Time-Restricted Eating and Metabolic Syndrome: Current Status and Future Perspectives
Abstract
1. Introduction
2. Diagnostic Criteria of MetS and Its Components
3. Impact of Circadian Rhythms and Circadian Rhythm Disruption on Metabolic Homeostasis
4. Effects of TRE in Animals and Healthy Humans
5. Characterization of TRE Trials in Humans with MetS or Its Components
5.1. Study Design
5.2. Inclusion Criteria and Participants
5.3. TRE Window and Duration of Intervention
5.4. TRE Intervention
5.5. Use of the MyCircadianClock Application
5.6. Calorie Intake Assessment
5.7. Use of Continuous Glucose Monitor
5.8. Physical Activity and Sleep Assessment
5.9. Body Composition Analysis
5.10. Primary Outcomes
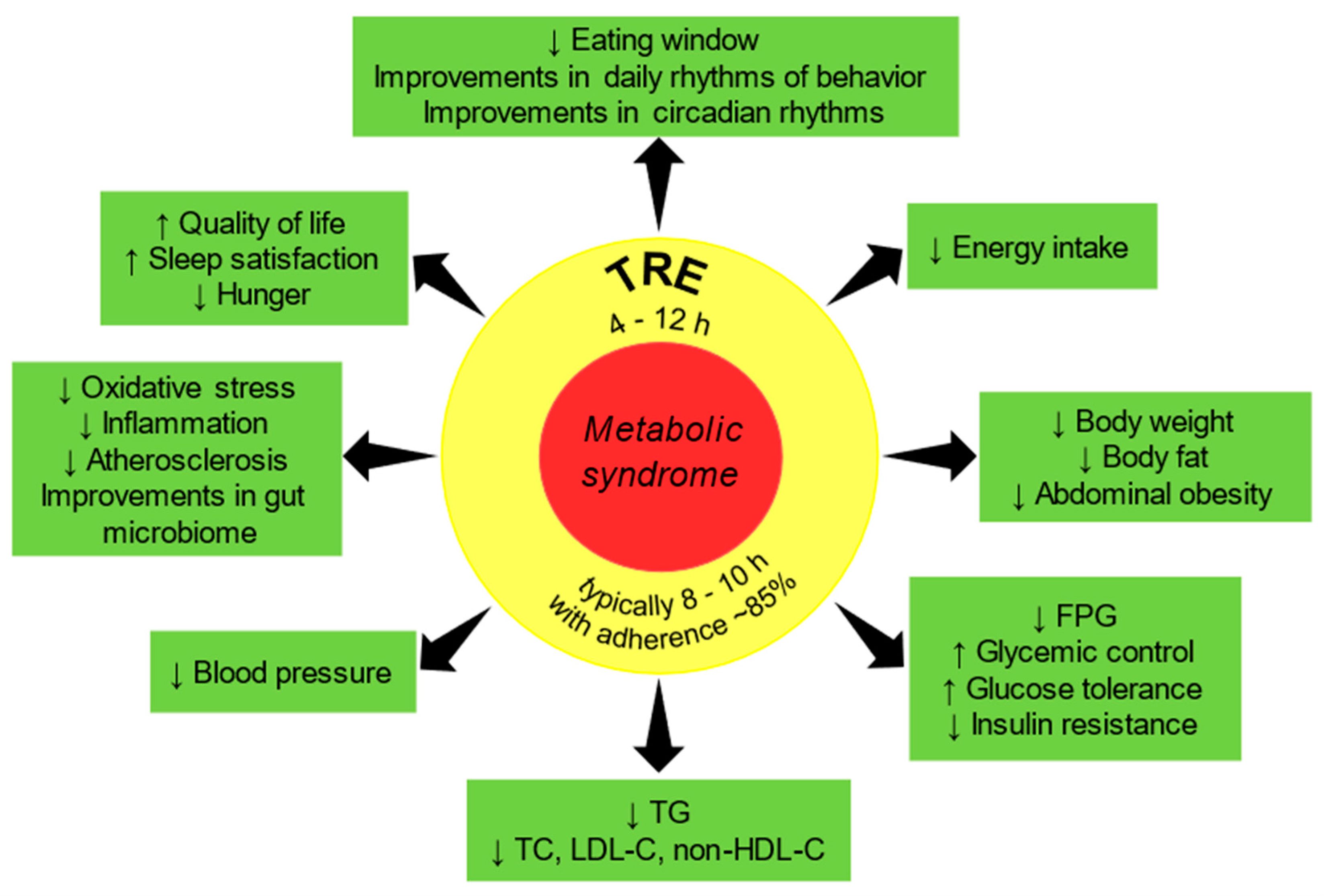
6. Major Findings of TRE Trials in Humans with MetS or Its Components
6.1. Adherence to TRE Intervention
6.2. Eating Pattern
6.3. Body Weight, Waist Circumference, and Body Composition
6.4. Calorie Intake
6.5. Glucose Metabolism
6.6. Lipid Metabolism
6.7. Neuroendocrine, Metabolic, Oxidative Stress, and Inflammation Biomarkers
6.8. Blood Pressure
6.9. Behavioral Effects and Safety
7. Effectiveness of TRE in Humans with MetS or Its Components: Summary of Clinical Evidence
8. Knowledge Gaps and Future Perspectives for TRE in MetS
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scuteri, A.; Laurent, S.; Cucca, F.; Cockcroft, J.; Cunha, P.G.; Mañas, L.R.; Raso, F.U.M.; Muiesan, M.L.; Ryliškytė, L.; Rietzschel, E.; et al. Metabolic syndrome across Europe: Different clusters of risk factors. Eur. J. Prev. Cardiol. 2015, 22, 486–491. [Google Scholar] [CrossRef]
- Virani, S.S.; Alonso, A.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Delling, F.N.; et al. On behalf of the American Heart Association Council on Epidemiology and Prevention statistics committee and stroke statistics subcommittee. Heart disease and stroke statistics—2020 update: A report from the American Heart Association. Circulation 2020, 141, e139–e596. [Google Scholar]
- Alberti, K.G.M.M.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.T.; Loria, C.M.; Smith, S.C., Jr.; et al. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar]
- Sygnowska, E.; Piwońska, A.; Waśkiewicz, A.; Broda, G. Socioeconomic factors and the risk of metabolic syndrome in the adult Polish population: The WOBASZ study. Kardiol. Pol. 2012, 70, 718–727. [Google Scholar]
- Eckel, R.H.; Alberti, K.G.M.M.; Grundy, S.M.; Zimmet, P.Z. The metabolic syndrome. Lancet 2010, 375, 181–183. [Google Scholar] [CrossRef]
- Suliga, E.; Kozieł, D.; Cieśla, E.; Rebak, D.; Głuszek, S. Dietary patterns in relation to metabolic syndrome among adults in Poland: A Cross-sectional study. Nutrients 2017, 9, 1366. [Google Scholar] [CrossRef]
- Kant, A.K.; Graubard, B.I. 40-year trends in meal and snack eating behaviors of American adults. J. Acad. Nutr. Diet. 2015, 115, 50–63. [Google Scholar] [CrossRef]
- Riou, J.; Lefevre, T.; Parizot, I.; Lhuissier, A.; Chauvin, P. Is there still a French eating model? A taxonomy of eating behaviors in adults living in the Paris metropolitan area in 2010. PLoS ONE 2015, 10, e0119161. [Google Scholar]
- Gupta, N.J.; Kumar, V.; Panda, S. A camera-phone based study reveals erratic eating pattern and disrupted daily eating-fasting cycle among adults in India. PLoS ONE 2017, 12, e0172852. [Google Scholar]
- Gill, S.; Panda, S. A Smartphone app reveals erratic diurnal eating patterns in humans that can be modulated for health benefits. Cell Metab. 2015, 22, 789–798. [Google Scholar] [CrossRef]
- Chow, L.S.; Manoogian, E.N.C.; Alvear, A.; Fleischer, J.G.; Thor, H.; Dietsche, K.; Wang, Q.; Hodges, J.S.; Esch, N.; Malaeb, S.; et al. Time-restricted eating effects on body composition and metabolic measures in humans who are overweight: A feasibility study. Obesity 2020, 28, 860–869. [Google Scholar] [CrossRef] [PubMed]
- Zarrinpar, A.; Chaix, A.; Panda, S. Daily eating patterns and their impact on health and disease. Trends Endocrinol. Metab. 2016, 27, 69–83. [Google Scholar] [CrossRef] [PubMed]
- Cahill, L.E.; Chiuve, S.E.; Mekary, R.A.; Jensen, M.K.; Flint, A.J.; Hu, F.B.; Rimm, E.B. Prospective study of breakfast eating and incident coronary heart disease in a cohort of male US health professionals. Circulation 2013, 128, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Pot, G.K.; Almoosawi, S.; Stephen, A.M. Meal irregularity and cardiometabolic consequences: Results from observational and intervention studies. Proc. Nutr. Soc. 2016, 75, 475–486. [Google Scholar] [CrossRef]
- Ha, K.; Song, Y. Associations of meal timing and frequency with obesity and metabolic syndrome among Korean adults. Nutrients 2019, 11, 2437. [Google Scholar] [CrossRef]
- Wilkinson, M.J.; Manoogian, E.N.; Zadourian, A.; Lo, H.; Fakhouri, S.; Shoghi, A.; Wang, X.; Fleischer, J.G.; Navlakha, S.; Panda, S.; et al. Ten-hour time-restricted eating reduces weight, blood pressure, and atherogenic lipids in patients with metabolic syndrome. Cell Metab. 2020, 31, 92–104.e5. [Google Scholar] [CrossRef]
- Xie, Y.; Tang, Q.; Chen, G.; Xie, M.; Yu, S.; Zhao, J.; Chen, L. New insights into the circadian rhythm and its related diseases. Front. Physiol. 2019, 10, 682. [Google Scholar] [CrossRef]
- Chaix, A.; Manoogian, E.N.; Melkani, G.C.; Panda, S. Time-restricted eating to prevent and manage chronic metabolic diseases. Annu. Rev. Nutr. 2019, 39, 291–315. [Google Scholar] [CrossRef]
- Mason, I.C.; Qian, J.; Adler, G.K.; Scheer, F.A.J.L. Impact of circadian disruption on glucose metabolism: Implications for type 2 diabetes. Diabetologia 2020, 63, 462–472. [Google Scholar] [CrossRef]
- Stenvers, D.J.; Scheer, F.A.J.L.; Schrauwen, P.; La Fleur, S.E.; Kalsbeek, A. Circadian clocks and insulin resistance. Nat. Rev. Endocrinol. 2019, 15, 75–89. [Google Scholar] [CrossRef]
- Chellappa, S.L.; Vujovic, N.; Williams, J.S.; Scheer, F.A.J.L. Impact of circadian disruption on cardiovascular function and disease. Trends Endocrinol. Metab. 2019, 30, 767–779. [Google Scholar] [CrossRef] [PubMed]
- Suliga, E.; Cieśla, E.; Rębak, D.; Kozieł, D.; Głuszek, S. Relationship between sitting time, physical activity, and metabolic syndrome among adults depending on body mass index (BMI). Med. Sci. Monit. 2018, 24, 7633–7645. [Google Scholar] [CrossRef] [PubMed]
- Sperling, L.C.; Mechanick, J.I.; Neeland, I.J.; Herrick, C.J.; Després, J.P.; Ndumele, C.E.; Vijayaraghavan, K.; Handelsman, Y.; Puckrein, G.A.; Araneta, M.R.G.; et al. The CardioMetabolic Health Alliance. Working toward a new care model for the metabolic syndrome. Am. Coll. Cardiol. 2015, 66, 1050–1067. [Google Scholar] [CrossRef] [PubMed]
- Vera, B.; Dashti, H.S.; Gómez-Abellán, P.; Hernández, A.M.; Esteban, A.; Scheer, F.A.J.L.; Saxena, R.; Garaulet, M. Modifiable lifestyle behaviors, but not a genetic risk score, associate with metabolic syndrome in evening chronotypes. Sci. Rep. 2018, 8, 945. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Martínez, P.; Mikhailidis, D.P.; Athyros, V.G.; Bullo, M.; Couture, P.; Covas, M.I.; de Koning, L.; Delgado-Lista, J.; Díaz-López, A.; Drevon, C.A.; et al. Lifestyle recommendations for the prevention and management of metabolic syndrome: An international panel recommendation. Nutr. Rev. 2017, 75, 307–326. [Google Scholar]
- Heymsfield, S.B.; Harp, J.B.; Reitman, M.L.; Beetsch, J.W.; Schoeller, D.A.; Erondu, N.; Pietrobelli, A. Why do obese patients not lose more weight when treated with low-calorie diets? A mechanistic perspective. Am. J. Clin. Nutr. 2007, 85, 346–354. [Google Scholar] [CrossRef]
- Feinman, R.D.; Pogozelski, W.K.; Astrup, A.; Bernstein, R.K.; Fine, E.J.; Westman, E.C.; Accurso, A.; Frassetto, L.; Gower, B.A.; McFarlane, S.I.; et al. Dietary carbohydrate restriction as the first approach in diabetes management: Critical review and evidence base. Nutrition 2015, 31, 1–13. [Google Scholar] [CrossRef]
- Sulli, G.; Manoogian, E.N.; Taub, P.R.; Panda, S. Training the circadian clock, clocking the drugs, and drugging the clock to prevent, manage, and treat chronic diseases. Trends Pharmacol. Sci. 2018, 39, 812–827. [Google Scholar] [CrossRef]
- Lopez-Minguez, J.; Gómez-Abellán, P.; Garaulet, M. Circadian rhythms, food timing and obesity. Proc. Nutr. Soc. 2016, 75, 501–511. [Google Scholar] [CrossRef]
- De Cabo, R.; Mattson, M.P. Effects of intermittent fasting on health, aging, and disease. N. Engl. J. Med. 2019, 381, 2541–2551. [Google Scholar] [CrossRef]
- Dashti, H.S.; Scheer, F.A.J.L.; Saxena, R.; Garaulet, M. Timing of food intake: Identifying contributing factors to design effective interventions. Adv. Nutr. 2019, 10, 606–620. [Google Scholar] [CrossRef] [PubMed]
- Dong, T.A.; Sandesara, P.B.; Dhindsa, D.S.; Quyyumi, A.A.; Arneson, L.C.; Dollar, A.L.; Taub, P.R.; Sperling, L.S. Intermittent fasting: A heart healthy dietary pattern? Am. J. Med. 2020, 133, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Panda, S. Circadian physiology of metabolism. Science 2016, 354, 1008–1015. [Google Scholar] [CrossRef]
- Antoni, R.; Robertson, T.M.; Robertson, M.D.; Johnston, J.D. A pilot feasibility study exploring the effects of a moderate time-restricted feeding intervention on energy intake, adiposity and metabolic physiology in free-living human subjects. J. Nutr. Sci. 2018, 7, 22. [Google Scholar] [CrossRef]
- Sutton, E.F.; Beyl, R.; Early, K.S.; Cefalu, W.T.; Ravussin, E.; Peterson, C.M. Early time-restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes. Cell Metab. 2018, 27, 1212–1221.e3. [Google Scholar] [CrossRef]
- Gabel, K.; Hoddy, K.K.; Haggerty, N.; Song, J.; Kroeger, C.M.; Trepanowski, J.F.; Panda, S.; Varady, K.A. Effects of 8-hour time restricted feeding on body weight and metabolic disease risk factors in obese adults: A pilot study. Nutr. Healthy Aging 2018, 4, 345–353. [Google Scholar] [CrossRef]
- Hutchison, A.T.; Regmi, P.; Manoogian, E.N.; Fleischer, J.G.; Wittert, G.A.; Panda, S.; Heilbronn, L.K. Time-restricted feeding improves glucose tolerance in men at risk for type 2 diabetes: A randomized crossover trial. Obesity 2019, 27, 724–732. [Google Scholar] [CrossRef]
- Anton, S.D.; Lee, S.A.; Donahoo, W.T.; McLaren, C.; Manini, T.M.; Leeuwenburgh, C.; Pahor, M. The effects of time restricted feeding on overweight, older adults: A Pilot Study. Nutrients 2019, 11, 1500. [Google Scholar] [CrossRef] [PubMed]
- Jamshed, H.; Beyl, R.A.; Della Manna, D.L.; Yang, E.S.; Ravussin, E.; Peterson, C.M. Early Time-restricted feeding improves 24-hour glucose levels and affects markers of the circadian clock, aging, and autophagy in humans. Nutrients 2019, 11, 1234. [Google Scholar] [CrossRef]
- Ravussin, E.; Beyl, R.A.; Poggiogalle, E.; Hsia, D.S.; Peterson, C.M. Early time-restricted feeding reduces appetite and increases fat oxidation but does not affect energy expenditure in humans. Obesity 2019, 27, 1244–1254. [Google Scholar] [CrossRef]
- Kesztyüs, D.; Cermak, P.; Gulich, M.; Kesztyüs, T. Adherence to time-restricted feeding and impact on abdominal obesity in primary care patients: Results of a pilot study in a pre–post design. Nutrients 2019, 11, 2854. [Google Scholar] [CrossRef] [PubMed]
- Cienfuegos, S.; Gabel, K.; Kalam, F.; Ezpeleta, M.; Wiseman, E.; Pavlou, V.; Lin, S.; Oliveira, M.L.; Varady, K.A. Effects of 4- and 6-h time-restricted feeding on weight and cardiometabolic health: A randomized controlled trial in adults with obesity. Cell Metab. 2020, 32, 366–378.e3. [Google Scholar] [CrossRef]
- Parr, E.B.; Devlin, B.L.; Radford, B.E.; Hawley, J.A. A delayed morning and earlier evening time-restricted feeding protocol for improving glycemic control and dietary adherence in men with overweight/obesity: A randomized controlled trial. Nutrients 2020, 12, 505. [Google Scholar] [CrossRef]
- Lowe, D.A.; Wu, N.; Rohdin-Bibby, L.; Moore, A.H.; Kelly, N.; Liu, Y.E.; Philip, E.; Vittinghoff, E.; Heymsfield, S.B.; Olgin, J.E.; et al. Effects of time-restricted eating on weight loss and other metabolic parameters in women and men with overweight and obesity. JAMA Intern. Med. 2020, 180, 1491. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.; Kang, J.; Kim, S.H.; Chung, H.S.; Kim, Y.J.; Yu, J.M.; Cho, S.T.; Oh, C.-M.; Kim, T. Beneficial effects of time-restricted eating on metabolic diseases: A systemic review and meta-analysis. Nutrients 2020, 12, 1267. [Google Scholar] [CrossRef]
- Queiroz, J.D.N.; Macedo, R.C.; Tinsley, G.M.; Reischak-Oliveira, A. Time-restricted eating and circadian rhythms: The biological clock is ticking. Crit. Rev. Food Sci. Nutr. 2020, 14, 1–13. [Google Scholar] [CrossRef]
- Regmi, P.; Heilbronn, L.K. Time-restricted eating: Benefits, mechanisms, and challenges in translation. iScience 2020, 23, 101161. [Google Scholar] [CrossRef]
- International Diabetes Federation. Consensus Statements—IDF Consensus Worldwide Definition of the Metabolic Syndrome. Available online: https://www.idf.org/e-library/consensus-statements/60-idfconsensus-worldwide-definitionof-the-metabolic-syndrome.html (accessed on 29 November 2020).
- The GBD 2015 Obesity Collaborators. Health effects of overweight and obesity in 195 countries over 25 years. N. Engl. J. Med. 2017, 377, 13–27. [Google Scholar]
- Ervin, R.B. Prevalence of metabolic syndrome among adults 20 years of age and over, by sex, age, race and ethnicity, and body mass index: United States, 2003–2006. Natl. Heal. Stat. Rep. 2009, 5, 1–7. [Google Scholar]
- Głuszek, S.; Ciesla, E.; Głuszek-Osuch, M.; Kozieł, D.; Kiebzak, W.; Wypchło, Ł.; Suliga, E. Anthropometric indices and cut-off points in the diagnosis of metabolic disorders. PLoS ONE 2020, 15, e0235121. [Google Scholar] [CrossRef]
- Suliga, E.; Kozieł, D.; Głuszek, S. Prevalence of metabolic syndrome in normal weight individuals. Ann. Agric. Environ. Med. 2016, 23, 631–635. [Google Scholar] [CrossRef]
- Bays, H.E.; Toth, P.P.; Kris-Etherton, P.M.; Abate, N.; Aronne, L.J.; Brown, W.V.; Gonzalez-Campoy, J.M.; Jones, S.R.; Kumar, R.; La Forge, R.; et al. Obesity, adiposity, and dyslipidemia: A consensus statement from the National Lipid Association. J. Clin. Lipidol. 2013, 7, 304–383. [Google Scholar] [CrossRef]
- Beltrán-Sánchez, H.; Harhay, M.O.; Harhay, M.M.; McElligott, S. Prevalence and trends of metabolic syndrome in the adult U.S. population, 1999–2010. J. Am. Coll. Cardiol. 2013, 62, 697–703. [Google Scholar] [CrossRef]
- Efeyan, A.; Comb, W.C.; Sabatini, D.M. Nutrient-sensing mechanisms and pathways. Nature 2015, 517, 302–310. [Google Scholar] [CrossRef]
- Maury, E.; Ramsey, K.M.; Bass, J. Circadian rhythms and metabolic syndrome: From experimental genetics to human disease. Circ. Res. 2010, 106, 447–462. [Google Scholar] [CrossRef]
- Szewczyk-Golec, K.; Woźniak, A.; Reiter, R.A. Inter-relationship of the chronobiotic, melatonin, with leptin and adiponectin: Implications for obesity. J. Pineal Res. 2015, 59, 277–291. [Google Scholar] [CrossRef]
- Szewczyk-Golec, K.; Rajewski, P.; Gackowski, M.; Mila-Kierzenkowska, C.; Wesołowski, R.; Sutkowy, P.; Pawłowska, M.; Woźniak, A. Melatonin supplementation lowers oxidative stress and regulates adipokines in obese patients on a calorie-restricted diet. Oxid. Med. Cell Longev. 2017, 2017, 8494107. [Google Scholar] [CrossRef]
- Qian, J.; Morris, C.J.; Caputo, R.; Garaulet, M.; Scheer, F.A.J.L. Ghrelin is impacted by the endogenous circadian system and by circadian misalignment in humans. Int. J. Obes. 2019, 43, 1644–1649. [Google Scholar] [CrossRef]
- Garaulet, M.; Qian, J.; Florez, J.C.; Arendt, J.; Saxena, R.; Scheer, F.A.J.L. Melatonin effects on glucose metabolism: Time to unlock the controversy. Trends Endocrinol. Metab. 2020, 31, 192–204. [Google Scholar] [CrossRef]
- Steppan, C.M.; Bailey, S.T.; Bhat, S.; Brown, E.J.; Banerjee, R.R.; Wright, C.M.; Patel, H.R.; Ahima, R.S.; Lazar, M.A. The hormone resistin links obesity to diabetes. Nature 2001, 409, 307–312. [Google Scholar] [CrossRef]
- Park, H.K.; Kwak, M.K.; Kim, H.J.; Ahima, R.S. Linking resistin, inflammation, and cardiometabolic diseases. Korean J. Intern. Med. 2017, 32, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Rutter, M.; Meigs, J.B.; Sullivan, L.M.; D’Agostino, R.B.; Wilson, P.W. C-reactive protein, the metabolic syndrome, and prediction of cardiovascular events in the framingham offspring study. Circulation 2004, 110, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Robertsa, C.K.; Sindhu, K.K. Oxidative stress and metabolic syndrome. Life Sci. 2009, 84, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Han, T.S.; Sattar, N.; Williams, K.; Gonzalez-Villalpando, C.; Lean, M.E.; Haffner, S.M. Prospective study of C-reactive protein in relation to the development of diabetes and metabolic syndrome in the Mexico City diabetes study. Diabetes Care 2002, 25, 2016–2021. [Google Scholar] [CrossRef] [PubMed]
- Sattar, N.; Gaw, A.; Scherbakova, O.; Ford, I.; O’Reilly, D.S.; Haffner, S.M.; Isles, C.; Macfarlane, P.W.; Packard, C.J.; Cobbe, S.M.; et al. Metabolic syndrome with and without C-reactive protein as a predictor of coronary heart disease and diabetes in the West of Scotland coronary prevention study. Circulation 2003, 108, 414–419. [Google Scholar] [CrossRef]
- Malik, S.; Wong, N.D.; Franklin, S.; Pio, J.; Fairchild, C.; Chen, R. Cardiovascular disease in U.S. patients with metabolic syndrome, diabetes, and elevated C-reactive protein. Diabetes Care 2005, 28, 690–693. [Google Scholar] [CrossRef]
- Swiatkiewicz, I.; Taub, P.R. The usefulness of C-reactive protein for the prediction of post-infarct left ventricular systolic dysfunction and heart failure. Kardiol. Pol. 2018, 76, 821–829. [Google Scholar] [CrossRef]
- Swiatkiewicz, I.; Magielski, P.; Kubica, A.; Zadourian, A.; DeMaria, A.N.; Taub, P.R. Enhanced inflammation is a marker for risk of post-infarct ventricular dysfunction and heart failure. Int. J. Mol. Sci. 2020, 21, 807. [Google Scholar] [CrossRef]
- Aronson, D.; Bartha, P.; Zinder, O.; Kerner, A.; Markiewicz, W.; Avizohar, O.; Brook, G.J.; Levy, Y. Obesity is the major determinant of elevated C-reactive protein in subjects with the metabolic syndrome. Int. J. Obes. 2004, 28, 674–679. [Google Scholar] [CrossRef]
- Aronson, D.; Sella, R.; Sheikh-Ahmad, M.; Kerner, A.; Avizohar, O.; Rispler, S.; Bartha, P.; Markiewicz, W.; Levy, Y.; Brook, G.J. The association between cardiorespiratory fitness and C-reactive protein in subjects with the metabolic syndrome. J. Am. Coll. Cardiol. 2004, 44, 2003–2007. [Google Scholar] [CrossRef]
- Houstis, N.; Rosen, E.D.; Lander, E.S. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature 2006, 440, 944–948. [Google Scholar] [CrossRef]
- Rains, J.L.; Jain, S.K. Oxidative stress, insulin signaling, and diabetes. Free Radic. Biol. Med. 2011, 50, 567–575. [Google Scholar] [CrossRef]
- Rania, V.; Deepb, G.; Singhc, R.K.; Palled, K.; Yadav, U.C.S. Oxidative stress and metabolic disorders: Pathogenesis and therapeutic strategies. Life Sci. 2016, 148, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Bass, J. Circadian topology of metabolism. Nature 2012, 491, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Asher, G.; Sassone-Corsi, P. Time for food: The intimate interplay between nutrition, metabolism, and the circadian clock. Cell 2015, 161, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Scheer, F.A.J.L.; Hu, K.; Evoniuk, H.; Kelly, E.E.; Malhotra, A.; Hilton, M.F.; Shea, S.A. Impact of the human circadian system, exercise, and their interaction on cardiovascular function. Proc. Natl. Acad. Sci. USA 2010, 107, 20541–20546. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Scheer, F.A.J.L. Circadian system and glucose metabolism: Implications for physiology and disease. Trends Endocrinol. Metab. 2016, 27, 282–293. [Google Scholar] [CrossRef] [PubMed]
- Mattis, J.; Sehgal, A. Circadian rhythms, sleep, and disorders of aging. Trends Endocrinol. Metab. 2016, 27, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Vollmers, C.; Gill, S.; DiTacchio, L.; Pulivarthy, S.R.; Le, H.D.; Panda, S. Time of feeding and the intrinsic circadian clock drive rhythms in hepatic gene expression. Proc. Natl. Acad. Sci. USA 2009, 106, 21453–21458. [Google Scholar] [CrossRef]
- Mattson, M.P.; Allison, D.B.; Fontana, L.; Harvie, M.; Longo, V.D.; Malaisse, W.J.; Mosley, M.; Notterpek, L.; Ravussin, E.; Scheer, F.A.J.L.; et al. Meal frequency and timing in health and disease. Proc. Natl. Acad. Sci. USA 2014, 111, 16647–16653. [Google Scholar] [CrossRef]
- Scheer, F.A.J.L.; Hilton, M.F.; Mantzoros, C.S.; Shea, S.A. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc. Natl. Acad. Sci. USA 2009, 106, 4453–4458. [Google Scholar] [CrossRef] [PubMed]
- Garaulet, M.; Gómez-Abellán, P.; Alburquerque-Béjar, J.J.; Lee, Y.-C.; Ordovás, J.M.; Scheer, F.A.J.L. Timing of food intake predicts weight loss effectiveness. Int. J. Obes. 2013, 37, 604–611. [Google Scholar] [CrossRef] [PubMed]
- McHill, A.W.; Wright, K.P. Role of sleep and circadian disruption on energy expenditure and in metabolic predisposition to human obesity and metabolic disease. Obes. Rev. 2017, 18, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Kohsaka, A.; Laposky, A.; Ramsey, K.M.; Estrada, C.; Joshu, C.; Kobayashi, Y.; Turek, F.W.; Bass, J. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab. 2007, 6, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Collet, T.-H.; van der Klaauw, A.A.; Henning, E.; Keogh, J.M.; Suddaby, D.; Dachi, S.V.; Dunbar, S.; Kelway, S.; Dickson, S.L.; Farooqi, I.S.; et al. The sleep/wake cycle is directly modulated by changes in energy balance. Sleep 2016, 39, 1691–1700. [Google Scholar] [CrossRef] [PubMed]
- Puttonen, S.; Härmä, M.; Hublin, C. Shift work and cardiovascular disease—Pathways from circadian stress to morbidity. Scand. J. Work. Environ. Health 2010, 36, 96–108. [Google Scholar] [CrossRef]
- McHill, A.W.; Melanson, E.L.; Higgins, J.; Connick, E.; Moehlman, T.M.; Stothard, E.R.; Wright, K.P. Impact of circadian misalignment on energy metabolism during simulated nightshift work. Proc. Natl. Acad. Sci. USA 2014, 111, 17302–17307. [Google Scholar] [CrossRef]
- Mukherji, A.; Kobiita, A.; Damara, M.; Misra, N.; Meziane, H.; Champy, M.-F.; Giangrande, A. Shifting eating to the circadian rest phase misaligns the peripheral clocks with the master SCN clock and leads to a metabolic syndrome. Proc. Natl. Acad. Sci. USA 2015, 112, E6691–E6698. [Google Scholar] [CrossRef]
- Mohebbi, I.; Shateri, K.; Seyedmohammadzad, M. The relationship between working schedule patterns and the markers of the metabolic syndrome: Comparison of shift workers with day workers. Int. J. Occup. Med. Environ. Health 2012, 25, 383–391. [Google Scholar] [CrossRef]
- Hall, M.H.; Muldoon, M.F.; Jennings, J.R.; Buysse, D.J.; Flory, J.D.; Manuck, S.B. Self-reported sleep duration is associated with the metabolic syndrome in midlife adults. Sleep 2008, 31, 635–643. [Google Scholar] [CrossRef]
- Markwald, R.R.; Melanson, E.L.; Smith, M.R.; Higgins, J.; Perreault, L.; Eckel, R.H.; Wright, K.P. Impact of insufficient sleep on total daily energy expenditure, food intake, and weight gain. Proc. Natl. Acad. Sci. USA 2013, 110, 5695–5700. [Google Scholar] [CrossRef] [PubMed]
- Hatori, M.; Vollmers, C.; Zarrinpar, A.; DiTacchio, L.; Bushong, E.A.; Gill, S.; Leblanc, M.; Chaix, A.; Joens, M.; Fitzpatrick, J.A.J.; et al. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 2012, 15, 848–860. [Google Scholar] [CrossRef]
- Chaix, A.; Zarrinpar, A.; Miu, P.; Panda, S. Time-restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges. Cell Metab. 2014, 20, 991–1005. [Google Scholar] [CrossRef]
- Zarrinpar, A.; Chaix, A.; Yooseph, S.; Panda, S. Diet and feeding pattern affect the diurnal dynamics of the gut microbiome. Cell Metab. 2014, 20, 1006–1017. [Google Scholar] [CrossRef]
- Chung, H.; Chou, W.; Sears, D.D.; Patterson, R.E.; Webster, N.J.; Ellies, L.G. Time-restricted feeding improves insulin resistance and hepatic steatosis in a mouse model of postmenopausal obesity. Metabolism 2016, 65, 1743–1754. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.; Le, H.D.; Melkani, G.C.; Panda, S. Time-restricted feeding attenuates age-related cardiac decline in Drosophila. Science 2015, 347, 1265–1269. [Google Scholar] [CrossRef]
- LeCheminant, J.D.; Christenson, E.; Bailey, B.W.; Tucker, L.A. Restricting night-time eating reduces daily energy intake in healthy young men: A short-term cross-over study. Br. J. Nutr. 2013, 110, 2108–2113. [Google Scholar] [CrossRef]
- McAllister, M.J.; Pigg, B.L.; Renteria, L.I.; Waldman, H.S. Time-restricted feeding improves markers of cardiometabolic health in physically active college-age men: A 4-week randomized pre-post pilot study. Nutr. Res. 2020, 75, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Moro, T.; Tinsley, G.; Bianco, A.; Marcolin, G.; Pacelli, Q.F.; Battaglia, G.; Palma, A.; Gentil, P.; Neri, M.; Paoli, A. Effects of eight weeks of time-restricted feeding (16/8) on basal metabolism, maximal strength, body composition, inflammation, and cardiovascular risk factors in resistance-trained males. J. Transl. Med. 2016, 14, 290. [Google Scholar] [CrossRef] [PubMed]
- Tinsley, G.M.; Forsse, J.S.; Butler, N.K.; Paoli, A.; Bane, A.A.; La Bounty, P.M.; Morgan, G.B.; Grandjean, P.W. Time-restricted feeding in young men performing resistance training: A randomized controlled trial. Eur. J. Sport Sci. 2017, 17, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Tinsley, G.M.; Moore, M.L.; Graybeal, A.J.; Paoli, A.; Kim, Y.; Gonzales, J.U.; Harry, J.R.; VanDusseldorp, T.A.; Kennedy, D.N.; Cruz, M.R. Time-restricted feeding plus resistance training in active females: A randomized trial. Am. J. Clin. Nutr. 2019, 110, 628–640. [Google Scholar] [CrossRef]
- Carlson, O.; Martin, B.; Stote, K.S.; Golden, E.; Maudsley, S.; Najjar, S.S.; Ferrucci, L.; Ingram, D.K.; Longo, D.L.; Rumpler, W.V.; et al. Impact of reduced meal frequency without caloric restriction on glucose regulation in healthy, normal-weight middle-aged men and women. Metabolism 2007, 56, 1729–1734. [Google Scholar] [CrossRef] [PubMed]
- Stote, K.S.; Baer, D.J.; Spears, K.; Paul, D.R.; Harris, G.K.; Rumpler, W.V.; Strycula, P.; Najjar, S.S.; Ferrucci, L.; Ingram, D.K.; et al. A controlled trial of reduced meal frequency without caloric restriction in healthy, normal-weight, middle-aged adults. Am. J. Clin. Nutr. 2007, 85, 981–988. [Google Scholar] [CrossRef] [PubMed]
- Martens, C.R.; Rossman, M.J.; Mazzo, M.R.; Jankowski, L.R.; Nagy, E.E.; Denman, B.A.; Richey, J.J.; Johnson, S.A.; Ziemba, B.P.; Wang, Y.; et al. Short-term time-restricted feeding is safe and feasible in non-obese healthy midlife and older adults. Geroscience 2020, 42, 667–686. [Google Scholar] [CrossRef] [PubMed]
- Gabel, K.; Hoddy, K.K.; Varady, K.A. Safety of 8-h time restricted feeding in adults with obesity. Appl. Physiol. Nutr. Metab. 2019, 44, 107–109. [Google Scholar] [CrossRef] [PubMed]
- Gabel, K.; Hoddy, K.K.; Burgess, H.J.; Varady, K.A. Effect of 8-h time-restricted feeding on sleep quality and duration in adults with obesity. Appl. Physiol. Nutr. Metab. 2019, 44, 903–906. [Google Scholar] [CrossRef] [PubMed]
- Goris, A.H.C.; Westerterp-Plantenga, M.S.; Westerterp, K.R. Undereating and underrecording of habitual food intake in obese men: Selective underreporting of fat intake. Am. J. Clin. Nutr. 2000, 71, 130–134. [Google Scholar] [CrossRef]
- Kubica, A.; Kasprzak, M.; Fabiszak, T.; Laskowska, E.; Navarese, E.P.; Sztuba, B.; Swiatkiewicz, I.; Kubica, J.; Obonska, K.; Kozinski, M.; et al. Discrepancies in assessment of adherence to antiplatelet treatment after myocardial infarction. Pharmacology 2015, 95, 50–58. [Google Scholar] [CrossRef]
- Ackermann, R.T.; Liss, D.T.; Finch, E.A.; Schmidt, K.K.; Hays, L.M.; Marrero, D.G.; Saha, C. A randomized comparative effectiveness trial for preventing type 2 diabetes. Am. J. Public Health 2015, 105, 2328–2334. [Google Scholar] [CrossRef]
- Katula, J.A.; Vitolins, M.Z.; Morgan, T.M.; Lawlor, M.S.; Blackwell, C.S.; Isom, S.P.; Pedley, C.F.; Goff, D.C. The healthy living partnerships to prevent diabetes study: 2-year outcomes of a randomized controlled trial. Am. J. Prev. Med. 2013, 44, S324–S332. [Google Scholar] [CrossRef]
- Jacobson, T.A.; Maki, K.C.; Orringer, C.E.; Jones, P.H.; Krisetherton, P.M.; Sikand, G.; La Forge, R.; Daniels, S.R.; Wilson, D.P.; Morris, P.B.; et al. National Lipid Association recommendations for patient-centered management of dyslipidemia: Part 2. J. Clin. Lipidol. 2015, 9, S1–S122.e1. [Google Scholar] [CrossRef] [PubMed]
- Zaulkffali, A.S.; Md Razip, N.N.; Syed Alwi, S.S.; Abd Jalil, A.; Abd Mutalib, M.S.; Gopalsamy, B.; Chang, S.K.; Zainal, Z.; Ibrahim, N.N.; Zakaria, Z.A. Vitamins D and E stimulate the PI3K-AKT signalling pathway in insulin-resistant SK-N-SH neuronal cells. Nutrients 2019, 11, 2525. [Google Scholar] [CrossRef] [PubMed]
- Zeb, F.; Wu, X.; Chen, L.; Fatima, S.; Haq, I.-U.; Chen, A.; Majeed, F.; Feng, Q.; Li, M. Effect of time-restricted feeding on metabolic risk and circadian rhythm associated with gut microbiome in healthy males. Br. J. Nutr. 2020, 123, 1216–1226. [Google Scholar] [CrossRef] [PubMed]
- Zomer, E.; Gurusamy, K.; Leach, R.; Trimmer, C.; Lobstein, T.; Morris, S.; James, W.; Finer, N. Interventions that cause weight loss and the impact on cardiovascular risk factors: A systematic review and meta-analysis. Obes. Rev. 2016, 17, 1001–1011. [Google Scholar] [CrossRef]
- Heran, B.S.; Galm, B.P.; Wright, J.M. Blood pressure lowering efficacy of alpha blockers for primary hypertension. Cochrane Database Syst. Rev. 2012, 7, CD004643. [Google Scholar] [CrossRef]
- Johnston, J.G.; Speed, J.S.; Jin, C.; Pollock, D.M. Loss of endothelin B receptor function impairs sodium excretion in a time- and sex-dependent manner. Am. J. Physiol. Physiol. 2016, 311, F991–F998. [Google Scholar] [CrossRef]
- Cederroth, C.R.; Albrecht, U.; Bass, J.; Brown, S.A.; Dyhrfjeld-Johnsen, J.; Gachon, F.; Green, C.B.; Hastings, M.H.; Helfrich-Förster, C.; HogenEsch, J.B.; et al. Medicine in the fourth dimension. Cell Metab. 2019, 30, 238–250. [Google Scholar] [CrossRef]
- St-Onge, M.-P.; Jamy American Heart Association Obesity Committee of the Council on Lifestyle and Cardiometabolic Health; Council on Cardiovascular Disease in the Young; Council on Clinical Cardiology; Stroke Council; Baskin, M.L.; Chiuve, S.E.; Johnson, H.M.; Kris-Etherton, P.; Varady, K. Meal timing and frequency: Implications for cardiovascular disease prevention: A scientific statement from the American heart association. Circulation 2017, 135, e96–e121. [Google Scholar] [CrossRef]
- Tinsley, G.M.; Paoli, A. Time-restricted eating and age-related muscle loss. Aging 2019, 11, 8741–8742. [Google Scholar] [CrossRef]
- Quist, J.S.; Jensen, M.M.; Clemmensen, K.K.B.; Pedersen, H.; Bjerre, N.; Størling, J.; Blond, M.B.; Albrechtsen, N.W.; Holst, J.J.; Torekov, S.S.; et al. Protocol for a single-centre, parallel-group, randomised, controlled, superiority trial on the effects of time-restricted eating on body weight, behaviour and metabolism in individuals at high risk of type 2 diabetes: The REStricted Eating Time (RESET) study. BMJ Open 2020, 10, e037166. [Google Scholar] [CrossRef]
| Reference and Study Design | Inclusion Criteria | Participants | TRE Duration and EW | Meal Timing During TRE | Primary Outcome |
|---|---|---|---|---|---|
| Gill and Panda [10] One group (pre-post design), pilot feasibility study | Healthy adults BMI > 25 kg/m2 EW ≥ 14 h | n = 8 (5 M, 3 F) Overweight/obese EW 14.8 h Age 34.4 ± 2.9 y (M) 36.3 ± 4.3 y (F) | 16 weeks EW 10–12 h | Self-selected EW | Change in body weight |
| Antoni et al. [34] Randomized controlled trial, pilot feasibility study | Healthy adults BMI 20–39 kg/m2 | n = 13 (12 F, 1 M) Overweight/obese TRE: n = 7 (6 F, 1 M) Age 47 ± 3 y Control: n = 6 (F) Age 45 ± 4 y | 10 weeks EW 8 h 37 min ± 22 min | TRE: breakfast delayed and dinner advanced by 1.5 h each Control: AL | Feasibility of TRE in reducing EW |
| Sutton et al. [35] Crossover | Males Age 35–70 y BMI 20–39 kg/m2 Prediabetes (based on HbA1c and OGTT) | n = 8 (M) Overweight/obese and prediabetic Age 56 ± 9 y | 5 weeks in each condition EW 6 or 12 h | eTRE: 8 a.m.–2 p.m. (dinner before 3 p.m.) Control: 8 a.m.–8 p.m. 3 meals provided and matched across arms | Changes in glucose tolerance, postprandial insulin, and insulin sensitivity |
| Gabel et al. [36,106,107] Matched historical controls, pilot study | Age 25–65 y BMI 30–45 kg/m2 Sedentary to lightly active | n = 46 (41 F, 5 M) Obese TRE: n = 23 (20 F, 3 M) Age 50 ± 2 y Control: n = 23 (21 F, 2 M) Age 48 ± 2 y | 12 weeks EW 8 h | TRE: 10 a.m.–6 p.m. Control: AL | Change in body weight |
| Hutchison et al. [37] Crossover | Age 30–70 y WC ≥ 102 cm High risk of T2DM | n = 15 (M) Obese (abdominal obesity) Age 55 ± 3 y | 7 days in each condition EW 9 h | eTRE: 8 a.m.–5 p.m. dTRE: 12 p.m.–9 p.m. | Glucose response to a mixed-nutrient meal in men at risk for T2DM |
| Anton et al. [38] One group (pre-post design), pilot feasibility study | Age ≥ 65 y BMI 25–40 kg/m2 Sedentary | n = 10 (6 F, 4 M) Overweight/obese Age ≥ 65 y | 4 weeks EW 8 h | Self-selected EW | Feasibility and safety of TRE in overweight, older adults |
| Jamshed et al. [39], Ravussin et al. [40] Crossover | Healthy adults Age 20–45 y BMI 25–35 kg/m2 | n = 11 (4 F, 7 M) Overweight/obese Age 32 ± 7 y | 4 days EW 6 or 12 h | eTRE: 8 a.m.–2 p.m. Control: 8 a.m.–8 p.m. 3 meals provided and matched across arms | Change in energy expenditure |
| Kesztyüs et al. [41] One group (pre-post design), pilot feasibility study | One or more components of MetS, including T2DM non requiring insulin | n = 40 (31 F, 9 M) Abdominal obesity Age 49.1 ± 12.4 y | 12 weeks EW 8–9 h | Self-selected EW | Adherence to TRE intervention (proportion of days with fasting ≥15 h) |
| Wilkinson et al. [16] One group (pre-post design), pilot study | Diagnosed MetS (≥3 components) EW ≥ 14 h | n = 19 (6 F, 13 M) Obese with MetS EW 15.1 ± 1.1 h Age 59 ± 11.1 y | 12 weeks EW 10 h | Self-selected EW | Change in mean blood glucose (CGM) |
| Chow et al. [11] Randomized controlled trial, feasibility study | Age 18–65 y BMI ≥ 25 kg/m2 EW ≥ 14 h | n = 20 (17 F, 3 M) Overweight/obese EW 15.2 ± 0.7 h TRE: n = 11 (9 F, 2 M) Age 46.5 ± 12.4 y Control: n = 9 (8 F, 1 M) Age 44.2 ± 12.3 y | 12 weeks EW 8 h | TRE: Self-selected EW Control: AL | Change in body weight |
| Cienfuegos et al. [42] Randomized controlled trial | Age 18–65 y BMI 30–49.9 kg/m2 Sedentary or moderately active | n = 58 (53 F, 5 M) Obese 4-h TRE: n = 19 (17 F, 2 M) Age 47 ± 2 y 6-h TRE: n = 20 (19 F, 1 M) Age 47 ± 3 y Control: n = 19 (17 F, 2 M) Age 45 ± 2 y | 8 weeks EW 4 or 6 h | 4-h TRE: 3 p.m.–7 p.m. 6-h TRE: 1 p.m.–7 p.m. Control: AL | Change in body weight |
| Parr et al. [43] Randomized controlled crossover trial | Males Age 30–45 y BMI 27–35 kg/m2 Sedentary lifestyle | n = 11 (M) Overweight/obese Age 38 ± 5 y Group 1: n = 6 Group 2: n = 5 | 5 days EW 8 h | TRE: 10 a.m.–6 p.m. Control: 7 a.m.–10 p.m. Isoenergetic diet protocols | Circadian blood profiles of glucose and insulin |
| Lowe [44] Randomized controlled trial | Age 18–64 y BMI 27–43 kg/m2 | n = 116 (46 F, 70 M) Overweight/obese Age 46.5 ± 10.5 y In-person TRE group: n = 25 (12 F, 13 M) Age 43.3 ± 11.8 y | 12 weeks EW 8 h | TRE: 12 p.m.–8 p.m. CMT (consistent meal timing): 3 structured meals per day | Change in body weight |
| Reference and Study Design | MetS Components | Results of TRE |
|---|---|---|
| Gill and Panda [10] One group (pre-post design), pilot feasibility study | Mean BMI > 30 kg/m2 | ↓ Body weight ↓ Hunger at night ↑ Morning and overall energy levels and sleep satisfaction ↓ Energy intake |
| Antoni et al. [34] Randomized controlled trial, pilot feasibility study | BMI 29 ± 2 kg/m2 | ↔ Body weight ↓ Body fat ↓ FPG ↔ Fasting insulin, lipids ↓ Energy intake ↓ EW by ~4.5 h |
| Sutton et al. [35] Crossover | BMI 32 ± 4 kg/m2 FPG 102 ± 9 mg/dL | ↔ Body weight ↑ Insulin sensitivity, β-cell function, TG ↔ FPG, lipids, TNF-α, IL-6 ↓ BP, fasting and postprandial insulin, evening appetite, oxidative stress Adherence to TRE was 98% (rigorously controlled trial) |
| Gabel et al. [36,106,107] Matched historical controls, pilot study | BMI ≥ 30 kg/m2 HDL-C 48 ± 2 mg/dL | ↓ Body weight ↔ Body fat, FPG, fasting insulin, insulin resistance, lipids, homocysteine, sleep quality/duration ↓ Energy intake ↓ Systolic BP |
| Hutchison et al. [37] Crossover | WC 115 ± 2 cm BP 141 ± 3/87 ± 2 mmHg FPG 105 ± 2 mg/dL | ↓ Body weight ↔ Body fat, FPG ↓ Mean fasting glucose (CGM) in eTRE ↑ Glucose tolerance ↓ Fasting TG, hunger |
| Anton et al. [38] One group (pre-post design), pilot feasibility study | WC 109 ± 13 cm BP 146 ± 16/78 ± 12 mmHg FPG 106 ± 28 mg/dL BMI 34 ± 3 kg/m2 | ↓ Body weight ↔ Blood glucose, WC, physical and cognitive function ↑ Quality of life Adherence to TRE was 84% |
| Jamshed et al. [39] Ravussin et al. [40] Crossover | BMI 30 ± 3 kg/m2 | ↓ Mean 24 h glucose and glycemic excursions (CGM) ↓ Morning FPG, insulin and HOMA-IR; mean and morning ghrelin, mean appetite ↑ Morning ketones, TC, LDL-C and HDL-C; metabolic flexibility, fullness, fat oxidation ↔ Energy expenditure Altered cortisol patterns and circadian clock genes expression related to aging, stress, autophagy, oxidative stress |
| Kesztyüs et al. [41] One group (pre-post design), pilot feasibility study | WC 107 ± 13 cm | ↓ Body weight ↓ WC ↓ HbA1c ↔ Lipids Adherence to TRE was 86% |
| Wilkinson et al. [16] One group (pre-post design), pilot study | Diagnosed MetS WC 109 ± 11 cm FPG 107 ± 15 mg/dL TG 161 ± 87 mg/dL HDL-C 47 ± 13 mg/dL BMI 30 ± 5 kg/m2 | ↓ Body weight ↓ WC ↓ Body fat ↔ Mean glucose (CGM), FPG, fasting insulin, HOMA-IR, HbA1c, HDL-C, TG, sleep quality ↓ TC, LDL-C, non-HDL-C ↓ Energy intake ↓ BP |
| Chow et al. [11] Randomized controlled trial, feasibility study | BMI 34 ± 8 kg/m2 BP 132 ± 13/85 ± 4 mmHg | ↓ Body weight ↓ Visceral fat mass, lean mass ↓ Fasting glucose (CGM), TG ↔ Mean glucose (CGM), glucose tolerance, HbA1c, lipids No significant differences in changes of fasting glucose and TG between TRE and non-TRE groups |
| Cienfuegos et al. [42] Randomized controlled trial | BMI 36 ± 1 kg/m2 BP 135 ± 5/88 ± 2 mmHg | ↓ Body weight ↓ Fat mass ↓ Energy intake ↓ Insulin resistance ↓ Oxidative stress ↔ TNF-α, IL-6 |
| Parr et al. [43] Randomized controlled crossover trial | BMI 32 ± 2 kg/m2 | ↔ Peak and waking glucose, AUC 24 h glucose (CGM) and insulin ↓ AUC nocturnal glucose (CGM) ↑ Feeling of well-being, TG |
| Lowe [44] Randomized controlled trial | BMI 31 ± 5 kg/m2 | ↓ Body weight, lean mass, energy expenditure, diastolic BP ↔ Fat mass, FPG, fasting insulin, HOMA-IR, HbA1c, lipids, systolic BP No significant differences in changes of outcomes between TRE and non-TRE groups |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Świątkiewicz, I.; Woźniak, A.; Taub, P.R. Time-Restricted Eating and Metabolic Syndrome: Current Status and Future Perspectives. Nutrients 2021, 13, 221. https://doi.org/10.3390/nu13010221
Świątkiewicz I, Woźniak A, Taub PR. Time-Restricted Eating and Metabolic Syndrome: Current Status and Future Perspectives. Nutrients. 2021; 13(1):221. https://doi.org/10.3390/nu13010221
Chicago/Turabian StyleŚwiątkiewicz, Iwona, Alina Woźniak, and Pam R. Taub. 2021. "Time-Restricted Eating and Metabolic Syndrome: Current Status and Future Perspectives" Nutrients 13, no. 1: 221. https://doi.org/10.3390/nu13010221
APA StyleŚwiątkiewicz, I., Woźniak, A., & Taub, P. R. (2021). Time-Restricted Eating and Metabolic Syndrome: Current Status and Future Perspectives. Nutrients, 13(1), 221. https://doi.org/10.3390/nu13010221






