25-Hydroxycholecalciferol Concentration Is Associated with Protein Loss and Serum Albumin Level during the Acute Phase of Burn Injury
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Vitamin D and Other Biochemical Parameters Measurements
2.3. Statistical Analysis
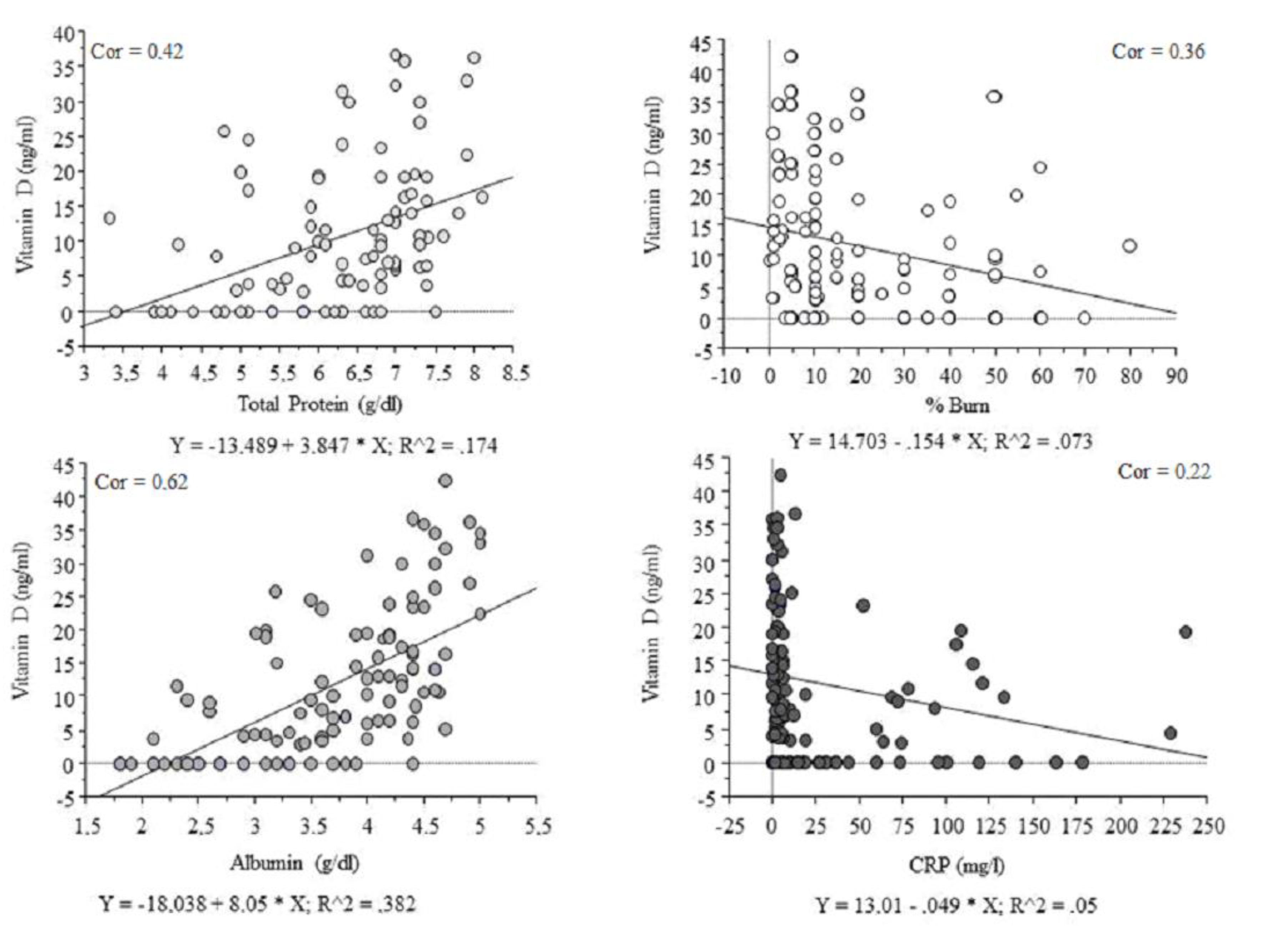
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chrzanowska-Wąsik, M.; Chemperek, E.; Sokołowski, D.; Goniewicz, M.; Badnarz, K.; Rzońca, P. Burn analysis in adult patients hospitalized in the East Centre of Burn Treatment and Reconstructive Surgery in Łęczna. J. Educ. Health Sport 2017, 7, 410–419. [Google Scholar]
- Klein, G.L. The interaction between burn injury and vitamin D metabolism and consequences for the patient. Curr. Clin. Pharmacol. 2008, 3, 204–210. [Google Scholar] [CrossRef][Green Version]
- Lips, P. Interaction between vitamin D and calcium. Scand. J. Clin. Lab. Investig. 2012, 243, 60–64. [Google Scholar]
- Klein, G.L.; Herndon, D.N.; Rutan, T.C.; Sherrard, D.J.; Coburn, J.W.; Langman, C.B.; Thomas, M.L.; Haddad, J.G., Jr.; Cooper, C.W.; Miller, N.L.; et al. Bone disease in burn patients. J. Bone Miner. Res. 1993, 8, 337–345. [Google Scholar] [CrossRef]
- Ding, J.; Kwan, P.; Ma, Z.; Iwashina, T.; Wang, J.; Shankowsky, H.A.; Tredget, E.E. Synergistic effect of vitamin D and low concentration of transforming growth factor beta 1, a potential role in dermal wound healing. Burns 2016, 42, 1277–1286. [Google Scholar] [CrossRef]
- Dickerson, R.N.; Van Cleve, J.R.; Swanson, J.M.; Maish, G.O.; Minard, G.; Croce, M.A.; Brown, R.O. Vitamin D deficiency in critically ill patients with traumatic injuries. Burns Trauma 2016, 4, 1–9. [Google Scholar] [CrossRef]
- Alizadeh, N.; Khalili, H.; Mohammadi, M.; Abdollahi, A. Serum Vitamin D levels at admission predict the length of intensive care unit stay but not in-hospital mortality of critically ill surgical patients. J. Res. Pharm. Pract. 2015, 4, 193. [Google Scholar]
- Płudowski, P.; Ducki, C.; Konstantynowicz, J.; Jaworski, M. Vitamin D status in Poland. Pol. Arch Med. Wewn 2016, 126, 530–539. [Google Scholar] [CrossRef]
- Rech, M.A.; Hidalgo, D.C.; Larson, J.; Zavala, S.; Mosier, M. Vitamin D in burn-injured patients. Burns 2019, 45, 32–41. [Google Scholar] [CrossRef]
- Rousseau, A.-F.; Losser, M.-R.; Ichai, C.; Berger, M.M. ESPEN endorsed recommendations: Nutritional therapy in major burns. Clin. Nutr. 2013, 32, 497–502. [Google Scholar] [CrossRef]
- Matyjaszek-Matuszek, B.; Lenart-Lipińska, M.; Woźniakowska, E. Clinical implications of vitamin D deficiency. Menopause Rev. 2015, 14, 75. [Google Scholar] [CrossRef] [PubMed]
- Prietl, B.; Treiber, G.; Pieber, T.R.; Amrein, K. Vitamin D and immune function. Nutrients 2013, 5, 2502–2521. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, M.G. The Hepatic Response to Thermal Injury: Is the Liver Important for Postburn Outcomes? Mol. Med. 2009, 15, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Al-Tarrah, K.; Hewison, M.; Moiemen, N.; Lord, J.M. Vitamin D status and its influence on outcomes following major burn injury and critical illness. Burns Trauma 2018, 6, s41038-018-0113-4. [Google Scholar] [CrossRef]
- Herndon, D.N.; Tompkins, R.G. Support of the metabolic response to burn injury. Lancet 2004, 363, 1895–1902. [Google Scholar] [CrossRef]
- Lagunova, Z.; Porojnicu, A.C.; Lindberg, F.; Hexeberg, S.; Moan, J. The dependency of vitamin D status on body mass index, gender, age and season. Anticancer. Res. 2009, 29, 3713–3720. [Google Scholar] [CrossRef]
- Klein, G.L. Burns: Where Has All the Calcium (and Vitamin D) Gone? Adv. Nutr. 2011, 2, 457–462. [Google Scholar] [CrossRef]
- Rousseau, A.-F.; Foidart-Desalle, M.; Ledoux, D.; Remy, C.; Croisier, J.-L.; Damas, P.; Cavalier, E. Effects of cholecalciferol supplementation and optimized calcium intakes on vitamin D status, muscle strength and bone health: A one-year pilot randomized controlled trial in adults with severe burns. Burns 2015, 41, 317–325. [Google Scholar] [CrossRef]
- Klein, G.L.; Herndon, D.N.; Chen, T.C.; Kulp, G.; Holick, M.F. Standard multivitamin supplementation does not improve vitamin D insufficiency after burns. J. Bone Miner. Metab. 2009, 27, 502–506. [Google Scholar] [CrossRef]
- Quraishi, S.A.; Camargo, C.A. Vitamin D in acute stress and critical illness. Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 625–634. [Google Scholar] [CrossRef]
- Lehnhardt, M.; Jafari, H.J.; Druecke, D.; Steinstraesser, L.; Steinau, H.U.; Klatte, W.; Homann, H.H. A qualitative and quantitative analysis of protein loss in human burn wounds. Burns 2005, 31, 159–167. [Google Scholar] [CrossRef]
- Caironi, P.; Gattinoni, L. The clinical use of albumin: The point of view of a specialist in intensive care. Blood Transfus. 2009, 7, 259–267. [Google Scholar]
- Lyons, O.; Whelan, B.; Bennett, K.; O’Riordan, D.; Silke, B. Serum albumin as an outcome predictor in hospital emergency medical admissions. Eur. J. Intern. Med. 2010, 21, 17–20. [Google Scholar] [CrossRef]
- Pérez-Guisado, J.; de Haro-Padilla, J.M.; Rioja, L.F.; DeRosier, L.C.; de la Torre, J.I. Serum albumin levels in burn people are associated to the total body surface burned and the length of hospital stay but not to the initiation of the oral/enteral nutrition. Int. J. Burn. Trauma 2013, 3, 159–163. [Google Scholar]
- Robinson, R. Low serum albumin and total lymphocyte count as predictors of 30 day hospital readmission in patients 65 years of age or older. PeerJ 2015, 3, e1181. [Google Scholar] [CrossRef]
- Bikle, D.; Bouillon, R.; Thadhani, R.; Schoenmakers, I. Vitamin D metabolites in captivity? Should we measure free or total 25(OH)D to assess vitamin D status? J. Steroid Biochem. Mol. Biol. 2017, 173, 105–116. [Google Scholar] [CrossRef]
- Yonemura, K.; Fujimoto, T.; Fujigaki, Y.; Hishida, A. Vitamin D deficiency is implicated in reduced serum albumin concentrations in patients with end-stage renal disease. Am. J. Kidney Dis. 2000, 36, 337–344. [Google Scholar] [CrossRef]
- Klein, G.L.; Chen, T.C.; Holick, M.F.; Langman, C.B.; Price, H.; Celis, M.M.; Herndon, D.N. Synthesis of vitamin D in skin after burns. Lancet 2004, 363, 291–292. [Google Scholar] [CrossRef]
| Burn Degree | Amount of Patients (n = 126) |
|---|---|
| I/II | 9 (7%) |
| II | 13 (10.3%) |
| II/III | 61 (48.5%) |
| III | 35 (27.8%) |
| III/IV | 7 (5.6%) |
| IV | 1 (0.8%) |
| Patient Characteristics | Mean | ±SD |
|---|---|---|
| Age [years] | 49.06 | 17.53 |
| BMI [kg/m2] | 24.59 | 3.76 |
| Percentage of body burns [%] | 21.11 | 20.80 |
| Day after burn [day] | 1.66 | 5.06 |
| Phosphate [mmol/L] | 1.18 | 0.22 |
| Calcium [mmol/L] | 2.29 | 0.26 |
| 25-Hydroxycholecalciferol Concentration [ng/mL] | Mean | ±SD |
|---|---|---|
| Whole cohort | 11.6 | 10.7 |
| Superficial | 18.2 | 13.5 |
| Superficial partial thickness | 13.08 | 9.9 |
| Superficial deep dermal | 11.7 | 13.4 |
| Full thickness | 12.8 | 11.7 |
| Full thickness with catastrophic | 8.45 | 8.3 |
| Catastrophic | 7.4 | 6.2 |
| 25-Hydroxycholecalciferol [ng/mL] vs., n = 126 | r | p Value | FDR * p Value |
|---|---|---|---|
| BMI [kg/m2] | 0.18 | NS | 0.43 |
| Total protein [g/dL] | 0.42 | p < 0.01 | p < 0.01 |
| Albumin [g/dL] | 0.62 | p < 0.01 | p < 0.01 |
| Percentage of body burns [%] | (−) 0.36 | p < 0.05 | 0.10 |
| AST [U/L] | (−) 0.21 | p < 0.05 | 0.07 |
| ALT [U/L] | (−) 0.08 | NS | 0.43 |
| CRP [mg/L] | (−) 0.22 | p < 0.05 | 0.07 |
| IL-6 [pg/mL] | (−) 0.16 | NS | 0.20 |
| Creatinine [mg/dL] | (−) 0.18 | p < 0.055 # | 0.10 |
| Percentage of Body Burns [%] | r | p Value |
|---|---|---|
| Total protein [g/dL] | (−) 0.58 | p < 0.01 |
| Albumin [g/dL] | (−) 0.62 | p < 0.01 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krajewski, A.; Piorun, K.; Maciejewska-Markiewicz, D.; Markowska, M.; Skonieczna-Żydecka, K.; Stachowska, E.; Polakowska, Z.; Mazurek, M.; Szczuko, M. 25-Hydroxycholecalciferol Concentration Is Associated with Protein Loss and Serum Albumin Level during the Acute Phase of Burn Injury. Nutrients 2020, 12, 2780. https://doi.org/10.3390/nu12092780
Krajewski A, Piorun K, Maciejewska-Markiewicz D, Markowska M, Skonieczna-Żydecka K, Stachowska E, Polakowska Z, Mazurek M, Szczuko M. 25-Hydroxycholecalciferol Concentration Is Associated with Protein Loss and Serum Albumin Level during the Acute Phase of Burn Injury. Nutrients. 2020; 12(9):2780. https://doi.org/10.3390/nu12092780
Chicago/Turabian StyleKrajewski, Andrzej, Krzysztof Piorun, Dominika Maciejewska-Markiewicz, Marta Markowska, Karolina Skonieczna-Żydecka, Ewa Stachowska, Zofia Polakowska, Maciej Mazurek, and Małgorzata Szczuko. 2020. "25-Hydroxycholecalciferol Concentration Is Associated with Protein Loss and Serum Albumin Level during the Acute Phase of Burn Injury" Nutrients 12, no. 9: 2780. https://doi.org/10.3390/nu12092780
APA StyleKrajewski, A., Piorun, K., Maciejewska-Markiewicz, D., Markowska, M., Skonieczna-Żydecka, K., Stachowska, E., Polakowska, Z., Mazurek, M., & Szczuko, M. (2020). 25-Hydroxycholecalciferol Concentration Is Associated with Protein Loss and Serum Albumin Level during the Acute Phase of Burn Injury. Nutrients, 12(9), 2780. https://doi.org/10.3390/nu12092780








