Current State of Evidence: Influence of Nutritional and Nutrigenetic Factors on Immunity in the COVID-19 Pandemic Framework
Abstract
1. Introduction
2. Background
2.1. The European Framework of Essential Micronutrient Requirements
2.2. Factors Affecting Bioavailability of Immune Related Micronutrients
2.2.1. Genetic Factors
2.2.2. Air Pollution, Food Mixture Interaction, and Other Factors
3. Materials and Methods
3.1. Information Sources
3.1.1. Literature Search Criteria for the 10 Nutrients, Immunity, and COVID-19
3.1.2. COVID-19 Epidemiological Indicators
3.1.3. Population Nutrition Data and Genetic Risk Assessment
3.1.4. Correlation Map Performance
4. Association between 10 Critical Micronutrients and Prevalence of COVID-19 in Europe
4.1. Vitamin D
4.2. Vitamin A
4.3. Vitamin C
4.4. Folate
4.5. Vitamin B6
4.6. Vitamin B12
4.7. Zinc
4.8. Iron
4.9. Copper
4.10. Selenium
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization WHO. Coronavirus Disease (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 10 June 2020).
- Gombart, A.F.; Pierre, A.; Maggini, S. A Review of micronutrients and the immune system–Working in harmony to reduce the risk of infection. Nutrients 2020, 12, 236. [Google Scholar] [CrossRef] [PubMed]
- Panel, E.; Nda, A. Scientific opinion on the substantiation of a health claim related to vitamin D and contribution to the normal function of the immune system pursuant to Article 14 of Regulation (EC) No 1924/2006. EFSA J. 2015, 13, 1–7. [Google Scholar] [CrossRef][Green Version]
- Panel, E.; Nda, A. Scientific Opinion on the substantiation of health claims related to vitamin D and normal function of the immune system and inflammatory response (ID 154, 159), maintenance of normal muscle function (ID 155) and maintenance of normal cardiovascular functi. EFSA J. 2010, 8, 1–17. [Google Scholar] [CrossRef]
- Opinion, S. Scientific opinion on the substantiation of health claims related to vitamin B6 and protein and glycogen metabolism (ID 65, 70, 71), function of the nervous system (ID 66), red blood cell formation (ID 67, 72, 186), function of the immune system (ID 68). EFSA J. 2009, 7, 1225. [Google Scholar] [CrossRef]
- Opinion, S. Scientific opinion on the substantiation of health claims related to zinc and function of the immune system (ID 291, 1757), DNA synthesis and cell division (ID 292, 1759), protection of DNA, proteins and lipids from oxidative damage (ID 294, 1758), mainte. EFSA J. 2009, 7, 1229. [Google Scholar] [CrossRef]
- Opinion, S. Scientific opinion on the substantiation of health claims related to copper and protection of DNA, proteins and lipids from oxidative damage (ID 263, 1726), function of the immune system (ID 264), maintenance of connective tissues (ID 265, 271, 1722), ene. EFSA J. 2009, 7, 1211. [Google Scholar] [CrossRef]
- Panel, E.; Nda, A. Scientific opinion on the substantiation of health claims related to copper and reduction of tiredness and fatigue (ID 272), maintenance of the normal function of the nervous system (ID 1723), maintenance of the normal function of the immune system (ID 17). EFSA J. 2011, 9, 2079. [Google Scholar] [CrossRef]
- Panel, E.; Nda, A. Scientific opinion on the substantiation of health claims related to iron and formation of red blood cells and haemoglobin (ID 249, ID 1589), oxygen transport (ID 250, ID 254, ID 256), energy-yielding metabolism (ID 251, ID 1589), function of the immune s. EFSA J. 2009, 7, 1215. [Google Scholar] [CrossRef]
- Opinion, S. Scientific opinion on the substantiation of health claims related to selenium and protection of DNA, proteins and lipids from oxidative damage (ID 277, 283, 286, 1289, 1290, 1291, 1293, 1751), function of the immune system (ID 278), thyroid function (ID 2). EFSA J. 2009, 7, 1220. [Google Scholar] [CrossRef]
- Kaur, B.; Henry, J. Micronutrient Status in Type 2 Diabetes: A review, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2014; Volume 71, ISBN 9780128002704. [Google Scholar]
- Sobczak, A.I.S.; Stefanowicz, F.; Pitt, S.J.; Ajjan, R.A.; Stewart, A.J. Total plasma magnesium, zinc, copper and selenium concentrations in type-I and type-II diabetes. BioMetals 2019, 32, 123–138. [Google Scholar] [CrossRef]
- Farhat, G.; Lees, E.; Macdonald-Clarke, C.; Amirabdollahian, F. Inadequacies of micronutrient intake in normal weight and overweight young adults aged 18–25 years: A cross-sectional study. Public Health 2019, 167, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Thomas-Valdés, S.; Tostes, M.d.G.V.; Anunciação, P.C.; da Silva, B.P.; Sant’Ana, H.M.P. Association between vitamin deficiency and metabolic disorders related to obesity. Crit. Rev. Food Sci. Nutr. 2017, 57, 3332–3343. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA). DRV Finder. Available online: https://www.efsa.europa.eu/en/interactive-pages/drvs (accessed on 21 July 2020).
- Moy, K.A.; Mondul, A.M.; Zhang, H.; Weinstein, S.J.; Wheeler, W.; Chung, C.C.; Männistö, S.; Yu, K.; Chanock, S.J.; Albanes, D. Genome-wide association study of circulating vitamin D-binding protein. Am. J. Clin. Nutr. 2014, 99, 1424–1431. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Hatchell, K.E.; Bradfield, J.P.; Bjonnes, A.; Chesi, A.; Lai, C.Q.; Langefeld, C.D.; Lu, L.; Lu, Y.; Lutsey, P.L.; et al. Transethnic Evaluation Identifies Low-Frequency Loci Associated with 25-Hydroxyvitamin D Concentrations. J. Clin. Endocrinol. Metab. 2018, 103, 1380–1392. [Google Scholar] [CrossRef]
- Kämpe, A.; Enlund-Cerullo, M.; Valkama, S.; Holmlund-Suila, E.; Rosendahl, J.; Hauta-Alus, H.; Pekkinen, M.; Andersson, S.; Mäkitie, O. Genetic variation in GC and CYP2R1 affects 25-hydroxyvitamin D concentration and skeletal parameters: A genome-wide association study in 24-month-old Finnish children. PLoS Genet. 2019, 15, 1–22. [Google Scholar] [CrossRef]
- Wang, T.J.; Zhang, F.; Richards, J.B.; Kestenbaum, B.; van Meurs, J.B.; Berry, D.; Kiel, D.P.; Streeten, E.A.; Ohlsson, C.; Koller, D.L.; et al. Common genetic determinants of vitamin D insufficiency: A genome-wide association study. Lancet 2010, 376, 180–188. [Google Scholar] [CrossRef]
- Ferrucci, L.; Perry, J.R.B.; Matteini, A.; Perola, M.; Tanaka, T.; Silander, K.; Rice, N.; Melzer, D.; Murray, A.; Cluett, C.; et al. Common variation in the β-carotene 15,15′-monooxygenase 1 gene affects circulating levels of carotenoids: A genome-wide association study. Am. J. Hum. Genet. 2008, 84, 123–133. [Google Scholar] [CrossRef]
- Kobylecki, C.J.; Afzal, S.; Nordestgaard, B.G. Genetically high plasma vitamin C and urate: A Mendelian randomization study in 106 147 individuals from the general population. Rheumatology 2018, 57, 1769–1776. [Google Scholar] [CrossRef]
- Shane, B.; Pangilinan, F.; Mills, J.L.; Fan, R.; Gong, T.; Cropp, C.D.; Kim, Y.; Ueland, P.M.; Bailey-Wilson, J.E.; Wilson, A.F.; et al. The 677C→T variant of MTHFR is the major genetic modifier of biomarkers of folate status in a young, healthy Irish population. Am. J. Clin. Nutr. 2018, 108, 1334–1341. [Google Scholar] [CrossRef]
- Tanaka, T.; Scheet, P.; Giusti, B.; Bandinelli, S.; Piras, M.G.; Usala, G.; Lai, S.; Mulas, A.; Corsi, A.M.; Vestrini, A.; et al. Genome-wide Association Study of Vitamin B6, Vitamin B12, Folate, and Homocysteine Blood Concentrations. Am. J. Hum. Genet. 2009, 84, 477–482. [Google Scholar] [CrossRef]
- Nongmaithem, S.S.; Joglekar, C.V.; Krishnaveni, G.V.; Sahariah, S.A.; Ahmad, M.; Ramachandran, S.; Gandhi, M.; Chopra, H.; Pandit, A.; Potdar, R.D.; et al. GWAS identifies population-specific new regulatory variants in FUT6 associated with plasma B12 concentrations in Indians. Hum. Mol. Genet. 2017, 26, 2551–2564. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.M.; Zhu, G.; Dy, V.; Heath, A.C.; Madden, P.A.F.; Kemp, J.P.; McMahon, G.; Pourcain, B.S.; Timpson, N.J.; Golding, J.; et al. Genome-wide association study identifies loci affecting blood copper, selenium and zinc. Hum. Mol. Genet. 2013, 22, 3998–4006. [Google Scholar] [CrossRef] [PubMed]
- McLaren, C.E.; Garner, C.P.; Constantine, C.C.; McLachlan, S.; Vulpe, C.D.; Snively, B.M.; Gordeuk, V.R.; Nickerson, D.A.; Cook, J.D.; Leiendecker-Foster, C.; et al. Genome-wide association study identifies genetic loci associated with iron deficiency. PLoS ONE 2011, 6. [Google Scholar] [CrossRef]
- Benyamin, B.; Esko, T.; Ried, J.S.; Radhakrishnan, A.; Vermeulen, S.H.; Traglia, M.; Gögele, M.; Anderson, D.; Broer, L.; Podmore, C.; et al. Novel loci affecting iron homeostasis and their effects in individuals at risk for hemochromatosis. Nat. Commun. 2014, 5, 4926. [Google Scholar] [CrossRef] [PubMed]
- Raffield, L.M.; Louie, T.; Sofer, T.; Jain, D.; Ipp, E.; Taylor, K.D.; Papanicolaou, G.J.; Avilés-Santa, L.; Lange, L.A.; Laurie, C.C.; et al. Genome-wide association study of iron traits and relation to diabetes in the Hispanic Community Health Study/Study of Latinos (HCHS/SOL): Potential genomic intersection of iron and glucose regulation? Hum. Mol. Genet. 2017, 26, 1966–1978. [Google Scholar] [CrossRef] [PubMed]
- Pichler, I.; Minelli, C.; Sanna, S.; Tanaka, T.; Schwienbacher, C.; Naitza, S.; Porcu, E.; Pattaro, C.; Busonero, F.; Zanon, A.; et al. Identification of a common variant in the TFR2 gene implicated in the physiological regulation of serum iron levels. Hum. Mol. Genet. 2011, 20, 1232–1240. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Hsu, L.; Harrison, T.; King, I.; Stürup, S.; Song, X.; Duggan, D.; Liu, Y.; Hutter, C.; Chanock, S.; et al. Genome-Wide Association Study of Serum Selenium Concentrations. Nutrients 2013, 5, 1706–1718. [Google Scholar] [CrossRef]
- Cornelis, M.C.; Fornage, M.; Foy, M.; Xun, P.; Gladyshev, V.N.; Morris, S.; Chasman, D.I.; Hu, F.B.; Rimm, E.B.; Kraft, P.; et al. Genome-wide association study of selenium concentrations. Hum. Mol. Genet. 2015, 24, 1469–1477. [Google Scholar] [CrossRef]
- NDA. EFSA Panel on Dietetic Products, N. and A. Scientific opinion on principles for deriving and applying dietary reference values. EFSA J. 2010, 8. [Google Scholar] [CrossRef]
- NDA. EFSA Panel on Dietetic Products, N. and A. Dietary reference values for vitamin D. EFSA J. 2016, 14, e04547. [Google Scholar] [CrossRef]
- Rousseau, S.; Kyomugasho, C.; Celus, M.; Hendrickx, M.E.G.; Grauwet, T. Barriers impairing mineral bioaccessibility and bioavailability in plant-based foods and the perspectives for food processing. Crit. Rev. Food Sci. Nutr. 2020, 60, 826–843. [Google Scholar] [CrossRef] [PubMed]
- Panel, E.; Nda, A. Scientific opinion on dietary reference values for iron. EFSA J. 2015, 13, 4254. [Google Scholar] [CrossRef]
- Sobiecki, J.G.; Appleby, P.N.; Bradbury, K.E.; Key, T.J. High compliance with dietary recommendations in a cohort of meat eaters, fish eaters, vegetarians, and vegans: Results from the European prospective investigation into cancer and Nutrition-Oxford study. Nutr. Res. 2016, 36, 464–477. [Google Scholar] [CrossRef] [PubMed]
- Rippin, H.; Hutchinson, J.; Jewell, J.; Breda, J.; Cade, J. Adult nutrient intakes from current national dietary surveys of European populations. Nutrients 2017, 9, 1288. [Google Scholar] [CrossRef]
- Mensink, G.B.M.; Fletcher, R.; Gurinovic, M.; Huybrechts, I.; Lafay, L.; Serra-Majem, L.; Szponar, L.; Tetens, I.; Verkaik-Kloosterman, J.; Baka, A.; et al. Mapping low intake of micronutrients across Europe. Br. J. Nutr. 2013, 110, 755–773. [Google Scholar] [CrossRef] [PubMed]
- Moyersoen, I.; Devleesschauwer, B.; Dekkers, A.; de Ridder, K.; Tafforeau, J.; van Camp, J.; van Oyen, H.; Lachat, C. Intake of fat-soluble vitamins in the belgian population: Adequacy and contribution of foods, fortified foods and supplements. Nutrients 2017, 9. [Google Scholar] [CrossRef]
- Vural, Z.; Avery, A.; Kalogiros, D.I.; Coneyworth, L.J.; Welham, S.J.M. Trace mineral intake and deficiencies in older adults living in the community and institutions: A systematic review. Nutrients 2020, 12. [Google Scholar] [CrossRef]
- Buniello, A.; MacArthur, J.A.L.; Cerezo, M.; Harris, L.W.; Hayhurst, J.; Malangone, C.; McMahon, A.; Morales, J.; Mountjoy, E.; Sollis, E.; et al. The NHGRI-EBI GWAS Catalog of published genome-wide association studies, targeted arrays and summary statistics 2019. Nucleic Acids Res. 2019, 47, D1005–D1012. [Google Scholar] [CrossRef] [PubMed]
- Auton, A.; Abecasis, G.; Altshuler, D.M.; Durbin, R.M. A global reference for human genetic variation. Nature 2015, 526, 68–74. [Google Scholar] [CrossRef]
- STHDA—Home. Available online: http://www.sthda.com/english/ (accessed on 2 July 2020).
- Sassi, F.; Tamone, C.; D’Amelio, P. Vitamin D: Nutrient, hormone, and immunomodulator. Nutrients 2018, 10, 1656. [Google Scholar] [CrossRef]
- Kehoe, L.; Walton, J.; Flynn, A. Nutritional challenges for older adults in Europe: Current status and future directions. Proc. Nutr. Soc. 2019, 78, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B.; Lahore, H.; McDonnell, S.L.; Baggerly, C.A.; French, C.B.; Aliano, J.L.; Bhattoa, H.P. Evidence that vitamin d supplementation could reduce risk of influenza and covid-19 infections and deaths. Nutrients 2020, 12, 988. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Ilie, P.C.; Stefanescu, S.; Smith, L. The role of vitamin D in the prevention of coronavirus disease 2019 infection and mortality. Aging Clin. Exp. Res. 2020, 32, 1195–1198. [Google Scholar] [CrossRef]
- Ruan, Q.; Yang, K.; Wang, W.; Jiang, L.; Song, J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensiv. Care Med. 2020, 46, 846–848. [Google Scholar] [CrossRef]
- Alipio, M. Vitamin D supplementation could possibly improve clinical outcomes of patients infected with Coronavirus-2019 (COVID-2019). SSRN Electron. J. 2020. [Google Scholar] [CrossRef]
- Daneshkhah, A.; Eshein, A.; Subramanian, H.; Roy, H.K.; Backman, V. The role of vitamin D in suppressing cytokine storm in COVID-19 patients and associated mortality. medRxiv 2020. [Google Scholar] [CrossRef]
- Bertoldi, G.; Gianesello, L.; Calò, L.A. ACE2, Rho kinase inhibition and the potential role of vitamin D against COVID-19. Aliment. Pharmacol. Ther. 2020, 52, 577–578. [Google Scholar] [CrossRef]
- Xu, J.; Yang, J.; Chen, J.; Luo, Q.; Zhang, Q.; Zhang, H. Vitamin D alleviates lipopolysaccharide-induced acute lung injury via regulation of the renin-angiotensin system. Mol. Med. Rep. 2017, 16, 7432–7438. [Google Scholar] [CrossRef]
- Hadizadeh, F. Supplementation with vitamin D in the COVID-19 pandemic? Nutr. Rev. 2020, 1–9. [Google Scholar] [CrossRef]
- Amrein, K.; Scherkl, M.; Hoffmann, M.; Neuwersch-Sommeregger, S.; Köstenberger, M.; Tmava Berisha, A.; Martucci, G.; Pilz, S.; Malle, O. Vitamin D deficiency 2.0: An update on the current status worldwide. Eur. J. Clin. Nutr. 2020. [Google Scholar] [CrossRef] [PubMed]
- Hastie, C.E.; Mackay, D.F.; Ho, F.; Celis-Morales, C.A.; Katikireddi, S.V.; Niedzwiedz, C.L.; Jani, B.D.; Welsh, P.; Mair, F.S.; Gray, S.R.; et al. Vitamin D concentrations and COVID-19 infection in UK Biobank. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 561–565. [Google Scholar] [CrossRef] [PubMed]
- Weir, E.K.; Thenappan, T.; Bhargava, M.; Chen, Y. Does vitamin D deficiency increase the severity of COVID-19? Clin. Med. 2020. [Google Scholar] [CrossRef] [PubMed]
- Berry, D.J.; Vimaleswaran, K.S.; Whittaker, J.C.; Hingorani, A.D.; Hyppönen, E. Evaluation of genetic markers as instruments for Mendelian randomization studies on vitamin D. PLoS ONE 2012, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; O’Reilly, P.F.; Aschard, H.; Hsu, Y.H.; Richards, J.B.; Dupuis, J.; Ingelsson, E.; Karasik, D.; Pilz, S.; Berry, D.; et al. Genome-wide association study in 79,366 European-ancestry individuals informs the genetic architecture of 25-hydroxyvitamin D levels. Nat. Commun. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Bonet, M.L.; Ribot, J.; Galmés, S.; Serra, F.; Palou, A. Carotenoids and carotenoid conversion products in adipose tissue biology and obesity: Pre-clinical and human studies. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 158676. [Google Scholar] [CrossRef]
- Chew, B.P. Role of carotenoids in the immune response. J. Dairy Sci. 1993, 76, 2804–2811. [Google Scholar] [CrossRef]
- Toti, E.; Oliver Chen, C.Y.; Palmery, M.; Valencia, D.V.; Peluso, I. Non-provitamin A and provitamin A carotenoids as immunomodulators: Recommended dietary allowance, therapeutic index, or personalized nutrition? Oxid. Med. Cell. Longev. 2018, 2018. [Google Scholar] [CrossRef]
- Maggini, S.; Wintergerst, E.S.; Beveridge, S.; Hornig, D.H. Selected vitamins and trace elements support immune function by strengthening epithelial barriers and cellular and humoral immune responses. Br. J. Nutr. 2007, 98, S29–S35. [Google Scholar] [CrossRef]
- García, O.P. Micronutrients, immunology and inflammation: Effect of vitamin A deficiency on the immune response in obesity. Proc. Nutr. Soc. 2012, 71, 290–297. [Google Scholar] [CrossRef]
- Kańtoch, M.; Litwińska, B.; Szkoda, M.; Siennicka, J. Importance of vitamin A deficiency in pathology and immunology of viral infections. Rocz. Panstw. Zakl. Hig. 2002, 53, 385–392. [Google Scholar] [PubMed]
- Semba, R.D. Vitamin A and immunity to viral, bacterial and protozoan infections. Proc. Nutr. Soc. 1999, 58, 719–727. [Google Scholar] [CrossRef]
- Villamor, E.; Mbise, R.; Spiegelman, D.; Hertzmark, E.; Fataki, M.; Peterson, K.E.; Ndossi, G.; Fawzi, W.W. Vitamin A supplements ameliorate the adverse effect of HIV-1, malaria, and diarrheal infections on child growth. Pediatrics 2002, 109, e6. [Google Scholar] [CrossRef] [PubMed]
- Stockman, L.J.; Bellamy, R.; Garner, P. SARS: Systematic review of treatment effects. PLoS Med. 2006, 3, e343. [Google Scholar] [CrossRef] [PubMed]
- Trasino, S.E. A role for retinoids in the treatment of COVID-19? Clin. Exp. Pharmacol. Physiol. 2020, 47, 1765–1767. [Google Scholar] [CrossRef]
- Chen, K.-H.; Wang, S.-F.; Wang, S.-Y.; Yang, Y.-P.; Wang, M.-L.; Chiou, S.-H.; Chang, Y.-L. Pharmacological development of the potential adjuvant therapeutic agents against coronavirus disease 2019. J. Chin. Med. Assoc. 2020. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Dietary reference values: Vitamin A advice published | European Food Safety Authority. Available online: https://www.efsa.europa.eu/en/press/news/150305 (accessed on 12 June 2017).
- D’Adamo, C.R.; D’Urso, A.; Ryan, K.A.; Yerges-Armstrong, L.M.; Semba, R.D.; Steinle, N.I.; Mitchell, B.D.; Shuldiner, A.R.; McArdle, P.F. A common variant in the SETD7 gene predicts serum lycopene concentrations. Nutrients 2016, 8, 82. [Google Scholar] [CrossRef]
- D’Adamo, C.R.; Dawson, V.J.; Ryan, K.A.; Yerges-Armstrong, L.M.; Semba, R.D.; Steinle, N.I.; Mitchell, B.D.; Shuldiner, A.R.; McArdle, P.F. The CAPN2/CAPN8 locus on chromosome 1q is associated with variation in serum alpha-carotene concentrations. J. Nutr. Nutr. 2017, 9, 254–264. [Google Scholar] [CrossRef]
- Mondul, A.M.; Yu, K.; Wheeler, W.; Zhang, H.; Weinstein, S.J.; Major, J.M.; Cornelis, M.C.; Männistö, S.; Hazra, A.; Hsing, A.W.; et al. Genome-wide association study of circulating retinol levels. Hum. Mol. Genet. 2011. [Google Scholar] [CrossRef]
- Goodman, D.S.; Huang, H.S.; Shiratori, T. Mechanism of the biosynthesis of vitamin A from beta-carotene. J. Biol. Chem. 1966, 241, 1929–1932. [Google Scholar]
- Wyss, A.; Wirtz, G.M.; Woggon, W.D.; Brugger, R.; Wyss, M.; Friedlein, A.; Riss, G.; Bachmann, H.; Hunziker, W. Expression pattern and localization of beta,beta-carotene 15,15′-dioxygenase in different tissues. Biochem. J. 2001, 354, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Lietz, G.; Oxley, A.; Leung, W.; Hesketh, J. Single nucleotide polymorphisms upstream from the β-carotene 15,15′-monoxygenase gene influence provitamin a conversion efficiency in female volunteers. J. Nutr. 2012, 142, 161S–165S. [Google Scholar] [CrossRef] [PubMed]
- Hemilä, H. Vitamin C and infections. Nutrients 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Hemilä, H. Vitamin C intake and susceptibility to pneumonia. Pediatr. Infect. Dis. J. 1997, 16, 836–837. [Google Scholar] [CrossRef] [PubMed]
- Atherton, J.G.; Kratzing, C.C.; Fisher, A. The effect of ascorbic acid on infection of chick-embryo ciliated tracheal organ cultures by coronavirus. Arch. Virol. 1978, 56, 195–199. [Google Scholar] [CrossRef]
- Simonson, W. Vitamin C and coronavirus. Geriatr. Nurs. 2020, 41, 331–332. [Google Scholar] [CrossRef]
- Carr, A.C. Vitamin C administration in the critically ill: A summary of recent meta-analyses. Crit. Care 2019, 23, 265. [Google Scholar] [CrossRef]
- Fowler, A.A.; Truwit, J.D.; Hite, R.D.; Morris, P.E.; DeWilde, C.; Priday, A.; Fisher, B.; Thacker, L.R.; Natarajan, R.; Brophy, D.F.; et al. Effect of vitamin C infusion on organ failure and biomarkers of inflammation and vascular injury in patients with sepsis and severe acute respiratory failure. JAMA 2019, 322, 1261. [Google Scholar] [CrossRef]
- Hiedra, R.; Lo, K.B.; Elbashabsheh, M.; Gul, F.; Wright, R.M.; Albano, J.; Azmaiprashvili, Z.; Patarroyo Aponte, G. The use of IV vitamin C for patients with COVID-19: A single center observational study. Expert Rev. Anti. Infect. Ther. 2020. [Google Scholar] [CrossRef]
- Carr, A.C. A new clinical trial to test high-dose vitamin C in patients with COVID-19. Crit. Care 2020, 24, 1–2. [Google Scholar] [CrossRef]
- Boretti, A.; Banik, B.K. Intravenous vitamin C for reduction of cytokines storm in acute respiratory distress syndrome. PharmaNutrition 2020, 12, 100190. [Google Scholar] [CrossRef] [PubMed]
- Panel, E.; Nda, A. Scientific opinion on dietary reference values for vitamin C 1. EFSA J. 2013, 11, 1–68. [Google Scholar] [CrossRef]
- Timpson, N.J.; Forouhi, N.G.; Brion, M.J.; Harbord, R.M.; Cook, D.G.; Johnson, P.; McConnachie, A.; Morris, R.W.; Rodriguez, S.; Luan, J. Erratum: Genetic variation at the SLC23A1 locus is associated with circulating concentrations of L-ascorbic acid (vitamin C): Evidence from 5 independent studies with > 15,000 participant (American Journal of Clinical Nutrition (2010) 92 (375–382)). Am. J. Clin. Nutr. 2013, 98, 253–254. [Google Scholar] [CrossRef]
- May, J.M. The SLC23 family of ascorbate transporters: Ensuring that you get and keep your daily dose of vitamin C. Br. J. Pharmacol. 2011, 164, 1793–1801. [Google Scholar] [CrossRef]
- Ducker, G.S.; Rabinowitz, J.D. One-Carbon metabolism in health and disease. Cell Metab. 2017, 25, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Saeed, F.; Nadeem, M.; Ahmed, R.S.; Tahir Nadeem, M.; Arshad, M.S.; Ullah, A. Studying the impact of nutritional immunology underlying the modulation of immune responses by nutritional compounds—A review. Food Agric. Immunol. 2016, 27, 205–229. [Google Scholar] [CrossRef]
- Troen, A.M.; Mitchell, B.; Sorensen, B.; Wener, M.H.; Johnston, A.; Wood, B.; Selhub, J.; McTiernan, A.; Yasui, Y.; Oral, E.; et al. Unmetabolized folic acid in plasma is associated with reduced natural killer cell cytotoxicity among postmenopausal women. J. Nutr. 2006, 136, 189–194. [Google Scholar] [CrossRef]
- Sheybani, Z.; Dokoohaki, M.H.; Negahdaripour, M.; Dehdashti, M.; Zolghadr, H.; Moghadami, M.; Masoom Masoompour, S.; Zolghadr, A.R. The Role of Folic Acid in the Management of Respiratory Disease Caused by COVID-19. ChemRxiv 2020, 12034980. [Google Scholar] [CrossRef]
- Singh, Y.; Gupta, G.; Kazmi, I.; Al-Abbasi, F.A.; Negi, P.; Chellappan, D.; Dua, K. SARS CoV-2 aggravates cellular metabolism mediated complications in COVID-19 infection. Dermatol. Ther. 2020, e13871. [Google Scholar] [CrossRef]
- Panel, E.; Nda, A. Scientific opinion on dietary reference values for folate. EFSA J. 2014, 12, 1–76. [Google Scholar] [CrossRef]
- Hiraoka, M.; Kagawa, Y. Genetic polymorphisms and folate status. Congenit. Anom. 2017, 57, 142–149. [Google Scholar] [CrossRef] [PubMed]
- van Meurs, J.B.J.; Pare, G.; Schwartz, S.M.; Hazra, A.; Tanaka, T.; Vermeulen, S.H.; Cotlarciuc, I.; Yuan, X.; Mälarstig, A.; Bandinelli, S.; et al. Common genetic loci influencing plasma homocysteine concentrations and their effect on risk of coronary artery disease. Am. J. Clin. Nutr. 2013, 98, 668–676. [Google Scholar] [CrossRef] [PubMed]
- Baum, L.; Wong, K.S.; Ng, H.K.; Tomlinson, B.; Rainer, T.H.; Chan, D.K.Y.; Thomas, G.N.; Chen, X.; Poon, P.; Cheung, W.S.; et al. Methylenetetrahydrofolate reductase gene A222V polymorphism and risk of ischemic stroke. Clin. Chem. Lab. Med. 2004, 42, 1370–1376. [Google Scholar] [CrossRef]
- Maggini, S. Feeding the immune system: The role of micronutrients in restoring resistance to infections. CAB Rev. Perspect. Agric. Vet. Sci. Nutr. Nat. Resour. 2008, 3. [Google Scholar] [CrossRef]
- Axelrod, A.E. Role of the B vitamins in the immune response. In Diet and Resistance to Disease; Springer: Boston, MA, USA, 1981; pp. 93–106. [Google Scholar]
- Wishart, K. Increased micronutrient requirements during physiologically demanding situations: Review of the current evidence. Vitam. Miner. 2017, 6, 1–16. [Google Scholar] [CrossRef]
- EFSA. Dietary Reference Values for vitamin B6. EFSA J. 2016, 14. [Google Scholar] [CrossRef]
- Kandeel, M.; Al-Nazawi, M. Virtual screening and repurposing of FDA approved drugs against COVID-19 main protease. Life Sci. 2020, 251, 117627. [Google Scholar] [CrossRef]
- Hilgenfeld, R. From SARS to MERS: Crystallographic studies on coronaviral proteases enable antiviral drug design. FEBS J. 2014, 281, 4085–4096. [Google Scholar] [CrossRef]
- Opinion, S. Scientific opinion on dietary reference values for cobalamin (vitamin B12). EFSA J. 2015, 13, 1–64. [Google Scholar] [CrossRef]
- Topilski, I.; Flaishon, L.; Naveh, Y.; Harmelin, A.; Levo, Y.; Shachar, I. The anti-inflammatory effects of 1,25-dihydroxyvitamin D3 on Th2 cellsin vivo are due in part to the control of integrin-mediated T lymphocyte homing. Eur. J. Immunol. 2004, 34, 1068–1076. [Google Scholar] [CrossRef]
- de Almeida Brasiel, P.G. The key role of zinc in elderly immunity: A possible approach in the COVID-19 crisis. Clin. Nutr. ESPEN 2020, 38, 65–66. [Google Scholar] [CrossRef] [PubMed]
- Tuerk, M.J.; Fazel, N. Zinc deficiency. Curr. Opin. Gastroenterol. 2009, 25, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Te Velthuis, A.J.W.; van den Worm, S.H.E.; Sims, A.C.; Baric, R.S.; Snijder, E.J.; van Hemert, M.J. Zn2+ inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathog. 2010, 6, e1001176. [Google Scholar] [CrossRef] [PubMed]
- Doboszewska, U.; Wlaź, P.; Nowak, G.; Młyniec, K. Targeting zinc metalloenzymes in COVID-19. Br. J. Pharmacol. 2020. [Google Scholar] [CrossRef]
- Razzaque, M.S. COVID-19 pandemic: Can maintaining optimal zinc balance enhance host resistance? Tohoku J. Exp. Med. 2020, 251, 175–181. [Google Scholar] [CrossRef]
- Derwand, R.; Scholz, M. Does zinc supplementation enhance the clinical efficacy of chloroquine/hydroxychloroquine to win today’s battle against COVID-19? Med. Hypotheses 2020, 142, 109815. [Google Scholar] [CrossRef]
- Finzi, E. Treatment of SARS-CoV-2 with high dose oral zinc salts: A report on four patients. Int. J. Infect. Dis. 2020. [Google Scholar] [CrossRef]
- NDA. EFSA Panel on Dietetic Products, N. and A. Scientific opinion on dietary reference values for zinc. EFSA J. 2014, 12, 3844. [Google Scholar] [CrossRef]
- Alvarez, B.V.; Quon, A.L.; Mullen, J.; Casey, J.R. Quantification of carbonic anhydrase gene expression in ventricle of hypertrophic and failing human heart. BMC Cardiovasc. Disord. 2013, 13, 1–10. [Google Scholar] [CrossRef]
- Brown, G.M. The metabolism of pantothenic acid. J. Biol. Chem. 1959, 234, 370–378. [Google Scholar]
- Lima, N.G.D.; Ma, J.; Winkler, L.; Chu, Q.; Loh, K.H.; Elizabeth, O.; Budnik, B.A.; Lykke-andersen, J.; Saghatelian, A.; Slavoff, S.A. A human microprotein that interacts with the mRNA decapping complex. Nat. Chem. Biol. 2017, 13, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Strahle, W.C.; Handley, J.C. Prediction of combustion induced vibration in rocket motors. Cell Host Microbe 1978, 13, 509–519. [Google Scholar] [CrossRef]
- Agoro, R.; Taleb, M.; Quesniaux, V.F.J.; Mura, C. Cell iron status influences macrophage polarization. PLoS ONE 2018, 13, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Minandri, F.; Imperi, F.; Frangipani, E.; Bonchi, C.; Visaggio, D.; Facchini, M.; Pasquali, P.; Bragonzi, A.; Visca, P. Role of iron uptake systems in pseudomonas aeruginosa virulence and airway infection. Infect. Immun. 2016, 84, 2324–2335. [Google Scholar] [CrossRef]
- Institute of Medicine (US) Panel on Micronutrients. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; National Academies Press (US): Washington, DC, USA, 2001. [Google Scholar]
- Bolondi, G.; Russo, E.; Gamberini, E.; Circelli, A.; Meca, M.C.C.; Brogi, E.; Viola, L.; Bissoni, L.; Poletti, V.; Agnoletti, V. Iron metabolism and lymphocyte characterisation during Covid-19 infection in ICU patients: An observational cohort study. World J. Emerg. Surg. 2020, 15, 41. [Google Scholar] [CrossRef]
- D’Amico, F.; Peyrin-Biroulet, L.; Danese, S. Oral iron for IBD patients: Lessons learned at time of COVID-19 pandemic. J. Clin. Med. 2020, 9, 1536. [Google Scholar] [CrossRef]
- Zhao, K.; Huang, J.; Dai, D.; Feng, Y.; Liu, L.; Nie, S. Serum iron level as a potential predictor of COVID-19 severity and mortality: A retrospective study. Open Forum Infect. Dis. 2020, 1–8. [Google Scholar] [CrossRef]
- Perricone, C.; Bartoloni, E.; Bursi, R.; Cafaro, G.; Guidelli, G.M.; Shoenfeld, Y.; Gerli, R. COVID-19 as part of the hyperferritinemic syndromes: The role of iron depletion therapy. Immunol. Res. 2020. [Google Scholar] [CrossRef]
- Abobaker, A. Can iron chelation as an adjunct treatment of COVID-19 improve the clinical outcome? Eur. J. Clin. Pharmacol. 2020, 395, 1–2. [Google Scholar] [CrossRef]
- Brigham, E.P.; McCormack, M.C.; Takemoto, C.M.; Matsui, E.C. Iron Status is associated with asthma and lung function in US women. PLoS ONE 2015, 10, e0117545. [Google Scholar] [CrossRef]
- Katsarou, M.S.; Papasavva, M.; Latsi, R.; Drakoulis, N. Hemochromatosis: Hereditary Hemochromatosis and HFE Gene, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; Volume 110, ISBN 9780128178423. [Google Scholar]
- Pilling, L.C.; Tamosauskaite, J.; Jones, G.; Wood, A.R.; Jones, L.; Kuo, C.L.; Kuchel, G.A.; Ferrucci, L.; Melzer, D. Common conditions associated with hereditary haemochromatosis genetic variants: Cohort study in UK biobank. BMJ 2019, 364. [Google Scholar] [CrossRef] [PubMed]
- Astle, W.J.; Elding, H.; Jiang, T.; Allen, D.; Ruklisa, D.; Mann, A.L.; Mead, D.; Bouman, H.; Riveros-Mckay, F.; Kostadima, M.A.; et al. The allelic landscape of human blood cell trait variation and links to common complex disease. Cell 2016, 167, 1415–1429.e19. [Google Scholar] [CrossRef] [PubMed]
- International Consortium for Blood Pressure Genome-Wide Association Studies; Ehret, G.B.; Munroe, P.B.; Rice, K.M.; Bochud, M.; Johnson, A.D.; Chasman, D.I.; Smith, A.V.; Tobin, M.D.; Verwoert, G.C.; et al. Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature 2011, 478, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Besold, A.N.; Culbertson, E.M.; Culotta, V.C. The Yin and Yang of copper during infection. JBIC J. Biol. Inorg. Chem. 2016, 21, 137–144. [Google Scholar] [CrossRef]
- Percival, S.S. Copper and immunity. Am. J. Clin. Nutr. 1998, 67, 1064S–1068S. [Google Scholar] [CrossRef]
- Báez-Santos, Y.M.; St. John, S.E.; Mesecar, A.D. The SARS-coronavirus papain-like protease: Structure, function and inhibition by designed antiviral compounds. Antiviral Res. 2015, 115, 21–38. [Google Scholar] [CrossRef]
- van Doremalen, N.; Bushmaker, T.; Morris, D.H.; Holbrook, M.G.; Gamble, A.; Williamson, B.N.; Tamin, A.; Harcourt, J.L.; Thornburg, N.J.; Gerber, S.I.; et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020, 382, 1564–1567. [Google Scholar] [CrossRef]
- Raha, S.; Mallick, R.; Basak, S.; Duttaroy, A.K. Is copper beneficial for COVID-19 patients? Med. Hypotheses 2020, 142, 109814. [Google Scholar] [CrossRef]
- Andreou, A.; Trantza, S.; Filippou, D.; Sipsas, N.; Tsiodras, S. COVID-19: The potential role of copper and N-acetylcysteine (NAC) in a combination of candidate antiviral treatments against SARS-CoV-2. In Vivo 2020, 34, 1567–1588. [Google Scholar] [CrossRef]
- Panel, E.; Nda, A. Scientific opinion on dietary reference values for copper. EFSA J. 2015, 13, 1–51. [Google Scholar] [CrossRef]
- Arnaud, L.; Kelley, L.P.; Helias, V.; Cartron, J.-P.; Ballif, B.A. SMIM1 is a type II transmembrane phosphoprotein and displays the Vel blood group antigen at its carboxyl-terminus. FEBS Lett. 2015, 589, 3624–3630. [Google Scholar] [CrossRef] [PubMed]
- Jayawardena, R.; Sooriyaarachchi, P.; Chourdakis, M.; Jeewandara, C.; Ranasinghe, P. Enhancing immunity in viral infections, with special emphasis on COVID-19: A review. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 367–382. [Google Scholar] [CrossRef]
- Zhang, J.; Taylor, E.W.; Bennett, K.; Saad, R.; Rayman, M.P. Association between regional selenium status and reported outcome of COVID-19 cases in China. Am. J. Clin. Nutr. 2020, 111, 1297–1299. [Google Scholar] [CrossRef] [PubMed]
- Beck, M.A.; Levander, O.A.; Handy, J. Selenium deficiency and viral infection. J. Nutr. 2003, 133, 1463S–1467S. [Google Scholar] [CrossRef] [PubMed]
- Kuras, R.; Reszka, E.; Wieczorek, E.; Jablonska, E.; Gromadzinska, J.; Malachowska, B.; Kozlowska, L.; Stanislawska, M.; Janasik, B.; Wasowicz, W. Biomarkers of selenium status and antioxidant effect in workers occupationally exposed to mercury. J. Trace Elem. Med. Biol. 2018, 49, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Seale, L.A.; Torres, D.J.; Berry, M.J.; Pitts, M.W. A role for selenium-dependent GPX1 in SARS-CoV-2 virulence. Am. J. Clin. Nutr. 2020, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Du, X.; Xu, Y.; Deng, Y.; Liu, M.; Zhao, Y.; Zhang, B.; Li, X.; Zhang, L.; Peng, C.; et al. Structure of Mpro from SARS-CoV-2 and discovery of its inhibitors. Nature 2020, 582, 289–293. [Google Scholar] [CrossRef]
- Vavougios, G.D. Selenium—Associated gene signatures within the SARS-CoV-2—Host genomic interaction interface. Free Radic. Biol. Med. 2020. [Google Scholar] [CrossRef]
- Kieliszek, M.; Lipinski, B. Selenium supplementation in the prevention of coronavirus infections (COVID-19). Med. Hypotheses 2020, 143, 109878. [Google Scholar] [CrossRef]
- Fogarty, H.; Townsend, L.; Ni Cheallaigh, C.; Bergin, C.; Martin-Loeches, I.; Browne, P.; Bacon, C.L.; Gaule, R.; Gillett, A.; Byrne, M.; et al. COVID19 coagulopathy in Caucasian patients. Br. J. Haematol. 2020, 189, 1044–1049. [Google Scholar] [CrossRef]
- Panel, E.; Nda, A. Scientific opinion on dietary reference values for selenium. EFSA J. 2014, 12, 1–67. [Google Scholar] [CrossRef]
- Satia, J.A.; King, I.B.; Morris, J.S.; Stratton, K.; White, E. Toenail and plasma levels as biomarkers of selenium exposure. Ann. Epidemiol. 2006, 16, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Elmadfa, I.; Meyer, A.L.; Nowak, V.; Nations, U.; Food, W.; Hasenegger, V. European nutrition and health report 2009. Ann. Nutr. Metab. 2009, 55, I–IV. [Google Scholar] [CrossRef]
- Roman Viñas, B.; Ribas Barba, L.; Ngo, J.; Gurinovic, M.; Novakovic, R.; Cavelaars, A.; de Groot, L.C.P.G.M.; van’t Veer, P.; Matthys, C.; Serra Majem, L. Projected prevalence of inadequate nutrient intakes in Europe. Ann. Nutr. Metab. 2011, 59, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Allayee, H.; Roth, N.; Hodis, H.N. Polyunsaturated fatty acids and cardiovascular disease: Implications for nutrigenetics. J. Nutr. 2009, 2, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Galmés, S.; Cifre, M.; Palou, A.; Oliver, P.; Serra, F. A genetic score of predisposition to low-grade inflammation associated with obesity may contribute to discern population at risk for metabolic syndrome. Nutrients 2019, 11, 298. [Google Scholar] [CrossRef] [PubMed]
- Cifre, M.; Díaz-Rúa, R.; Varela-Calviño, R.; Reynés, B.; Pericás-Beltrán, J.; Palou, A.; Oliver, P. Human peripheral blood mononuclear cell in vitro system to test the efficacy of food bioactive compounds: Effects of polyunsaturated fatty acids and their relation with BMI. Mol. Nutr. Food Res. 2017, 61, 1600353. [Google Scholar] [CrossRef]
- NDA. EFSA Panel on Dietetic Products, N. and A. Scientific opinion on the tolerable upper intake level of eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA) and docosapentaenoic acid (DPA). EFSA J. 2012, 10. [Google Scholar] [CrossRef]
- Rogero, M.M.; Leão, M.d.C.; Santana, T.M.; Pimentel, M.V.d.M.B.; Carlini, G.C.G.; da Silveira, T.F.F.; Gonçalves, R.C.; Castro, I.A. Potential benefits and risks of omega-3 fatty acids supplementation to patients with COVID-19. Free Radic. Biol. Med. 2020, 156, 190–199. [Google Scholar] [CrossRef]
- Galmés, S.; Serra, F.; Palou, A. Vitamin E metabolic effects and genetic variants: A challenge for precision nutrition in obesity and associated disturbances. Nutrients 2018, 10, 1919. [Google Scholar] [CrossRef]
- Wallace, T.C. Combating COVID-19 and building immune resilience: A potential role for magnesium nutrition? J. Am. Coll. Nutr. 2020, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zahedipour, F.; Hosseini, S.A.; Sathyapalan, T.; Majeed, M.; Jamialahmadi, T.; Al-Rasadi, K.; Banach, M.; Sahebkar, A. Potential effects of curcumin in the treatment of COVID -19 infection. Phyther. Res. 2020, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Rocha, F.A.C.; de Assis, M.R. Curcumin as a potential treatment for COVID-19. Phyther. Res. 2020, 10–12. [Google Scholar] [CrossRef]
- Wahedi, H.M.; Ahmad, S.; Abbasi, S.W. Stilbene-based natural compounds as promising drug candidates against COVID-19. J. Biomol. Struct. Dyn. 2020, 1–10. [Google Scholar] [CrossRef]
- Colunga Biancatelli, R.M.L.; Berrill, M.; Catravas, J.D.; Marik, P.E. Quercetin and vitamin C: An experimental, synergistic therapy for the prevention and treatment of SARS-CoV-2 related disease (COVID-19). Front. Immunol. 2020, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Dhar, D.; Mohanty, A. Gut microbiota and Covid-19- possible link and implications. Virus Res. 2020, 285, 198018. [Google Scholar] [CrossRef]
- Stubbs, B.J.; Koutnik, A.P.; Goldberg, E.L.; Upadhyay, V.; Turnbaugh, P.J.; Verdin, E.; Newman, J.C. Investigating ketone bodies as immunometabolic countermeasures against respiratory viral infections. Med 2020, 1–23. [Google Scholar] [CrossRef]
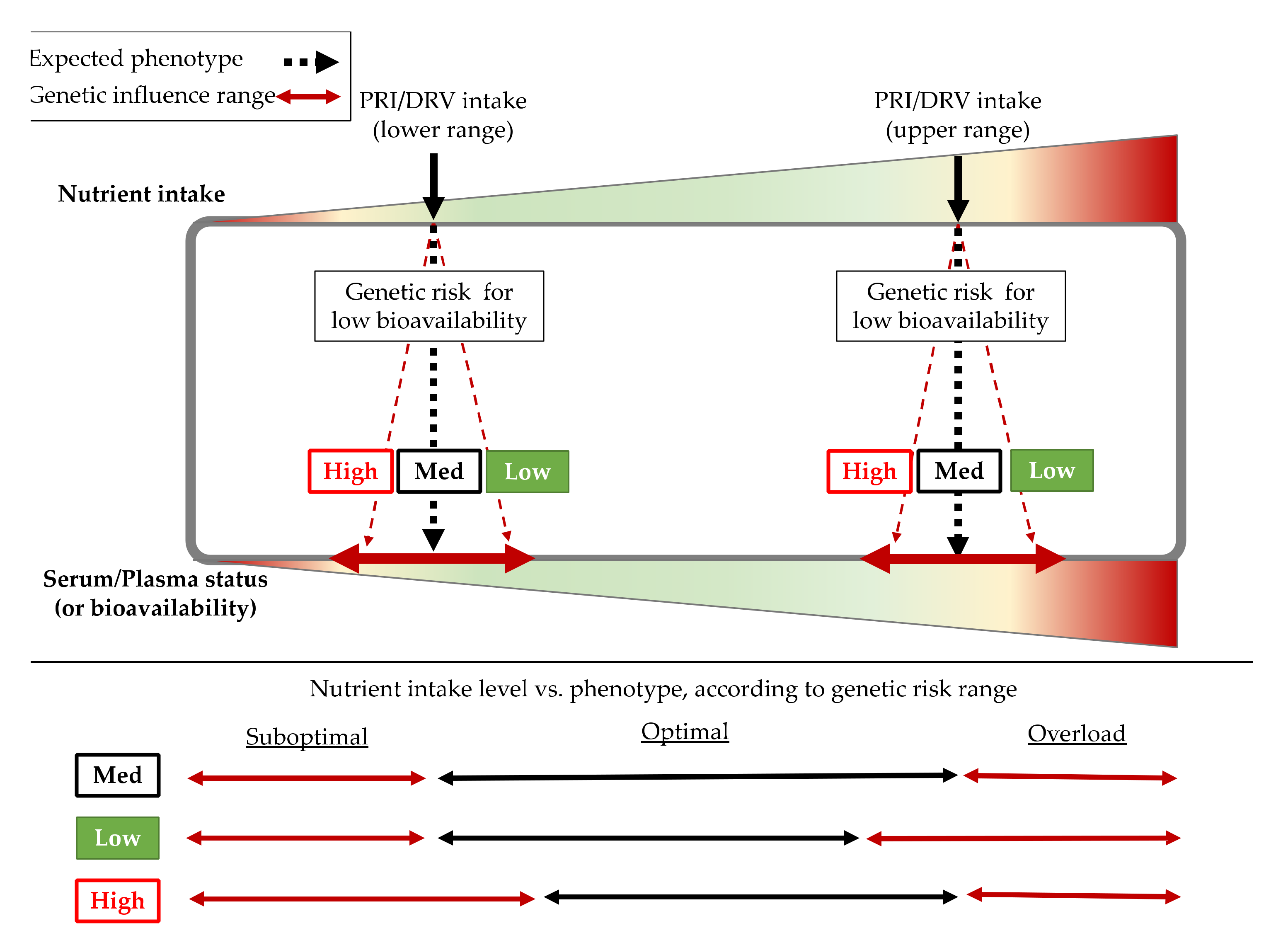
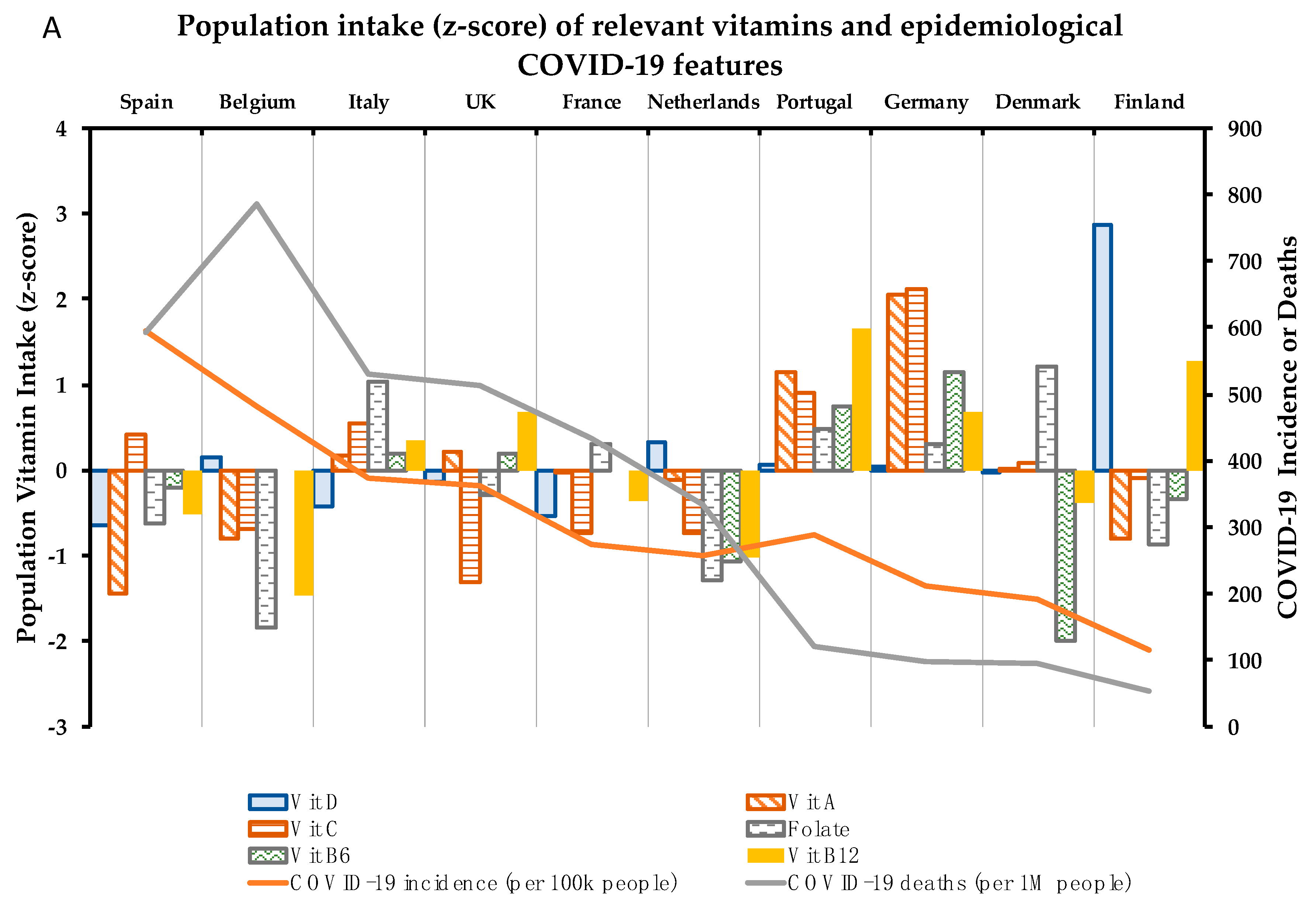
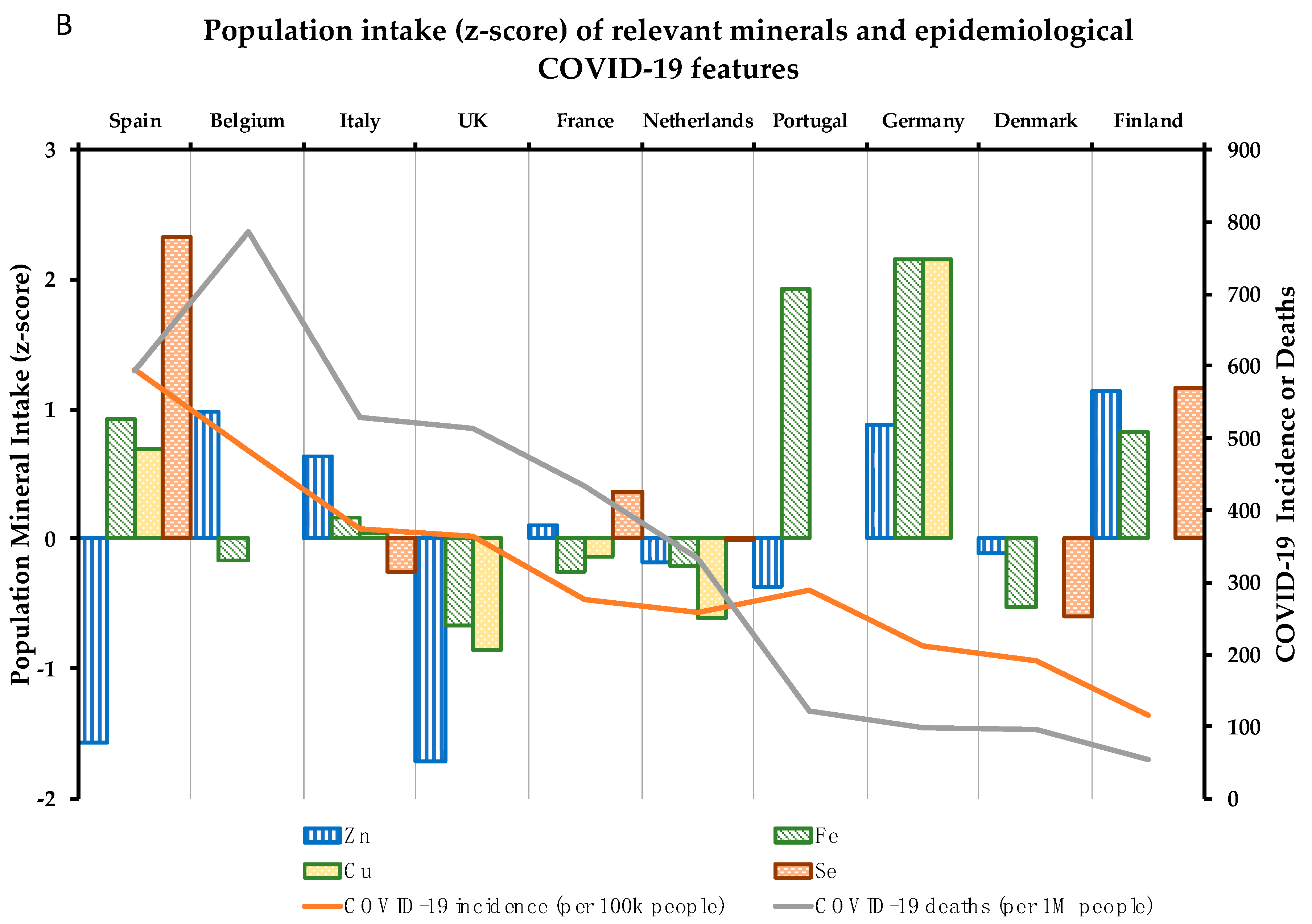
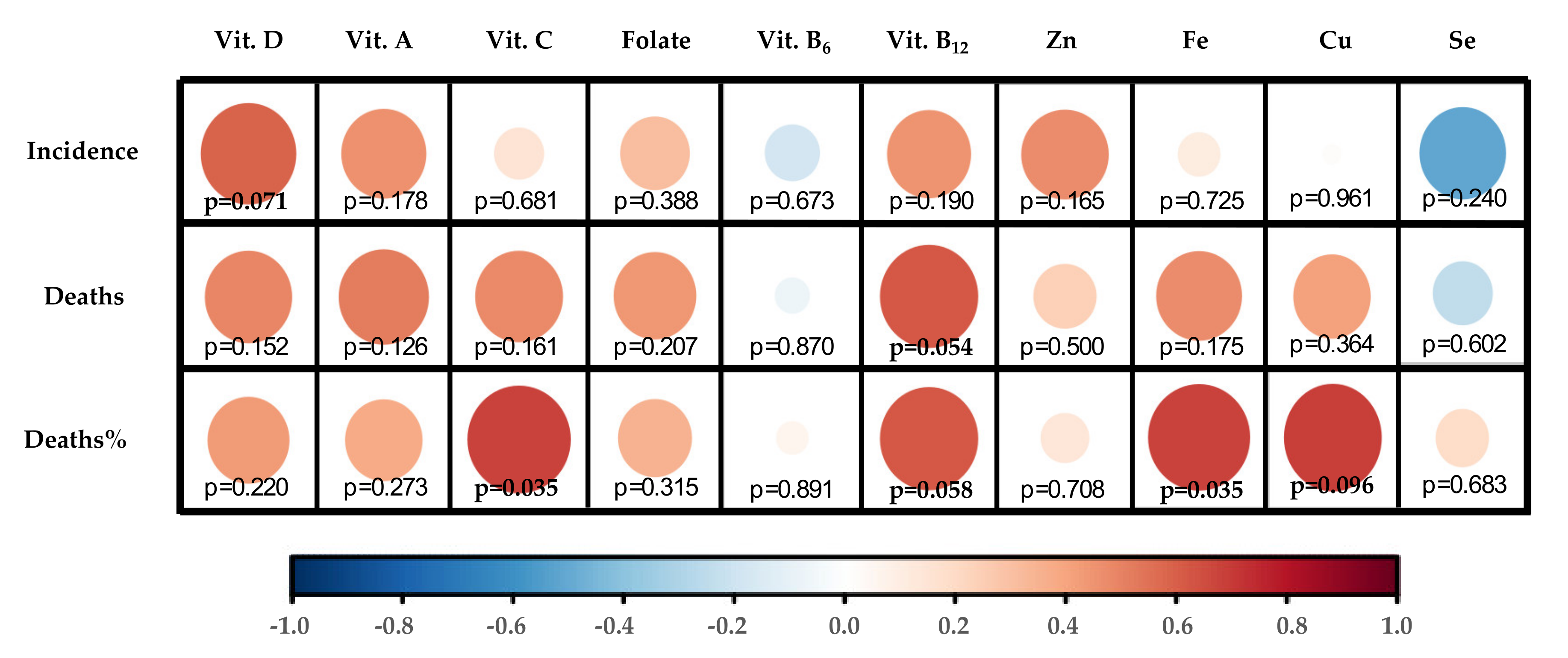
| Micronutrient [EFSA Cite] | EFSA DRVs | SNP Affecting Status (Gene) | Associated Trait | Risk Allele | Population Based Discovery | Cite |
|---|---|---|---|---|---|---|
| Vitamin D [3,4] | AI: 15 μg/day UL: 100 μg/day | rs7041 (GC) | ↑ vitamin D-binding protein ↓ Serum Vitamin D ** | C C | 1380 European 8541 African American or Afro-Caribbean | [16,17] |
| rs1155563 (GC) | ↓ Serum Vitamin D | C | 761 European | [18] | ||
| rs12785878 (NADSYN1) | Vitamin D Deficiency | C | 16125 European | [19] | ||
| Vitamin A (and ß-carotene) [3] | PRI: 650 (w)/50 (m) μg RE/day UL: 3000 μg RE/day | rs6564851 (BCO1) | ↓ Serum Carotenoids | T | 1191 European | [20] |
| Vitamin C [3,4] | PRI: 95 (w)/110 (m) mg/day UL: ND | rs33972313 (SLC23A1) | ↓ Serum Vitamin C | A | 9234 European | [21] |
| Folate [5] | PRI: 330 μg DFE/day UL: 1000 μg DFE /day | rs1801133 (MTHFR) | ↓ Folic acid in red blood cells | T | 2232 European | [22] |
| Vitamin B6 [5] | AI: 1.6 (w)/1.7 (m) mg/day UL: 25 mg/day | rs4654748 (NBPF3) | ↓ Serum vitamin B6 | C | 2934 European | [23] |
| Vitamin B12 [5] | PRI: 4 μg/day UL: ND | rs11254363 (CUBN) | ↓ Serum vitamin B12 | A | 2934 European | [23] |
| rs526934 (TCN1) | ↓ Serum vitamin B12 | G | 2934 European | |||
| rs602662 (FUT2) | ↓ Serum vitamin B12 | G | 2934 European | |||
| ↓ Serum vitamin B12 | G | 1001 South Asian | [24] | |||
| Zinc [6] | PRI*: 10.1 (w)/12.9 (m) mg/day UL: 25 mg/day | rs2120019 (PPCDC) | ↓ Serum Zinc | C | 2603 European | [25] |
| rs1532423 (CA1) | ↓ Serum Zinc | C | 2603 European | |||
| rs4826508 (NBDY) | ↓ Serum Zinc | C | 2603 European | |||
| Iron [9] | PRI: 11 (w + m)/16 (w#) mg/day UL: ND | rs1800562 (HFE) | ↑ Unsaturated iron-binding capacity | G | 679 European | [26] |
| ↑ Total iron-binding capacity | G | 679 European | ||||
| ↓ Transferrin saturation | G | 23986 European | [27] | |||
| ↑ Transferrin levels | G | 23986 European | ||||
| ↓ Serum iron | G | 23986 European | ||||
| ↓ Ferritin levels | G | 23986 European | ||||
| ↓ Hemoglobin | G | 4818 European | ||||
| rs1799945 (HFE) | ↓ Transferrin saturation | C | 12375 Hispanic or Latin American | [28] | ||
| ↓ Serum iron | C | 5633 European | [29] | |||
| ↓ Serum iron | C | 12375 Hispanic or Latin American | [28] | |||
| ↓ Hemoglobin | C | |||||
| rs3811647 (TF) | ↓ Unsaturated iron-binding capacity | G | 679 European | [26] | ||
| ↓ Total iron-binding capacity | G | 679 European | ||||
| ↓ Transferrin | G | 5633 European | [29] | |||
| rs7385804 (TFR2) | ↓ Serum iron | C | 23986 European | [27] | ||
| Copper [7,8] | AI: 1.3 (w)/1.6 (m) mg/day UL: 5 mg/day | rs2769264 (SELENBP1) | ↓ Serum copper | T | 2603 European | [30] |
| rs1175550 (SMIM1) | ↓ Serum copper | A | 2603 European | [31] | ||
| Selenium [10] | AI: 70 µg/day UL: 300 µg/day | rs891684 (SLC39A11) | ↓ Serum Selenium | A | 1203 European | [25] |
| rs17823744 (DMGDH) | ↓ Toenail Selenium | A | 4162 European |
| COVID-19 Parameters | Vitamin Intake (% vs. Requirements) | Mineral Intake (% vs. Requirements) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Country | I | M | D% | D | A | C | Folate | B6 | B12 | Zn | Fe | Cu | Se |
| Spain | 595.0 | 593.0 | 10.0 | 14.1 | 77.2 | 109.9 | 74.9 | 112.2 | 128.1 | 81.2 | 110.9 | 115.4 | 108.2 |
| Belgium | 481.6 | 786.0 | 16.3 | 25.0 | 110.0 | 87.8 | 63.0 | ND | 111.3 | 112.4 | 94.5 | ND | ND |
| Italy | 373.5 | 529.0 | 14.2 | 17.0 | 160.0 | 112.5 | 91.1 | 117.9 | 143.8 | 108.2 | 99.5 | 96.5 | 59.6 |
| UK | 363.2 | 513.0 | 14.1 | 21.0 | 162.5 | 75.3 | 78.1 | 117.8 | 149.6 | 79.4 | 86.9 | 70.2 | 64.3 |
| Portugal | 288.5 | 122.0 | 4.2 | 23.9 | 210.0 | 119.4 | 85.8 | 125.8 | 166.9 | 95.9 | 126.1 | ND | ND |
| France | 275.7 | 433.0 | 15.7 | 15.7 | 150.0 | 86.9 | 84.0 | ND | 130.8 | 101.7 | 93.3 | 90.8 | 71.2 |
| Netherlands | 258.3 | 334.0 | 12.9 | 27.6 | 144.9 | 86.6 | 68.5 | 99.7 | 119.1 | 98.2 | 93.9 | 77.0 | 64.2 |
| Germany | 211.9 | 97.0 | 4.6 | 23.5 | 256.0 | 143.9 | 83.9 | 131.4 | 149.4 | 111.2 | 129.6 | 157.8 | ND |
| Denmark | 190.7 | 95.0 | 5.0 | 22.5 | 152.0 | 103.2 | 92.8 | 86.2 | 130.6 | 99.1 | 89.2 | ND | 53.2 |
| Finland | 115.5 | 54.0 | 4.7 | 62.7 | 110.0 | 99.8 | 72.5 | 110.3 | 160.0 | 114.3 | 109.5 | 95.0 | 86.4 |
| Finnish | British | Italian | Spanish | |
|---|---|---|---|---|
| Vitamin D | ||||
| Low | 6.1 | 16.5 | 19.6 | 10.3 |
| Medium | 77.8 | 75.8 | 75.7 | 77.6 |
| High | 16.2 | 7.7 | 4.7 | 12.1 |
| χ2 (p-value) | Ref. | 0.000 | 0.000 | 0.217 |
| Vitamin A | ||||
| Low | 31.3 | 28.6 | 13.1 | 18.7 |
| Medium | 56.6 | 48.4 | 54.2 | 46.7 |
| High | 12.1 | 23.1 | 32.7 | 34.6 |
| χ2 (p-value) | Ref. | 0.032 | 0.000 | 0.000 |
| Vitamin C | ||||
| Low | 96.0 | 90.1 | 95.3 | 99.1 |
| Medium | 4.0 | 7.7 | 4.7 | 0.9 |
| High | 0.0 | 2.2 | 0.0 | 0.0 |
| χ2 (p-value) | Ref. | 0.146 | 0.764 | 0.001 |
| Folate | ||||
| Low | 51.5 | 44.0 | 30.8 | 28.0 |
| Medium | 42.4 | 47.3 | 44.9 | 55.1 |
| High | 6.1 | 8.8 | 24.3 | 16.8 |
| χ2 (p-value) | Ref. | 0.267 | 0.000 | 0.000 |
| Vitamin B6 | ||||
| Low | 21.2 | 18.7 | 29.0 | 23.4 |
| Medium | 54.5 | 51.6 | 50.5 | 46.7 |
| High | 24.2 | 29.7 | 20.6 | 29.9 |
| χ2 (p-value) | Ref. | 0.473 | 0.216 | 0.275 |
| Vitamin B12 | ||||
| Low | 5.1 | 4.4 | 15.9 | 13.1 |
| Medium | 80.8 | 86.8 | 78.5 | 77.6 |
| High | 14.1 | 8.8 | 5.6 | 9.3 |
| χ2 (p-value) | Ref. | 0.152 | 0.000 | 0.023 |
| Zinc | ||||
| Low | 29.3 | 31.9 | 21.5 | 21.5 |
| Medium | 62.6 | 59.3 | 55.1 | 59.8 |
| High | 8.1 | 8.8 | 23.4 | 18.7 |
| χ2 (p-value) | Ref. | 0.800 | 0.001 | 0.011 |
| iron | ||||
| Low | 7.1 | 17.6 | 17.8 | 19.6 |
| Medium | 55.6 | 69.2 | 61.7 | 59.8 |
| High | 37.4 | 13.2 | 20.6 | 20.6 |
| χ2 (p-value) | Ref. | 0.000 | 0.000 | 0.000 |
| Copper | ||||
| Low | 17.2 | 15.4 | 19.6 | 15.9 |
| Medium | 38.4 | 45.1 | 38.3 | 44.9 |
| High | 44.4 | 39.6 | 42.1 | 39.3 |
| χ2 (p-value) | Ref. | 0.407 | 0.801 | 0.422 |
| Selenium | ||||
| Low | 26.3 | 20.9 | 24.3 | 17.8 |
| Medium | 69.7 | 67.0 | 69.2 | 73.8 |
| High | 4.0 | 12.1 | 6.5 | 8.4 |
| χ2 (p-value) | Ref. | 0.033 | 0.571 | 0.037 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galmés, S.; Serra, F.; Palou, A. Current State of Evidence: Influence of Nutritional and Nutrigenetic Factors on Immunity in the COVID-19 Pandemic Framework. Nutrients 2020, 12, 2738. https://doi.org/10.3390/nu12092738
Galmés S, Serra F, Palou A. Current State of Evidence: Influence of Nutritional and Nutrigenetic Factors on Immunity in the COVID-19 Pandemic Framework. Nutrients. 2020; 12(9):2738. https://doi.org/10.3390/nu12092738
Chicago/Turabian StyleGalmés, Sebastià, Francisca Serra, and Andreu Palou. 2020. "Current State of Evidence: Influence of Nutritional and Nutrigenetic Factors on Immunity in the COVID-19 Pandemic Framework" Nutrients 12, no. 9: 2738. https://doi.org/10.3390/nu12092738
APA StyleGalmés, S., Serra, F., & Palou, A. (2020). Current State of Evidence: Influence of Nutritional and Nutrigenetic Factors on Immunity in the COVID-19 Pandemic Framework. Nutrients, 12(9), 2738. https://doi.org/10.3390/nu12092738







