Protective Effect of Spore Powder of Antrodia camphorata ATCC 200183 on CCl4-Induced Liver Fibrosis in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Preparation Spore Powder of A. camphorata
2.3. Proximate Analysis and Triterpenoids Analysis
2.4. Animals and Treatment
2.5. Serum and Tissue Biomakers
2.6. Histopathological Study
2.7. RNA Extraction and RT-qPCR Analysis
2.8. Protein Extraction and Western Blot Analysis
2.9. Statistical Analysis
3. Results
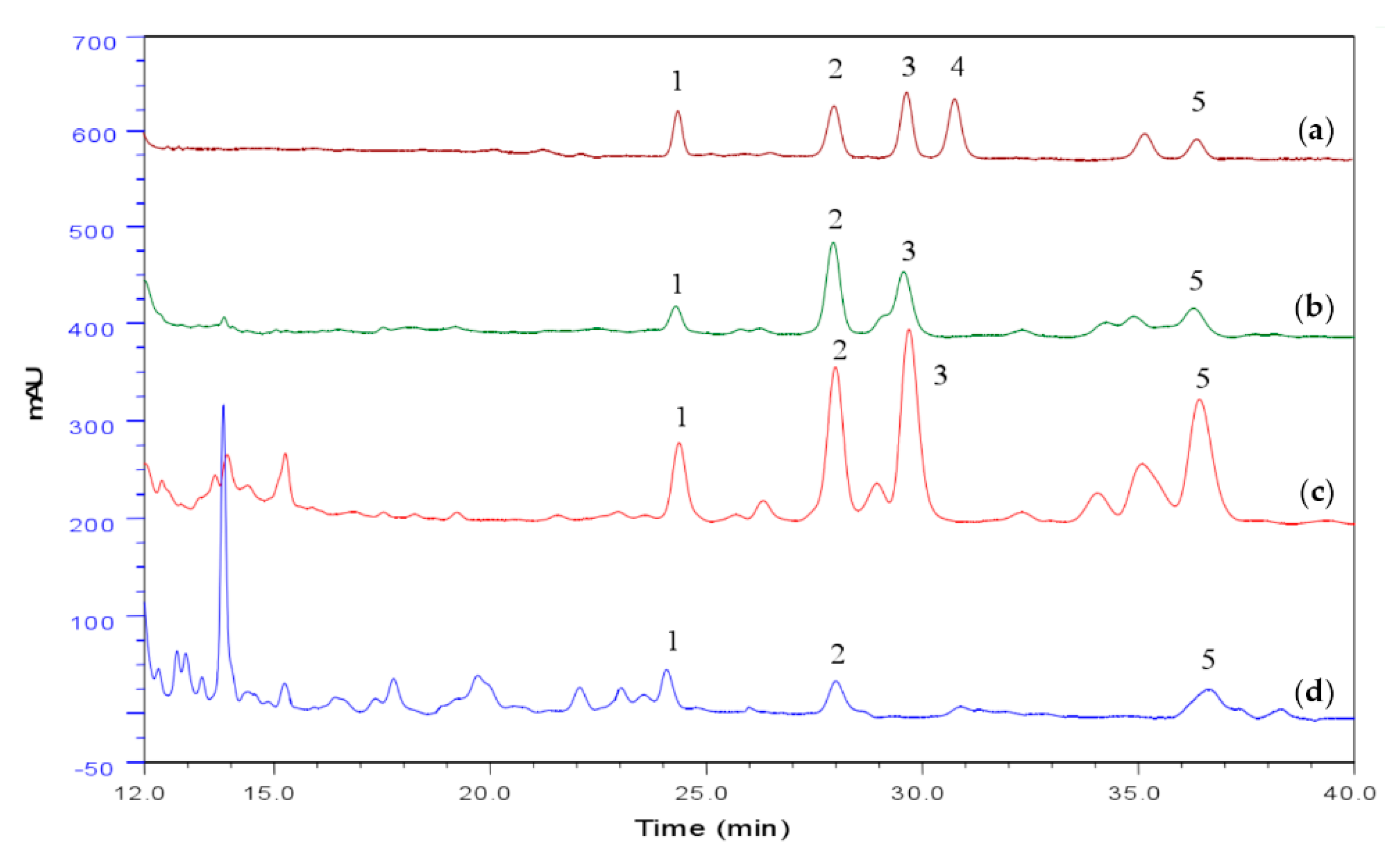
3.1. Chemical Characteristics of Spore, Mycelia and Cultural Broth of A. camphorata
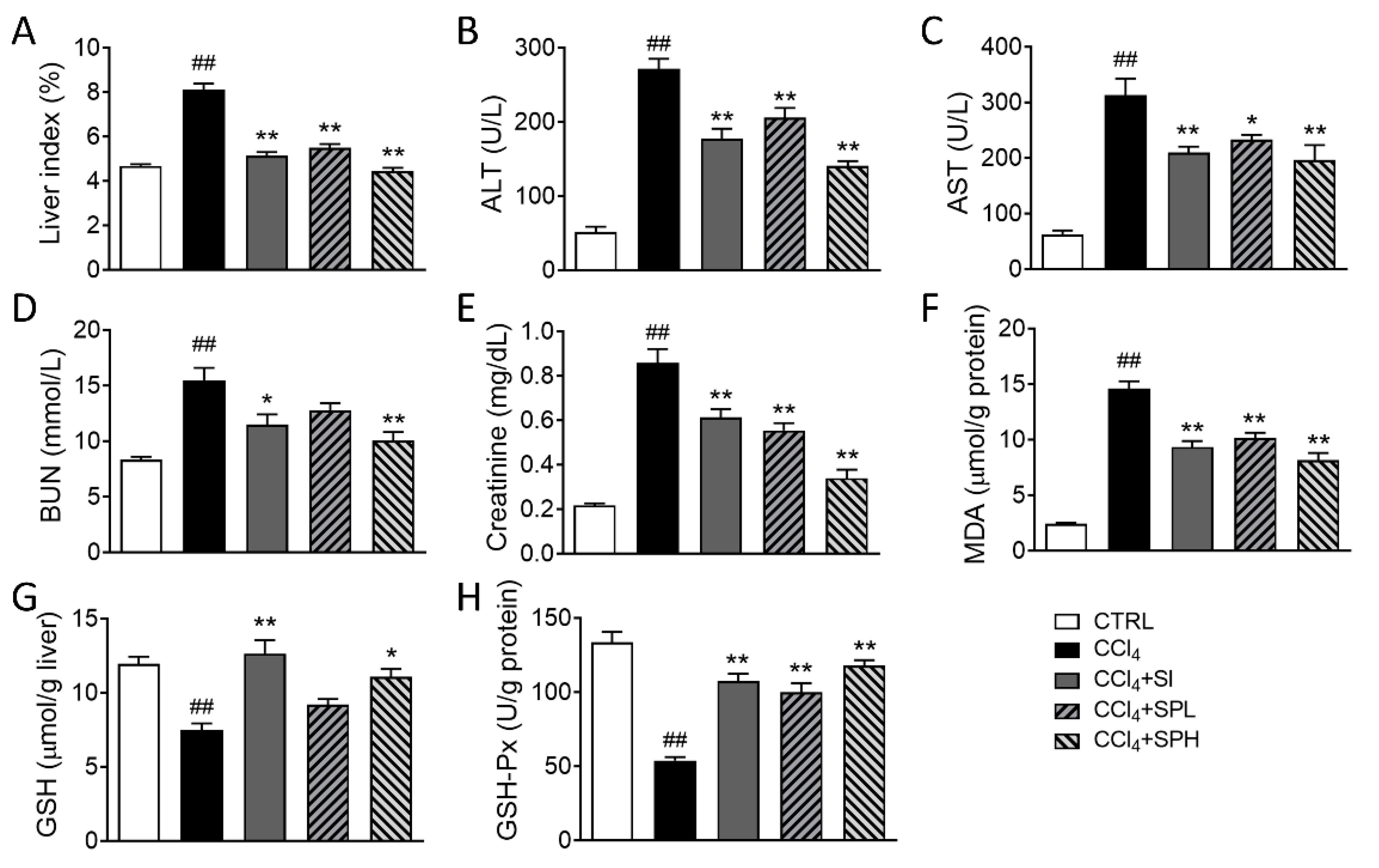
3.2. Effects of Spore Powder of A. camphorata on Serum and Tissue Biomarkers in CCl4-Induced Liver Fibrosis in Mice
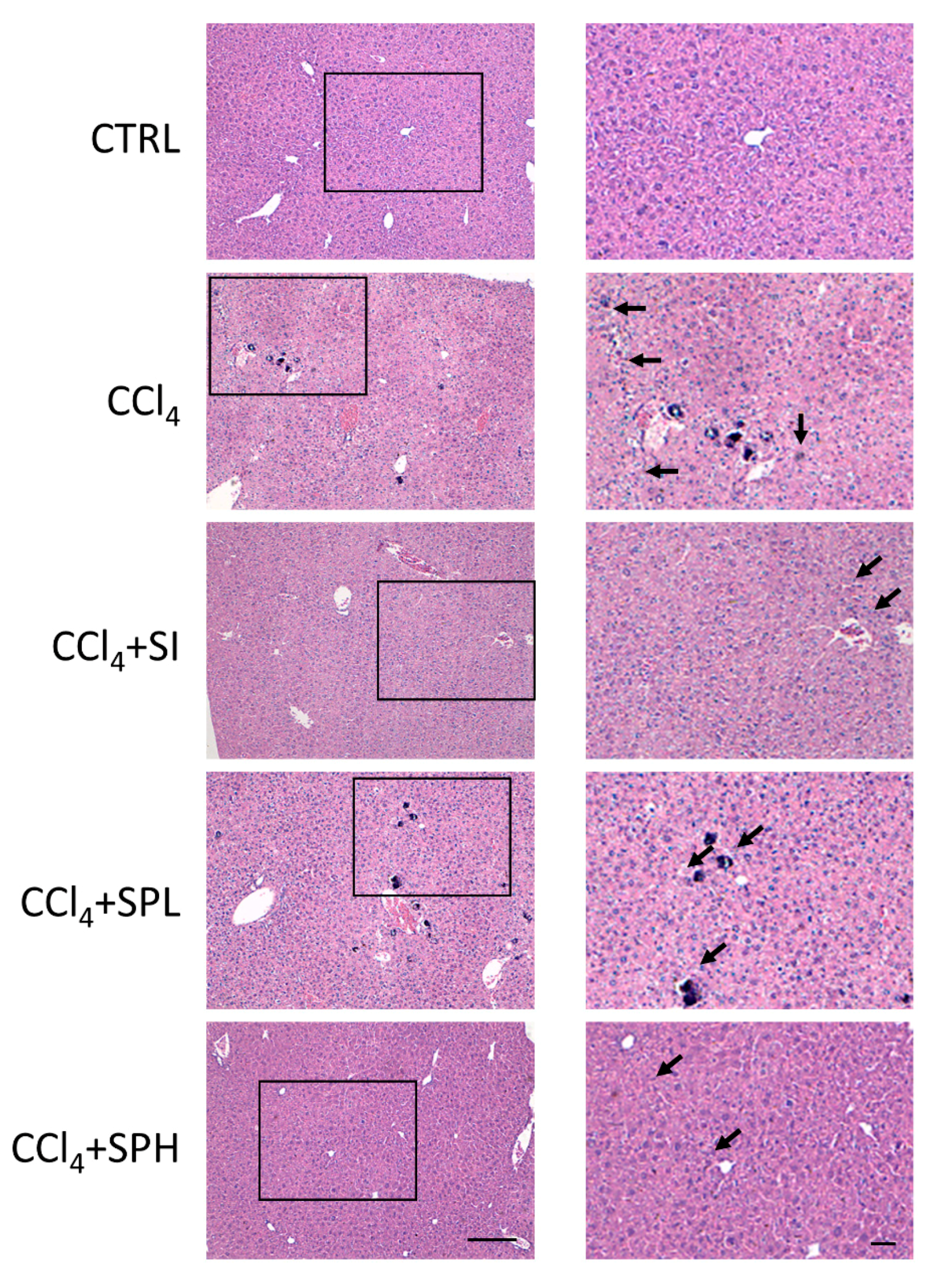
3.3. Effects of Spore Powder of A. camphorata on Liver Pathology on CCl4-Induced Liver Fibrosis in Mice
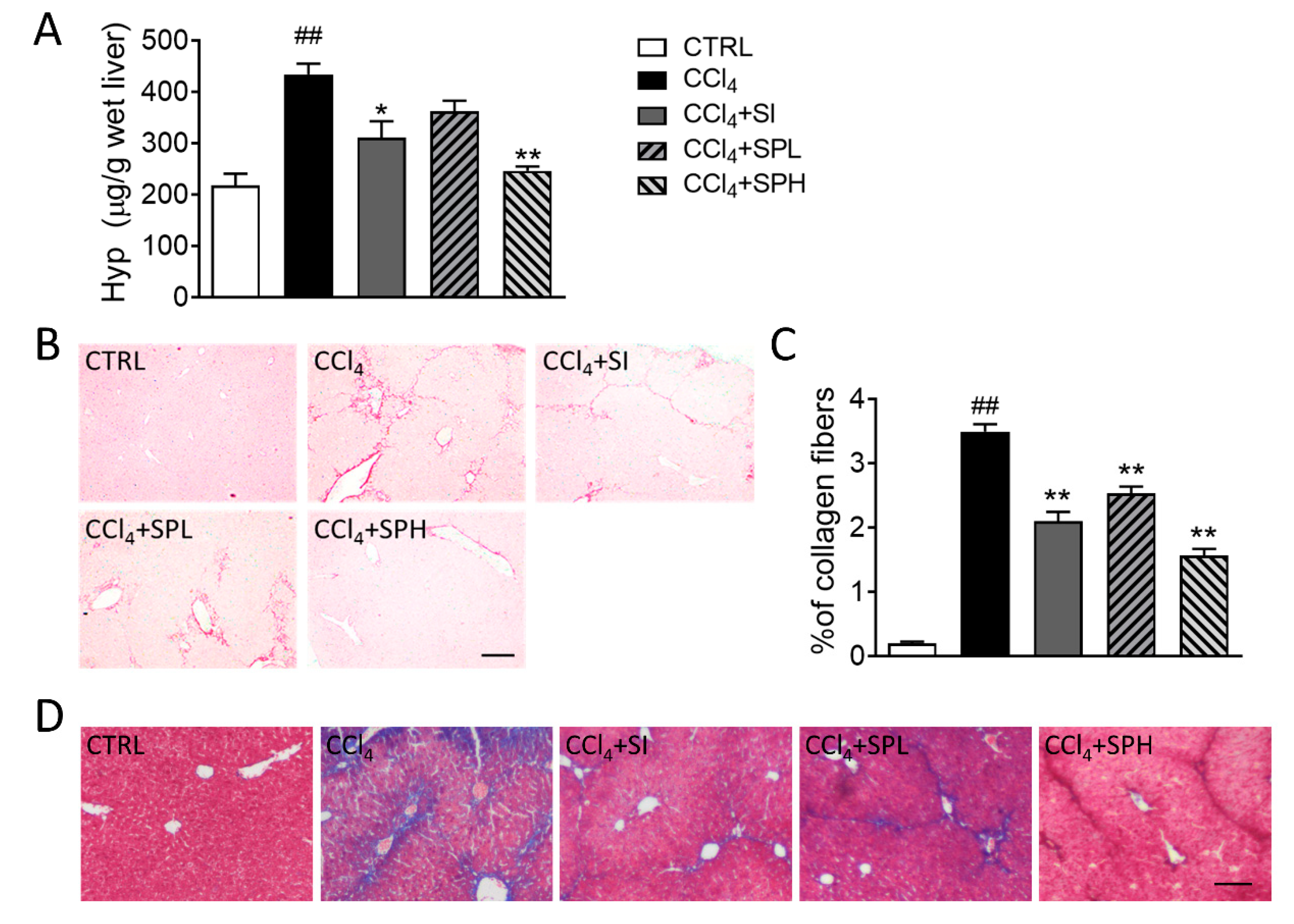
3.4. Effects of Spore Powder of A. camphorata on Collagen Content in LiverTissues
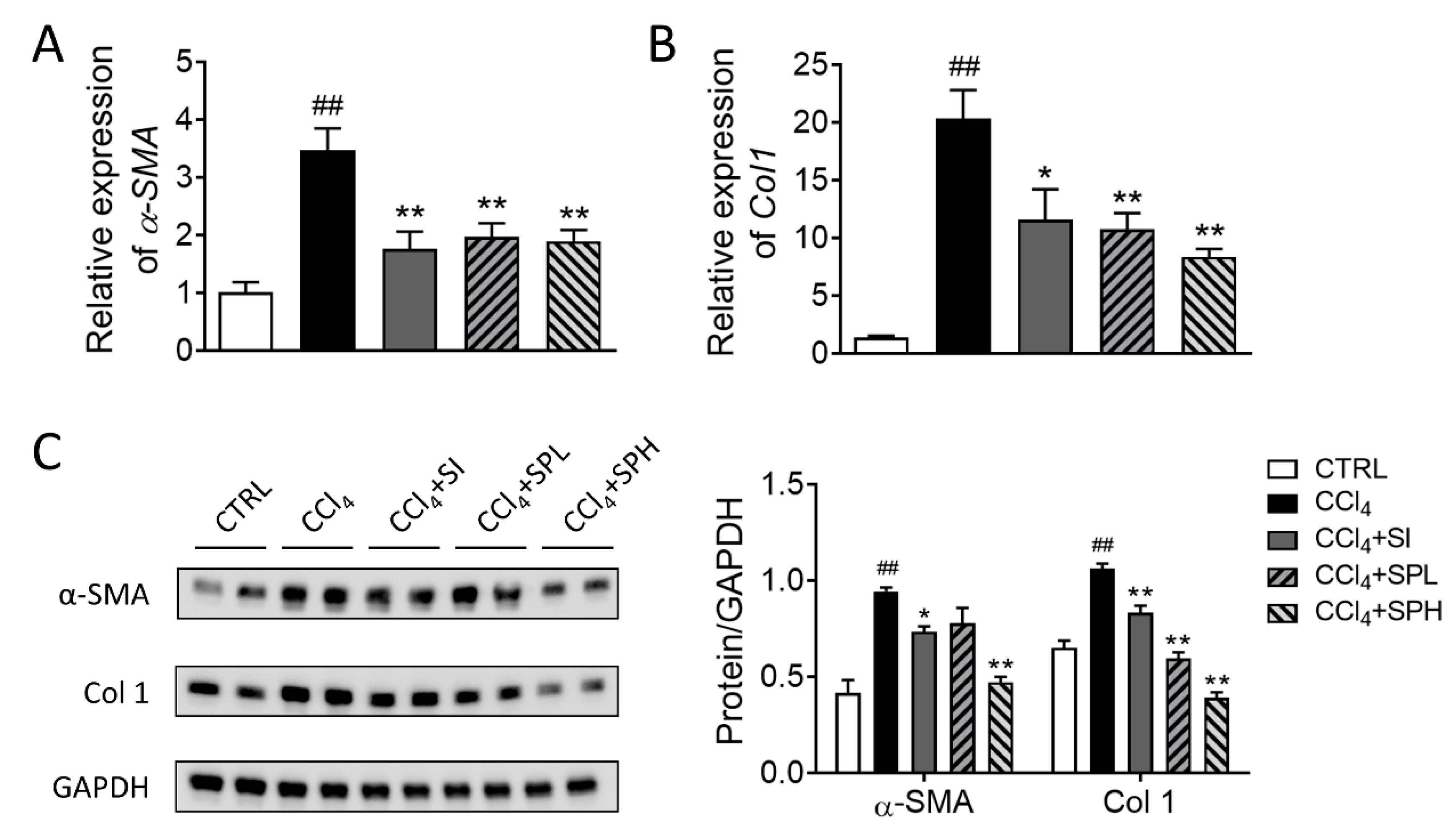
3.5. Effects of Spore Powder of A. camphorata on α-SMA and Col 1 Expression in Liver Tissues
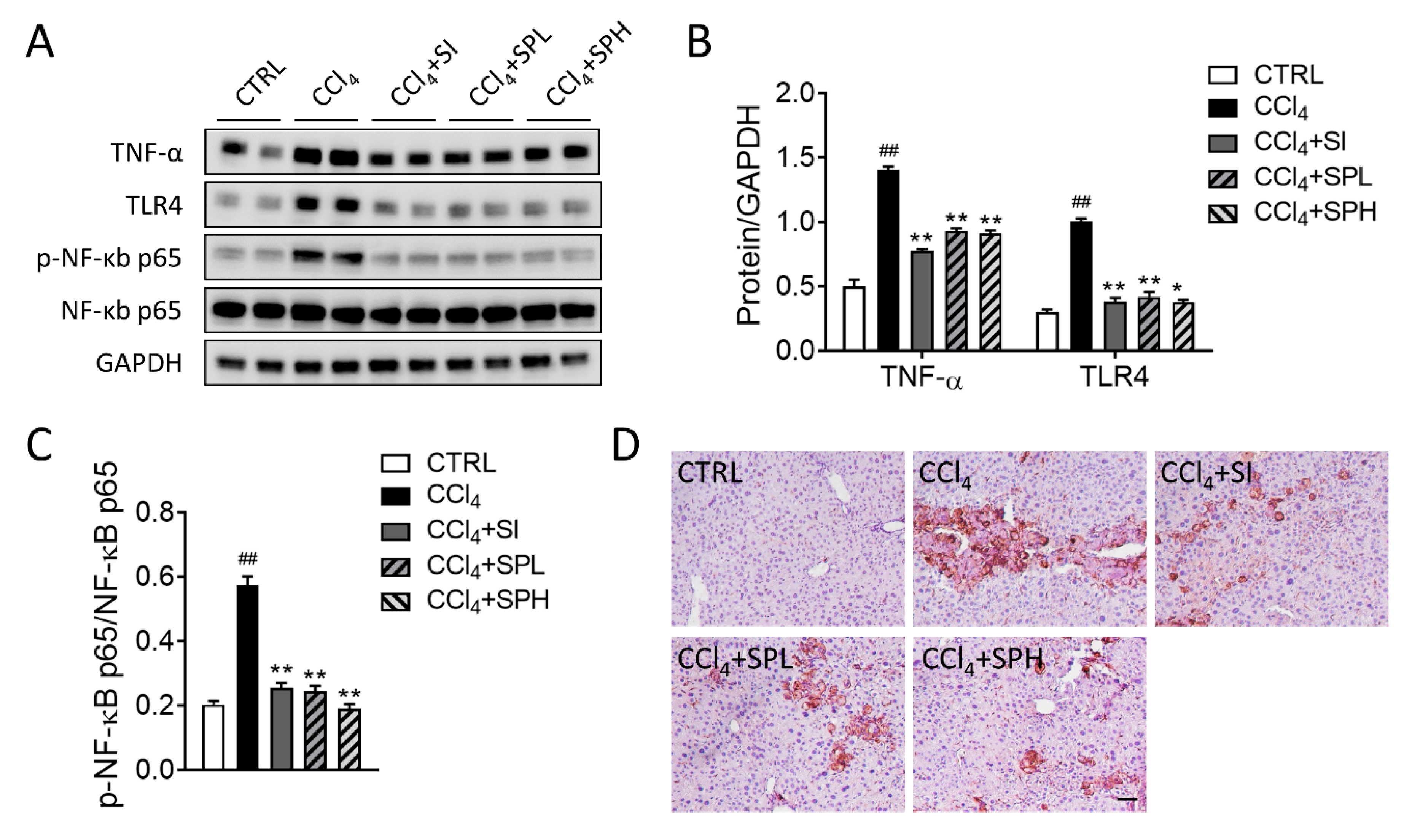
3.6. Effects of Spore Powder of A. camphorata on TNF-α, TLR4, NF-κB and F4/80 Protein Expression in Liver Tissues
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Orman, E.S.; Odena, G.; Bataller, R. Alcoholic liver disease: Pathogenesis, management, and novel targets for therapy. J. Gastroenterol. Hepatol. 2013, 28 (Suppl. S1), 77–84. [Google Scholar] [CrossRef] [PubMed]
- Schuppan, D. Liver fibrosis: Common mechanisms and antifibrotic therapies. Clin. Res. Hepatol. Gastroenterol. 2015, 39 (Suppl. S1), S51–S59. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.K.; Yim, H.J. Reversal of liver cirrhosis: Current evidence and expectations. Korean J. Intern. Med. 2017, 32, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-L.; Lin, Y.-S.; Liu, K.-F.; Peng, W.-H.; Hsu, C.-M. Hepatoprotective mechanisms of taxifolin on carbon tetrachloride-induced acute liver injury in mice. Nutrients 2019, 11, 2655. [Google Scholar] [CrossRef]
- Pan, Y.; Long, X.; Yi, R.; Zhao, X. Polyphenols in Liubao Tea Can Prevent CCl4-Induced Hepatic Damage in Mice through Its Antioxidant Capacities. Nutrients 2018, 10, 1280. [Google Scholar] [CrossRef]
- Lin, T.-A.; Ke, B.-J.; Cheng, C.-S.; Wang, J.-J.; Wei, B.-L.; Lee, C.-L. Red Quinoa bran extracts protects against carbon tetrachloride-induced liver injury and fibrosis in mice via activation of antioxidative enzyme systems and blocking TGF-1 pathway. Nutrients 2019, 11, 395. [Google Scholar] [CrossRef]
- Lee, H.-Y.; Lee, G.-H.; Yoon, Y.; Chae, H.-J. R. verniciflua and E. ulmoides extract (ILF-RE) protects against chronic CCl4-Induced liver damage by enhancing antioxidation. Nutrients 2019, 11, 382. [Google Scholar] [CrossRef]
- Go, J.; Kim, J.E.; Koh, E.K.; Song, S.H.; Sung, J.E.; Lee, H.A.; Lee, Y.H.; Lim, Y.; Hong, J.T.; Hwang, D.Y. Protective effect of gallotannin-enriched extract isolated from Galla Rhois against CCl4-induced hepatotoxicity in ICR mice. Nutrients 2016, 8, 107. [Google Scholar] [CrossRef]
- Dong, D.; Yin, L.; Qi, Y.; Xu, L.; Peng, J. Protective effect of the total saponins from Rosa laevigata Michx fruit against carbon tetrachloride-induced liver fibrosis in rats. Nutrients 2015, 7, 4829–4850. [Google Scholar] [CrossRef]
- Wu, S.H.; Ryvarden, L.; Chang, T.T. Antrodia camphorata (“niu-chang-chih”), new combination of a medicinal fungus in Taiwan. Bot. Bull. Acad. Sin. 1997, 38, 273–275. [Google Scholar]
- Balusikova, K.; Kovar, J. Alcohol dehydrogenase and cytochrome P450 2E1 can be induced by long-term exposure to ethanol in cultured liver HEP-G2 cells. In Vitro Cell. Dev. Biol. Anim. 2013, 49, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Geethangili, M.; Tzeng, Y.M. Review of Pharmacological effects of Antrodia camphorata and its bioactive compounds. Evid. Based Complement. Altern. Med. 2011, 2011, 212641. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; Wang, J.; Sun, Q.; Xie, M.; Lu, Z.M.; Xu, H.Y.; Shi, J.S.; Xu, Z.H. Identification of antrodin B from Antrodia camphorata as a new anti-hepatofibrotic compound using a rapid cell screening method and biological evaluation. Hepatol. Res. 2016, 46, E15–E25. [Google Scholar] [CrossRef]
- Geng, Y.; He, Z.; Lu, Z.M.; Xu, H.Y.; Xu, G.H.; Shi, J.S.; Xu, Z.H. Antrodia camphorata ATCC 200183 sporulates asexually in submerged culture. Appl. Microbiol. Biotechnol. 2013, 97, 2851–2858. [Google Scholar] [CrossRef]
- Li, H.-X.; Lu, Z.-M.; Geng, Y.; Gong, J.-S.; Zhang, X.-J.; Shi, J.-S.; Xu, Z.-H.; Ma, Y.-H. Efficient production of bioactive metabolites from Antrodia camphorata ATCC 200183 by asexual reproduction-based repeated batch fermentation. Bioresour. Technol. 2015, 194, 334–343. [Google Scholar] [CrossRef]
- Lu, Z.-M.; He, Z.; Li, H.-X.; Gong, J.-S.; Geng, Y.; Xu, H.-Y.; Xu, G.-H.; Shi, J.-S.; Xu, Z.-H. Modified arthroconidial inoculation method for the efficient fermentation of Antrodia camphorata ATCC 200183. Biochem. Eng. J. 2014, 87, 41–49. [Google Scholar] [CrossRef]
- Schuppan, D.; Kim, Y.O. Evolving therapies for liver fibrosis. J. Clin. Investig. 2013, 123, 1887–1901. [Google Scholar] [CrossRef]
- Wang, C.C.; Zhang, W.W.; Wong, J.H.; Ng, T.; Ye, X.J. Diversity of potentially exploitable pharmacological activities of the highly prized edible medicinal fungus Antrodia camphorata. Appl. Microbiol. Biotechnol. 2019, 103, 7843–7867. [Google Scholar] [CrossRef]
- Kuang, Y.; Li, B.; Wang, Z.; Qiao, X.; Ye, M. Terpenoids from the medicinal mushroom Antrodia camphorata: Chemistry and medicinal potential. Nat. Prod. Rep. 2020. [Google Scholar] [CrossRef]
- Huo, Y.; Win, S.; Than, T.A.; Yin, S.; Ye, M.; Hu, H.; Kaplowitz, N. Antcin H protects against acute liver injury through disruption of the interaction of c-Jun-N-Terminal kinase with mitochondria. Antioxid. Redox Signal. 2017, 26, 207–220. [Google Scholar] [CrossRef]
- Li, Z.W.; Kuang, Y.; Tang, S.N.; Li, K.; Huang, Y.; Qiao, X.; Yu, S.W.; Tzeng, Y.M.; Lo, J.Y.; Ye, M. Hepatoprotective activities of Antrodia camphorata and its triterpenoid compounds against CCl(4)-induced liver injury in mice. J. Ethnopharmacol. 2017, 206, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.C.; Song, T.Y.; Shih, P.H.; Yen, G.C. Antioxidant properties of water-soluble polysaccharides from Antrodia cinnamomea in submerged culture. Food Chem. 2007, 104, 1115–1122. [Google Scholar] [CrossRef]
- Kuo, P.L.; Hsu, Y.L.; Cho, C.Y.; Ng, L.T.; Kuo, Y.H.; Lin, C.C. Apoptotic effects of Antrodia cinnamomea fruiting bodies extract are mediated through calcium and calpain-dependent pathways in Hep 3B cells. Food Chem. Toxicol. 2006, 44, 1316–1326. [Google Scholar] [CrossRef] [PubMed]
- Song, T.Y.; Yen, G.C. Protective effects of fermented filtrate from Antrodia camphorata in submerged culture against CCl4-induced hepatic toxicity in rats. J. Agric. Food Chem. 2003, 51, 1571–1577. [Google Scholar] [CrossRef]
- Hsiao, G.; Shen, M.-Y.; Lin, K.-H.; Lan, M.-H.; Wu, L.-Y.; Chou, D.-S.; Lin, C.-H.; Su, C.-H.; Sheu, J.-R. Antioxidative and hepatoprotective effects of Antrodia camphorata Extract. J. Agric. Food Chem. 2003, 51, 3302–3308. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.C.; Lin, M.T.; Duan, K.J.; Chen, Y.S. The hepatoprotective activity against ethanol-induced cytotoxicity by aqueous extract of Antrodia cinnamomea. J. Chin. Inst. Chem. Eng. 2008, 39, 441–447. [Google Scholar] [CrossRef]
- Lin, W.C.; Kuo, S.C.; Lin, W.L.; Fang, H.L.; Wang, B.C. Filtrate of fermented mycelia from Antrodia camphorata reduces liver fibrosis induced by carbon tetrachloride in rats. World J. Gastroenterol. 2006, 12, 2369–2374. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Liu, Y.; Gui, J.; Zhou, X.; Yuan, X.; Chu, Y.; Liu, H. Effects of human truncated variant of hepatocyte growth factor (tvNK1) on carbon tetrachloride-induced liver fibrosis in rat. Chin. Biotechnol. 2016, 36, 18–23. [Google Scholar]
- Bomble, M.; Tacke, F.; Rink, L.; Kovalenko, E.; Weiskirchen, R. Analysis of antigen-presenting functionality of cultured rat hepatic stellate cells and transdifferentiated myofibroblasts. Biochem. Biophys. Res. Commun. 2010, 396, 342–347. [Google Scholar] [CrossRef]
- Muhanna, N.; Abu Tair, L.; Doron, S.; Amer, J.; Azzeh, M.; Mahamid, M.; Friedman, S.; Safadi, R. Amelioration of hepatic fibrosis by NK cell activation. Gut 2011, 60, 90–98. [Google Scholar] [CrossRef]
- Wasmuth, H.E.; Tacke, F.; Trautwein, C. Chemokines in liver inflammation and fibrosis. Semin. Liver Dis. 2010, 30, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Paik, Y.H.; Schwabe, R.F.; Bataller, R.; Russo, M.P.; Jobin, C.; Brenner, D.A. Toll-like receptor 4 mediates inflammatory signaling by bacterial lipopolysaccharide in human hepatic stellate cells. Hepatology 2003, 37, 1043–1055. [Google Scholar] [CrossRef]
- Zhu, Q.; Zou, L.; Jagavelu, K.; Simonetto, D.A.; Huebert, R.C.; Jiang, Z.D.; DuPont, H.L.; Shah, V.H. Intestinal decontamination inhibits TLR4 dependent fibronectin-mediated cross-talk between stellate cells and endothelial cells in liver fibrosis in mice. J. Hepatol. 2012, 56, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.J.; Lu, C.C.; Lin, C.S.; Martel, J.; Ko, Y.F.; Ojcius, D.M.; Wu, T.R.; Tsai, Y.H.; Yeh, T.S.; Lu, J.J.; et al. Antrodia cinnamomea reduces obesity and modulates the gut microbiota in high-fat diet-fed mice. Int. J. Obes. 2018, 42, 231–243. [Google Scholar] [CrossRef] [PubMed]
| Spore (g/100 g) | Mycelia (g/100 g) | Cultural Broth (g/100 g) | |
|---|---|---|---|
| HPLC | 1.31 | 1.07 | 0.00051 |
| vanillin–acetic acid system | 1.68 | 1.59 | 0.097 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, Y.; Li, H.-X.; Zhou, L.; Lu, Z.-M.; Shi, J.; Geng, Y.; Xu, Z.-H. Protective Effect of Spore Powder of Antrodia camphorata ATCC 200183 on CCl4-Induced Liver Fibrosis in Mice. Nutrients 2020, 12, 2778. https://doi.org/10.3390/nu12092778
Ren Y, Li H-X, Zhou L, Lu Z-M, Shi J, Geng Y, Xu Z-H. Protective Effect of Spore Powder of Antrodia camphorata ATCC 200183 on CCl4-Induced Liver Fibrosis in Mice. Nutrients. 2020; 12(9):2778. https://doi.org/10.3390/nu12092778
Chicago/Turabian StyleRen, Yilin, Hua-Xiang Li, Lingxi Zhou, Zhen-Ming Lu, Jinsong Shi, Yan Geng, and Zheng-Hong Xu. 2020. "Protective Effect of Spore Powder of Antrodia camphorata ATCC 200183 on CCl4-Induced Liver Fibrosis in Mice" Nutrients 12, no. 9: 2778. https://doi.org/10.3390/nu12092778
APA StyleRen, Y., Li, H.-X., Zhou, L., Lu, Z.-M., Shi, J., Geng, Y., & Xu, Z.-H. (2020). Protective Effect of Spore Powder of Antrodia camphorata ATCC 200183 on CCl4-Induced Liver Fibrosis in Mice. Nutrients, 12(9), 2778. https://doi.org/10.3390/nu12092778







