Feasibility, Process, and Effects of Short-Term Calorie Reduction in Cancer Patients Receiving Chemotherapy: An Integrative Review
Abstract
1. Introduction
2. Materials and Methods
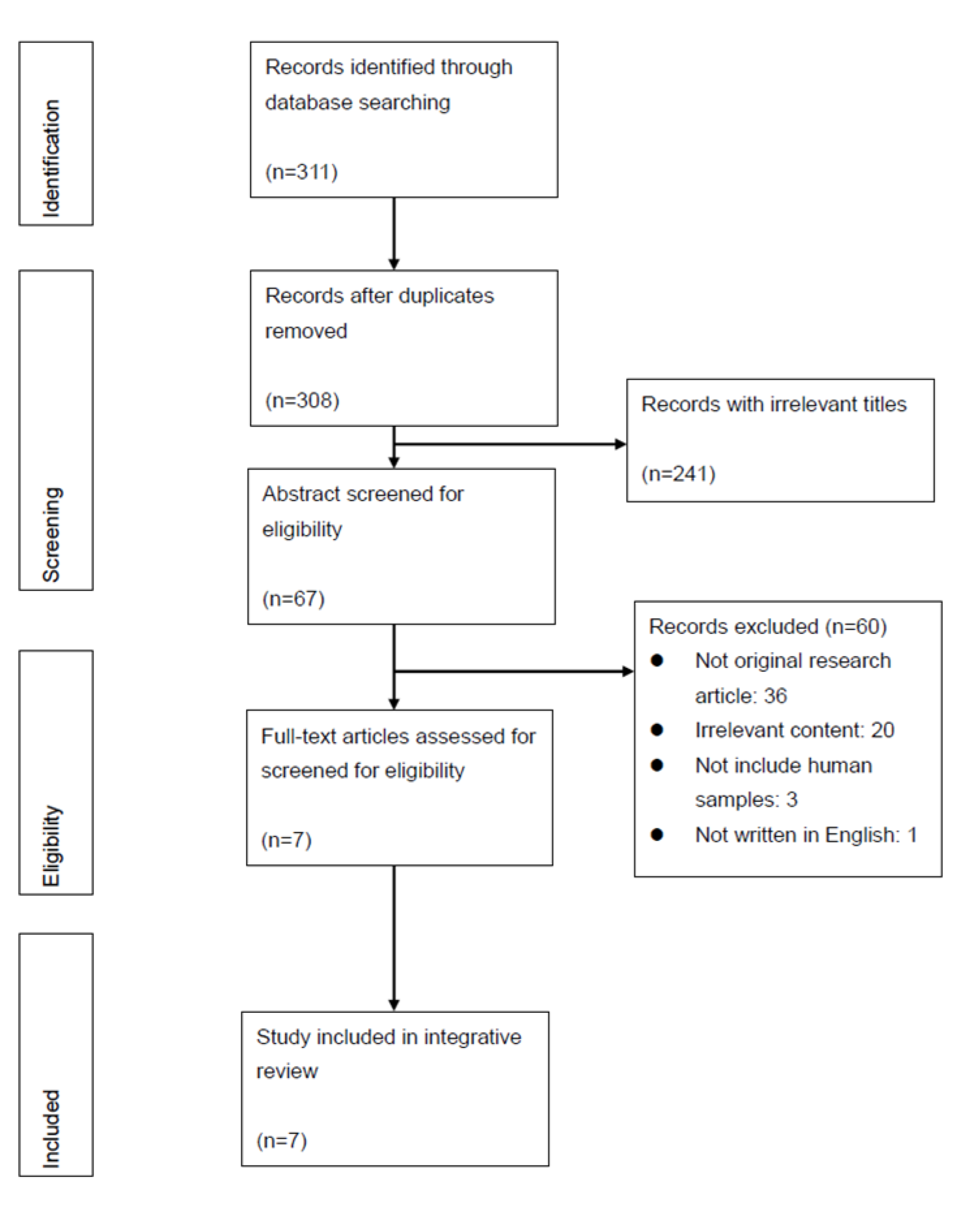
2.1. Literature Search
2.2. Data Evaluation and Analysis
3. Results
3.1. Study Characteristics
3.2. Outcome Measurements
3.2.1. Safety and Tolerance of SCR
3.2.2. Effects of SCR
SCR’s Protective or Regenerative Effect on Normal Cells
Sensitizing Tumor Cells to Chemotherapy
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- De Cabo, R.; Mattson, M.P. Effects of intermittent fasting on health, aging, and disease. N. Engl. J. 2019, 381, 2541–2551. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P.; Longo, V.D.; Harvie, M. Impact of intermittent fasting on health and disease processes. Ageing Res. Rev. 2017, 39, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Anton, S.D.; Moehl, K.; Donahoo, W.T.; Marosi, K.; Lee, S.A.; Mainous, A.G.; Leeuwenburgh, C.; Mattson, M.P. Flipping the metabolic switch: Understanding and applying the health benefits of fasting. Obesity (Silver Spring Md.) 2018, 26, 254–268. [Google Scholar] [CrossRef] [PubMed]
- Kanarek, N.; Petrova, B.; Sabatini, D.M. Dietary modifications for enhanced cancer therapy. Nature 2020, 579, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Hammer, M.J.; Voss, J.G. Malglycemia and cancer: Introduction to a conceptual model. Proc. Oncol. Nurs. Forum. 2012, 39, E275–E287. [Google Scholar] [CrossRef] [PubMed]
- Storey, S.; Von Ah, D. Impact of malglycemia on clinical outcomes in hospitalized patients with cancer: A review of the literature. Proc. Oncol. Nurs. Forum. 2012, 39, 458–465. [Google Scholar] [CrossRef]
- Brandhorst, S.; Longo, V.D. Fasting and caloric restriction in cancer prevention and treatment. Recent Results Cancer Res. 2016, 207, 241–266. [Google Scholar] [CrossRef]
- Nencioni, A.; Caffa, I.; Cortellino, S.; Longo, V.D. Fasting and cancer: Molecular mechanisms and clinical application. Nat. Rev. Cancer 2018, 18, 707–719. [Google Scholar] [CrossRef]
- O’Flanagan, C.H.; Smith, L.A.; McDonell, S.B.; Hursting, S.D. When less may be more: Calorie restriction and response to cancer therapy. BMC Med. 2017, 15, 106. [Google Scholar] [CrossRef]
- Lee, C.; Longo, V. Fasting vs dietary restriction in cellular protection and cancer treatment: From model organisms to patients. Oncogene 2011, 30, 3305–3316. [Google Scholar] [CrossRef]
- Icard, P.; Teboul, B.; El Baze, P. A Simple method to optimize the effectiveness of chemotherapy: Modulation of glucose intake during chemotherapy. Anticancer Res. 2017, 37, 6199–6202. [Google Scholar] [PubMed]
- D’Aronzo, M.; Vinciguerra, M.; Mazza, T.; Panebianco, C.; Saracino, C.; Pereira, S.P.; Graziano, P.; Pazienza, V. Fasting cycles potentiate the efficacy of gemcitabine treatment in in vitro and in vivo pancreatic cancer models. Oncotarget 2015, 6, 18545. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Yan, Y.; Gius, D.R.; Vassilopoulos, A. Metabolic regulation of Sirtuins upon fasting and the implication for cancer. Curr. Opin. Oncol. 2013, 25, 630. [Google Scholar] [CrossRef] [PubMed]
- Lo Re, O.; Panebianco, C.; Porto, S.; Cervi, C.; Rappa, F.; Di Biase, S.; Caraglia, M.; Pazienza, V.; Vinciguerra, M. Fasting inhibits hepatic stellate cells activation and potentiates anti-cancer activity of sorafenib in hepatocellular cancer cells. J. Cell. Physiol. 2018, 233, 1202–1212. [Google Scholar] [CrossRef] [PubMed]
- Panebianco, C.; Adamberg, K.; Adamberg, S.; Saracino, C.; Jaagura, M.; Kolk, K.; Di Chio, A.G.; Graziano, P.; Vilu, R.; Pazienza, V. Engineered resistant-starch (ERS) diet shapes colon microbiota profile in parallel with the retardation of tumor growth in in vitro and in vivo pancreatic cancer models. Nutrients 2017, 9, 331. [Google Scholar] [CrossRef]
- Di Biase, S.; Shim, H.S.; Kim, K.H.; Vinciguerra, M.; Rappa, F.; Wei, M.; Brandhorst, S.; Cappello, F.; Mirzaei, H.; Lee, C. Fasting regulates EGR1 and protects from glucose-and dexamethasone-dependent sensitization to chemotherapy. PLoS Biol. 2017, 15, e2001951. [Google Scholar] [CrossRef]
- De las Peñas, R.; Majem, M.; Perez-Altozano, J.; Virizuela, J.; Diz, P.; Donnay, O.; Hurtado, A.; Jimenez-Fonseca, P.; Ocon, M. SEOM clinical guidelines on nutrition in cancer patients (2018). Clin. Transl. Oncol. 2019, 21, 87–93. [Google Scholar] [CrossRef]
- American Institute for Cancer Research; LIVESTRONG Foundation; Savor Health™. HEAL Well: A Cancer Nutrition Guide; 2015; p. 26. [Google Scholar]
- Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Fearon, K.; Hütterer, E.; Isenring, E.; Kaasa, S. ESPEN guidelines on nutrition in cancer patients. Clin. Nutr. 2017, 36, 11–48. [Google Scholar] [CrossRef]
- Maschke, J.; Kruk, U.; Kastrati, K.; Kleeberg, J.; Buchholz, D.; Erickson, N.; Huebner, J. Nutritional care of cancer patients: A survey on patients’ needs and medical care in reality. Int. J. Clin. Oncol. 2017, 22, 200–206. [Google Scholar] [CrossRef]
- Keinki, C.; Seilacher, E.; Ebel, M.; Ruetters, D.; Kessler, I.; Stellamanns, J.; Rudolph, I.; Huebner, J. Information needs of cancer patients and perception of impact of the disease, of self-efficacy, and locus of control. J. Cancer Educ. 2016, 31, 610–616. [Google Scholar] [CrossRef]
- Amano, K.; Maeda, I.; Morita, T.; Tatara, R.; Katayama, H.; Uno, T.; Takagi, I. Need for nutritional support, eating-related distress and experience of terminally ill patients with cancer: A survey in an inpatient hospice. BMJ Supportive Palliat. Care 2016, 6, 373–376. [Google Scholar] [CrossRef] [PubMed]
- Whittemore, R.; Knafl, K. The integrative review: Updated methodology. J. Adv. Nurs. 2005, 52, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.G.; Swiontkowski, M.F.; Heckman, J.D. Introducing levels of evidence to the journal. JBJS 2003, 85, 1–3. [Google Scholar] [CrossRef]
- National Heart Lung and Blood Institute; National Institutes of Health; US Department of Health and Human Services. Study Quality Assessment Tools. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 14 August 2020).
- Cesario, S.; Morin, K.; Santa-Donato, A. Evaluating the level of evidence of qualitative research. J. Obstet. Gynecol. Neonatal Nurs. JOGNN/NAACOG 2002, 31, 708–714. [Google Scholar] [CrossRef]
- National Heart Lung and Blood Institute; National Institutes of Health; US Department of Health and Human Services. Quality Assessment of Controlled Intervention Studies. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 14 August 2020).
- National Heart Lung and Blood Institute; National Institutes of Health; US Department of Health and Human Services. Quality Assessment Tool for Case Series Studies. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 14 August 2020).
- Bauersfeld, S.P.; Kessler, C.S.; Wischnewsky, M.; Jaensch, A.; Steckhan, N.; Stange, R.; Kunz, B.; Brückner, B.; Sehouli, J.; Michalsen, A. The effects of short-term fasting on quality of life and tolerance to chemotherapy in patients with breast and ovarian cancer: A randomized cross-over pilot study. BMC Cancer 2018, 18, 476. [Google Scholar] [CrossRef]
- De Groot, S.; Vreeswijk, M.P.; Welters, M.J.; Gravesteijn, G.; Boei, J.J.; Jochems, A.; Houtsma, D.; Putter, H.; van der Hoeven, J.J.; Nortier, J.W. The effects of short-term fasting on tolerance to (neo) adjuvant chemotherapy in HER2-negative breast cancer patients: A randomized pilot study. BMC Cancer 2015, 15, 652. [Google Scholar] [CrossRef]
- Mas, S.; Le Bonniec, A.; Cousson-Gélie, F. Why do women fast during breast cancer chemotherapy? A qualitative study of the patient experience. Br. J. Health Psychol. 2019, 24, 381–395. [Google Scholar] [CrossRef]
- De Groot, S.; Lugtenberg, R.T.; Cohen, D.; Welters, M.J.; Ehsan, I.; Vreeswijk, M.P.; Smit, V.T.; de Graaf, H.; Heijns, J.B.; Portielje, J.E. Fasting mimicking diet as an adjunct to neoadjuvant chemotherapy for breast cancer in the multicentre randomized phase 2 DIRECT trial. Nat. Commun. 2020, 11, 1–9. [Google Scholar] [CrossRef]
- Zorn, S.; Ehret, J.; Schäuble, R.; Rautenberg, B.; Ihorst, G.; Bertz, H.; Urbain, P.; Raynor, A. Impact of modified short-term fasting and its combination with a fasting supportive diet during chemotherapy on the incidence and severity of chemotherapy-induced toxicities in cancer patients-a controlled cross-over pilot study. BMC Cancer 2020, 20, 1–14. [Google Scholar] [CrossRef]
- Dorff, T.B.; Groshen, S.; Garcia, A.; Shah, M.; Tsao-Wei, D.; Pham, H.; Cheng, C.-W.; Brandhorst, S.; Cohen, P.; Wei, M. Safety and feasibility of fasting in combination with platinum-based chemotherapy. BMC Cancer 2016, 16, 360. [Google Scholar] [CrossRef]
- Safdie, F.M.; Dorff, T.; Quinn, D.; Fontana, L.; Wei, M.; Lee, C.; Cohen, P.; Longo, V.D. Fasting and cancer treatment in humans: A case series report. Aging (Albany NY) 2009, 1, 988. [Google Scholar] [CrossRef] [PubMed]
- Levine, C.S.; Miyamoto, Y.; Markus, H.R.; Rigotti, A.; Boylan, J.M.; Park, J.; Kitayama, S.; Karasawa, M.; Kawakami, N.; Coe, C.L. Culture and healthy eating: The role of independence and interdependence in the United States and Japan. Pers. Soc. Psychol. Bull. 2016, 42, 1335–1348. [Google Scholar] [CrossRef]
- Bruggeman, A.R.; Kamal, A.H.; LeBlanc, T.W.; Ma, J.D.; Baracos, V.E.; Roeland, E.J. Cancer cachexia: Beyond weight loss. J. Oncol. Pract. 2016, 12, 1163–1171. [Google Scholar] [CrossRef]
- De Groot, S.; Lugtenberg, R.; Welters, M.; Ehsan, I.; Vreeswijk, M.; Smit, V.; de Graaf, H.; Heijns, J.; Portielje, J.; van de Wouw, A. Abstract P1-15-20: DIetary REstriction as an Adjunct to Neoadjuvant ChemoTherapy for HER2-Negative Breast Cancer: Final Results from the DIRECT Trial (BOOG 2013-04); AACR: Philadelphia, PA, USA, 2019. [Google Scholar]
- Oliveira, C.L.; Mattingly, S.; Schirrmacher, R.; Sawyer, M.B.; Fine, E.J.; Prado, C.M. A nutritional perspective of ketogenic diet in cancer: A narrative review. J. Acad. Nutr. Diet. 2018, 118, 668–688. [Google Scholar] [CrossRef] [PubMed]
- Lv, M.; Zhu, X.; Wang, H.; Wang, F.; Guan, W. Roles of caloric restriction, ketogenic diet and intermittent fasting during initiation, progression and metastasis of cancer in animal models: A systematic review and meta-analysis. PLoS ONE 2014, 9, e115147. [Google Scholar] [CrossRef] [PubMed]
- Mendelsohn, A.R.; Larrick, J.W. Prolonged fasting/refeeding promotes hematopoietic stem cell regeneration and rejuvenation. Rejuvenation Res. 2014, 17, 385–389. [Google Scholar] [CrossRef] [PubMed]
- White, E.; DiPaola, R.S. The double-edged sword of autophagy modulation in cancer. Clin Cancer Res. 2009, 15, 5308–5316. [Google Scholar] [CrossRef] [PubMed]
- Watkins, E.; Serpell, L. The psychological effects of short-term fasting in healthy women. Front Nutr. 2016, 3, 27. [Google Scholar] [CrossRef] [PubMed]
- Dorff, T.B.; Shelechi, M.; Kang, I.; Morgan, T.E.; Groshen, S.G.; Yennu, S.; Garcia, A.A.; Quinn, D.I.; Longo, V. A Randomized Phase II Clinical Trial of a Fasting-Mimic Diet Prior to Chemotherapy to Evaluate the Impact on Toxicity and Efficacy; American Society of Clinical Oncology: Alexandria, VA, USA, 2018. [Google Scholar]
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/results?term=chemotherapy%2C+fasting&cond=Cancer&Search=Apply&recrs=b&recrs=a&recrs=f&recrs=d&recrs=e&recrs=m&age_v=&age=1&gndr=&type=&rslt= (accessed on 14 August 2020).
- Gutiérrez-Salmeán, G.; Ceballos, G.; Meaney, E. Anthracyclines and cardiotoxicity. Int. J. Cancer Res. Prev. 2015, 8, 515. [Google Scholar]
| Databases | Searching Strategies: Combination of Medical Subheadings | Initial Results |
|---|---|---|
| PubMed | (“fasting” OR “calorie restricted”) AND “chemotherapy” | 238 |
| Ovid Medline | (“fasting” OR “diet, carbohydrate-restricted” OR “calorie restriction”) AND (“maintenance chemotherapy” OR “induction chemotherapy” OR “consolidation chemotherapy” OR “chemotherapy, adjuvant” OR “chemotherapy, cancer, regional perfusion”) | 9 |
| CINAHL | (“fasting” OR (“preprocedural Fasting” OR “restricted diet” OR “diet, reducing” OR “diet, low carbohydrate”) AND (“chemotherapy, cancer” OR “chemotherapy, adjuvant” OR “chemotherapy care (Saba CCC)” OR “chemotherapy management (Iowa NIC)” OR “antineoplastic agents, combined”) | 7 |
| PsychINFO | (“calories” OR “dietary restraint”) AND “chemotherapy” | 38 |
| Embase | “caloric restriction” AND “cancer chemotherapy” | 19 |
| Level | Definition |
|---|---|
| I | Randomized controlled trial |
| II | Prospective cohort study or Poor-quality randomized controlled trial |
| III | Case-control study or Retrospective cohort study |
| IV | Case series |
| V | Expert opinion |
| First Author, Year (Country) | Goal: Research Design | Sample Size, Population, Exclusion Criteria | Calorie Reduction Plan | Chemotherapy Regimen | Measuring Time and Outcome Measurements | Main Results |
|---|---|---|---|---|---|---|
| Dorff, 2016 (USA) | Determine the safety/feasibility of fasting prior to C/T: Cohort study (24/48/72 h.) |
| Dose/time: escalating fast, up to 72 h (24 h before C/T completion → 48 h before C/T completion → if safe/feasible, then continue with 72 h (48 h before and 24 h after); if not, then try 48 h with specific low-calorie diet (repeat for at least 2 C/T cycles)) Content: NPO except for water and non-caloric beverage and rescue (<200 kcal/24 h. if fasting symptoms present) |
|
|
|
| Safdie, 2009 (USA) | Examine the safety of fasting before and after chemotherapy: Case study |
| (Vary by cases) Does/time: 48–140 h prior to and/or 5–56 h following C/T (self-selected C/T cycles) Content: Some NPO except for water and vitamin, others unspecified Control: self-control | Individualized |
|
|
| Bauersfeld, 2018 (Germany) | Examine the feasibility and effects of QOL of short-term fasting during C/T: Randomized, individually controlled trial |
| Dose/time: 60 h (36 h before and 24 h after C/T) Content: Unrestricted amounts of water, herbal tea, 2 × 100 cL vegetable juice and small standardizes quantities of light vegetable broth with a maximum total energy intake of 350 kcal/day Control group: self-controlled (group A: fast for the first half of C/T cycles (2 or 3 cycles) followed by normal diet); group B: vice versa sequence) |
|
|
|
| de Groot, 2015 (The Netherlands) | Identify the effects of 48 h fasting on C/T, including side effects, hematological parameters in breast cancer patient receiving TAC: Randomized controlled trial |
| Dose/time: 48 h fasting (24 h before and after starting C/T) Content: NPO except for water or coffee/tea without sugar Control group: Eat according to the guidelines for healthy nutrition (n = 6, minimum of 2 pieces of fruit per day) |
|
|
|
| de Groot, 2020 (The Netherlands) | Evaluate the impact of FMD on toxicity as well as on the radiological and pathological response to chemotherapy for breast cancer: Randomized controlled trial |
| Dose/time: 4-day plant-based low amino-acid substitution diet (FMD, 3 days prior to and on the day of C/T) Content: decreased calorie intake from FMD (1200 kcal at day 1, 100 kcal at day 2–4) Control group: regular diet |
|
|
|
| Zorn, 2020 (German) | Evaluate the influence of 96-h fasting on chemotherapy-induced toxicities in patients with gynecological cancer: controlled cross-over trial |
| Dose/time: 96 h fasting (72 h before and 24 h after starting C/T) or 6-day normocaloric ketogenic diet plus 96 h fasting Content: 25% of daily calorie requirement (400–600 kcal/day) with macronutrients revealed to a ketogenic composition Control group: everyone served as their own controls (2–3 cycles of SCR and 2–3 cycles of normal diet) |
|
|
|
| Mas, 2019 (France) | Explore the motivations to fast among cancer patients: Qualitative study |
| Dose/time: Having performed at least one 24 h fast before C/T within a year (duration ranges from a day and half to 7 days; C/T cycles range from one to 10 months). Content: not specified | Not mentioned |
|
|
| Quality Rating Criteria for Cohort Study | Study | Quality Rating Criteria for Case Report | Study |
|---|---|---|---|
| Dorff, 2016 | Safdie, 2009 | ||
| Research question/objective was clearly stated | Yes | Research question/objective was clearly stated | No |
| Study population was clearly specified/defined | Yes | Study population was clearly specified/defined | Yes |
| Participation rate of eligible persons was ≥50% | Unclear | Cases were consecutive | No |
| Prespecified Inclusion/exclusion criteria | Yes | Subjects were comparable | No |
| Justification of sample size/power/variance/effect size | No | Intervention was clearly described | Yes |
| Exposure(s) measured prior to outcome(s) evaluation | Yes | Clearly defined, valid and reliable outcome measures | No |
| Sufficient timeframe to see a possible association | Yes | Adequate length of follow-up | Yes |
| Examine different exposure levels as related to the outcome | Yes | Well-described statistical methods | Not applicable |
| Clearly defined, valid and reliable exposure measures | Yes | Well-described results | Yes |
| Assessed the exposure(s) more than once over time | Yes | ||
| Clearly defined valid and reliable outcome measures | Yes | ||
| Outcome assessors were blinded to the exposure status of participants | Unclear | ||
| Loss to follow-up was 20% or less | Yes | ||
| Key potential confounding variables measured and adjusted statistically | No | ||
| Suggesting Quality (% of criteria met) | Fair (71%) | Suggesting Quality (% of criteria met) | Fair (50%) |
| Level of Evidence | II | Level of Evidence | IV |
| Quality Rating Criteria | Studies | |||
|---|---|---|---|---|
| Bauersfeld, 2018 | de Groot, 2015 | de Groot, 2020 | Zorn, 2020 | |
| Study was described as randomized or an RCT | Yes | Yes | Yes | No |
| Adequate randomization | Yes | Yes | Yes | No |
| Concealed treatment allocation | Yes | Unclear | Yes | Unclear |
| Study participants and providers were blinded to group assignment | Not applicable | Not applicable | Not applicable | Not applicable |
| People assessing the outcomes were blinded to the assignments | Unclear | Unclear | Yes | Unclear |
| Groups were similar at baseline on important characteristics | No | Yes | Yes | No |
| Overall drop-out rate at endpoint was ≤20% for treatment group | No | No | No | No |
| Differential drop-out rate between groups at endpoint was ≤15% or lower | Yes | Yes | No | No |
| Adherence to the intervention protocols were high | No | Yes | No | No |
| Other interventions were avoided or similar in the groups | Yes | Yes | Yes | Yes |
| Outcomes were assessed using valid and reliable measures | Yes | Yes | Yes | Yes |
| Sufficient sample size to be able to detect a difference with ≥80% power | Yes | No | Yes | Yes |
| Outcomes reported or subgroups analyzed were prespecified | Unclear | Unclear | Yes | Yes |
| All randomized participants were analyzed in the original group (intention-to-treat analysis) | Yes | Yes | Yes | Yes |
| Suggesting Level of Quality (% of criteria met) | Fair (62%) | Fair (62%) | Good (77%) | Poor (38%) |
| Level of Evidence | II | II | I | II |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, C.-C.; Chen, H.; Huang, T.-C.; Wu, W.-W.; Lin, J.-M.; Tien, F.-M. Feasibility, Process, and Effects of Short-Term Calorie Reduction in Cancer Patients Receiving Chemotherapy: An Integrative Review. Nutrients 2020, 12, 2823. https://doi.org/10.3390/nu12092823
Tang C-C, Chen H, Huang T-C, Wu W-W, Lin J-M, Tien F-M. Feasibility, Process, and Effects of Short-Term Calorie Reduction in Cancer Patients Receiving Chemotherapy: An Integrative Review. Nutrients. 2020; 12(9):2823. https://doi.org/10.3390/nu12092823
Chicago/Turabian StyleTang, Chia-Chun, Hsi Chen, Tai-Chung Huang, Wei-Wen Wu, Jing-Mei Lin, and Feng-Ming Tien. 2020. "Feasibility, Process, and Effects of Short-Term Calorie Reduction in Cancer Patients Receiving Chemotherapy: An Integrative Review" Nutrients 12, no. 9: 2823. https://doi.org/10.3390/nu12092823
APA StyleTang, C.-C., Chen, H., Huang, T.-C., Wu, W.-W., Lin, J.-M., & Tien, F.-M. (2020). Feasibility, Process, and Effects of Short-Term Calorie Reduction in Cancer Patients Receiving Chemotherapy: An Integrative Review. Nutrients, 12(9), 2823. https://doi.org/10.3390/nu12092823





