Nanotechnol. 2016, 7, 1918–1927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belorio, M.; Gómez, M. Psyllium: A Useful Functional Ingredient in Food Systems. Crit. Rev. Food Sci. Nutr. 2020, 1–12. [Google Scholar] [CrossRef]
- Mehrinejad Choobari, S.Z.; Sari, A.A.; Daraei Garmakhany, A. Effect of Plantago ovata Forsk Seed Mucilage on Survivability of Lactobacillus Acidophilus, Physicochemical and Sensory Attributes of Produced Low-fat Set Yoghurt. Food Sci. Nutr. 2021, 9, 1040–1049. [Google Scholar] [CrossRef]
- Jones, B.O.; John, O.O.; Luke, C.; Ochieng, A.; Bassey, B.J. Application of Mucilage from Dicerocaryum eriocarpum Plant as Biosorption Medium in the Removal of Selected Heavy Metal Ions. J. Environ. Manage. 2016, 177, 365–372. [Google Scholar] [CrossRef]
- Basiri, S.; Shekarforoush, S.S.; Mazkour, S.; Modabber, P.; Kordshouli, F.Z. Evaluating the Potential of Mucilaginous Seed of Psyllium (Plantago ovata) as a New Lead Biosorbent. Bioact. Carbohydr. Diet. Fibre 2020, 24, 100242. [Google Scholar] [CrossRef]
- Mehrafarin, A.; Rezazadeh, S.; Naghdi Badi, H.; Noormohammadi, G.; Zand, E.; Qaderi, A. A Review on Biology, Cultivation and Biotechnology of Fenugreek (Trigonella foenum-graecum L.) as a Valuable Medicinal Plant and Multipurpose. J. Med. Plants. 2011, 10, 6–24. [Google Scholar]
- Kilar, M.; Kilar, J.H. Wykorzystanie Kozieradki Pospolitej (Trigonella foenum-graecum L.) w Zielarstwie i Fitoterapii (Use of fenugreek (Trigonella foenum-graecum L.) is a herbaceous annual plant). Herbalism 2016, 1, 89–106. [Google Scholar] [CrossRef]
- Syed, Q.A.; Zainab, R.; Ahmad, M.A.; Shukat, R.; Ishaq, R.; Niaz, M.; Rahmad, H.U.U. Nutritional and Therapeutic Properties of Fenugreek (Trigonella foenum-graecum): A Review. Int. J. Food Prop. 2020, 23, 1777–1791. [Google Scholar] [CrossRef]
- Sindhu, G.; Shyni, G.L.; Pushpan, C.K.; Nambisan, B.; Helen, A. Evaluation of Anti-Arthritic Potential of Trigonella foenum graecum L. (Fenugreek) Mucilage against Rheumatoid Arthritis. Prostaglandins Other Lipid Mediat. 2018, 138, 48–53. [Google Scholar] [CrossRef]
- Al-Jasass, F.M.; Al-Jasser, M.S. Chemical Composition and Fatty Acid Content of Some Spices and Herbs under Saudi Arabia Conditions. Sci. World J. 2012, 2012, 859892. [Google Scholar] [CrossRef] [Green Version]
- Iurian, S.; Dinte, E.; Iuga, C.; Bogdan, C.; Spiridon, I.; Barbu-Tudoran, L.; Bodoki, A.; Tomuţă, I.; Leucuţa, S.E. The Pharmaceutical Applications of a Biopolymer Isolated from Trigonella foenum-graecum Seeds: Focus on the Freeze-Dried Matrix Forming Capacity. Saudi Pharm. J. 2017, 25, 1217–1225. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Kumar, N.; Sharma, P.K. Extraction and Evaluation of Trigonella foenum graecum Linn. and Linum usitatissimum Seed Mucilage. Glob. J. Pharmacol. 2014, 8, 510–514. [Google Scholar]
- Youssef, M.K.; Wang, Q.; Cui, S.W.; Barbut, S. Purification and Partial Physicochemical Characteristics of Protein Free Fenugreek Gums. Food Hydrocoll. 2009, 23, 2049–2053. [Google Scholar] [CrossRef]
- Salarbashi, D.; Bazeli, J.; Fahmideh-Rad, E. Fenugreek Seed Gum: Biological Properties, Chemical Modifications, and Structural Analysis—A Review. Int. J. Biol. Macromol. 2019, 138, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Memiş, S.; Tornuk, F.; Bozkurt, F.; Durak, M.Z. Production and Characterization of a New Biodegradable Fenugreek Seed Gum Based Active Nanocomposite Film Reinforced with Nanoclays. Int. J. Biol. Macromol. 2017, 103, 669–675. [Google Scholar] [CrossRef]
- Wu, T.; Yan, M.H.; Zhang, Y.; Miao, Y.Q.; Lu, C.M.; Wu, G.R. Antioxidant and Antimicrobial Activity of Acidolysis and Enzymolysis Products of Fenugreek Polysaccharides. J. Food. Sci. 2007, 28, 509–543. [Google Scholar]
- Kumar, D.; Singhal, A.; Bansal, S.; Gupta, S. Extraction, Isolation and Evaluation Trigonella foenum-graecum as Mucoadhesive Agent for Nasal Gel Drug Delivery. J. Nepal Pharm. Assoc. 2015, 27, 40–45. [Google Scholar] [CrossRef] [Green Version]
- Haghshenas, B.; Abdullah, N.; Nami, Y.; Radiah, D.; Rosli, R.; Yari Khosroushahi, A. Microencapsulation of Probiotic Bacteria Lactobacillus plantarum 15HN Using Alginate-Psyllium-Fenugreek Polymeric Blends. J. Appl. Microbiol. 2015, 118, 1048–1057. [Google Scholar] [CrossRef]
- Zemzmi, J.; Ródenas, L.; Blas, E.; Najar, T.; Pascual, J.J. Characterisation and In Vitro Evaluation of Fenugreek (Trigonella foenum-graecum) Seed Gum as a Potential Prebiotic in Growing Rabbit Nutrition. Animals 2020, 10, 1041. [Google Scholar] [CrossRef]
- Deshmukh, A.S.; Barge, S.H.; Gaikwad, D.K. Palyno-Morphometric Studies in Some Cassia L. Species From Maharashtra. Indian J. Plant. Sci. 2014, 4, 71–78. [Google Scholar]
- Vadivel, V.; Kunyanga, C.N.; Biesalski, H.K. Antioxidant Potential and Type II Diabetes-Related Enzyme Inhibition of Cassia obtusifolia L.: Effect of Indigenous Processing Methods. Food Bioprocess. Technol. 2011, 5, 2687–2696. [Google Scholar] [CrossRef]
- Deore, U.V.; Mahajan, H.S. Isolation and Characterization of Natural Polysaccharide from Cassia obtustifolia Seed Mucilage as Film Forming Material for Drug Delivery. Int. J. Biol. Macromol. 2018, 115, 1071–1078. [Google Scholar] [CrossRef]
- Dong, X.; Fu, J.; Yin, X.; Yang, C.; Zhang, X.I.N.; Wang, W.; Du, X.; Wang, Q.; Ni, J. Cassiae Semen: A Review of Its Phytochemistry and Pharmacology (Review). Mol. Med. Rep. 2017, 16, 2331–2346. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Bothara, S.B.; Singh, S.; Patel, R.D.; Mahobia, N.K. Pharmaceutical Characterization of Cassia tora of Seed Mucilage in Tablet Formulations. Der Pharm. Lett. 2010, 2, 54–61. [Google Scholar]
- Yadav, S.; Sharma, P.K.; Goyal, N.K.; Patel, D.K. Extraction and Characterization of Mucilage from Cassia fistula Seeds. Am. J. Sci. Res. 2015, 10, 68–72. [Google Scholar] [CrossRef]
- Pareek, V.; Singh, M.; Bhat, Z.A.; Singh, P.; Kumar, D.; Sheela, S. Studies on Mucilage of Basella alba Linn. J. Pharm Res. 2010, 3, 1892–1894. [Google Scholar]
- Chatchawal, C.; Nualkaew, N.; Preeprame, S.; Porasuphatana, S.; Priprame, A. Physical and Biological Properties of Mucilage from Basella alba L. Stem and Its Gel Formulation. Isan J. Pharm. Sci. 2010, 6, 104–112. [Google Scholar]
- Ramu, G.; Mohan, G.K.; Jayaveera, K. Preliminary Investigation of Patchaippasali Mucilage (Basella alba) as Tablet Binder. Int. J. Green Pharm. 2011, 5, 24. [Google Scholar] [CrossRef]
- Das, S.; Alam, M.N.; Batuta, S.; Ahamed, G.; Fouzder, C.; Kundu, R.; Mandal, D.; Begum, N.A. Exploring the Efficacy of Basella alba Mucilage towards the Encapsulation of the Hydrophobic Antioxidants for Their Better Performance. Process. Biochem. 2017, 61, 178–188. [Google Scholar] [CrossRef]
- Gutierrez, R.M.P.; Velazquez, E.G.; Carrera, S.P.P. Spinacia oleracea Linn Considered as One of the Most Perfect Foods: A Pharmacological and Phytochemical Review. Mini-Rev. Med. Chem. 2019, 19, 1666–1680. [Google Scholar] [CrossRef]
- Nor, H.I.; Tengku, N.; Yusnita, H. Optimization of Extraction Conditions on Yield, Crude Protein Content and Emulsifying Capacity of Mucilage from Talinum paniculatum. Asian J. Agric. Biol. 2019, 7, 156–165. [Google Scholar]
- Reyes-Reyes, M.; Salazar-Montoya, J.A.; Rodríguez-Páez, L.I.; Ramos-Ramírez, E.G. In Vitro Fermentation of Oligosaccharides Obtained from Enzymatic Hydrolysis of Opuntia streptacantha Mucilage. J. Sci. Food Agric. 2019, 99, 2883–2891. [Google Scholar] [CrossRef] [PubMed]
- Espino-Díaz, M.; De Jesús Ornelas-Paz, J.; Martínez-Téllez, M.A.; Santillán, C.; Barbosa-Cánovas, G.V.; Zamudio-Flores, P.B.; Olivas, G.I. Development and Characterization of Edible Films Based on Mucilage of Opuntia ficus-indica (L.). J. Food Sci. 2010, 75, E347–E352. [Google Scholar] [CrossRef]
- Cruz-Rubio, J.M.; Mueller, M.; Loeppert, R.; Viernstein, H.; Praznik, W. The Effect of Cladode Drying Techniques on the Prebiotic Potential and Molecular Characteristics of the Mucilage Extracted from Opuntia ficus-indica and Opuntia Joconostle. Sci. Pharm. 2020, 88, 43. [Google Scholar] [CrossRef]
- du Toit, A.; de Wit, M.; Hugo, A. Cultivar and Harvest Month Influence the Nutrient Content of Opuntia spp. Cactus Pear Cladode Mucilage Extracts. Molecules 2018, 23, 916. [Google Scholar] [CrossRef] [Green Version]
- Liguori, G.; Gentile, C.; Gaglio, R.; Perrone, A.; Guarcello, R.; Francesca, N.; Fretto, S.; Inglese, P.; Settanni, L. Effect of Addition of Opuntia ficus-indica Mucilage on the Biological Leavening, Physical, Nutritional, Antioxidant and Sensory Aspects of Bread. J. Biosci. Bioeng. 2020, 129, 184–191. [Google Scholar] [CrossRef]
- de Andrade Vieira, É.; Alves Alcântara, M.; Albuquerque dos Santos, N.; Duarte Gondim, A.; Iacomini, M.; Mellinger, C.; Tribuzy de Magalhães Cordeiro, A.M. Mucilages of Cacti from Brazilian Biodiversity: Extraction, Physicochemical and Technological Properties. Food Chem. 2021, 346, 128892. [Google Scholar] [CrossRef]
- Cruz-Rubio, J.M.; Mueller, M.; Viernstein, H.; Loeppert, R.; Praznik, W. Prebiotic Potential and Chemical Characterization of the Poly and Oligosaccharides Present in the Mucilage of Opuntia ficus-indica and Opuntia joconostle. Food Chem. 2021, 362, 130167. [Google Scholar] [CrossRef]
- Dominguez-Martinez, B.M.; Martínez-Flores, H.E.; Berrios, J.D.J.; Otoni, C.G.; Wood, D.F.; Velazquez, G. Physical Characterization of Biodegradable Films Based on Chitosan, Polyvinyl Alcohol and Opuntia Mucilage. J. Polym. Environ. 2017, 25, 683–691. [Google Scholar] [CrossRef]
- Allegra, A.; Sortino, G.; Inglese, P.; Settanni, L.; Todaro, A.; Gallotta, A. The Effectiveness of Opuntia ficus-indica Mucilage Edible Coating on Post-Harvest Maintenance of ‘Dottato’ Fig (Ficus Carica L.) Fruit. Food Packag. Shelf Life 2017, 12, 135–141. [Google Scholar] [CrossRef]
- de Campo, C.; Dick, M.; Pereira dos Santos, P.; Haas Costa, T.M.; Paese, K.; Stanisçuaski Guterres, S.; de Oliveira Rios, A.; Hickmann Flôres, S. Zeaxanthin Nanoencapsulation with Opuntia Monacantha Mucilage as Structuring Material: Characterization and Stability Evaluation under Different Temperatures. Colloids Surfaces A Physicochem. Eng. Asp. 2018, 558, 410–421. [Google Scholar] [CrossRef]
- Otálora, M.C.; Carriazo, J.G.; Iturriaga, L.; Nazareno, M.A.; Osorio, C. Microencapsulation of Betalains Obtained from Cactus Fruit (Opuntia ficus-indica) by Spray Drying Using Cactus Cladode Mucilage and Maltodextrin as Encapsulating Agents. Food Chem. 2015, 187, 174–181. [Google Scholar] [CrossRef]
- Adjeroud, N.; Elabbas, S.; Merzouk, B.; Hammoui, Y.; Felkai-Haddache, L.; Remini, H.; Leclerc, J.-P.; Madani, K. Effect of Opuntia ficus-indica Mucilage on Copper Removal from Water by Electrocoagulation-Electroflotation Technique. J. Electroanal. Chem. 2018, 811, 26–36. [Google Scholar] [CrossRef]
- Tannous, S.; Haykal, T.; Dhaini, J.; Hodroj, M.H.; Rizk, S. The Anti-Cancer Effect of Flaxseed Lignan Derivatives on Different Acute Myeloid Leukemia Cancer Cells. Biomed. Pharmacother. 2020, 132, 110884. [Google Scholar] [CrossRef]
- Thakur, G.; Mitra, A.; Pal, K.; Rousseau, D. Effect of Flaxseed Gum on Reduction of Blood Glucose and Cholesterol in Type 2 Diabetic Patients. Int. J. Food Sci. Nutr. 2009, 60, 126–136. [Google Scholar] [CrossRef]
- Prasad, K. Flaxseed and Cardiovascular Health. J. Cardiovasc. Pharmacol. 2009, 54, 369–377. [Google Scholar] [CrossRef] [Green Version]
- Kaneda, T.; Nakajima, Y.; Koshikawa, S.; Nugroho, A.E.; Morita, H. Cyclolinopeptide F, a Cyclic Peptide from Flaxseed Inhibited RANKL-Induced Osteoclastogenesis via Downergulation of RANK Expression. J. Nat. Med. 2019, 73, 504–512. [Google Scholar] [CrossRef]
- Hu, Y.; Shim, Y.Y.; Reaney, M.J.T. Flaxseed Gum Solution Functional Properties. Foods 2020, 9, 681. [Google Scholar] [CrossRef]
- Fabre, J.-F.; Lacroux, E.; Valentin, R.; Mouloungui, Z. Ultrasonication as a Highly Efficient Method of Flaxseed Mucilage Extraction. Ind. Crops Prod. 2015, 65, 354–360. [Google Scholar] [CrossRef] [Green Version]
- Cowley, J.M.; Herliana, L.; Neumann, K.A.; Ciani, S.; Cerne, V.; Burton, R.A. A Small-Scale Fractionation Pipeline for Rapid Analysis of Seed Mucilage Characteristics. Plant. Methods 2020, 16, 20. [Google Scholar] [CrossRef]
- Akl, E.M.; Abdelhamid, S.M.; Wagdy, S.M.; Salama, H.H. Manufacture of Functional Fat-Free Cream Cheese Fortified with Probiotic Bacteria and Flaxseed Mucilage as a Fat Replacing Agent. Curr. Nutr. Food Sci. 2020, 16, 1393–1403. [Google Scholar] [CrossRef]
- Bustamante, M.; Villarroel, M.; Rubilar, M.; Shene, C. Lactobacillus acidophilus La-05 Encapsulated by Spray Drying: Effect of Mucilage and Protein from Flaxseed (Linum usitatissimum L.). LWT Food Sci. Technol. 2015, 62, 1162–1168. [Google Scholar] [CrossRef]
- Nerkar, P.P.; Gattani, S. In Vivo, in Vitro Evaluation of Linseed Mucilage Based Buccal Mucoadhesive Microspheres of Venlafaxine. Drug Deliv. 2011, 18, 111–121. [Google Scholar] [CrossRef]
- Tee, Y.B.; Wong, J.; Tan, M.C.; Talib, R.A. Development of Edible Film from Flaxseed Mucilage. BioResources 2016, 11, 10286–11029. [Google Scholar] [CrossRef] [Green Version]
- de Paiva, P.H.E.N.; Correa, L.G.; Paulo, A.F.S.; Balan, G.C.; Ida, E.I.; Shirai, M.A. Film Production with Flaxseed Mucilage and Polyvinyl Alcohol Mixtures and Evaluation of Their Properties. J. Food Sci. Technol. 2021, 58, 3030–3038. [Google Scholar] [CrossRef]
- Gao, Y.; Kuok, K.I.; Jin, Y.; Wang, R. Biomedical Applications of Aloe vera. Crit. Rev. Food Sci. Nutr. 2019, 59, S244–S256. [Google Scholar] [CrossRef]
- Hęś, M.; Dziedzic, K.; Górecka, D.; Jędrusek-Golińska, A.; Gujska, E. Aloe vera (L.) Webb.: Natural Sources of Antioxidants— Review. Plant Foods Hum. Nutr. 2019, 74, 255–265. [Google Scholar] [CrossRef] [Green Version]
- Sánchez, M.; González-Burgos, E.; Iglesias, I.; Gómez-Serranillos, M.P. Pharmacological Update Properties of Aloe vera and Its Major Active Constituents. Molecules 2020, 25, 1324. [Google Scholar] [CrossRef] [Green Version]
- Tornero-Martínez, A.; Cruz-Ortiz, R.; Jaramillo-Flores, M.E.; Osorio-Díaz, P.; Ávila-Reyes, S.V.; Alvarado-Jasso, G.M.; Mora-Escobedo, R. In Vitro Fermentation of Polysaccharides from Aloe vera and the Evaluation of Antioxidant Activity and Production of Short Chain Fatty Acids. Molecules 2019, 24, 3605. [Google Scholar] [CrossRef] [Green Version]
- Hamman, J. Composition and Applications of Aloe vera Leaf Gel. Molecules 2008, 13, 1599–1616. [Google Scholar] [CrossRef] [Green Version]
- Sumi, F.A.; Sikder, B.; Rahman, M.M.; Lubna, S.R.; Ulla, A.; Hossain, M.H.; Jahan, I.A.; Alam, M.A.; Subhan, N. Phenolic Content Analysis of Aloe vera Gel and Evaluation of the Effect of Aloe Gel Supplementation on Oxidative Stress and Fibrosis in Isoprenaline-Administered Cardiac Damage in Rats. Prev. Nutr. Food Sci. 2019, 24, 254–264. [Google Scholar] [CrossRef]
- Rahman, M.S.; Islam, R.; Rana, M.M.; Spitzhorn, L.-S.; Rahman, M.S.; Adjaye, J.; Asaduzzaman, S.M. Characterization of Burn Wound Healing Gel Prepared from Human Amniotic Membrane and Aloe vera Extract. BMC Complement. Altern. Med. 2019, 19, 115. [Google Scholar] [CrossRef] [Green Version]
- Jain, S. Antibacterial Effect of Aloe Vera Gel against Oral Pathogens: An In Vitro Study. J. Clin. Diagn. Res. 2016, 10, ZC41–ZC44. [Google Scholar] [CrossRef]
- Otálora, M.C.; Wilches-Torres, A.; Castaño, J.A.G. Extraction and Physicochemical Characterization of Dried Powder Mucilage from Opuntia ficus-indica Cladodes and Aloe vera Leaves: A Comparative Study. Polymers 2021, 13, 1689. [Google Scholar] [CrossRef]
- Liu, C.; Du, P.; Guo, Y.; Xie, Y.; Yu, H.; Yao, W.; Cheng, Y.; Qian, H. Extraction, Characterization of Aloe Polysaccharides and the in-Depth Analysis of Its Prebiotic Effects on Mice Gut Microbiota. Carbohydr. Polym. 2021, 261, 117874. [Google Scholar] [CrossRef]
- Saini, D.K.; Saini, M.R. Evaluation of Radioprotective Efficacy and Possible Mechanism of Action of Aloe Gel. Environ. Toxicol. Pharmacol. 2011, 31, 427–435. [Google Scholar] [CrossRef]
- Im, S.-A.; Kim, J.-W.; Kim, H.-S.; Park, C.-S.; Shin, E.; Do, S.-G.; Park, Y.I.; Lee, C.-K. Prevention of Azoxymethane/Dextran Sodium Sulfate-Induced Mouse Colon Carcinogenesis by Processed Aloe vera Gel. Int. Immunopharmacol. 2016, 40, 428–435. [Google Scholar] [CrossRef]
- Hussain, A.; Sharma, C.; Khan, S.; Shah, K.; Haque, S. Aloe Vera Inhibits Proliferation of Human Breast and Cervical Cancer Cells and Acts Synergistically with Cisplatin. Asian Pacific J. Cancer Prev. 2015, 16, 2939–2946. [Google Scholar] [CrossRef] [Green Version]
- Pandey, R.; Mishra, A. Antibacterial Activities of Crude Extract of Aloe barbadensis to Clinically Isolated Bacterial Pathogens. Appl. Biochem. Biotechnol. 2010, 160, 1356–1361. [Google Scholar] [CrossRef]
- Goudarzi, M.; Fazeli, M.; Azad, M.; Seyedjavadi, S.S.; Mousavi, R. Aloe Vera Gel: Effective Therapeutic Agent against Multidrug-Resistant Pseudomonas aeruginosa Isolates Recovered from Burn Wound Infections. Chemother. Res. Pract. 2015, 39806, 639806. [Google Scholar] [CrossRef] [Green Version]
- Quezada, M.P.; Salinas, C.; Gotteland, M.; Cardemil, L. Acemannan and Fructans from Aloe vera (Aloe barbadensis Miller) Plants as Novel Prebiotics. J. Agric. Food Chem. 2017, 65, 10029–10039. [Google Scholar] [CrossRef]
- Gullón, B.; Gullón, P.; Tavaria, F.; Alonso, J.L.; Pintado, M. In Vitro Assessment of the Prebiotic Potential of Aloe vera Mucilage and Its Impact on the Human Microbiota. Food Funct. 2015, 6, 525–531. [Google Scholar] [CrossRef]
- Passafiume, R.; Gaglio, R.; Sortino, G.; Farina, V. Effect of Three Different Aloe vera Gel-Based Edible Coatings on the Quality of Fresh-Cut “Hayward” Kiwifruits. Foods 2020, 9, 939. [Google Scholar] [CrossRef] [PubMed]
- Gannasin, S.P.; Adzahan, N.M.; Mustafa, S.; Muhammad, K. Techno-Functional Properties and in Vitro Bile Acid-Binding Capacities of Tamarillo (Solanum betaceum Cav.) Hydrocolloids. Food Chem. 2016, 196, 903–909. [Google Scholar] [CrossRef] [PubMed]
- Do Nascimento, G.E.; Corso, C.R.; De Paula Werner, M.F.; Baggio, C.H.; Iacomini, M.; Cordeiro, L.M.C. Structure of an Arabinogalactan from the Edible Tropical Fruit Tamarillo (Solanum betaceum) and Its Antinociceptive Activity. Carbohydr. Polym. 2015, 116, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Gannasin, S.P.; Mustafa, S.; Adzahan, N.M.; Muhammad, K. In Vitro Prebiotic Activities of Tamarillo (Solanum betaceum Cav.) Hydrocolloids. J. Funct. Foods 2015, 19, 10–19. [Google Scholar] [CrossRef]
- Acosta-Quezada, P.G.; Raigón, M.D.; Riofrío-Cuenca, T.; García-Martínez, M.D.; Plazas, M.; Burneo, J.I.; Figueroa, J.G.; Vilanova, S.; Prohens, J. Diversity for Chemical Composition in a Collection of Different Varietal Types of Tree Tomato (Solanum betaceum Cav.), an Andean Exotic Fruit. Food Chem. 2015, 169, 327–335. [Google Scholar] [CrossRef]
- Ordóñez, R.M.; Cardozo, M.L.; Zampini, I.C.; Isla, M.I. Evaluation of Antioxidant Activity and Genotoxicity of Alcoholic and Aqueous Beverages and Pomace Derived from Ripe Fruits of Cyphomandra betacea Sendt. J. Agric. Food Chem. 2010, 58, 331–337. [Google Scholar] [CrossRef]
- Osorio, C.; Hurtado, N.; Dawid, C.; Hofmann, T.; Heredia-Mira, F.J.; Morales, A.L. Chemical Characterisation of Anthocyanins in Tamarillo (Solanum betaceum Cav.) and Andes Berry (Rubus glaucus Benth.) Fruits. Food Chem. 2012, 132, 1915–1921. [Google Scholar] [CrossRef]
- Gannasin, S.P.; Adzahan, N.M.; Hamzah, M.Y.; Mustafa, S.; Muhammad, K. Physicochemical Properties of Tamarillo (Solanum betaceum Cav.) Hydrocolloid Fractions. Food Chem. 2015, 182, 292–301. [Google Scholar] [CrossRef]
- Gunness, P.; Gidley, M.J. Mechanisms Underlying the Cholesterol-Lowering Properties of Soluble Dietary Fibre Polysaccharides. Food Funct. 2010, 1, 149–155. [Google Scholar] [CrossRef]
- Jouki, M.; Tabatabaei Yazdi, F.; Mortazavi, S.A.; Koocheki, A. Physical, Barrier and Antioxidant Properties of a Novel Plasticized Edible Film from Quince Seed Mucilage. Int. J. Biol. Macromol. 2013, 62, 500–507. [Google Scholar] [CrossRef]
- Hussain, M.A.; Gulzar, M.; Haseeb, M.T.; Tahir, M.N. Quince Seed Mucilage: A Stimuli-Responsive/Smart Biopolymer. In Functional Biopolymers; Mazumder, A.M.J., Sheardown, H., Al-Ahmed, A., Eds.; Springer: Basel, Switzerland, 2018; pp. 127–148. [Google Scholar]
- Rahimi, R.; Shams-Ardekani, M.R.; Abdollahi, M. A Review of the Efficacy of Traditional Iranian Medicine for Inflammatory Bowel Disease. World J. Gastroenterol. 2010, 16, 4504–4514. [Google Scholar] [CrossRef]
- Silva, B.M.; Andrade, P.; Ferreres, F.; National, S.; Oliveira, M. Composition of Quince (Cydonia oblonga Miller) Seeds: Phenolics, Organic Acids and Free Amino Acids. Nat. Prod. Res. 2005, 19, 273–281. [Google Scholar] [CrossRef]
- Lindberg, B.; Mosihuzzaman, M.; Nahar, N.; Abeysekera, R.M.; Brown, R.G.; Willison, J.H.M. An Unusual (4-O-Methyl-d-Glucurono)-d-Xylan Isolated from the Mucilage of Seeds of the Quince Tree (Cydonia oblonga). Carbohydr. Res. 1990, 207, 307–310. [Google Scholar] [CrossRef]
- Rezagholi, F.; Hashemi, S.M.B.; Gholamhosseinpour, A.; Sherahi, M.H.; Hesarinejad, M.A.; Ale, M.T. Characterizations and Rheological Study of the Purified Polysaccharide Extracted from Quince Seeds. J. Sci. Food Agric. 2019, 99, 143–151. [Google Scholar] [CrossRef] [Green Version]
- Nikoofar, E.; Hojjatoleslamy, M.; Shakerian, A.; Molavi, H. Surveying the Effect of Oat Beta Glucan As a Fat Replacer on Rheological and Physicochemical Characteristics of Non Fat Set Yoghurt. Int. J. Farming Allied Sci. 2013, 2, 861–865. [Google Scholar]
- Farahmandfar, R.; Mohseni, M.; Asnaashari, M. Effects of Quince Seed, Almond, and Tragacanth Gum Coating on the Banana Slices Properties during the Process of Hot Air Drying. Food Sci. Nutr. 2017, 5, 1057–1064. [Google Scholar] [CrossRef] [PubMed]
- Jouki, M.; Mortazavi, S.A.; Yazdi, F.T.; Koocheki, A.; Khazaei, N. Use of Quince Seed Mucilage Edible Films Containing Natural Preservatives to Enhance Physico-Chemical Quality of Rainbow Trout Fillets during Cold Storage. Food Sci. Hum. Wellness 2014, 3, 65–72. [Google Scholar] [CrossRef] [Green Version]
- Jouki, M.; Yazdi, F.T.; Mortazavi, S.A.; Koocheki, A.; Khazaei, N. Effect of Quince Seed Mucilage Edible Films Incorporated with Oregano or Thyme Essential Oil on Shelf Life Extension of Refrigerated Rainbow Trout Fillets. Int. J. Food Microbiol. 2014, 174, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Jouki, M.; Mortazavi, S.A.; Yazdi, F.T.; Koocheki, A. Characterization of Antioxidant–Antibacterial Quince Seed Mucilage Films Containing Thyme Essential Oil. Carbohydr. Polym. 2014, 99, 537–546. [Google Scholar] [CrossRef] [PubMed]
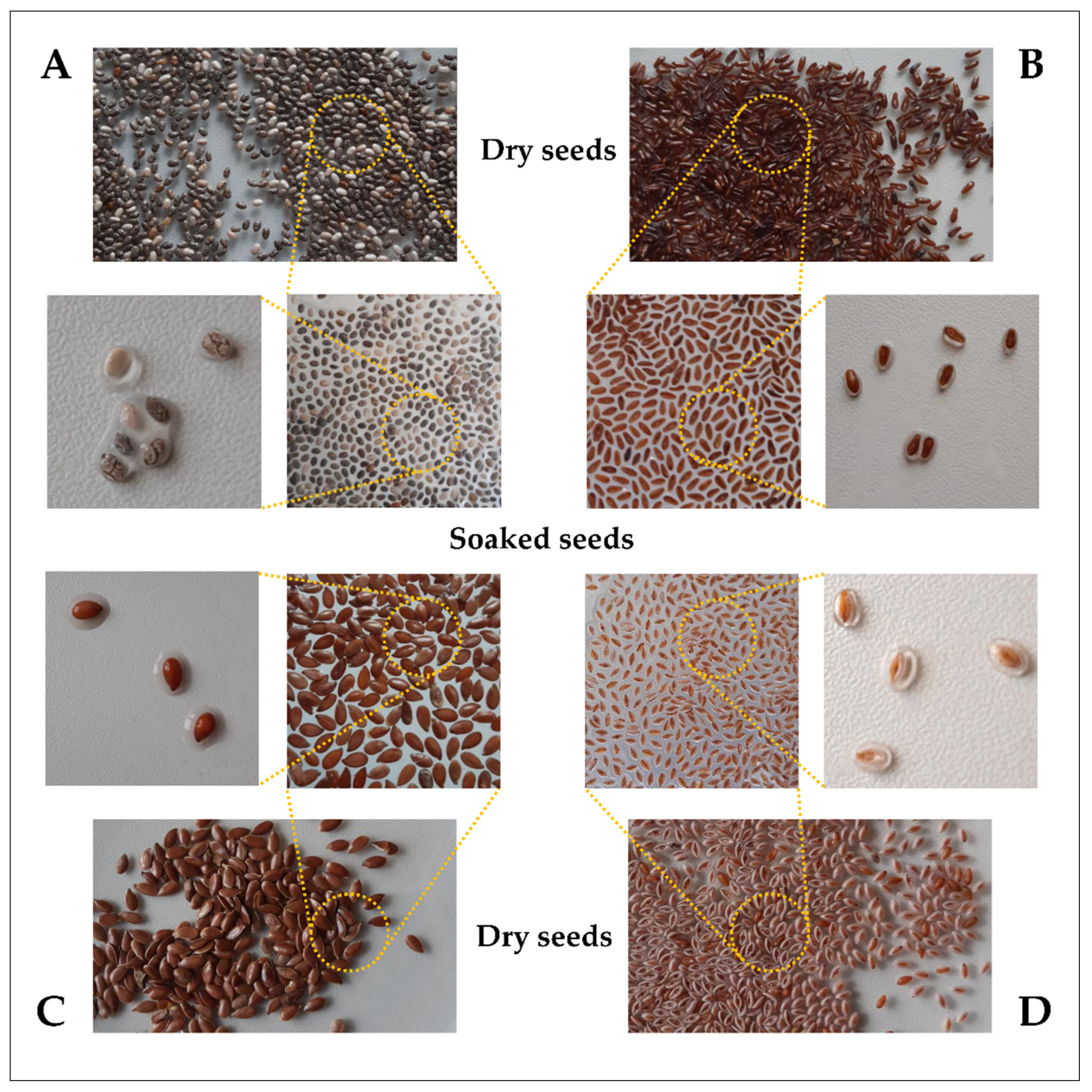

| Family | Species | Common Name | Part of Plant | Mucilage Chemical Composition (Key Polysaccharides) | References |
|---|---|---|---|---|---|
| Lamiales order | |||||
| Lamiaceae | Salvia hispanica * Salvia macrosiphon * | Chia | Seed | Tetrasaccharide consisting of β-D-xylopyranosyl, α-D-glucopyranosyl, and 4-O-methyl-α-D-glucopyranosyluronic acid | [4] |
| Lamiaceae | Ocimum basilicum * | Great basil | Seed | Two major fractions of glucomannan and (1 → 4)-linked xylan and a minor fraction of glucan | [5,6] |
| Lamiaceae | Hyptis suaveolens * | Pignut, chan, bushmint, sangura, wilaiti tuls | Seed | Neutral fraction: D-galactopyranosyl-, D-glucopyranosyl- and D-mannopyranosyl-units; the acid polysaccharide: L-fucopyranosyl, D-xylopyranosyl and 4-O-methyl-D-glucuronic acid units | [7,8] |
| Plantaginaceae | Plantago psyllium * Plantago major * Plantago ovata * | Psyllium, ispaghula | Seed husk | Xylan with 1 → 3 and 1 → 4 linkages containing arabinose and xylose on the sides; other units found: arabinose, rhamnose, galactose, glucose and small amounts of mannose, galactose, galacturonic and glucuronic acids; branched structure | [9,10] |
| Scrophulariaceae | Verbascum spp. | Mullein | Flower | Pectic polysaccharide containing the rhamnogalacturonan | [11,12] |
| Fabales order | |||||
| Fabaceae | Glycyrrhiza glabra | Licorice root | Root | Arabinogalactan with saponins (including glycyrrhizin), flavonoids, isoflavones, coumarins, lactones, sterols | [13,14] |
| Fabaceae | Cyamopsis tetragonoloba | Guar, cluster bean, gavar, guvar bean | Seed | Complex polymer of galactose and mannose (galactomannan) | [15] |
| Fabaceae | Trigonella foenum-graecum * | Fenugreek | Seed | Galactomannan | [16] |
| Fabaceae | Cassia obtusifolia * Cassia fistula * | Sicklepod | Seed | Glucomannan | [17,18,19] |
| Brassicales order | |||||
| Moringaceae | Moringa oleifera | Kelor horseradish tree, drumstick, sajna | Bark | Arabinogalactan | [20,21] |
| Brassicaceae | Arabidopsis thaliana | Thale cress, mouse-ear cress | Seed | Unbranched rhamnogalacturonan with small quantities of homogalacturonan, cellulose, and arabinoxylan | [22] |
| Brassicaceae | Sinapis alba | Yellow mustard | Seed | Pectic polysaccharides and a small portion of β-1,4 linked glucosyl backbone | [23] |
| Brassicaceae | Eruca sativa | Arugula, garden rocket, rocket salad, roka, roquette, rucola or rugula | Seed | Polysaccharides built with mannose and galactose as main components with minor amount of fructose, glucose and arabinose | [24,25] |
| Malvales order | |||||
| Malvaceae | Althaea officinalis | Marshmallow | Root | Neutral and acidic polysaccharides, rhamnogalacturonan | [26,27] |
| Malvaceae | Corchorus olitorius | Jew’s mallow, molokhiajute plant | Leaf | Rhamnogalacturonan | [28,29,30] |
| Malvaceae | Hibiscus rosa-sinensis | Chinese hibiscus, China rose, rosemallow, shoeblackplant | Leaf | Backbone chain composed of alpha-1,4-linked D-galactosyl α-1,2-linked L-rhamnosyl α-1,4-linked D-galacturonic acid units | [31,32] |
| Malvaceae | Abelmoschus esculentus | Okra, Lady’s finger, green ginseng | Fruit | Rhamnogalacturonan | [33,34] |
| Caryophyllales order | |||||
| Basellaceae | Basella alba * | Malabar spinach | Leaf, stem, flower | Polysaccharide, containing D-galactose (as major monosaccharide) and L-arabinose (in minor amounts) | [35] |
| Amaranthaceae | Spinacia oleracea * | Spinach | Leaf | Polysaccharide, containing neutral sugar (arabinose, galactose, mannose, glucose, rhamnose and xylose) and uronic acids | [36,37] |
| Talinaceae | Talinum triangulare syn. T fructiosum * | Surinum purslane, Ceylon spinach, waterleaf | Leaf | Polysaccharide-proteins and polysaccharide-triterpenoids | [38,39] |
| Cactaceae | Opuntia spp. * | Nopal | Leaflike steam | Backbone of α-D-galacturonic acid units linked 1 → 2 to β-L-rhamnose units linked 1 → 4 with branching on C-4 | [40,41] |
| Other orders (Boraginales, Ericales, Malpighiales, Asparagales, Solanales, Dioscoreales, Rosales) | |||||
| Boraginaceae | Cordia dichotoma | Indian cherry, fragrant manjack, snotty gobbles, cummingcordia, glue berry, pink pearl, bird lime tree | Fruit | Polysaccharide built of galactose, arabinose and glucuronic acid with traces of rhamnose | [42,43] |
| Ebenaceae | Diospyros melanoxylon | Coromandel ebony East Indian ebony | Seed, bark | Neutral arabinoxylans and acidic pectin-like rhamnogalacturonan | [44,45] |
| Ebenaceae | Diospyros peregrina | Gaub persimmon, Malabar ebony, Wild mangosteen, Indian persimmon | Fruit | Neutral arabinoxylans and acidic pectin-like rhamnogalacturonan | [45] |
| Linaceae | Linum usitatissimum * | Flaxseed, linseed | Seed | Neutral arabinoxylans and acidic pectin-like rhamnogalacturonan | [46,47] |
| Asphodelaceae | Aloe barbadensis * | Aloe vera burn plant, medicinal aloe or Barbados aloe | Leaf | Backbone of α-D-galacturonic acid units linked 1 → 2 to β-L-rhamnose units linked 1 → 4 with branching on C-4 | [48] |
| Solanaceae | Solanum betaceum, syn. Cyphomandra betacea * | Tamarillo | Fruit | Methoxylated homogalacturonans mixed with type I arabinogalactans, a linear (1 → 5)-linked α-L-arabinan, and a linear (1 → 4)-β-D-xylan | [49] |
| Dioscoreaceae | Dioscorea polystachya | Chinese yam, cinnamon-vine | Bulb | Poly(β1-4) mannose with additional linkages and proteins mixed composition of mannose, glucose, galactose and glucuronic acid | [50] |
| Rosaceae | Cydonia oblonga syn. Cydonia vulgaris * | Quince | Seed | Glucuronoxylan based polysaccharides | [51,52] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dybka-Stępień, K.; Otlewska, A.; Góźdź, P.; Piotrowska, M. The Renaissance of Plant Mucilage in Health Promotion and Industrial Applications: A Review. Nutrients 2021, 13, 3354. https://doi.org/10.3390/nu13103354
Dybka-Stępień K, Otlewska A, Góźdź P, Piotrowska M. The Renaissance of Plant Mucilage in Health Promotion and Industrial Applications: A Review. Nutrients. 2021; 13(10):3354. https://doi.org/10.3390/nu13103354
Chicago/Turabian StyleDybka-Stępień, Katarzyna, Anna Otlewska, Patrycja Góźdź, and Małgorzata Piotrowska. 2021. "The Renaissance of Plant Mucilage in Health Promotion and Industrial Applications: A Review" Nutrients 13, no. 10: 3354. https://doi.org/10.3390/nu13103354
APA StyleDybka-Stępień, K., Otlewska, A., Góźdź, P., & Piotrowska, M. (2021). The Renaissance of Plant Mucilage in Health Promotion and Industrial Applications: A Review. Nutrients, 13(10), 3354. https://doi.org/10.3390/nu13103354







