The Feasibility of the “Omega Kid” Study Protocol: A Double-Blind, Randomised, Placebo-Controlled Trial Investigating the Effect of Omega-3 Supplementation on Self-Regulation in Preschool-Aged Children
Abstract
1. Introduction
Hypothesis
2. Materials and Methods
2.1. Study Design
2.2. Ethics Approval and Clinical Trial Registration
2.3. Participants, Inclusion and Exclusion Criteria
2.4. Recruitment
2.5. Randomisation and Blinding of Study Intervention
2.6. Data Collection
2.7. Blood Collection
2.8. Feasibility of Finger-Prick Blood Sample
2.9. Compliance to Study Protocol
2.10. Acceptability of Study Intervention
2.11. Completion Rate of Children and Parent/Caregiver Tasks
2.11.1. Self-Regulation
2.11.2. Executive Function
2.11.3. EEG Measures
2.11.4. ADHD
2.11.5. N-3 LCPUFA Dietary Intake
2.12. Statistical Analysis
3. Results
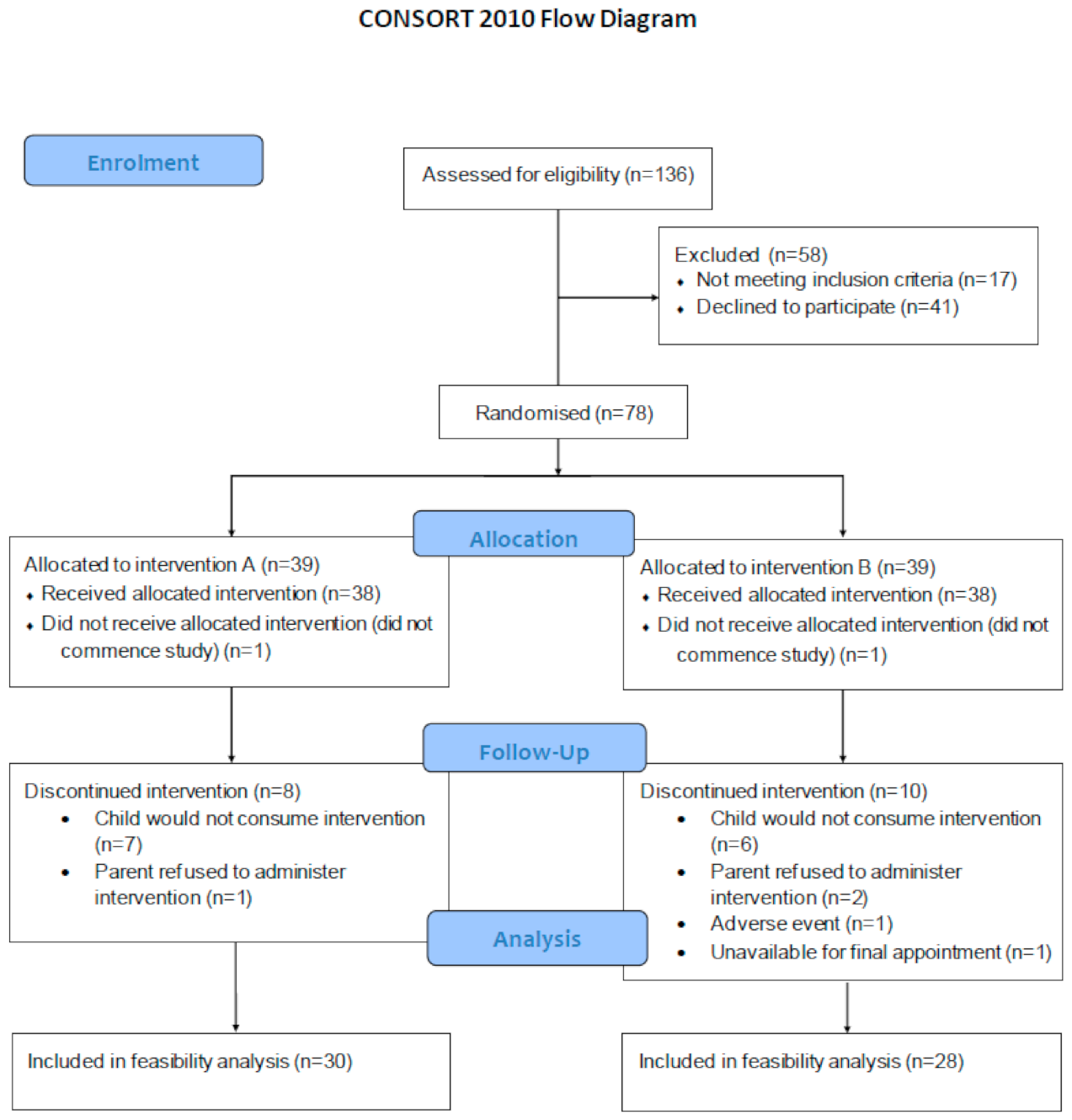
3.1. Recruitment and Retention
3.2. Enrolment and Randomisation to Intervention
3.3. Blood Collection
3.4. Compliance to Treatment
3.5. Acceptability of Study Intervention
3.6. Collection of Outcome Measures
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Murray, D.W.; Rosanbalm, K.; Christopoulos, C.; Hamoudi, A. Self-Regulation and Toxic Stress: Foundations for Under-Standing Self-Regulation from an Applied Developmental Perspective, in OPRE Report# 2015-21; Office of Planning, Research and Evaluation, Administration for Children and Families, U.S. Department of Health and Human Services: Washington, DC, USA, 2015.
- Shiels, K.; Hawk, L.W., Jr. Self-regulation in ADHD: The role of error processing. Clin. Psychol. Rev. 2010, 30, 951–961. [Google Scholar] [CrossRef]
- Mazefsky, C.A.; Herrington, J.; Siegel, M.; Scarpa, A.; Maddox, B.B.; Scahill, L.; White, S.W. The Role of Emotion Regu-lation in Autism Spectrum Disorder. J. Am. Acad. Child Adolesc. Psychiatry 2013, 52, 679–688. [Google Scholar] [CrossRef]
- Skibbe, L.E.; Montroy, J.J.; Bowles, R.P.; Morrison, F.J. Self-regulation and the development of literacy and language achievement from preschool through second grade. Early Child. Res. Q. 2019, 46, 240–251. [Google Scholar] [CrossRef]
- Lonigan, C.J.; Spiegel, J.A.; Goodrich, J.M.; Morris, B.M.; Osborne, C.M.; Lerner, M.D.; Phillips, B.M. Does Preschool Self-Regulation Predict Later Behavior Problems in General or Specific Problem Behaviors? J. Abnorm. Child Psychol. 2017, 45, 1491–1502. [Google Scholar] [CrossRef]
- Robson, D.A.; Allen, M.S.; Howard, S.J. Self-regulation in childhood as a predictor of future outcomes: A meta-analytic review. Psychol. Bull. 2020, 146, 324–354. [Google Scholar] [CrossRef]
- Moffitt, T.E.; Arseneault, L.; Belsky, D.W.; Dickson, N.; Hancox, R.J.; Harrington, H.; Houts, R.; Poulton, R.; Roberts, B.W.; Ross, S.A.; et al. A gradient of childhood self-control predicts health, wealth, and public safety. Proc. Natl. Acad. Sci. USA 2011, 108, 2693–2698. [Google Scholar] [CrossRef] [PubMed]
- Howard, S.J.; Vasseleu, E.; Neilsen-Hewett, C.; Cliff, K. Evaluation of the Preschool Situational Self-Regulation Toolkit (PRSIST) Program for Supporting children’s early self-regulation development: Study protocol for a cluster randomized con-trolled trial. Trials 2018, 19, 64. [Google Scholar] [CrossRef] [PubMed]
- Miyake, A.; Friedman, N.P.; Emerson, M.J.; Witzki, A.H.; Howerter, A.; Wager, T.D. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cogn. Psychol. 2000, 41, 49–100. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Hale, D.; Das, S.; Goddings, A.-L.; Blakemore, S.-J.; Viner, R.M. Effectiveness of universal self-regulation–based interventions in children and adolescents: A systematic review and meta-analysis. JAMA Pediatr. 2018, 172, 566–575. [Google Scholar] [CrossRef]
- Montroy, J.J.; Bowles, R.P.; Skibbe, L.E.; McClelland, M.M.; Morrison, F.J. The development of self-regulation across early childhood. Dev. Psychol. 2016, 52, 1744. [Google Scholar]
- Meyer, B.J.; Onyiaodike, C.C.; Brown, E.A.; Jordan, F.; Murray, H.; Nibbs, R.J.B.; Sattar, N.; Lyall, H.; Nelson, S.M.; Freeman, D.J. Maternal Plasma DHA Levels Increase Prior to 29 Days Post-LH Surge in Women Undergoing Frozen Embryo Transfer: A Prospective, Observational Study of Human Pregnancy. J. Clin. Endocrinol. Metab. 2016, 101, 1745–1753. [Google Scholar] [CrossRef] [PubMed]
- Stonehouse, W. Does consumption of LC omega-3 PUFA enhance cognitive performance in healthy school-aged children and throughout adulthood? Evidence from clinical trials. Nutrients 2014, 6, 2730–2758. [Google Scholar] [CrossRef] [PubMed]
- Weiser, M.J.; Butt, C.M.; Mohajeri, M.H. Docosahexaenoic Acid and Cognition throughout the Lifespan. Nutrients 2016, 8, 99. [Google Scholar] [CrossRef] [PubMed]
- Chung, W.-L.; Chen, J.-J.; Su, H.-M. Fish Oil Supplementation of Control and (n-3) Fatty Acid-Deficient Male Rats Enhances Reference and Working Memory Performance and Increases Brain Regional Docosahexaenoic Acid Levels. J. Nutr. 2008, 138, 1165–1171. [Google Scholar] [CrossRef] [PubMed]
- Meyer, B.J.; Mann, N.J.; Lewis, J.L.; Milligan, G.C.; Sinclair, A.J.; Howe, P.R.C. Dietary intakes and food sources of omega-6 and omega-3 polyunsaturated fatty acids. Lipids 2003, 38, 391–398. [Google Scholar] [CrossRef]
- Meyer, B.J.; Kolanu, N. Australian children are not consuming enough long-chain omega-3 polyunsaturated fatty acids for optimal health. Nutrients 2011, 27, 1136–1140. [Google Scholar] [CrossRef]
- Ryan, A.S.; Nelson, E.B. Assessing the Effect of Docosahexaenoic Acid on Cognitive Functions in Healthy, Preschool Children: A Randomized, Placebo-Controlled, Double-Blind Study. Clin. Pediatr. 2008, 47, 355–362. [Google Scholar] [CrossRef]
- Leutgeb, V.; Koechel, A.; Lang, L.; Koch, J. Effects of Omega-3 Fatty Acids on Cognitive, Emotional, and Social Behavioral Parameters in Kindergarten Children: A Pilot Study. Kindh. Entwickl. 2015, 24, 86–93. [Google Scholar] [CrossRef]
- Øyen, J.; Kvestad, I.; Midtbø, L.K.; Graff, I.E.; Hysing, M.; Stormark, K.M.; Markhus, M.W.; Baste, V.; Frøyland, L.; Koletzko, B.; et al. Fatty fish intake and cognitive function: FINS-KIDS, a randomized controlled trial in preschool children. BMC Med. 2018, 16, 41. [Google Scholar] [CrossRef]
- Widenhorn-Müller, K.; Schwanda, S.; Scholz, E.; Spitzer, M.; Bode, H. Effect of supplementation with long-chain ω-3 polyunsaturated fatty acids on behavior and cognition in children with attention deficit/hyperactivity disorder (ADHD): A randomized placebo-controlled intervention trial. Prostaglandins Leukot. Essent. Fat. Acids 2014, 91, 49–60. [Google Scholar] [CrossRef]
- Stevens, L.; Zhang, W.; Peck, L.; Kuczek, T.; Grevstad, N.; Mahon, A.; Zentall, S.S.; Arnold, L.E.; Burgess, J.R. EFA supplementation in children with inattention, hyperactivity, and other disruptive behaviors. Lipids 2003, 38, 1007–1021. [Google Scholar] [CrossRef] [PubMed]
- Richardson, A.J.; Puri, B.K. A randomized double-blind, placebo-controlled study of the effects of supplementation with highly unsaturated fatty acids on ADHD-related symptoms in children with specific learning difficulties. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2002, 26, 233–239. [Google Scholar] [CrossRef]
- Richardson, A.J.; Montgomery, P. The Oxford-Durham study: A randomized, controlled trial of dietary supplementation with fatty acids in children with developmental coordination disorder. Pediatrics 2005, 115, 1360–1366. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.; Fransson, G.; Östlund, S.; Areskoug, B.; Gillberg, C. Omega 3/6 fatty acids for reading in children: A randomized, double-blind, placebo-controlled trial in 9-year-old mainstream schoolchildren in Sweden. J. Child Psychol. Psychiatry 2017, 58, 83–93. [Google Scholar] [CrossRef]
- Kirby, A.; Woodward, A.; Jackson, S.; Wang, Y.; Crawford, M. A double-blind, placebo-controlled study investigating the effects of omega-3 supplementation in children aged 8–10 years from a mainstream school population. Res. Dev. Disabil. 2010, 31, 718–730. [Google Scholar] [CrossRef] [PubMed]
- National Health and Medical Research Council. Nutrient Reference Values for Australia and New Zealand. 2014. Available online: https://www.nrv.gov.au/nutrients/fats-total-fat-fatty-acids (accessed on 29 March 2019).
- McClelland, M.M.; Cameron, C.E. Self-regulation in early childhood: Improving conceptual clarity and developing eco-logically valid measures. Child Dev. Perspect. 2012, 6, 136–142. [Google Scholar] [CrossRef]
- Howard, S.J.; Melhuish, E. An Early Years Toolbox for Assessing Early Executive Function, Language, Self-Regulation, and Social Development: Validity, Reliability, and Preliminary Norms. J. Psychoeduc. Assess. 2016, 35, 255–275. [Google Scholar] [CrossRef] [PubMed]
- Sherman, E.M.S.; Brooks, B.L. Behavior Rating Inventory of Executive Function—Preschool Version (BRIEF-P): Test Review and Clinical Guidelines for Use. Child Neuropsychol. 2010, 16, 503–519. [Google Scholar] [CrossRef]
- Purpura, D.J.; Lonigan, C.J. Conners’ Teacher Rating Scale for Preschool Children: A Revised, Brief, Age-Specific Measure. J. Clin. Child Adolesc. Psychol. 2009, 38, 263–272. [Google Scholar] [CrossRef]
- Swierk, M.; Williams, P.G.; Wilcox, J.; Russell, K.G.; Meyer, B.J. Validation of an Australian electronic food frequency questionnaire to measure polyunsaturated fatty acid intake. Nutrition 2011, 27, 641–646. [Google Scholar] [CrossRef][Green Version]
- Harris, W.S.; Von Schacky, C. The Omega-3 Index: A new risk factor for death from coronary heart disease? Prev. Med. 2004, 39, 212–220. [Google Scholar] [CrossRef]
- Van der Wurff, I.; Von Schacky, C.; Berge, K.; Zeegers, M.; Kirschner, P.; De Groot, R. Association between blood omega-3 index and cognition in typically developing Dutch adolescents. Nutrients 2016, 8, 13. [Google Scholar] [CrossRef]
- Johnstone, S.J.; Jiang, H.; Sun, L.; Rogers, J.M.; Valderrama, J.; Zhang, D. Development of Frontal EEG Differences Between Eyes-Closed and Eyes-Open Resting Conditions in Children: Data From a Single-Channel Dry-Sensor Portable Device. Clin. EEG Neurosci. 2020. [CrossRef]
- Rogers, J.M.; Johnstone, S.J.; Aminov, A.; Donnelly, J.F.; Wilson, P. Test-retest reliability of a single-channel, wireless EEG system. Int. J. Psychophysiol. 2016, 106, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, S.J.; Blackman, R.; Bruggemann, J.M. EEG From a Single-Channel Dry-Sensor Recording Device. Clin. EEG Neurosci. 2012, 43, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Hayman, R.; Taylor, B.J.; Peart, N.; Galland, B.C.; Sayers, R. Participation in research: Informed consent, motivation and influence. J. Paediatr. Child Health 2001, 37, 51–54. [Google Scholar] [CrossRef]
- De Groot, R.H.; Meyer, B.J. ISSFAL Official Statement Number 6: The importance of measuring blood omega-3 long chain polyunsaturated fatty acid levels in research. Prostaglandins Leukot. Essent. Fatty Acids 2019, 157, 102029. [Google Scholar] [CrossRef]
- Yoo, H.; Kim, S.; Hur, H.-K.; Kim, H.-S. The effects of an animation distraction intervention on pain response of pre-school children during venipuncture. Appl. Nurs. Res. 2011, 24, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Birnie, K.A.; Noel, M.; Chambers, C.T.; Uman, L.S.; Parker, J.A. Psychological interventions for needle-related procedural pain and distress in children and adolescents. Cochrane Database Syst. Rev. 2018. [Google Scholar] [CrossRef] [PubMed]
- Cohen, L.L.; Blount, R.L.; Cohen, R.J.; Schaen, E.R.; Zaff, J.F. Comparative study of distraction versus topical anesthesia for pediatric pain management during immunizations. Health Psychol. 1999, 18, 591. [Google Scholar] [CrossRef] [PubMed]
- Megel, M.E.; Heser, R.; Matthews, K. Parents’assistance to Children Having Immunizations. Issues Compr. Pediatr. Nurs. 2002, 25, 151–165. [Google Scholar] [PubMed]
- Cavender, K.; Goff, M.D.; Hollon, E.C.; Guzzetta, C.E. Parents’ positioning and distracting children during venipuncture: Effects on children’s pain, fear, and distress. J. Holist. Nurs. 2004, 22, 32–56. [Google Scholar] [CrossRef] [PubMed]
- Fard, S.G.; Loh, S.P.; Turchini, G.M.; Wang, B.; Elliott, G.; Sinclair, A.J. Microencapsulated Tuna Oil Results in Higher Absorption of DHA in Toddlers. Nutrients 2020, 12, 248. [Google Scholar] [CrossRef]
| Variables | Methods | Who Completes the Task? |
|---|---|---|
| Self-regulation | ||
| Head-Toes-Knees-Shoulders task | Child to follow instruction and enact opposite action to what was instructed (e.g., touch their knees when told to touch your head) [28] | Children during clinic visits |
| Child Self-Regulation and Behaviour questionnaire | 34-item questionnaire [29] | Child’s parent or caregiver during clinic visits |
| Executive function | ||
| Go/No-Go task | The “Go/No-Go task” is an iPad based measure of inhibition [29] | Children during clinic visits |
| “Mr Ant” task | The “Mr Ant” task is an iPad based measure of working memory [29] | Children during clinic visits |
| Behaviour Rating Inventory of Executive Functioning—Preschool Version (BRIEF-P) | The BRIEF-P is a 73-item questionnaire [30] | Child’s parent/caregiver during clinic visits |
| Other assessments | ||
| EEG | A wireless, single-channel, dry-sensor, portable, measurement device (the Neurosky® MindWave Mobile 2TM headset) | A trained research assistant during clinic visits |
| ADHD questionnaire | Conners’ Teacher Rating Scale-15 (CTRS-15) [31] | Parent/caregiver during clinic visits |
| PUFA food frequency questionnaire | A link was provided and was completed online [32] | Parent/caregiver at home |
| Acceptability of finger-prick blood sampling | A single question Likert Scale A pictorial “smiley face” Likert Scale | Parent/caregiver during clinic visits Children during clinic visits |
| End-of-trial questionnaire | Open-ended questionnaire to assess experience with the study | Parent/caregiver during clinic visits |
| Manipulation check Whole blood fatty acids | Capillary blood using gas chromatography HS–Omega-3 Index® [33] | Analysis conducted by Omegametrix |
| Enrolment | Allocation | Postallocation | |||
|---|---|---|---|---|---|
| TIMEPOINT | −t2 | −t1 | 0 | tbaseline | T12weeks |
| ENROLMENT: | |||||
| Eligibility screen | X | ||||
| Informed consent | X | ||||
| Exclusions 1 | X | ||||
| Allocation | X | ||||
| INTERVENTIONS: | |||||
| Omega-3 |  | ||||
| Placebo |  | ||||
| ASSESSMENTS: | |||||
| Self-regulation | |||||
| Head, toes, knees, shoulder task | X | X | |||
| Child self-regulation and behavioural questionnaire | X | X | |||
| Additional outcomes Executive function | |||||
| Go/No-Go task | X | X | |||
| “Mr Ant” task | X | X | |||
| Behaviour rating inventory of executive functioning | X | X | |||
| EEG | X | X | |||
| Other assessments ADHD 2 questionnaire | X | X | |||
| PUFA 3 food frequency questionnaire | X | ||||
| Manipulation check—whole-blood fatty acids | X | X | |||
| Acceptability of finger-prick blood sampling | X | X | |||
| End-of-trial questionnaire | X | ||||
| Adverse events |  | ||||
| Sad Face | Neutral | Happy Face | |
|---|---|---|---|
| Number of responses (n = 69) | 14 | 8 | 47 |
| % of total responses | 20.3 | 11.6 | 68.1 |
| Completely Disagree | Somewhat Disagree | Not Sure | Somewhat Agree | Completely Agree | |
|---|---|---|---|---|---|
| Number of responses (n = 70) | 1 | 1 | 2 | 10 | 56 |
| % of total responses | 1.4 | 1.4 | 2.9 | 14.3 | 80.0 |
| Outcome Measure | Completed Assessment n (%) | Of Completers, Those Who Also Complied n (%) | Completed Assessment n (%) | Of Completers, Those Who Also Complied n (%) |
|---|---|---|---|---|
| Baseline | Post-Intervention | |||
| HTKS | 71/76 (93) | 71/71 (100) | 56/58 (97) | 56/56 (100) |
| Mr Ant | 74/76 (97) | 74/74 (100) | 57/58 (98) | 57/57 (100) |
| Go/No-Go | 74/76 (97) | 72/74 (97) | 56/58 (97) | 56/56 (100) |
| EEG Go/No-Go | 68/76 (89) | 66/68 (97) | 52/58 (90) | 52/52 (100) |
| EEG Eyes-Open | 69/76 (91) | 67/69 (97) | 52/58 (90) | 52/52 (100) |
| EEG Eyes-Closed | 66/76 (87) | 66/66 (100) | 52/58 (90) | 52/52 (100) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roach, L.A.; Byrne, M.K.; Howard, S.J.; Johnstone, S.J.; Batterham, M.; Wright, I.M.R.; Okely, A.D.; de Groot, R.H.M.; van der Wurff, I.S.M.; Jones, A.; et al. The Feasibility of the “Omega Kid” Study Protocol: A Double-Blind, Randomised, Placebo-Controlled Trial Investigating the Effect of Omega-3 Supplementation on Self-Regulation in Preschool-Aged Children. Nutrients 2021, 13, 213. https://doi.org/10.3390/nu13010213
Roach LA, Byrne MK, Howard SJ, Johnstone SJ, Batterham M, Wright IMR, Okely AD, de Groot RHM, van der Wurff ISM, Jones A, et al. The Feasibility of the “Omega Kid” Study Protocol: A Double-Blind, Randomised, Placebo-Controlled Trial Investigating the Effect of Omega-3 Supplementation on Self-Regulation in Preschool-Aged Children. Nutrients. 2021; 13(1):213. https://doi.org/10.3390/nu13010213
Chicago/Turabian StyleRoach, Lauren A., Mitchell K. Byrne, Steven J. Howard, Stuart J. Johnstone, Marijka Batterham, Ian M. R. Wright, Anthony D. Okely, Renate H. M. de Groot, Inge S. M. van der Wurff, Alison Jones, and et al. 2021. "The Feasibility of the “Omega Kid” Study Protocol: A Double-Blind, Randomised, Placebo-Controlled Trial Investigating the Effect of Omega-3 Supplementation on Self-Regulation in Preschool-Aged Children" Nutrients 13, no. 1: 213. https://doi.org/10.3390/nu13010213
APA StyleRoach, L. A., Byrne, M. K., Howard, S. J., Johnstone, S. J., Batterham, M., Wright, I. M. R., Okely, A. D., de Groot, R. H. M., van der Wurff, I. S. M., Jones, A., & Meyer, B. J. (2021). The Feasibility of the “Omega Kid” Study Protocol: A Double-Blind, Randomised, Placebo-Controlled Trial Investigating the Effect of Omega-3 Supplementation on Self-Regulation in Preschool-Aged Children. Nutrients, 13(1), 213. https://doi.org/10.3390/nu13010213




_Okely.png)



