Effects of Dietary and Lifestyle Interventions on Liver, Clinical and Metabolic Parameters in Children and Adolescents with Non-Alcoholic Fatty Liver Disease: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction
2.4. Outcome Measures
2.5. Study Quality
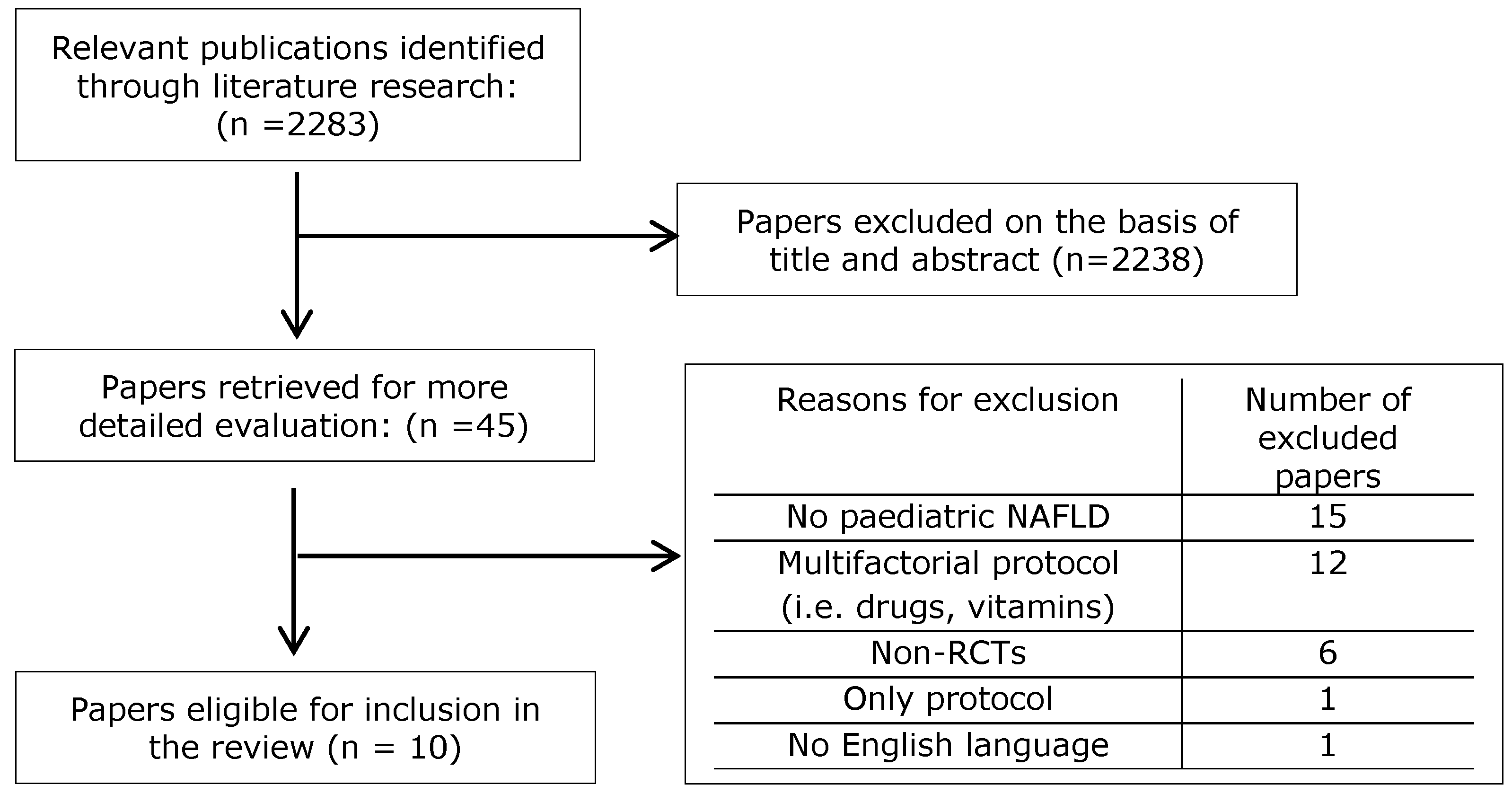
3. Results
3.1. Interventions Based on Diet
3.1.1. Effects of Diet on Liver Characteristics
3.1.2. Effects of Diet on Anthropometric Characteristics
3.1.3. Effects of Diet on Glucose Metabolism
3.1.4. Effects of Diet on Lipid Profile
3.2. Interventions Based on Diet Plus Physical Activity
3.2.1. Effects of Diet Plus Exercise on Liver Characteristics
3.2.2. Effects of Diet Plus Exercise on Anthropometric Characteristics
3.2.3. Effects of Diet Plus Exercise on Glucose Metabolism
3.2.4. Effects of Diet Plus Exercise on Lipid Profile
3.3. Interventions Evaluating the Effects of Physical Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kleiner, D.E. Histopathology, grading and staging of nonalcoholic fatty liver disease. Minerva Gastroenterol. Dietol. 2018, 64, 28–38. [Google Scholar] [CrossRef]
- Shah, J.; Okubote, T.; Alkhouri, N. Overview of updated practice guidelines for pediatric nonalcoholic fatty liver disease. Gastroenterol. Hepatol. 2018, 14, 407–414. [Google Scholar]
- Africa, J.A.; Newton, K.P.; Schwimmer, J.B. Lifestyle interventions including nutrition, exercise, and supplements for nonalcoholic fatty liver disease in children. Dig. Dis. Sci. 2016, 61, 1375–1386. [Google Scholar] [CrossRef] [PubMed]
- Draijer, L.; Benninga, M.; Koot, B. Pediatric NAFLD: An overview and recent developments in diagnostics and treatment. Expert Rev. Gastroenterol. Hepatol. 2019, 13, 447–461. [Google Scholar] [CrossRef]
- Nobili, V.; Socha, P. Pediatric nonalcoholic fatty liver disease: Current thinking. J. Pediatr. Gastroenterol. Nutr. 2018, 66, 188–192. [Google Scholar] [CrossRef]
- Mann, J.P.; Tang, G.Y.; Nobili, V.; Armstrong, M.J. Evaluations of lifestyle, dietary, and pharmacologic treatments for pediatric nonalcoholic fatty liver disease: A systematic review. Clin. Gastroenterol. Hepatol. 2019, 17, 1457–1476. [Google Scholar] [CrossRef]
- Schwimmer, J.B.; Deutsch, R.; Kahen, T.; LaVine, J.E.; Stanley, C.; Behling, C.; Rasmussen, S.A.; Qin, C.; Lackritz, E.M.; Callaghan, W.M.; et al. Prevalence of fatty liver in children and adolescents. Pediatrics 2006, 118, 1388–1393. [Google Scholar] [CrossRef]
- Vittorio, J.; LaVine, J.E. Recent advances in understanding and managing pediatric nonalcoholic fatty liver disease. F1000Research 2020, 9, 377. [Google Scholar] [CrossRef]
- Fitzpatrick, E.; Dhawan, A. Childhood and adolescent nonalcoholic fatty liver disease: Is it different from adults? J. Clin. Exp. Hepatol. 2019, 9, 716–722. [Google Scholar] [CrossRef]
- Gibson, P.S.; Lang, S.; Gilbert, M.; Kamat, D.; Bansal, S.; Ford-Adams, M.E.; Desai, A.P.; Dhawan, A.; Fitzpatrick, E.; Moore, J.B.; et al. Assessment of diet and physical activity in paediatric non-alcoholic fatty liver disease patients: A United Kingdom case control study. Nutrients 2015, 7, 9721–9733. [Google Scholar] [CrossRef]
- Papandreou, D.; Karabouta, Z.; Pantoleon, A.; Rousso, I. Investigation of anthropometric, biochemical and dietary parameters of obese children with and without non-alcoholic fatty liver disease. Appetite 2012, 59, 939–944. [Google Scholar] [CrossRef] [PubMed]
- Oddy, W.H.; Herbison, C.E.; Jacoby, P.; Ambrosini, G.L.; O’Sullivan, T.A.; Ayonrinde, O.T.; Olynyk, J.K.; Black, L.J.; Beilin, L.J.; Mori, T.A.; et al. The western dietary pattern is prospectively associated with nonalcoholic fatty liver disease in adolescence. Am. J. Gastroenterol. 2013, 108, 778–785. [Google Scholar] [CrossRef] [PubMed]
- Deldin, A.R.; Lee, S. Role of physical activity in the treatment of nonalcoholic fatty liver disease in children and adolescents. Appl. Physiol. Nutr. Metab. 2013, 38, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Rinella, M.E.; Tacke, F.; Sanyal, A.J.; Anstee, Q.M. Report on the AASLD/EASL joint workshop on clinical trial endpoints in NAFLD. Hepatology 2019, 70, 1424–1436. [Google Scholar] [CrossRef]
- Vos, M.B.; Abrams, S.H.; Barlow, S.E.; Caprio, S.; Daniels, S.R.; Kohli, R.; Mouzaki, M.; Sathya, P.; Schwimmer, J.B.; Sundaram, S.S.; et al. NASPGHAN clinical practice guideline for the diagnosis and treatment of nonalcoholic fatty liver disease in children: Recommendations from the expert committee on nafld (econ) and the north american society of pediatric gastroenterology, hepatology and nutrition (naspghan). J. Pediatr. Gastroenterol. Nutr. 2017, 64, 319–334. [Google Scholar] [CrossRef] [PubMed]
- Chalasani, N.P.; Younossi, Z.M.; LaVine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef]
- Awai, H.I.; Newton, K.P.; Schwimmer, J.B. Nonalcoholic fatty liver disease in children. In Alcoholic and Non-Alcoholic Fatty Liver Disease; Springer: Berlin/Heidelberg, Germany, 2016; pp. 339–362. [Google Scholar]
- Goldner, D.; Lavine, J.E. Nafld in children: Unique considerations and challenges. Gastroenterology 2020, 158, 1967–1983. [Google Scholar] [CrossRef]
- Vukovic, R.; Dos Santos, T.J.; Ybarra, M.; Atar, M. Children with metabolically healthy obesity: A review. Front. Endocrinol. 2019, 10, 865. [Google Scholar] [CrossRef] [PubMed]
- Gibson, P.S.; Lang, S.; Dhawan, A.; Fitzpatrick, E.; Blumfield, M.L.; Truby, H.; Hart, K.H.; Moore, J.B. Systematic review: Nutrition and physical activity in the management of paediatric nonalcoholic fatty liver disease. J. Pediatr. Gastroenterol. Nutr. 2017, 65, 141–149. [Google Scholar] [CrossRef]
- Utz-Melere, M.; Targa-Ferreira, C.; Lessa-Horta, B.; Epifanio, M.; Mouzaki, M.; Mattos, A.A. Non-alcoholic fatty liver disease in children and adolescents: lifestyle change—A systematic review and meta-analysis. Ann. Hepatol. 2018, 17, 345–354. [Google Scholar] [CrossRef]
- Medrano, M.; Cadenas-Sanchez, C.; Alvarez-Bueno, C.; Cavero-Redondo, I.; Ruiz, J.R.; Ortega, F.B.; Labayen, I. Evidence-based exercise recommendations to reduce hepatic fat content in youth—A systematic review and meta-analysis. Prog. Cardiovasc. Dis. 2018, 61, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Ezaizi, Y.; Kabbany, M.N.; Selvakumar, P.C.; Sarmini, M.T.; Singh, A.; Lopez, R.; Nobili, V.; Alkhouri, N. Comparison between non-alcoholic fatty liver disease screening guidelines in children and adolescents. JHEP Rep. 2019, 1, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Koot, B.G.P.; Van Der Baan-Slootweg, O.H.; Vinke, S.; Bohte, A.E.; Tamminga-Smeulders, C.L.J.; Jansen, P.L.M.; Stoker, J.; Benninga, M.A. Intensive lifestyle treatment for non-alcoholic fatty liver disease in children with severe obesity: Inpatient versus ambulatory treatment. Int. J. Obes. 2016, 40, 51–57. [Google Scholar] [CrossRef]
- Vos, M.B.; Weber, M.B.; Welsh, J.; Khatoon, F.; Jones, D.P.; Whitington, P.F.; McClain, C.J. Fructose and oxidized low-density lipoprotein in pediatric nonalcoholic fatty liver disease: A pilot study. Arch. Pediatr. Adolesc. Med. 2009, 163, 674–675. [Google Scholar] [CrossRef] [PubMed]
- Ramon-Krauel, M.; Salsberg, S.L.; Ebbeling, C.B.; Voss, S.D.; Mulkern, R.V.; Apura, M.M.; Cooke, E.A.; Sarao, K.; Jonas, M.M.; Ludwig, D.S. A Low-glycemic-load versus low-fat diet in the treatment of fatty liver in obese children. Child. Obes. 2013, 9, 252–260. [Google Scholar] [CrossRef]
- Jin, R.; Welsh, J.A.; Le, N.A.; Holzberg, J.; Sharma, P.; Martin, D.R.; Vos, M.B. Dietary fructose reduction improves markers of cardiovascular disease risk in hispanic-american adolescents with NAFLD. Nutrients 2014, 6, 3187–3201. [Google Scholar] [CrossRef]
- Utari, A.; Maududi, M.S.; Kusumawati, N.R.D.; Mexitalia, M. Effects of low glycemic index diet on insulin resistance among obese adolescent with non-alcoholic fatty liver disease: A randomized controlled trial. Med. J. Indones. 2019, 28, 123–128. [Google Scholar] [CrossRef]
- Schwimmer, J.B.; Ugalde-Nicalo, P.; Welsh, J.A.; Angeles, J.E.; Cordero, M.; Harlow, K.E.; Alazraki, A.; Durelle, J.; Knight-Scott, J.; Newton, K.P.; et al. Effect of a low free sugar diet vs. usual diet on nonalcoholic fatty liver disease in adolescent Boys: A randomized clinical trial. JAMA 2019, 321, 256–265. [Google Scholar] [CrossRef]
- Goss, A.M.; Dowla, S.; Pendergrass, M.; Ashraf, A.; Bolding, M.; Morrison, S.; Amerson, A.; Soleymani, T.; Gower, B. Effects of a carbohydrate-restricted diet on hepatic lipid content in adolescents with non-alcoholic fatty liver disease: A pilot, randomized trial. Pediatr. Obes. 2020, 15, e12630. [Google Scholar] [CrossRef]
- Wang, C.L.; Liang, L.; Fu, J.F.; Zou, C.C.; Hong, F.; Xue, J.Z.; Lu, J.R.; Wu, X.M. Effect of lifestyle intervention on non-alcoholic fatty liver disease in Chinese obese children. World J. Gastroenterol. 2008, 14, 1598–1602. [Google Scholar] [CrossRef]
- Chan, D.F.Y.; So, H.K.; Hui, S.C.N.; Chan, R.; Li, A.M.; Sea, M.M.; Chu, W.C.W.; Chan, M.; Woo, J.; Nelson, E.A.S. Dietitian-led lifestyle modification programme for obese Chinese adolescents with non-alcoholic fatty liver disease: A randomized controlled study. Int. J. Obes. 2018, 42, 1680–1690. [Google Scholar] [CrossRef] [PubMed]
- Ganen, A.D.P.; De Mello, M.T.; Sanches, P.D.L.; Da Silva, P.L.; Campos, R.M.D.S.; Carnier, J.; Corgosinho, F.; Foschini, D.; Masquio, D.L.; Tock, L.; et al. Long-term effects of aerobic plus resistance training on the adipokines and neuropeptides in nonalcoholic fatty liver disease obese adolescents. Eur. J. Gastroenterol. Hepatol. 2012, 24, 1313–1324. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, H.J. Use of percentiles and Z-scores in anthropometry. In Handbook of Anthropometry; Springer: Berlin/Heidelberg, Germany, 2012; pp. 29–48. [Google Scholar]
- Kelly, A.S.; Daniels, S.R. Rethinking the use of body mass index z-score in children and adolescents with severe obesity: Time to kick it to the curb? J. Pediatr. 2017, 188, 7–8. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Bacha, F.; Tfayli, H.; Michaliszyn, S.F.; Yousuf, S.; Arslanian, S. Adipose tissue insulin resistance in youth on the spectrum from normal weight to obese and from normal glucose tolerance to impaired glucose tolerance to type 2 diabetes. Diabetes Care 2019, 42, 265–272. [Google Scholar] [CrossRef]
- Bland, M.; Altman, D.G. Comparisons against baseline within randomised groups are often used and can be highly misleading. Trials 2011, 12, 264. [Google Scholar] [CrossRef]
- Bland, M.; Altman, D.G. Best (but oft forgotten) practices: Testing for treatment effects in randomized trials by separate analyses of changes from baseline in each group is a misleading approach. Am. J. Clin. Nutr. 2015, 102, 991–994. [Google Scholar] [CrossRef]
- Jensen, T.; Abdelmalek, M.F.; Sullivan, S.; Nadeau, K.J.; Cree, M.G.; Roncal, C.; Nakagawa, T.; Kuwabara, M.; Sato, Y.; Kang, D.H.; et al. Fructose and sugar: A major mediator of non-alcoholic fatty liver disease. J. Hepatol. 2018, 68, 1063–1075. [Google Scholar] [CrossRef]
- Mager, D.R.; Iñiguez, I.R.; Gilmour, S.; Yap, J. The Effect of a low fructose and low glycemic index/load (FRAGILE) dietary intervention on indices of liver function, cardiometabolic risk factors, and body composition in children and adolescents with nonalcoholic fatty liver disease (NAFLD). J. Parenter. Enter. Nutr. 2015, 39, 73–84. [Google Scholar] [CrossRef]
- WHO. Guidelines: Sugars Intake for Adults and Children. Available online: https://www.who.int/nutrition/publications/guidelines/sugars_intake/en/ (accessed on 15 June 2020).
- Katsagoni, C.N.; Papatheodoridis, G.V.; Ioannidou, P.; Deutsch, M.; Alexopoulou, A.; Papadopoulos, N.; Papageorgiou, M.V.; Fragopoulou, E.; Kontogianni, M.D. Improvements in clinical characteristics of patients with non-alcoholic fatty liver disease, after an intervention based on the Mediterranean lifestyle: A randomised controlled clinical trial. Br. J. Nutr. 2018, 120, 164–175. [Google Scholar] [CrossRef]
- Ho, C.M.; Ho, S.L.; Jeng, Y.M.; Lai, Y.S.; Chen, Y.H.; Lu, S.C.; Chen, H.L.; Chang, P.Y.; Hu, R.H.; Lee, P.H. Accumulation of free cholesterol and oxidized low-density lipoprotein is associated with portal inflammation and fibrosis in nonalcoholic fatty liver disease. J. Inflamm. 2019, 16, 1–8. [Google Scholar] [CrossRef]
- Nobili, V.; Parola, M.; Alisi, A.; Marra, F.; Piemonte, F.; Mombello, C.; Sutti, S.; Povero, D.; Maina, V.; Novo, E.; et al. Oxidative stress parameters in paediatric non-alcoholic fatty liver disease. Int. J. Mol. Med. 2010, 26, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.C.; Ness, E.; Kowdley, K.V. Nutritional approaches to achieve weight loss in nonalcoholic fatty liver disease. Adv. Nutr. 2017, 8, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Romero-Gomez, M.; Zelber-Sagi, S.; Trenell, M.I. Treatment of NAFLD with diet, physical activity and exercise. J. Hepatol. 2017, 67, 829–846. [Google Scholar] [CrossRef] [PubMed]
- Zelber-Sagi, S.; Salomone, F.; Mlynarsky, L. The Mediterranean dietary pattern as the diet of choice for non-alcoholic fatty liver disease: Evidence and plausible mechanisms. Liver Int. 2017, 37, 936–949. [Google Scholar] [CrossRef]
- Della Corte, C.; Mosca, A.; Vania, A.; Alterio, A.; Iasevoli, S.; Nobili, V. Good adherence to the Mediterranean diet reduces the risk for NASH and diabetes in pediatric patients with obesity: The results of an Italian Study. Nutrients 2017, 39–40, 8–14. [Google Scholar] [CrossRef]
- WHO. Who Global Recommendations on Physical Activity for Health; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Van Der Heijden, G.J.; Wang, Z.; Chu, Z.; Toffolo, G.; Manesso, E.; Sauer, P.J.J.; Sunehag, A.L. Strength exercise improves muscle mass and hepatic insulin sensitivity in obese youth. Med. Sci. Sports Exerc. 2010, 42, 1973–1980. [Google Scholar] [CrossRef] [PubMed]
- Van Der Heijden, G.J.; Wang, Z.; Chu, Z.D.; Sauer, P.J.; Haymond, M.W.; Rodriguez, L.M.; Sunehag, A.L. A 12-week aerobic exercise program reduces hepatic fat accumulation and insulin resistance in obese, hispanic adolescents. Obesity 2010, 18, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Bacha, F.; Hannon, T.; Kuk, J.L.; Boesch, C.; Arslanian, S. Effects of aerobic versus resistance exercise without caloric restriction on abdominal fat, intrahepatic lipid, and insulin sensitivity in obese adolescent boys. Diabetes 2012, 61, 2787–2795. [Google Scholar] [CrossRef]
- Lee, S.; Deldin, A.R.; White, D.; Kim, Y.; Libman, I.; Rivera-Vega, M.; Kuk, J.L.; Sandoval, S.; Boesch, C.; Arslanian, S. Aerobic exercise but not resistance exercise reduces intrahepatic lipid content and visceral fat and improves insulin sensitivity in obese adolescent girls: A randomized controlled trial. Am. J. Physiol. Metab. 2013, 305, E1222–E1229. [Google Scholar] [CrossRef]
- González-Ruíz, K.; Ramírez-Vélez, R.; Correa-Bautista, J.E.; Peterson, M.; García-Hermoso, A. The effects of exercise on abdominal fat and liver enzymes in pediatric obesity: A systematic review and meta-analysis. Child. Obes. 2017, 13, 272–282. [Google Scholar] [CrossRef]
- Orci, L.A.; Gariani, K.; Oldani, G.; Delaune, V.; Morel, P.; Toso, C. Exercise-based interventions for nonalcoholic fatty liver disease: A meta-analysis and meta-regression. Clin. Gastroenterol. Hepatol. 2016, 14, 1398–1411. [Google Scholar] [CrossRef] [PubMed]
- Katsagoni, C.N.; Georgoulis, M.; Papatheodoridis, G.V.; Panagiotakos, D.; Kontogianni, M.D. Effects of lifestyle interventions on clinical characteristics of patients with non-alcoholic fatty liver disease: A meta-analysis. Metab. Clin. Exp. 2017, 68, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Liong, E.C.; So, K.F.; Fung, M.L.; Tipoe, G.L. Beneficial mechanisms of aerobic exercise on hepatic lipid metabolism in non-alcoholic fatty liver disease. Hepatobiliary Pancreat. Dis. Int. 2015, 14, 139–144. [Google Scholar] [CrossRef]
- Ye, Y.; Chen, L.; Yang, X. The efficacy of resistance training for non-alcoholic fatty-liver disease: A meta-analysis of randomized controlled trials. Int. J. Clin. Exp. Med. 2019, 12, 13188–13195. [Google Scholar]
- Hashida, R.; Kawaguchi, T.; Bekki, M.; Omoto, M.; Matsuse, H.; Nago, T.; Takano, Y.; Ueno, T.; Koga, H.; George, J.; et al. Aerobic vs. resistance exercise in non-alcoholic fatty liver disease: A systematic review. J. Hepatol. 2017, 66, 142–152. [Google Scholar] [CrossRef]
- Wang, S.T.; Zheng, J.; Peng, H.W.; Cai, X.L.; Pan, X.T.; Li, H.Q.; Hong, Q.Z.; Peng, X.-E. Physical activity intervention for non-diabetic patients with non-alcoholic fatty liver disease: A meta-analysis of randomized controlled trials. BMC Gastroenterol. 2020, 20, 66. [Google Scholar] [CrossRef]
- França, S.L.G.; Sahade, V.; Nunes, M.; Adan, L.F. Adherence to nutritional therapy in obese adolescents; a review. Nutr. Hosp. 2013, 28, 988–998. [Google Scholar]
- Wijarnpreecha, K.; Thongprayoon, C.; Panjawatanan, P.; Ungprasert, P. Short sleep duration and risk of nonalcoholic fatty liver disease: A systematic review and meta-analysis. J. Gastroenterol. Hepatol. 2016, 31, 1802–1807. [Google Scholar] [CrossRef]
- Katsagoni, C.N.; Papatheodoridis, G.V.; Papageorgiou, M.V.; Ioannidou, P.; Deutsch, M.; Alexopoulou, A.; Papadopoulos, N.; Fragopoulou, E.; Kontogianni, M.D. A “healthy diet–optimal sleep” lifestyle pattern is inversely associated with liver stiffness and insulin resistance in patients with nonalcoholic fatty liver disease. Appl. Physiol. Nutr. Metab. 2017, 42, 250–256. [Google Scholar] [CrossRef]
- Katsagoni, C.N.; Georgoulis, M.; Papatheodoridis, G.V.; Fragopoulou, E.; Ioannidou, P.; Papageorgiou, M.; Alexopoulou, A.; Papadopoulos, N.; Deutsch, M.; Kontogianni, M.D. Associations between lifestyle characteristics and the presence of nonalcoholic fatty liver disease: A case–Control study. Metab. Syndr. Relat. Disord. 2017, 15, 72–79. [Google Scholar] [CrossRef]
- Wang, A.Y.; Dhaliwal, J.; Mouzaki, M. Lean non-alcoholic fatty liver disease. Clin. Nutr. 2019, 38, 975–981. [Google Scholar] [CrossRef] [PubMed]
- Wattacheril, J.; Sanyal, A.J. Lean NAFLD: An underrecognized outlier. Curr. Hepatol. Rep. 2016, 15, 134–139. [Google Scholar] [CrossRef] [PubMed]
| Author [ref] | Journal | Country | Sample (n) | Intervention Group | Control Group | Nafld Definition | Age (y) (mean ± SD) | Sex (boys) | Type of Intervention | Dura-tion | Primary Outcomes | Secontary Outcomes | Completion Rates |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diet only interventions | |||||||||||||
| Vos et al, 2009 [26] | Arch Pediatr Adolesc Med | USA | 10 | 6 (Low-fructose group) | 4 (Low-fat group) | Liver biopsy or serology and US | Low-fructose group: 13.3 ± 0.65. Low-fat group: 12.5 ± 1.0. | NR | Low-fructose group: Diet with eliminated sugar containing beverages, fruit juice and food items with high-fructose corn syrup (HFCS) as one of the top 5 ingredients on the label. Low-fat group: Diet based on the American Heart Association recommendations. | 6mo | Oxidatized LDL-C | Changes in BMI z-score, BP, AST, ALT, HDL-C, LDL-C, TCHOL, TG, Urinary isoprostanes | NR |
| Ramon-Krauel et al, 2013 [27] | Child Obes. | USA | 17 | 8 (Low-glycemic-load group) | 9 (Low-fat diet group) | ≥9% liver fat content assessed by MRS | Low-GL group: 11.8 ± 3.0. Low-fat group: 13.8 ± 3.2. | Low-glycemic load group: 7. Low-fat group: 7. | Low-GL group: Ad libitum diet. C:F:P: 40:35-40:20–25. Low to moderate GL carbohydrate food items. Low-fat group: Ad libitum diet. C:F:P:55-60:20:20–25. Vitamin supplements provided in both groups. | 6mo | Intrahepatic tiglyceride content (IHTC) | Height, Body weight, waist/abdomen/hip circumferences, BP, TCHOL, HDL-C, LDL-C, TG, ALT, AST, Glucose, Insulin | 94% |
| Jin et al, 2014 [28] | Nutrients | USA | 24 | 13 (Glucose beverages group) | 11 (Fructose beverages group) | IHTG content >8% assessed by MRS | Glucose beverages group: 14.2 ± 0.88. Fructose beverages group: 13.0 ± 0.71. | Glucose beverages group: 3. Fructose beverages group: 8. | Glucose beverages group: Consumption of 3 servings (8 fl oz bottles) of glucose-containing beverages daily. No diet or physical activity modification. Fructose beverages group: onsumption of 3 servings (8 fl oz bottles) of fructose-containing beverages daily. No diet or physical activity modification. | 4wks | Body weight, Hepatic fat (%), ALT, AST, TG, FFAs, Glucose, Insulin, hs-CRP, LDL-C, lag time, oxLDL, PAI-1 | NR | 91.7% |
| Agustini et al, 2019 [29] | Med J Indones | Indo-nesia | 32 | 16 (low-energy, GI, and fat) | 16 (nutrition education) | US-diagnosed fatty liver | 12-14yrs | 24 | Intervention group: Nutrition education and lunch diet (lunch box provided consisted of low-fat, <25% of total calories, low cholesterol, <300 mg/day, and low-GI diet). Control group: Nutrition education | 12wks | FBG, Insulin, Body weight, Height, Dietary intake | NR | 100% |
| Schwimmer et al, 2019 [30] | JAMA | USA | 40 | 20 (Low-sugar diet group) | 20 (Usual diet group) | ≥10% of IHTG measured by MRI-PDFF and ALT≥45U/L | 11-16yrs old. Low-sugar diet group: 12.8 ± 1.87. Usual diet group: 13.4 ± 1.9. | 40 | Low-sugar diet group: Avoidance of sugar-containing foods and drinks. Ad libitum diet with >3% of free sugars. Usual diet group: maintainance of their habitual diet. | 8wks | Percentage of hepatic steatosis | Insulin resistance; ALT, AST, GGT, FBG, Insulin, TCHOL, LDL-C, HDL-C, TG, sweetness perception testing diet adherence, adverse events. | 100% |
| Goss et al, 2020 [31] | Pediatr Obes. | USA | 32 | 16 (CRD-group) | 16 (FRD-group) | ALT>45IU/L and/or indication of echogenic liver via US | 9–17 yrs old. CRD-group: 14.5 ± 2.6. FRD-group: 14.2 ± 2.1. | CRD-group: 9. RRD-group: 7. | CRD-group: Diet aiming at minimizing the intake of refined CHO. Meal plans provided. C:F:P:≤25:≥50:25. FRD-group: Diet promoting low-energy, high-quality foods, based on based on the USDA MyPlate Daily Food Plan. Meal plans provided. C:F:P:55:20:25. | 8wks | Hepatic fat content, Body composition, TCHOL, LDL-C, HDL-C, TG, Glucose, Insulin, CRP, ALT, AST, GGT | NR | 78% |
| Author [ref] | Journal | Country | Sample (n) | Intervention Group | Control Group | Nafld definition | Age (y) (mean ± SD) | Sex (boys) | Type of Intervention | Duration | Primary Outcomes | Secontary Outcomes | Completion Rates |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diet plus exercise interventions | |||||||||||||
| Wang et al, 2008 [32] | World J Gastroenterol | China | 57 | 19 (Group 2) | 38 (Group 1) | Liver fatty infiltration in US, abnormal ALT (1.5 times <50IU/L) | Group 1: 14.04 ± 1.8. Group 2: 13.4 ± 2.5. | Group 1: 26 Group 2: 13 | Group 1: no intervention; Group 2: Aerobic exercise: 3 hrs of free exercise daily; Diet: Low-calorie (↓ 250 kcal)- high carbohydrate (50%)- low fat (10%) diet. | 1mo | Height, Body weight, FBG, insulin, ALT, AST, TG, TCHOL, fatty liver indication in US | - | NR |
| Koot et al, 2015 [25] | International Journal of Obesity | The Netherlands | 55 | 23 at Inpatient group & 21 at Ambulatory group | 18 (Usual care group) | IHTG > 1.8% assesed by 1H-MRS | Inpatient treatment group: 14.9 ± 2.5. Ambulatory treatment group: 14.4 ± 2.1. Usual care group: 14.7 ± 2.4 | Inpatient treatment group: 10. Ambulatory treatment group: 13. Usual care group: 10 | Inpatient treatment group: High intensity aerobic exercise 4 days per wk (30–60min each); Nutrition (focused on dietary quality) and behavior (self-regulation, self-awareness, goal setting) modification sessions at weekly base (60min each). Ambulatory treatment group: Each session consisted of 60min high intensity exercise (plus advice to engage in exercise 3times/wk), an 1 hr-educational program and a 30min nutritional program Control group: Usual care | 6mo & 18mo follow-up | 1H-MRS determined liver steatosis, ALT | Length, Body weight, Waist circumference, BP, GGT, Insulin, HD-CL, LDL-C, Glucose | 91% (6mo) and 80% (24mo) |
| Chan et al, 2018 [33] | Int J Obes (Lond) | China | 52 | 26 | 26 | IHTG≥5% measured by 1H-MRS | Intervention group: 15.3 ± 3.4. Control group: 13.8 ± 5.3. | Intervention group: 16. Control group: 17. | Intervention group: Individualized menu plan (balanced diet with a relative increase in protein) provided by a dietitian and exercise plan aiming at 30min aerobic exercise 2-3times/wk. Control group: Usual diet and exercise advice (↓ high-glycemic index carbohydrateand animal fat, and exercise for at least 2-3 times/wk for 30min per session. | 16wks & 52wks follow-up | Changes in IHTC assessed by 1H-MRS at 16 wks | Maintanance of changes in IHTC at 52 wks, FBG, Lipid profile, ALT, AST, Serum insulin, Anthropometric measurements | 80.8% |
| Exercise-focused interventions | |||||||||||||
| de Piano et al, 2012 [34] | Eur J Gastroenterol Hepatol | Brazil | 28 | 14 aerobic training plus resistance training group (AT+RT) | 14 aerobic training group (AT) | US-diagnosed fatty liver | 16.48 ± 1.42 | 27 | AT+RT group: Aerobic and resistance exercise training 3 times/wk for 1 h each time (30min aerobic training: running on treadmill, and 30min resistance trianing). AT-group: Aerobic exercise training 3 times/wk for 1 h each. Psychological intervention, Nutritional intervention (Nutritional education on a balanced diet), clinical evaluation once a week for both groups. | 1yr | Body weight, Height, Body composition, FBG, Insulin, TCHOL, LDL-C, VLDL, HDL-C, TG, AST, ALT, GGT, Adipokines, Neuropeptides, | NR | NR |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katsagoni, C.N.; Papachristou, E.; Sidossis, A.; Sidossis, L. Effects of Dietary and Lifestyle Interventions on Liver, Clinical and Metabolic Parameters in Children and Adolescents with Non-Alcoholic Fatty Liver Disease: A Systematic Review. Nutrients 2020, 12, 2864. https://doi.org/10.3390/nu12092864
Katsagoni CN, Papachristou E, Sidossis A, Sidossis L. Effects of Dietary and Lifestyle Interventions on Liver, Clinical and Metabolic Parameters in Children and Adolescents with Non-Alcoholic Fatty Liver Disease: A Systematic Review. Nutrients. 2020; 12(9):2864. https://doi.org/10.3390/nu12092864
Chicago/Turabian StyleKatsagoni, Christina N., Eleftheria Papachristou, Amalia Sidossis, and Labros Sidossis. 2020. "Effects of Dietary and Lifestyle Interventions on Liver, Clinical and Metabolic Parameters in Children and Adolescents with Non-Alcoholic Fatty Liver Disease: A Systematic Review" Nutrients 12, no. 9: 2864. https://doi.org/10.3390/nu12092864
APA StyleKatsagoni, C. N., Papachristou, E., Sidossis, A., & Sidossis, L. (2020). Effects of Dietary and Lifestyle Interventions on Liver, Clinical and Metabolic Parameters in Children and Adolescents with Non-Alcoholic Fatty Liver Disease: A Systematic Review. Nutrients, 12(9), 2864. https://doi.org/10.3390/nu12092864





