Lactobacillus rhamnosus GG Reduces β-conglycinin-Allergy-Induced Apoptotic Cells by Regulating Bacteroides and Bile Secretion Pathway in Intestinal Contents of BALB/c Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Allergen
2.2. LGG
2.3. Animals
2.4. Experimental Design
2.5. Allergy Symptom Score
2.6. Analysis of Immunoglobulin E (IgE) and Histamine (HIS) in Mice Serum
2.7. Detection of Apoptotic Cells by TUNEL Assay
2.8. 16S rRNA Gene Sequencing Analysis
2.9. Metabolomics Analysis
2.10. Statistical Analysis
3. Results
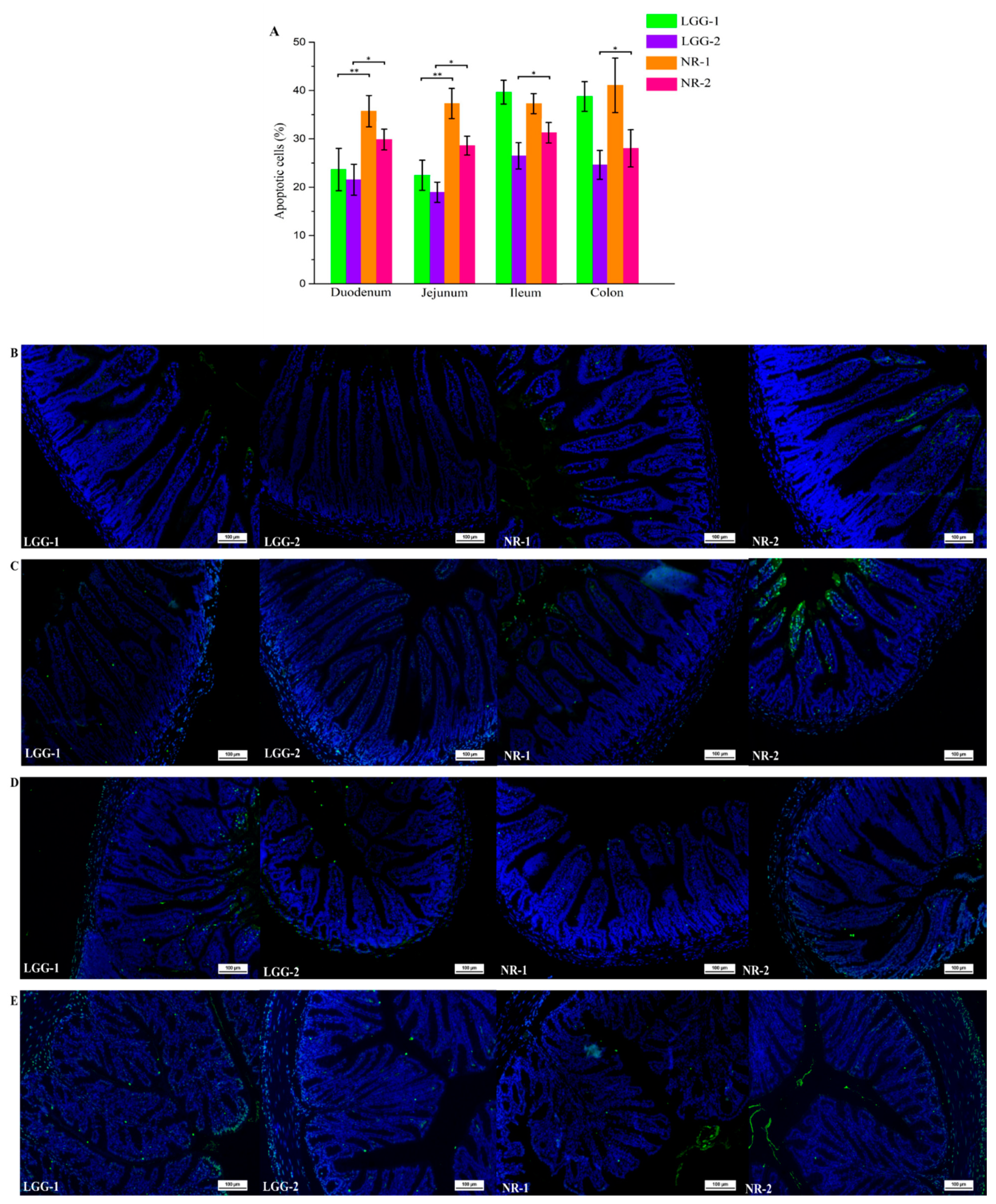
3.1. β-CG Induced Intestinal Cell Apoptosis in Allergic Mice
3.2. LGG Reduces Intestinal Apoptotic Cells Induced by β-CG Allergy
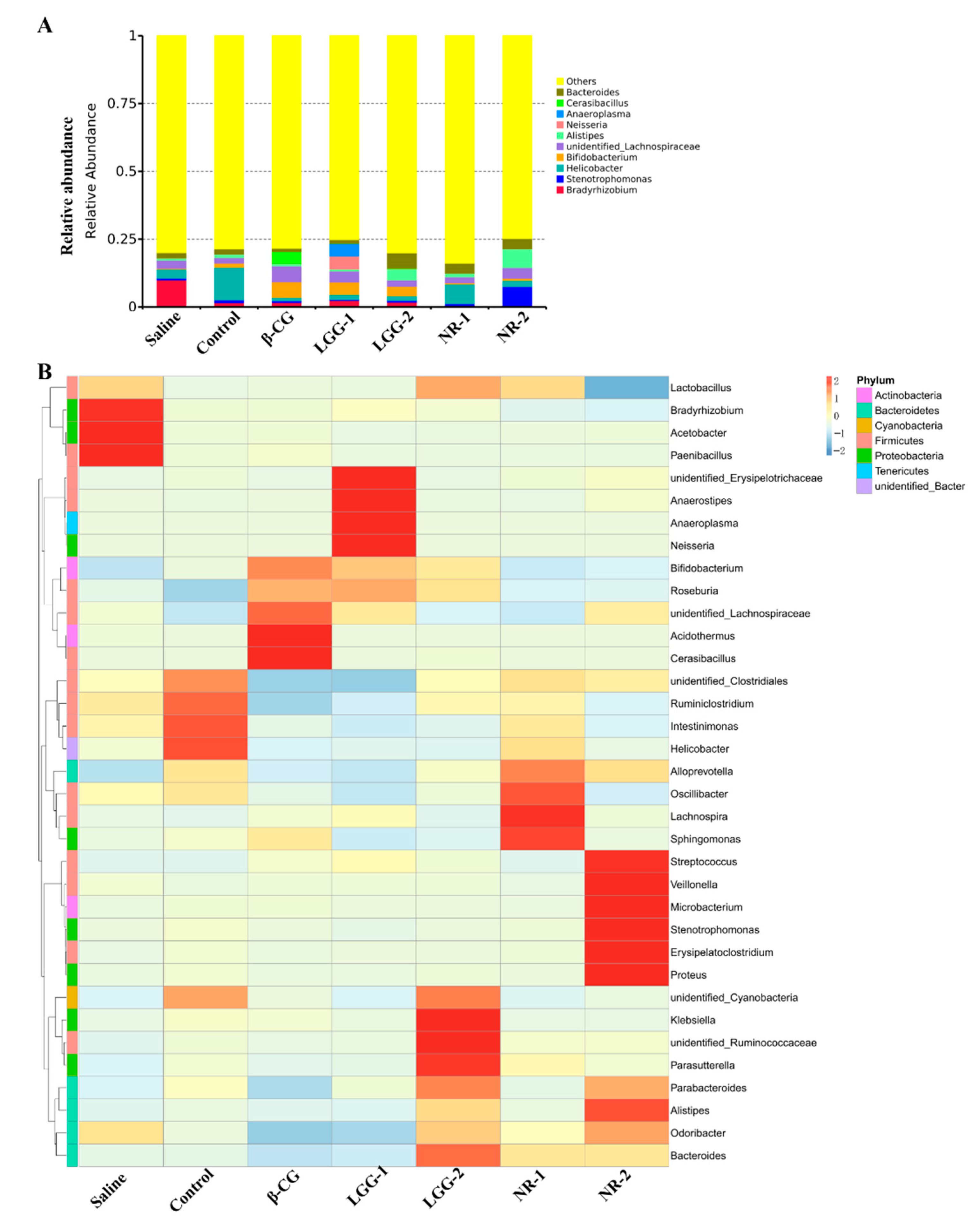
3.3. LGG Regulates the Imbalance of Gut Microbiota Induced by β-CG Allergy
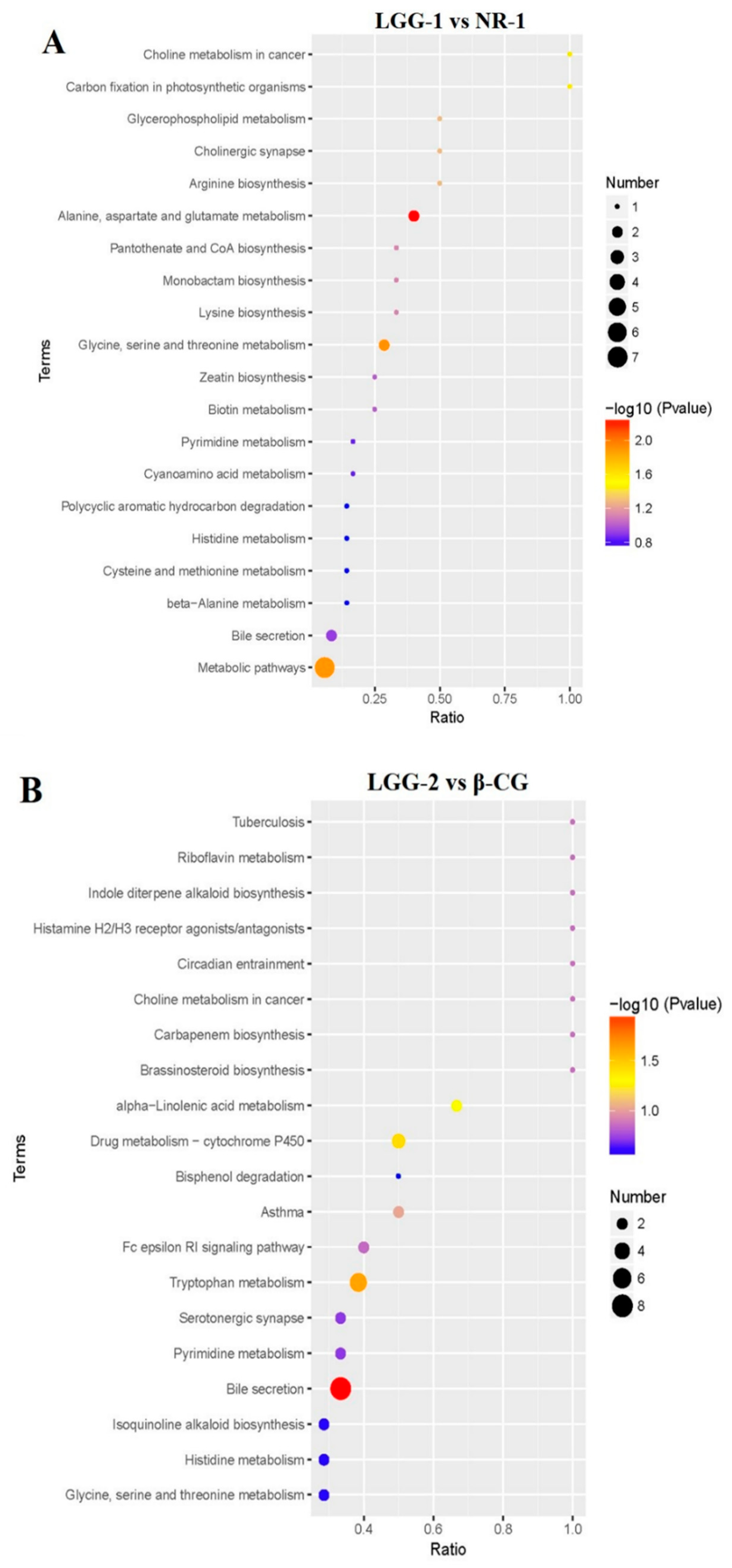
3.4. LGG Alleviates Intestinal Metabolites Disorder Induced by β-CG Allergy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bu, G.; Li, T.; Zhao, Y.; Chen, F. Effects of high hydrostatic pressure combined with heat treatment on the antigenicity and conformation of β-conglycinin. Eur. Food Res. Technol. 2020, 246, 1065–1072. [Google Scholar] [CrossRef]
- Ippoushi, K.; Wakagi, M.; Hashimoto, N.; Takano-Ishikawa, Y. Absolute quantification of the a, a′, and β subunits of β-conglycinin from soybeans by liquid chromatography/tandem mass spectrometry using stable isotope-labelled peptides. Food Res. Int. 2019, 116, 1223–1228. [Google Scholar] [CrossRef] [PubMed]
- Sastre, B.; Cañas, J.A.; Rodrigo-Muñoz, J.M.; Fernandez-Nieto, M.; Barranco, P.; Quirce, S.; Sastre, J.; Del Pozo, V. Eosinophil-Derived Exosomes Contribute to Asthma Remodeling by Activating Structural Lung Cells. Clin. Exp. Allergy 2018, 141, AB72. [Google Scholar] [CrossRef]
- Gao, H.; Feng, B.S.; Liu, J.Q.; Mo, L.H.; Geng, X.R.; Xiao, Y.; Zhang, Y.Y.; Hong, J.Y.; Liu, Z.J.; Liu, Z.G. Survivin induces defects in apoptosis in eosinophils in intestine with food allergy. Innate Immun. 2019, 25, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Cui, H.; Peng, X.; Fang, J.; Zuo, Z.; Deng, J.; Huang, J. Dietary nickel chloride induces oxidative stress, apoptosis and alters Bax/Bcl-2 and caspase-3 mRNA expression in the cecal tonsil of broilers. Food Chem. Toxicol. 2014, 63, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Santos, F.D.S.; Mazzoli, A.; Maia, A.R.; Saggese, A.; Isticato, R.; Leite, F.; Iossa, S.; Ricca, E.; Baccigalupi, L. A probiotic treatment increases the immune response induced by the nasal delivery of spore-adsorbed TTFC. Microb. Cell Fact. 2020, 19, 1–13. [Google Scholar] [CrossRef]
- Hamsah, H.; Widanarni, W.; Alimuddin, A.; Yuhana, M.; Junior, M.Z.; Hidayatullah, D. Immune response and resistance of Pacific white shrimp larvae administered probiotic, prebiotic, and synbiotic through the bio-encapsulation of Artemia sp. Aquac. Int. 2019, 27, 567–580. [Google Scholar] [CrossRef]
- Maruščáková, I.C.; Schusterová, P.; Bielik, B.; Toporčák, J.; Mudroňová, D. Effect of Application of Probiotic Pollen Suspension on Immune Response and Gut Microbiota of Honey Bees (Apis mellifera). Probiotics Antimicrob. Proteins 2020, 12, 929–936. [Google Scholar] [CrossRef]
- Ankita, S.; Sarangi, A.N.; Amit, G.; Rajni, S.; Rajat, B.; Priyanka, G.; Amita, A.; Rakesh, A. Effect of administration of a probiotic preparation on gut microbiota and immune response in healthy women in India: An open-label, single-arm pilot study. BMC Gastroenterol. 2018, 18, 85. [Google Scholar]
- Ramírez, C.; Rojas, R.; Romero, J. Partial Evaluation of Autochthonous Probiotic Potential of the Gut Microbiota of Seriola lalandi. Probiotics Antimicrob. Proteins 2020, 12, 672–682. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, J.; Li, Q.; Shi, Z.; Wu, H.; Zhang, H.; Tang, L.; Yi, R.; Su, H.; Sun, X. Oral administration of a mixture of probiotics protects against food allergy via induction of CD103+ dendritic cells and modulates the intestinal microbiota. J. Funct. Foods 2019, 55, 65–75. [Google Scholar] [CrossRef]
- Nie, Y.F.; Yan, X.H. Cross-talk between bile acids and intestinal microbiota in host metabolism and health. J. Zhejiang Univ. Sci. B 2015, 16, 436–446. [Google Scholar] [CrossRef] [PubMed]
- Wahlstrom, A.; Sayin, S.I.; Marschall, H.; Backhed, F. Intestinal Crosstalk between Bile Acids and Microbiota and Its Impact on Host Metabolism. Cell Metab. 2016, 24, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Enright, E.F.; Griffin, B.T.; Gahan, C.G.M.; Joyce, S.A. Microbiome-mediated bile acid modification: Role in intestinal drug absorption and metabolism. Pharmacol. Res. 2018, 133, 170–186. [Google Scholar] [CrossRef] [PubMed]
- Dawson, P.A.; Shneider, B.L.; Hofmann, A.F. Bile formation and the enterohepatic circulation. In Physiology of the Gastrointestinal Tract; Elsevier: Amsterdam, The Netherlands, 2018; pp. 931–956. [Google Scholar]
- Duboc, H.; Rajca, S.; Rainteau, D.; Benarous, D.; Maubert, M.; Quervain, E.; Thomas, G.; Barbu, V.; Humbert, L.; Despras, G. Connecting dysbiosis, bile-acid dysmetabolism and gut inflammation in inflammatory bowel diseases. Gut 2013, 62, 531–539. [Google Scholar] [CrossRef]
- Irina, S.; Petrova, M.I.; Astrid, F.; Lore, P.; Jeroen, V.; Ceuppens, J.L.; Sven, S.; Sarah, L. Intranasal administration of probiotic Lactobacillus rhamnosus GG prevents birch pollen-induced allergic asthma in a murine model. Allergy 2019, 74, 100–110. [Google Scholar]
- Yazdi, F.G.; Zakeri, A.; Ark, I.V.; Leusink-Muis, T.; Folkerts, G. Crude Turmeric Extract Improves the Suppressive Effects of Lactobacillus rhamnosus GG on Allergic Inflammation in a Murine Model of House Dust Mite-Induced Asthma. Front. Immunol. 2020, 11, 1092. [Google Scholar] [CrossRef]
- Juan, Z.; Jing-Yi, M.; Qiu-Hong, L.; Hui, S.; Xin, S. Lactobacillus rhamnosus GG induced protective effect on allergic airway inflammation is associated with gut microbiota. Cell. Immunol. 2018, 332, 77–84. [Google Scholar]
- Basturk, A.; Isik, İ.; Atalay, A.; Yılmaz, A. Investigation of the Efficacy of Lactobacillus rhamnosus GG in Infants with Cow’s Milk Protein Allergy: A Randomised Double-Blind Placebo-Controlled Trial. Probiotics Antimicrob. Proteins 2020, 12, 138–143. [Google Scholar] [CrossRef]
- Guadamuro, L.; Diaz, M.; Jiménez, S.; Molinos-Norniella, C.; Pérez-Solis, D.; Rodríguez, J.M.; Bousoo, C.; Gueimonde, M.; Margolles, A.; Delgado, S. Fecal Changes following Introduction of Milk in Infants with Outgrowing Non-IgE Cow’s Milk Protein Allergy Are Influenced by Previous Consumption of the Probiotic LGG. Front. Immunol. 2019, 10, 1819. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, X.; Hu, Y.; Zhang, B.; Zhang, Y.; Wang, S. Lactobacillus rhamnosus GG alleviates β-conglycinin induced allergy by regulating the T cell receptor signalling pathway. Food Funct. 2020, 11, 10554–10567. [Google Scholar] [CrossRef] [PubMed]
- Ippoushi, K.; Tanaka, Y.; Wakagi, M.; Hashimoto, N. Evaluation of protein extraction methods for β-conglycinin quantification in soybeans and soybean products. LWT Food Sci. Technol. 2020, 132, 109871. [Google Scholar] [CrossRef]
- Mendoza-Reinoso, V.; Baek, D.Y.; Kurutz, A.; Rubin, J.R.; Koh, A.J.; Mccauley, L.K.; Roca, H. Unique Pro-Inflammatory Response of Macrophages during Apoptotic Cancer Cell Clearance. Cells 2020, 9, 429. [Google Scholar] [CrossRef] [PubMed]
- Welcome, M.O. Gut Microbiota Disorder, Gut Epithelial and Blood–Brain Barrier Dysfunctions in Etiopathogenesis of Dementia: Molecular Mechanisms and Signaling Pathways. Neuromol. Med. 2019, 21, 205–226. [Google Scholar] [CrossRef]
- Wang, H.; Gao, K.; Wen, K.; Allen, I.C.; Li, G.; Zhang, W.; Kocher, J.; Yang, X.; Giri-Rachman, E.; Li, G.-H.; et al. Lactobacillus rhamnosus GG modulates innate signaling pathway and cytokine responses to rotavirus vaccine in intestinal mononuclear cells of gnotobiotic pigs transplanted with human gut microbiota. BMC Microbiol. 2016, 16, 109. [Google Scholar] [CrossRef]
- Wu, Y.; Zhen, W.; Geng, Y.; Wang, Z.; Guo, Y. Pretreatment with probiotic Enterococcus faecium NCIMB 11181 ameliorates necrotic enteritis-induced intestinal barrier injury in broiler chickens. Sci. Rep. 2019, 9, 1–17. [Google Scholar] [CrossRef]
- Al-Hisnawi, A.; Rodiles, A.; Rawling, M.D.; Castex, M.; Waines, P.; Gioacchini, G.; Carnevali, O.; Merrifield, D.L. Dietary probiotic Pediococcus acidilactici MA18/5M modulates the intestinal microbiota and stimulates intestinal immunity in rainbow trout (Oncorhynchus mykiss). J. World Aquac. Soc. 2019, 50, 1133–1151. [Google Scholar] [CrossRef]
- Li, X.; Hu, D.; Tian, Y.; Song, Y.; Hou, Y.; Sun, L.; Zhang, Y.; Man, C.; Zhang, W.; Jiang, Y. Protective effects of a novel Lactobacillus rhamnosus strain with probiotic characteristics against lipopolysaccharide-induced intestinal inflammation in vitro and in vivo. Food Funct. 2020, 11, 5799–5814. [Google Scholar] [CrossRef]
- Cukrowska, B.; Biera, J.B.; Zakrzewska, M.; Klukowski, M.; Maciorkowska, E. The Relationship between the Infant Gut Microbiota and Allergy. The Role of Bifidobacterium breve and Prebiotic Oligosaccharides in the Activation of Anti-Allergic Mechanisms in Early Life. Nutrients 2020, 12, 946. [Google Scholar] [CrossRef]
- Bridgman, S.L.; Kozyrskyj, A.L.; Scott, J.A.; Becker, A.B.; Azad, M.B. Gut microbiota and allergic disease in children. Ann. Allergy Asthma Immunol. 2016, 116, 99–105. [Google Scholar] [CrossRef]
- Reddel, S.; Mennini, M.; Del Chierico, F.; Vernocchi, P.; Valluzzi, R.; Fierro, V.; Riccardi, C.; Fiocchi, A.; Putignani, L. Gut microbiota profile in infants with milk and/or egg allergy and evaluation of intestinal colonization and persistence of a probiotic mixture. World Allergy Organ. J. 2020, 13, 100424. [Google Scholar] [CrossRef]
- Rooks, M.G.; Garrett, W.S. Gut microbiota, metabolites and host immunity. Nat. Rev. Immunol. 2016, 16, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H. Immune regulation by microbiome metabolites. Immunology 2018, 154, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.; Blacher, E.; Elinav, E. Microbiome, metabolites and host immunity. Curr. Opin. Microbiol. 2017, 35, 8–15. [Google Scholar] [CrossRef]
- Smolinska, S.; Jutel, M.; Crameri, R.; Omahony, L. Histamine and gut mucosal immune regulation. Allergy 2014, 69, 273–281. [Google Scholar] [CrossRef]
- Amo, G.; Cornejo-García, J.A.; García-Menaya, J.M.; Cordobes, C.; Torres, M.J.; Esguevillas, G.; Mayorga, C.; Martinez, C.; Blanca-Lopez, N.; Canto, G.; et al. FCERI and Histamine Metabolism Gene Variability in Selective Responders to NSAIDS. Front. Pharmacol. 2016, 7, 353. [Google Scholar] [CrossRef]
- Byun, S.; Kim, D.-H.; Ryerson, D.; Kim, Y.-C.; Sun, H.; Kong, B.; Yau, P.; Guo, G.; Xu, H.E.; Kemper, B. Postprandial FGF19-induced phosphorylation by Src is critical for FXR function in bile acid homeostasis. Nat. Commun. 2018, 9, 1–14. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Wu, Y.; Hu, Y.; Zhang, Y.; Wang, S. Lactobacillus rhamnosus GG Reduces β-conglycinin-Allergy-Induced Apoptotic Cells by Regulating Bacteroides and Bile Secretion Pathway in Intestinal Contents of BALB/c Mice. Nutrients 2021, 13, 55. https://doi.org/10.3390/nu13010055
Chen X, Wu Y, Hu Y, Zhang Y, Wang S. Lactobacillus rhamnosus GG Reduces β-conglycinin-Allergy-Induced Apoptotic Cells by Regulating Bacteroides and Bile Secretion Pathway in Intestinal Contents of BALB/c Mice. Nutrients. 2021; 13(1):55. https://doi.org/10.3390/nu13010055
Chicago/Turabian StyleChen, Xiaoxu, Yuekun Wu, Yaozhong Hu, Yan Zhang, and Shuo Wang. 2021. "Lactobacillus rhamnosus GG Reduces β-conglycinin-Allergy-Induced Apoptotic Cells by Regulating Bacteroides and Bile Secretion Pathway in Intestinal Contents of BALB/c Mice" Nutrients 13, no. 1: 55. https://doi.org/10.3390/nu13010055
APA StyleChen, X., Wu, Y., Hu, Y., Zhang, Y., & Wang, S. (2021). Lactobacillus rhamnosus GG Reduces β-conglycinin-Allergy-Induced Apoptotic Cells by Regulating Bacteroides and Bile Secretion Pathway in Intestinal Contents of BALB/c Mice. Nutrients, 13(1), 55. https://doi.org/10.3390/nu13010055



