Milk Consumption for the Prevention of Fragility Fractures
Abstract
:1. Introduction
2. Definition of Dairy and Milk Intake
3. Fragility Fractures
4. Potential Mechanisms
5. Interventional and Observational Studies
6. Meta-Analyses of Cohort Studies Examining Milk Intakes and Hip Fracture Risk
| Study: First Author (year) | Highest and Lowest Milk Intake Category | Hip Fracture Ascertainment | Number of Hip Fractures | SES Adjustment |
|---|---|---|---|---|
| Cumming (1997) [89] | 3 glasses/day vs. rarely/never | Self-report a | 306 | No |
| Fujiwara (1997) [84] | ≥5 vs. 1 glass/week | Self-report + medical records a | 55 | No |
| Meyer (1997) [88] | ≥5 vs. 1 glass/day | Self-report + medical records | 213 | Yes |
| Owusu (1997) [85] | 2.5 vs. ≤1 glass/week | Self-report a | 56 | No |
| Kanis (2005) (meta-analysis) [90] | Highest vs. lowest | Self-report/registers | 413 | Not reported |
| Feart (2013) [91] | Highest vs. lowest | Self-report | 57 | Yes |
| Feskanich (2014) [87] | ≥4 vs. 1 glass/day | Self-report a | 1716 | No |
| Michaëlsson (2014) [72] | ≥3 vs. <1 glass/day | Registers | 5425 | Yes |
| Sahni (2014) [86] | ≥7 vs. 1 glass/week | Self-report + medical records | 97 | No |
| Feskanich b (2018) [79] | ≥480 mL/day vs. <240 mL/week | Self-report + death records a | 2832 | No |
7. Hip Fracture Ascertainment Method (Outcome Assessment)
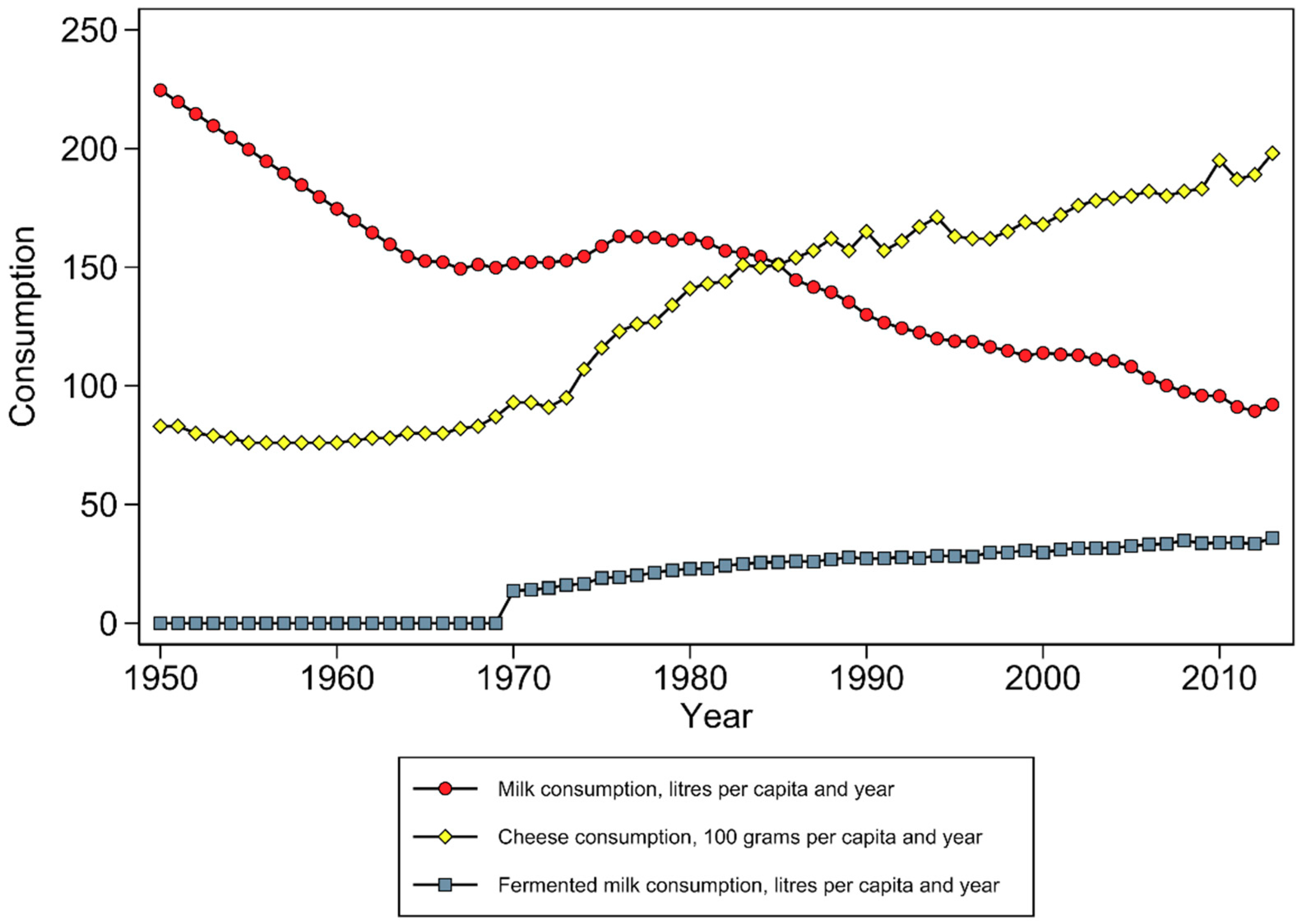
8. Cohort and Population-Specific Characteristics and Confounders
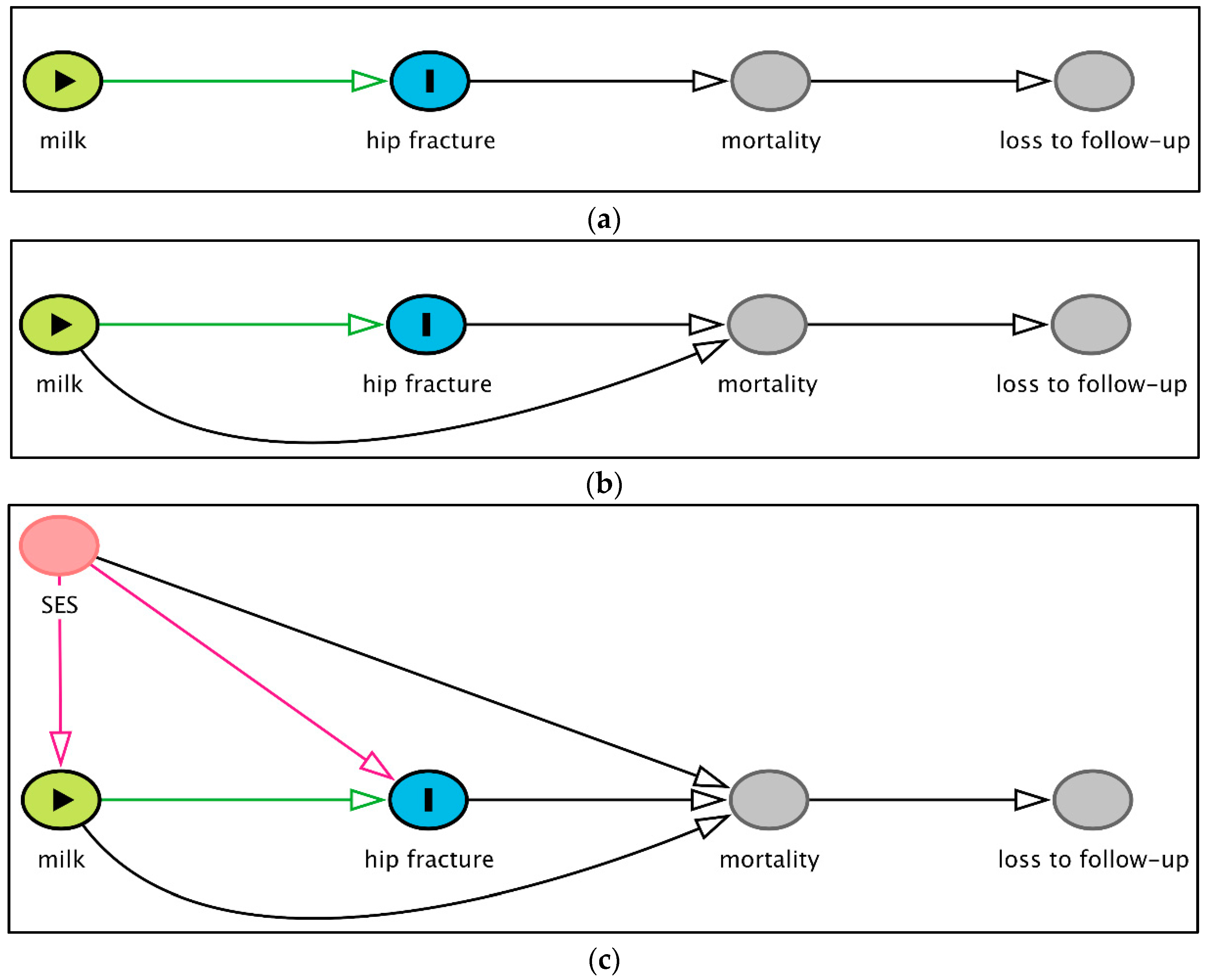
9. Epidemiological Considerations—The Combined Effect of the Different Biases
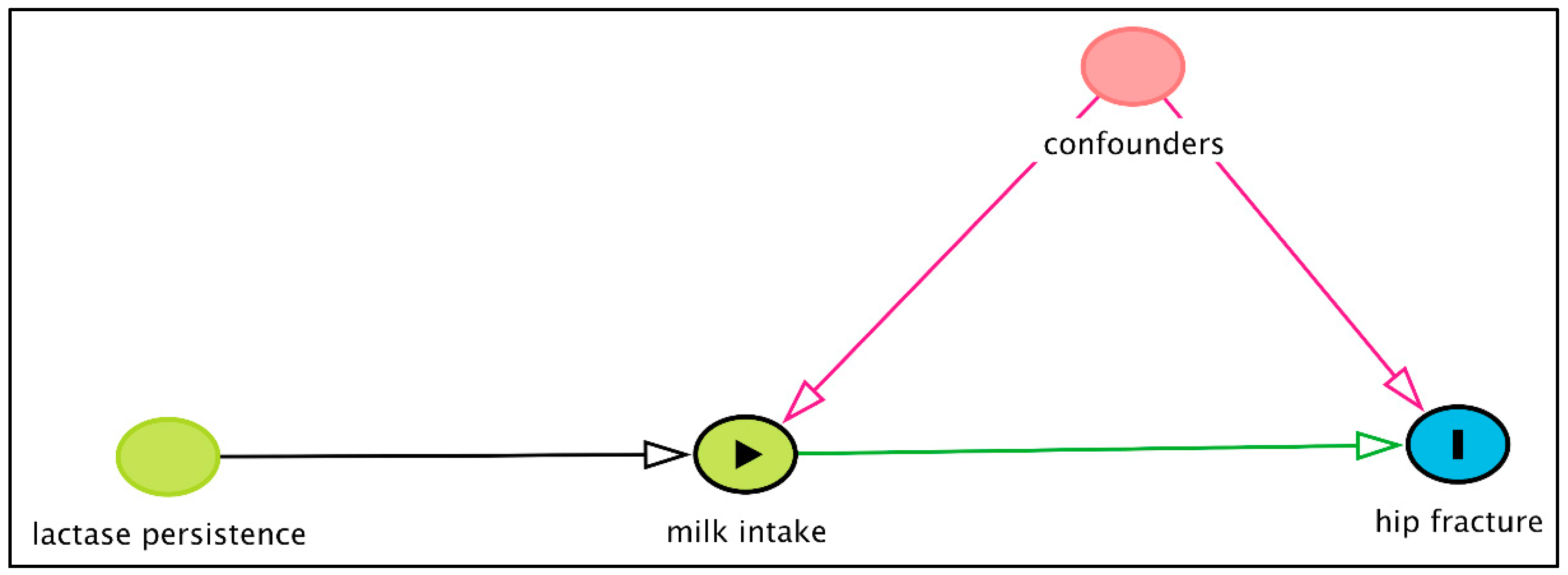
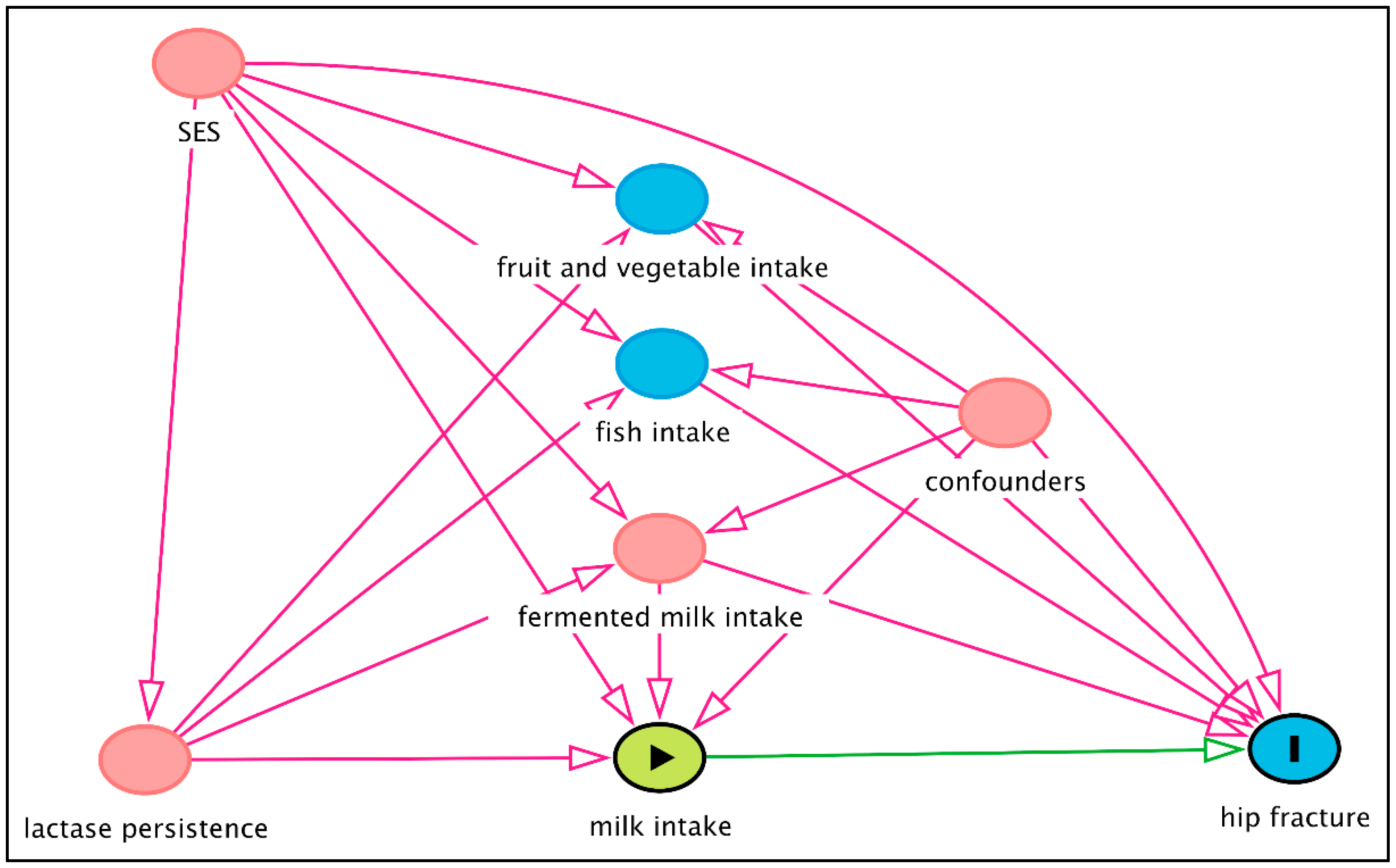
10. Mendelian Randomisation Studies
11. Author Autonomy from Dairy Industry
12. Summary and Recommendations for Future Research
Author Contributions
Funding
Conflicts of Interest
Disclaimer
References
- Heaney, R.P. Dairy and bone health. J. Am. Coll. Nutr. 2009, 28 (Suppl. 1), 82S–90S. [Google Scholar] [CrossRef]
- Sattui, S.E.; Saag, K.G. Fracture mortality: Associations with epidemiology and osteoporosis treatment. Nat. Rev. Endocrinol. 2014, 10, 592–602. [Google Scholar] [CrossRef]
- Michaëlsson, K.; Melhus, H.; Ferm, H.; Ahlbom, A.; Pedersen, N.L. Genetic liability to fractures in the elderly. Arch. Intern. Med. 2005, 165, 1825–1830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willett, W.C.; Ludwig, D.S. Milk and Health. N. Engl. J. Med. 2020, 382, 644–654. [Google Scholar] [CrossRef] [PubMed]
- Dimidi, E.; Cox, S.R.; Rossi, M.; Whelan, K. Fermented Foods: Definitions and Characteristics, Impact on the Gut Microbiota and Effects on Gastrointestinal Health and Disease. Nutrients 2019, 11, 1806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, M.; Kumar, A.; Nagpal, R.; Mohania, D.; Behare, P.; Verma, V.; Kumar, P.; Poddar, D.; Aggarwal, P.K.; Henry, C.J.; et al. Cancer-preventing attributes of probiotics: An update. Int. J. Food Sci. Nutr. 2010, 61, 473–496. [Google Scholar] [CrossRef] [PubMed]
- Nestel, P.J.; Mellett, N.; Pally, S.; Wong, G.; Barlow, C.K.; Croft, K.; Mori, T.A.; Meikle, P.J. Effects of low-fat or full-fat fermented and non-fermented dairy foods on selected cardiovascular biomarkers in overweight adults. Br. J. Nutr. 2013, 110, 2242–2249. [Google Scholar] [CrossRef] [Green Version]
- Sonestedt, E.; Wirfalt, E.; Wallstrom, P.; Gullberg, B.; Orho-Melander, M.; Hedblad, B. Dairy products and its association with incidence of cardiovascular disease: The Malmo diet and cancer cohort. Eur. J. Epidemiol. 2011, 26, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Bian, S.; Hu, J.; Zhang, K.; Wang, Y.; Yu, M.; Ma, J. Dairy product consumption and risk of hip fracture: A systematic review and meta-analysis. BMC Public Health 2018, 18, 165. [Google Scholar] [CrossRef] [Green Version]
- Malmir, H.; Larijani, B.; Esmaillzadeh, A. Consumption of milk and dairy products and risk of osteoporosis and hip fracture: A systematic review and Meta-analysis. Crit. Rev. Food Sci. Nutr. 2020, 60, 1722–1737. [Google Scholar] [CrossRef]
- Codex Alimentarius. International food standards. General standard for the use of dairy terms. 1999, CXS 206–1999. Available online: http://www.fao.org/fao-who-codexalimentarius/codex-texts/list-standards/en/ (accessed on 4 September 2020).
- David, S.D. Raw milk in court: Implications for public health policy and practice. Public Health Rep. 2012, 127, 598–601. [Google Scholar] [CrossRef] [Green Version]
- Mungai, E.A.; Behravesh, C.B.; Gould, L.H. Increased outbreaks associated with nonpasteurized milk, United States, 2007–2012. Emerg. Infect. Dis. 2015, 21, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Andreoletti, O.; Baggesen, D.; Bolton, D.; Butaye, P.; Cook, P.; Davies, R.; Fernández Escámex, P.; Griffin, J.; Hald, T.; Havelaar, A.; et al. EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards). Scientific Opinion on the public health risks related to the consumption of raw drinking milk. EFSA J. 2015, 13, 3940. [Google Scholar] [CrossRef] [Green Version]
- Portnoi, P.A.; MacDonald, A. The Lactose and Galactose Content of Cheese Suitable for Galactosaemia: New Analysis. JIMD Rep. 2016, 29, 85–87. [Google Scholar] [CrossRef]
- Changes in Canadians’ Prefereces for Milk and Dairy Products. Statistics Canada; 2017. Report No.: 21-004-X201700114786. Available online: https://www150.statcan.gc.ca/n1/en/catalogue/21-004-X201700114786 (accessed on 13 August 2020).
- Sebastian, R.; Goldman, J.; Wilkinson Enns, C.; LaComb, R. Fluid milk consumption in the United States: What we eat in America, NHANES 2005–2006. Food Surveys Research Group Dietary Data Brief No. 3. 2010. Available online: http://ars.usda.gov/Services/docs.htm?docid=19476 (accessed on 27 August 2020).
- Swedish Food Agency. Riksmaten (Swedish). Available online: https://www.livsmedelsverket.se/matvanor-halsa--miljo/matvanor---undersokningar (accessed on 27 August 2020).
- Willett, W. Nutritional Epidemiology, 3rd ed.; Oxford University Press: Oxford, UK, 2013. [Google Scholar]
- Borgström, F.; Karlsson, L.; Ortsäter, G.; Norton, N.; Halbout, P.; Cooper, C.; Lorentzon, M.; McCloskey, E.V.; Harvey, N.C.; Javaid, M.K.; et al. Fragility fractures in Europe: Burden, management and opportunities. Arch. Osteoporos. 2020, 15, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michaëlsson, K.; Nordström, P.; Nordström, A.; Garmo, H.; Byberg, L.; Pedersen, N.L.; Melhus, H. Impact of hip fracture on mortality: A cohort study in hip fracture discordant identical twins. J. Bone Miner. Res. 2014, 29, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Kanis, J.A.; Johnell, O.; De Laet, C.; Jonsson, B.; Oden, A.; Ogelsby, A.K. International variations in hip fracture probabilities: Implications for risk assessment. J. Bone Miner. Res. 2002, 17, 1237–1244. [Google Scholar] [CrossRef] [PubMed]
- Baker, P.N.; Salar, O.; Ollivere, B.J.; Forward, D.P.; Weerasuriya, N.; Moppett, I.K.; Moran, C.G. Evolution of the hip fracture population: Time to consider the future? A retrospective observational analysis. BMJ Open 2014, 4, e004405. [Google Scholar] [CrossRef]
- Karampampa, K.; Ahlbom, A.; Michaëlsson, K.; Andersson, T.; Drefahl, S.; Modig, K. Declining incidence trends for hip fractures have not been accompanied by improvements in lifetime risk or post-fracture survival—A nationwide study of the Swedish population 60 years and older. Bone 2015, 78, 55–61. [Google Scholar] [CrossRef]
- Sigurdsson, G.; Aspelund, T.; Chang, M.; Jonsdottir, B.; Sigurdsson, S.; Eiriksdottir, G.; Gudmundsson, A.; Harris, T.B.; Gudnason, V.; Lang, T.F. Increasing sex difference in bone strength in old age: The Age, Gene/Environment Susceptibility-Reykjavik study (AGES-REYKJAVIK). Bone 2006, 39, 644–651. [Google Scholar] [CrossRef]
- Finkelstein, J.S.; Brockwell, S.E.; Mehta, V.; Greendale, G.A.; Sowers, M.R.; Ettinger, B.; Lo, J.C.; Johnston, J.M.; Cauley, J.A.; Danielson, M.E.; et al. Bone mineral density changes during the menopause transition in a multiethnic cohort of women. J. Clin. Endocrinol. Metab. 2008, 93, 861–868. [Google Scholar] [CrossRef] [PubMed]
- Wagner, H.; Melhus, H.; Gedeborg, R.; Pedersen, N.L.; Michaelsson, K. Simply ask them about their balance--future fracture risk in a nationwide cohort study of twins. Am. J. Epidemiol. 2009, 169, 143–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, R.M.Y.; Wong, H.; Zhang, N.; Chow, S.K.H.; Chau, W.W.; Wang, J.; Chim, Y.N.; Leung, K.S.; Cheung, W.H. The relationship between sarcopenia and fragility fracture-a systematic review. Osteoporos. Int. 2019, 30, 541–553. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Curtis, E.M.; Cooper, C.; Harvey, N.C. State of the art in osteoporosis risk assessment and treatment. J. Endocrinol. Invest. 2019, 42, 1149–1164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cusack, S.; Cashman, K.D. Impact of genetic variation on metabolic response of bone to diet. Proc. Nutr. Soc. 2003, 62, 901–912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moayyeri, A.; Hammond, C.J.; Hart, D.J.; Spector, T.D. Effects of age on genetic influence on bone loss over 17 years in women: The Healthy Ageing Twin Study (HATS). J. Bone Miner. Res. 2012, 27, 2170–2178. [Google Scholar] [CrossRef]
- Siris, E.S.; Chen, Y.-T.; Abbott, T.A.; Barrett-Connor, E.; Miller, P.D.; Wehren, L.E.; Berger, M.L. Bone Mineral Density Thresholds for Pharmacological Intervention to Prevent Fractures. Arch. Intern. Med. 2004, 164, 1108–1112. [Google Scholar] [CrossRef] [Green Version]
- Järvinen, T.L.; Sievänen, H.; Khan, K.M.; Heinonen, A.; Kannus, P. Shifting the focus in fracture prevention from osteoporosis to falls. BMJ 2008, 336, 124–126. [Google Scholar] [CrossRef] [Green Version]
- Mackey, D.C.; Lui, L.Y.; Cawthon, P.M.; Bauer, D.C.; Nevitt, M.C.; Cauley, J.A.; Hillier, T.A.; Lewis, C.E.; Barrett-Connor, E.; Cummings, S.R.; et al. High-trauma fractures and low bone mineral density in older women and men. JAMA 2007, 298, 2381–2388. [Google Scholar] [CrossRef]
- Sanders, K.M.; Pasco, J.A.; Ugoni, A.M.; Nicholson, G.C.; Seeman, E.; Martin, T.J.; Skoric, B.; Panahi, S.; Kotowicz, M.A. The exclusion of high trauma fractures may underestimate the prevalence of bone fragility fractures in the community: The Geelong Osteoporosis Study. J. Bone Miner. Res. 1998, 13, 1337–1342. [Google Scholar] [CrossRef]
- Stone, K.L.; Seeley, D.G.; Lui, L.Y.; Cauley, J.A.; Ensrud, K.; Browner, W.S.; Nevitt, M.C.; Cummings, S.R.; Osteoporotic Fractures Research, G. BMD at multiple sites and risk of fracture of multiple types: Long-term results from the Study of Osteoporotic Fractures. J. Bone Miner. Res. 2003, 18, 1947–1954. [Google Scholar] [CrossRef] [PubMed]
- Bolland, M.J.; Leung, W.; Tai, V.; Bastin, S.; Gamble, G.D.; Grey, A.; Reid, I.R. Calcium intake and risk of fracture: Systematic review. BMJ 2015, 351, h4580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reid, I.R.; Bolland, M.J. Calcium and/or Vitamin D Supplementation for the Prevention of Fragility Fractures: Who Needs It? Nutrients 2020, 12, 1011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reid, I.R.; Bolland, M.J. Skeletal and nonskeletal effects of vitamin D: Is vitamin D a tonic for bone and other tissues? Osteoporos. Int. 2014, 25, 2347–2357. [Google Scholar] [CrossRef] [PubMed]
- Darling, A.L.; Manders, R.J.F.; Sahni, S.; Zhu, K.; Hewitt, C.E.; Prince, R.L.; Millward, D.J.; Lanham-New, S.A. Dietary protein and bone health across the life-course: An updated systematic review and meta-analysis over 40 years. Osteoporos. Int. 2019, 30, 741–761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durosier-Izart, C.; Biver, E.; Merminod, F.; van Rietbergen, B.; Chevalley, T.; Herrmann, F.R.; Ferrari, S.L.; Rizzoli, R. Peripheral skeleton bone strength is positively correlated with total and dairy protein intakes in healthy postmenopausal women. Am. J. Clin. Nutr. 2017, 105, 513–525. [Google Scholar] [CrossRef] [PubMed]
- Langsetmo, L.; Shikany, J.M.; Burghardt, A.J.; Cawthon, P.M.; Orwoll, E.S.; Cauley, J.A.; Taylor, B.C.; Schousboe, J.T.; Bauer, D.C.; Vo, T.N.; et al. High dairy protein intake is associated with greater bone strength parameters at the distal radius and tibia in older men: A cross-sectional study. Osteoporos. Int. 2018, 29, 69–77. [Google Scholar] [CrossRef]
- Morton, R.W.; Murphy, K.T.; McKellar, S.R.; Schoenfeld, B.J.; Henselmans, M.; Helms, E.; Aragon, A.A.; Devries, M.C.; Banfield, L.; Krieger, J.W.; et al. A systematic review, meta-analysis and meta-regression of the effect of protein supplementation on resistance training-induced gains in muscle mass and strength in healthy adults. Br. J. Sports Med. 2018, 52, 376–384. [Google Scholar] [CrossRef]
- Blair, M.; Kellow, N.J.; Dordevic, A.L.; Evans, S.; Caissutti, J.; McCaffrey, T.A. Health Benefits of Whey or Colostrum Supplementation in Adults ≥35 Years; a Systematic Review. Nutrients 2020, 12, 299. [Google Scholar] [CrossRef] [Green Version]
- Ticinesi, A.; Meschi, T.; Lauretani, F.; Felis, G.; Franchi, F.; Pedrolli, C.; Barichella, M.; Benati, G.; Di Nuzzo, S.; Ceda, G.P.; et al. Nutrition and Inflammation in Older Individuals: Focus on Vitamin D, n-3 Polyunsaturated Fatty Acids and Whey Proteins. Nutrients 2016, 8, 186. [Google Scholar] [CrossRef] [Green Version]
- De Noni, I.; FitzGerald, R.; Korhonen, H.; Le Roux, Y.; Livesey, C.; Thorsdottir, I.; Tomé, D.; Witkamp, R. Scientific Report of EFSA prepared by a DATEX Working Group on β-casomorphins. Review of the potential health impact of β-casomorphins and related peptides. EFSA Sci. Rep. 2009, 231, 1–107. [Google Scholar]
- Brooke-Taylor, S.; Dwyer, K.; Woodford, K.; Kost, N. Systematic Review of the Gastrointestinal Effects of A1 Compared with A2 β-Casein. Adv. Nutr. 2017, 8, 739–748. [Google Scholar] [CrossRef] [PubMed]
- Küllenberg de Gaudry, D.; Lohner, S.; Schmucker, C.; Kapp, P.; Motschall, E.; Hörrlein, S.; Röger, C.; Meerpohl, J.J. Milk A1 β-casein and health-related outcomes in humans: A systematic review. Nutr. Rev. 2019, 77, 278–306. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, C.; Mølgaard, C.; Michaelsen, K.F. Cow’s milk and linear growth in industrialized and developing countries. Annu. Rev. Nutr. 2006, 26, 131–173. [Google Scholar] [CrossRef]
- Crowe, F.L.; Key, T.J.; Allen, N.E.; Appleby, P.N.; Roddam, A.; Overvad, K.; Grønbæk, H.; Tjønneland, A.; Halkjær, J.; Dossus, L.; et al. The Association between Diet and Serum Concentrations of IGF-I, IGFBP-1, IGFBP-2, and IGFBP-3 in the European Prospective Investigation into Cancer and Nutrition. Cancer Epidemiol. Biomark. Prev. 2009, 18, 1333–1340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribot, C.; Tremollieres, F.; Pouilles, J.M.; Albarede, J.L.; Mansat, M.; Utheza, G.; Bonneu, M.; Bonnissent, P.; Ricoeur, C. Risk factors for hip fracture. MEDOS study: Results of the Toulouse Centre. Bone 1993, 14 (Suppl. 1), S77–S80. [Google Scholar] [CrossRef]
- Kalkwarf, H.J.; Khoury, J.C.; Lanphear, B.P. Milk intake during childhood and adolescence, adult bone density, and osteoporotic fractures in US women. Am. J. Clin. Nutr. 2003, 77, 257–265. [Google Scholar] [CrossRef] [Green Version]
- Moore, L.L.; Bradlee, M.L.; Gao, D.; Singer, M.R. Effects of average childhood dairy intake on adolescent bone health. J. Pediatr. 2008, 153, 667–673. [Google Scholar] [CrossRef] [Green Version]
- Hoeflich, A.; Meyer, Z. Functional analysis of the IGF-system in milk. Best Pract. Res. Clin. Endocrinol. Metab. 2017, 31, 409–418. [Google Scholar] [CrossRef]
- Da Silva, M.S.; Rudkowska, I. Dairy nutrients and their effect on inflammatory profile in molecular studies. Mol. Nutr. Food Res. 2015, 59, 1249–1263. [Google Scholar] [CrossRef]
- Khan, I.T.; Nadeem, M.; Imran, M.; Ullah, R.; Ajmal, M.; Jaspal, M.H. Antioxidant properties of Milk and dairy products: A comprehensive review of the current knowledge. Lipids Health Dis. 2019, 18, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manolagas, S.C.; Parfitt, A.M. What old means to bone. Trends Endocrinol. Metab. 2010, 21, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Michaëlsson, K.; Wolk, A.; Byberg, L.; Ärnlöv, J.; Melhus, H. Intake and serum concentrations of α-tocopherol in relation to fractures in elderly women and men: 2 cohort studies. Am. J. Clin. Nutr. 2014, 99, 107–114. [Google Scholar] [CrossRef] [Green Version]
- Jensen, G.L. Inflammation: Roles in aging and sarcopenia. JPEN J. Parenter. Enteral Nutr. 2008, 32, 656–659. [Google Scholar] [CrossRef]
- Klijn, N.; Weerkamp, A.H.; de Vos, W.M. Genetic marking of Lactococcus lactis shows its survival in the human gastrointestinal tract. Appl. Environ. Microbiol. 1995, 61, 2771–2774. [Google Scholar] [CrossRef] [Green Version]
- Swarte, J.C.; Eelderink, C.; Douwes, R.M.; Said, M.Y.; Hu, S.; Post, A.; Westerhuis, R.; Bakker, S.J.L.; Harmsen, H.J.M. Effect of High versus Low Dairy Consumption on the Gut Microbiome: Results of a Randomized, Cross-Over Study. Nutrients 2020, 12, 2129. [Google Scholar] [CrossRef]
- Alm, L. Effect of fermentation on lactose, glucose, and galactose content in milk and suitability of fermented milk products for lactose intolerant individuals. J. Dairy Sci. 1982, 65, 346–352. [Google Scholar] [CrossRef]
- Ohlsson, J.; Johansson, M.; Hansson, H.; Abrahamson, A.; Byberg, L.; Smedman, A.; Lindmark-Månsson, H.; Lundh, Å. Lactose, glucose and galactose content in milk, fermented milk and lactose-free milk products. Int. Dairy J. 2017, 73, 151–154. [Google Scholar] [CrossRef]
- Portnoi, P.A.; MacDonald, A. Determination of the lactose and galactose content of cheese for use in the galactosaemia diet. J. Hum. Nutr. Diet. 2009, 22, 400–408. [Google Scholar] [CrossRef]
- Michaëlsson, K.; Wolk, A.; Melhus, H.; Byberg, L. Milk, Fruit and Vegetable, and Total Antioxidant Intakes in Relation to Mortality Rates: Cohort Studies in Women and Men. Am. J. Epidemiol. 2017, 185, 345–361. [Google Scholar] [CrossRef] [Green Version]
- Hao, L.; Huang, H.; Gao, J.; Marshall, C.; Chen, Y.; Xiao, M. The influence of gender, age and treatment time on brain oxidative stress and memory impairment induced by D-galactose in mice. Neurosci. Lett. 2014, 571, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Bao, M.; Li, D.; Li, Y.M. Advanced glycation in D-galactose induced mouse aging model. Mech. Ageing Dev. 1999, 108, 239–251. [Google Scholar] [CrossRef]
- Cui, X.; Zuo, P.; Zhang, Q.; Li, X.; Hu, Y.; Long, J.; Packer, L.; Liu, J. Chronic systemic D-galactose exposure induces memory loss, neurodegeneration, and oxidative damage in mice: Protective effects of R-alpha-lipoic acid. J. Neurosci. Res. 2006, 83, 1584–1590. [Google Scholar] [CrossRef] [PubMed]
- Zarrati, M.; Salehi, E.; Nourijelyani, K.; Mofid, V.; Zadeh, M.J.H.; Najafi, F.; Ghaflati, Z.; Bidad, K.; Chamari, M.; Karimi, M.; et al. Effects of Probiotic Yogurt on Fat Distribution and Gene Expression of Proinflammatory Factors in Peripheral Blood Mononuclear Cells in Overweight and Obese People with or without Weight-Loss Diet. J. Am. Coll. Nutr. 2014, 33, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Barreto, F.M.; Colado Simao, A.N.; Morimoto, H.K.; Batisti Lozovoy, M.A.; Dichi, I.; da Silva Miglioranza, L.H. Beneficial effects of Lactobacillus plantarum on glycemia and homocysteine levels in postmenopausal women with metabolic syndrome. Nutrition 2014, 30, 939–942. [Google Scholar] [CrossRef] [PubMed]
- Kekkonen, R.A.; Lummela, N.; Karjalainen, H.; Latvala, S.; Tynkkynen, S.; Jarvenpaa, S.; Kautiainen, H.; Julkunen, I.; Vapaatalo, H.; Korpela, R. Probiotic intervention has strain-specific anti-inflammatory effects in healthy adults. World J. Gastroenterol. 2008, 14, 2029–2036. [Google Scholar] [CrossRef]
- Michaëlsson, K.; Wolk, A.; Langenskiöld, S.; Basu, S.; Warensjö Lemming, E.; Melhus, H.; Byberg, L. Milk intake and risk of mortality and fractures in women and men: Cohort studies. BMJ 2014, 349, g6015. [Google Scholar] [CrossRef] [Green Version]
- Warensjo, E.; Nolan, D.; Tapsell, L. Dairy food consumption and obesity-related chronic disease. Adv. Food Nutr. Res. 2010, 59, 1–41. [Google Scholar] [CrossRef]
- Satija, A.; Yu, E.; Willett, W.C.; Hu, F.B. Understanding nutritional epidemiology and its role in policy. Adv. Nutr. 2015, 6, 5–18. [Google Scholar] [CrossRef] [Green Version]
- Michaëlsson, K.; Holmberg, L.; Ljunghall, S.; Mallmin, H.; Persson, P.G.; Wolk, A. Effect of prefracture versus postfracture dietary assessment on hip fracture risk estimates. Int. J. Epidemiol. 1996, 25, 403–410. [Google Scholar] [CrossRef] [Green Version]
- Flather, M.D.; Farkouh, M.E.; Pogue, J.M.; Yusuf, S. Strengths and limitations of meta-analysis: Larger studies may be more reliable. Control. Clin. Trials 1997, 18, 568–579. [Google Scholar] [CrossRef]
- Barnard, N.D.; Willett, W.C.; Ding, E.L. The Misuse of Meta-analysis in Nutrition Research. JAMA 2017, 318, 1435–1436. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.C.; Crippa, A.; Orsini, N.; Wolk, A.; Michaelsson, K. Milk Consumption and Mortality from All Causes, Cardiovascular Disease, and Cancer: A Systematic Review and Meta-Analysis. Nutrients 2015, 7, 7749–7763. [Google Scholar] [CrossRef]
- Feskanich, D.; Meyer, H.E.; Fung, T.T.; Bischoff-Ferrari, H.A.; Willett, W.C. Milk and other dairy foods and risk of hip fracture in men and women. Osteoporos. Int. 2018, 29, 385–396. [Google Scholar] [CrossRef]
- Sahni, S.; Tucker, K.L.; Kiel, D.P.; Quach, L.; Casey, V.A.; Hannan, M.T. Milk and yogurt consumption are linked with higher bone mineral density but not with hip fracture: The Framingham Offspring Study. Arch. Osteoporos. 2013, 8, 119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michaëlsson, K.; Wolk, A.; Lemming, E.W.; Melhus, H.; Byberg, L. Intake of milk or fermented milk combined with fruit and vegetable consumption in relation to hip fracture rates: A cohort study of Swedish women. J. Bone Miner. Res. 2018, 33, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Holvik, K.; Meyer, H.E.; Laake, I.; Feskanich, D.; Omsland, T.K.; Sogaard, A.J. Milk drinking and risk of hip fracture. The Norwegian Epidemiologic Osteoporosis Studies (NOREPOS). Br. J. Nutr. 2018, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Aslam, H.; Holloway-Kew, K.L.; Mohebbi, M.; Jacka, F.N.; Pasco, J.A. Association between dairy intake and fracture in an Australian-based cohort of women: A prospective study. BMJ Open 2019, 9, e031594. [Google Scholar] [CrossRef] [Green Version]
- Fujiwara, S.; Kasagi, F.; Yamada, M.; Kodama, K. Risk factors for hip fracture in a Japanese cohort. J. Bone Miner. Res. 1997, 12, 998–1004. [Google Scholar] [CrossRef]
- Owusu, W.; Willett, W.C.; Feskanich, D.; Ascherio, A.; Spiegelman, D.; Colditz, G.A. Calcium intake and the incidence of forearm and hip fractures among men. J. Nutr. 1997, 127, 1782–1787. [Google Scholar] [CrossRef] [Green Version]
- Sahni, S.; Mangano, K.M.; Tucker, K.L.; Kiel, D.P.; Casey, V.A.; Hannan, M.T. Protective association of milk intake on the risk of hip fracture: Results from the Framingham Original Cohort. J. Bone Miner. Res. 2014, 29, 1756–1762. [Google Scholar] [CrossRef] [PubMed]
- Feskanich, D.; Bischoff-Ferrari, H.A.; Frazier, A.L.; Willett, W.C. Milk consumption during teenage years and risk of hip fractures in older adults. JAMA Pediatr. 2014, 168, 54–60. [Google Scholar] [CrossRef]
- Meyer, H.E.; Pedersen, J.I.; Løken, E.B.; Tverdal, A. Dietary factors and the incidence of hip fracture in middle-aged Norwegians. A prospective study. Am. J. Epidemiol. 1997, 145, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Cumming, R.G.; Cummings, S.R.; Nevitt, M.C.; Scott, J.; Ensrud, K.E.; Vogt, T.M.; Fox, K. Calcium intake and fracture risk: Results from the study of osteoporotic fractures. Am. J. Epidemiol. 1997, 145, 926–934. [Google Scholar] [CrossRef] [PubMed]
- Kanis, J.A.; Johansson, H.; Oden, A.; De Laet, C.; Johnell, O.; Eisman, J.A.; Mc Closkey, E.; Mellstrom, D.; Pols, H.; Reeve, J.; et al. A meta-analysis of milk intake and fracture risk: Low utility for case finding. Osteoporos. Int. 2005, 16, 799–804. [Google Scholar] [CrossRef] [PubMed]
- Feart, C.; Lorrain, S.; Ginder Coupez, V.; Samieri, C.; Letenneur, L.; Paineau, D.; Barberger-Gateau, P. Adherence to a Mediterranean diet and risk of fractures in French older persons. Osteoporos. Int. 2013, 24, 3031–3041. [Google Scholar] [CrossRef] [Green Version]
- Tognon, G.; Nilsson, L.M.; Shungin, D.; Lissner, L.; Jansson, J.H.; Renstrom, F.; Wennberg, M.; Winkvist, A.; Johansson, I. Nonfermented milk and other dairy products: Associations with all-cause mortality. Am. J. Clin. Nutr. 2017, 105, 1502–1511. [Google Scholar] [CrossRef]
- Tognon, G.; Rothenberg, E.; Petrolo, M.; Sundh, V.; Lissner, L. Dairy product intake and mortality in a cohort of 70-year-old Swedes: A contribution to the Nordic diet discussion. Eur. J. Nutr. 2018, 57, 2869–2876. [Google Scholar] [CrossRef] [Green Version]
- Ludvigsson, J.F.; Andersson, E.; Ekbom, A.; Feychting, M.; Kim, J.L.; Reuterwall, C.; Heurgren, M.; Olausson, P.O. External review and validation of the Swedish national inpatient register. BMC Public Health 2011, 11, 450. [Google Scholar] [CrossRef] [Green Version]
- Gjertsen, J.-E.; Engesæter, L.B.; Furnes, O.; Havelin, L.I.; Steindal, K.; Vinje, T.; Fevang, J.M. The Norwegian Hip Fracture Register: Experiences after the first 2 years and 15,576 reported operations. Acta Orthop. 2008, 79, 583–593. [Google Scholar] [CrossRef] [Green Version]
- Colditz, G.A.; Martin, P.; Stampfer, M.J.; Willett, W.C.; Sampson, L.; Rosner, B.; Hennekens, C.H.; Speizer, F.E. Validation of questionnaire information on risk factors and disease outcomes in a prospective cohort study of women. Am. J. Epidemiol. 1986, 123, 894–900. [Google Scholar] [CrossRef] [PubMed]
- Baleanu, F.; Moreau, M.; Kinnard, V.; Iconaru, L.; Karmali, R.; Paesmans, M.; Bergmann, P.; Body, J.J. What is the validity of self-reported fractures? Bone Rep. 2020, 12, 100256. [Google Scholar] [CrossRef] [PubMed]
- Brauer, C.A.; Coca-Perraillon, M.; Cutler, D.M.; Rosen, A.B. Incidence and Mortality of Hip Fractures in the United States. JAMA 2009, 302, 1573–1579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- von Friesendorff, M.; McGuigan, F.E.; Wizert, A.; Rogmark, C.; Holmberg, A.H.; Woolf, A.D.; Akesson, K. Hip fracture, mortality risk, and cause of death over two decades. Osteoporos. Int. 2016, 27, 2945–2953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joakimsen, R.M.; Fonnebo, V.; Sogaard, A.J.; Tollan, A.; Stormer, J.; Magnus, J.H. The Tromso study: Registration of fractures, how good are self-reports, a computerized radiographic register and a discharge register? Osteoporos. Int. 2001, 12, 1001–1005. [Google Scholar] [CrossRef] [PubMed]
- Schousboe, J.T.; Paudel, M.L.; Taylor, B.C.; Virnig, B.A.; Cauley, J.A.; Curtis, J.R.; Ensrud, K.E. Magnitude and consequences of misclassification of incident hip fractures in large cohort studies: The Study of Osteoporotic Fractures and Medicare claims data. Osteoporos. Int. 2013, 24, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Pemberton, J. Are hip fractures underestimated as a cause of death? The influence of coroners and pathologists on the death rate. Community Med. 1988, 10, 117–123. [Google Scholar] [CrossRef]
- Donaldson, L.J.; Parsons, L.; Cook, A.J. Death certification in fractured neck of femur. Public Health 1989, 103, 237–243. [Google Scholar] [CrossRef]
- Bergholdt, H.K.M.; Ellervik, C.; Nordestgaard, B.G. Response to letter: Observational studies investigating hip fracture risk: A fundamental methodological issue? J. Intern. Med. 2018, 284, 327. [Google Scholar] [CrossRef]
- Schisterman, E.F.; Cole, S.R.; Platt, R.W. Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidemiology 2009, 20, 488–495. [Google Scholar] [CrossRef] [Green Version]
- Darmon, N.; Drewnowski, A. Does social class predict diet quality? Am. J. Clin. Nutr. 2008, 87, 1107–1117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirkpatrick, S.I.; Dodd, K.W.; Reedy, J.; Krebs-Smith, S.M. Income and race/ethnicity are associated with adherence to food-based dietary guidance among US adults and children. J. Acad. Nutr. Diet 2012, 112, 624–635.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.; Kawachi, I.; Berkman, L.F.; Grodstein, F. Education, other socioeconomic indicators, and cognitive function. Am. J. Epidemiol. 2003, 157, 712–720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ervin, R.B.; Wright, J.D.; Kennedy-Stephenson, J. Use of Dietary Supplements in the United States, 1988–1994; National Ctr for Health Statistics: Hyattsville, MD, USA, 1999; Volume 244.
- Tajeu, G.S.; Delzell, E.; Smith, W.; Arora, T.; Curtis, J.R.; Saag, K.G.; Morrisey, M.A.; Yun, H.; Kilgore, M.L. Death, debility, and destitution following hip fracture. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 346–353. [Google Scholar] [CrossRef]
- Tosteson, A.N.; Gottlieb, D.J.; Radley, D.C.; Fisher, E.S.; Melton, L.J., 3rd. Excess mortality following hip fracture: The role of underlying health status. Osteoporos. Int. 2007, 18, 1463–1472. [Google Scholar] [CrossRef] [Green Version]
- Byberg, L.; Michaëlsson, K. Fourth response to commentaries regarding our article “Milk intake and risk of mortality and fractures in women and men: Cohort studies” BMJ 2014, 349, g6015. Available online: https://www.bmj.com/content/349/bmj.g6015/rr-0 (accessed on 12 August 2020).
- Khalili, H.; Huang, E.S.; Jacobson, B.C.; Camargo, C.A.; Feskanich, D.; Chan, A.T. Use of proton pump inhibitors and risk of hip fracture in relation to dietary and lifestyle factors: A prospective cohort study. BMJ 2012, 344, e372. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.-X.; Lewis, J.D.; Epstein, S.; Metz, D.C. Long-term Proton Pump Inhibitor Therapy and Risk of Hip Fracture. JAMA 2006, 296, 2947–2953. [Google Scholar] [CrossRef]
- Leontiadis, G.I.; Moayyedi, P. Proton pump inhibitors and risk of bone fractures. Curr. Treat. Options Gastroenterol. 2014, 12, 414–423. [Google Scholar] [CrossRef]
- Itoh, S.; Sekino, Y.; Shinomiya, K.; Takeda, S. The effects of risedronate administered in combination with a proton pump inhibitor for the treatment of osteoporosis. J. Bone Miner. Metab. 2013, 31, 206–211. [Google Scholar] [CrossRef]
- Byberg, L.; Michaëlsson, K. Second reply to commentaries regarding our manuscript “Milk intake and risk of mortality and fractures in women and men: Cohort studies”. BMJ 2014, 349, g6015. Available online: https://www.bmj.com/content/349/bmj.g6015/rr/779932 (accessed on 12 August 2020).
- Hernán, M.A.; Hernández-Díaz, S.; Robins, J.M. A structural approach to selection bias. Epidemiology 2004, 15, 615–625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greenland, S.; Pearl, J.; Robins, J.M. Causal Diagrams for Epidemiologic Research. Epidemiology 1999, 10, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Pearl, J. Causality: Models, Reasoning and Inference; Cambridge University Press: New York, NY, USA, 2009; p. 478. [Google Scholar]
- d-Separation Without Tears. Available online: http://dagitty.net/learn/dsep/index.html (accessed on 13 August 2020).
- VanderWeele, T.J. Commentary: Resolutions of the birthweight paradox: Competing explanations and analytical insights. Int. J. Epidemiol. 2014, 43, 1368–1373. [Google Scholar] [CrossRef] [PubMed]
- Vandenbroucke, J.P.; von Elm, E.; Altman, D.G.; Gotzsche, P.C.; Mulrow, C.D.; Pocock, S.J.; Poole, C.; Schlesselman, J.J.; Egger, M. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and elaboration. PLoS Med. 2007, 4, e297. [Google Scholar] [CrossRef] [Green Version]
- Textor, J.; van der Zander, B.; Gilthorpe, M.S.; Liśkiewicz, M.; Ellison, G.T. Robust causal inference using directed acyclic graphs: The R package ‘dagitty’. Int. J. Epidemiol. 2017, 45, 1887–1894. [Google Scholar] [CrossRef] [Green Version]
- Lawlor, D.A.; Harbord, R.M.; Sterne, J.A.C.; Timpson, N.; Davey Smith, G. Mendelian randomization: Using genes as instruments for making causal inferences in epidemiology. Stat. Med. 2008, 27, 1133–1163. [Google Scholar] [CrossRef]
- Gerbault, P.; Liebert, A.; Itan, Y.; Powell, A.; Currat, M.; Burger, J.; Swallow, D.M.; Thomas, M.G. Evolution of lactase persistence: An example of human niche construction. Philos. Trans. R. Soc. B Biol. Sci. 2011, 366, 863–877. [Google Scholar] [CrossRef] [Green Version]
- Forsgård, R.A. Lactose digestion in humans: Intestinal lactase appears to be constitutive whereas the colonic microbiome is adaptable. Am. J. Clin. Nutr. 2019, 110, 273–279. [Google Scholar] [CrossRef]
- Enattah, N.S.; Sahi, T.; Savilahti, E.; Terwilliger, J.D.; Peltonen, L.; Järvelä, I. Identification of a variant associated with adult-type hypolactasia. Nat. Genet. 2002, 30, 233–237. [Google Scholar] [CrossRef]
- Troelsen, J.T.; Olsen, J.; Møller, J.; Sjöström, H. An upstream polymorphism associated with lactase persistence has increased enhancer activity. Gastroenterology 2003, 125, 1686–1694. [Google Scholar] [CrossRef]
- Ding, M.; Huang, T.; Bergholdt, H.K.; Nordestgaard, B.G.; Ellervik, C.; Qi, L. Dairy consumption, systolic blood pressure, and risk of hypertension: Mendelian randomization study. BMJ 2017, 356, j1000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vissers, L.E.T.; Sluijs, I.; van der Schouw, Y.T.; Forouhi, N.G.; Imamura, F.; Burgess, S.; Barricarte, A.; Boeing, H.; Bonet, C.; Chirlaque, M.D.; et al. Dairy Product Intake and Risk of Type 2 Diabetes in EPIC-InterAct: A Mendelian Randomization Study. Diabetes Care 2019, 42, 568–575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergholdt, H.K.M.; Larsen, M.K.; Varbo, A.; Nordestgaard, B.G.; Ellervik, C. Lactase persistence, milk intake, hip fracture and bone mineral density: A study of 97 811 Danish individuals and a meta-analysis. J. Intern. Med. 2018, 284, 254–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burgess, S.; Small, D.S.; Thompson, S.G. A review of instrumental variable estimators for Mendelian randomization. Stat. Methods Med. Res. 2017, 26, 2333–2355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Enattah, N.; Pekkarinen, T.; Välimäki, M.J.; Löyttyniemi, E.; Järvelä, I. Genetically defined adult-type hypolactasia and self-reported lactose intolerance as risk factors of osteoporosis in Finnish postmenopausal women. Eur. J. Clin. Nutr. 2005, 59, 1105–1111. [Google Scholar] [CrossRef] [Green Version]
- Timpson, N.J.; Brennan, P.; Gaborieau, V.; Moore, L.; Zaridze, D.; Matveev, V.; Szeszenia-Dabrowska, N.; Lissowska, J.; Mates, D.; Bencko, V.; et al. Can lactase persistence genotype be used to reassess the relationship between renal cell carcinoma and milk drinking? Potentials and problems in the application of Mendelian randomization. Cancer Epidemiol. Biomark. Prev. 2010, 19, 1341–1348. [Google Scholar] [CrossRef] [Green Version]
- Burgess, S.; Thompson, S.G.; Collaboration, C.C.G. Avoiding bias from weak instruments in Mendelian randomization studies. Int. J. Epidemiol. 2011, 40, 755–764. [Google Scholar] [CrossRef] [Green Version]
- Spiller, W.; Slichter, D.; Bowden, J.; Davey Smith, G. Detecting and correcting for bias in Mendelian randomization analyses using Gene-by-Environment interactions. Int. J. Epidemiol. 2018, 48, 702–712. [Google Scholar] [CrossRef] [Green Version]
- Corella, D.; Arregui, M.; Coltell, O.; Portoles, O.; Guillem-Saiz, P.; Carrasco, P.; Sorli, J.V.; Ortega-Azorin, C.; Gonzalez, J.I.; Ordovas, J.M. Association of the LCT-13910C>T polymorphism with obesity and its modulation by dairy products in a Mediterranean population. Obesity 2011, 19, 1707–1714. [Google Scholar] [CrossRef] [Green Version]
- Wagh, K.; Bhatia, A.; Alexe, G.; Reddy, A.; Ravikumar, V.; Seiler, M.; Boemo, M.; Yao, M.; Cronk, L.; Naqvi, A.; et al. Lactase persistence and lipid pathway selection in the Maasai. PLoS ONE 2012, 7, e44751. [Google Scholar] [CrossRef] [Green Version]
- Nestle, M. Corporate Funding of Food and Nutrition Research: Science or Marketing? JAMA Intern. Med. 2016, 176, 13–14. [Google Scholar] [CrossRef] [PubMed]
- Ahn, R.; Woodbridge, A.; Abraham, A.; Saba, S.; Korenstein, D.; Madden, E.; Boscardin, W.J.; Keyhani, S. Financial ties of principal investigators and randomized controlled trial outcomes: Cross sectional study. BMJ 2017, 356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jørgensen, A.W.; Hilden, J.; Gøtzsche, P.C. Cochrane reviews compared with industry supported meta-analyses and other meta-analyses of the same drugs: Systematic review. BMJ 2006, 333, 782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lundh, A.; Lexchin, J.; Mintzes, B.; Schroll, J.B.; Bero, L. Industry sponsorship and research outcome. Cochrane Database Syst. Rev. 2017, MR000033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamarche, B.; Givens, D.I.; Soedamah-Muthu, S.; Krauss, R.M.; Jakobsen, M.U.; Bischoff-Ferrari, H.A.; Pan, A.; Despres, J.P. Does Milk Consumption Contribute to Cardiometabolic Health and Overall Diet Quality? Can. J. Cardiol. 2016, 32, 1026–1032. [Google Scholar] [CrossRef] [Green Version]
- Rozenberg, S.; Body, J.J.; Bruyere, O.; Bergmann, P.; Brandi, M.; Cooper, C.; Devogelaer, J.P.; Gielen, E.; Goemaere, S.; Kaufman, J.M.; et al. Effects of Dairy Products Consumption on Health: Benefits and Beliefs-A Commentary from the Belgian Bone Club and the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases. Calcif. Tissue Int. 2016, 98, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thorning, T.K.; Raben, A.; Tholstrup, T.; Soedamah-Muthu, S.S.; Givens, I.; Astrup, A. Milk and dairy products: Good or bad for human health? An assessment of the totality of scientific evidence. Food Nutr. Res. 2016, 60, 32527. [Google Scholar] [CrossRef] [Green Version]
- Fardellone, P.; Séjourné, A.; Blain, H.; Cortet, B.; Thomas, T. Osteoporosis: Is milk a kindness or a curse? Joint Bone Spine 2017, 84, 275–281. [Google Scholar] [CrossRef]
- Hiligsmann, M.; Neuprez, A.; Buckinx, F.; Locquet, M.; Reginster, J.Y. A scoping review of the public health impact of vitamin D-fortified dairy products for fracture prevention. Arch. Osteoporos. 2017, 12, 57. [Google Scholar] [CrossRef]
- van den Heuvel, E.; Steijns, J. Dairy products and bone health: How strong is the scientific evidence? Nutr. Res. Rev. 2018, 31, 164–178. [Google Scholar] [CrossRef]
- Fardellone, P. The effect of milk consumption on bone and fracture incidence, an update. Aging Clin. Exp. Res. 2019, 31, 759–764. [Google Scholar] [CrossRef] [PubMed]
- Marangoni, F.; Pellegrino, L.; Verduci, E.; Ghiselli, A.; Bernabei, R.; Calvani, R.; Cetin, I.; Giampietro, M.; Perticone, F.; Piretta, L.; et al. Cow’s Milk Consumption and Health: A Health Professional’s Guide. J. Am. Coll. Nutr. 2019, 38, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Matia-Martin, P.; Torrego-Ellacuria, M.; Larrad-Sainz, A.; Fernandez-Perez, C.; Cuesta-Triana, F.; Rubio-Herrera, M.A. Effects of Milk and Dairy Products on the Prevention of Osteoporosis and Osteoporotic Fractures in Europeans and Non-Hispanic Whites from North America: A Systematic Review and Updated Meta-Analysis. Adv. Nutr. 2019, 10, S120–S143. [Google Scholar] [CrossRef] [PubMed]
- Aslam, H.; Ruusunen, A.; Berk, M.; Loughman, A.; Rivera, L.; Pasco, J.A.; Jacka, F.N. Unravelled facets of milk derived opioid peptides: A focus on gut physiology, fractures and obesity. Int. J. Food Sci. Nutr. 2020, 71, 36–49. [Google Scholar] [CrossRef]
- Geiker, N.R.W.; Molgaard, C.; Iuliano, S.; Rizzoli, R.; Manios, Y.; van Loon, L.J.C.; Lecerf, J.M.; Moschonis, G.; Reginster, J.Y.; Givens, I.; et al. Impact of whole dairy matrix on musculoskeletal health and aging-current knowledge and research gaps. Osteoporos. Int. 2020, 31, 601–615. [Google Scholar] [CrossRef] [Green Version]
- Ong, A.M.; Kang, K.; Weiler, H.A.; Morin, S.N. Fermented Milk Products and Bone Health in Postmenopausal Women: A Systematic Review of Randomized Controlled Trials, Prospective Cohorts, and Case-Control Studies. Adv. Nutr. 2020, 11, 251–265. [Google Scholar] [CrossRef]
- Rizzoli, R.; Biver, E. Are Probiotics the New Calcium and Vitamin D for Bone Health? Curr. Osteoporos. Rep. 2020, 18, 273–284. [Google Scholar] [CrossRef]
- Mohanty, D.P.; Mohapatra, S.; Misra, S.; Sahu, P.S. Milk derived bioactive peptides and their impact on human health—A review. Saudi J. Biol. Sci. 2016, 23, 577–583. [Google Scholar] [CrossRef] [Green Version]
- Hill, D.; Sugrue, I.; Arendt, E.; Hill, C.; Stanton, C.; Ross, R.P. Recent advances in microbial fermentation for dairy and health. F1000Research 2017, 6, 751. [Google Scholar] [CrossRef]
- Hidayat, K.; Du, X.; Shi, B.M.; Qin, L.Q. Systematic review and meta-analysis of the association between dairy consumption and the risk of hip fracture: Critical interpretation of the currently available evidence. Osteoporos. Int. 2020, 31, 1411–1425. [Google Scholar] [CrossRef]
- Melnik, B.C.; Schmitz, G. Exosomes of pasteurized milk: Potential pathogens of Western diseases. J. Transl. Med. 2019, 17, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polzonetti, V.; Pucciarelli, S.; Vincenzetti, S.; Polidori, P. Dietary Intake of Vitamin D from Dairy Products Reduces the Risk of Osteoporosis. Nutrients 2020, 12, 1743. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Zhan, Y.; Chen, Y.; Jiang, Y. Effects of dairy products on bone mineral density in healthy postmenopausal women: A systematic review and meta-analysis of randomized controlled trials. Arch. Osteoporos. 2020, 15, 48. [Google Scholar] [CrossRef] [PubMed]
- Michaëlsson, K.; Byberg, L. Mixing of Apples and Oranges in Milk Research: A Cohort Analysis of Non-Fermented Milk Intake and All-Cause Mortality. Nutrients 2020, 12, 1393. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Byberg, L.; Warensjö Lemming, E. Milk Consumption for the Prevention of Fragility Fractures. Nutrients 2020, 12, 2720. https://doi.org/10.3390/nu12092720
Byberg L, Warensjö Lemming E. Milk Consumption for the Prevention of Fragility Fractures. Nutrients. 2020; 12(9):2720. https://doi.org/10.3390/nu12092720
Chicago/Turabian StyleByberg, Liisa, and Eva Warensjö Lemming. 2020. "Milk Consumption for the Prevention of Fragility Fractures" Nutrients 12, no. 9: 2720. https://doi.org/10.3390/nu12092720
APA StyleByberg, L., & Warensjö Lemming, E. (2020). Milk Consumption for the Prevention of Fragility Fractures. Nutrients, 12(9), 2720. https://doi.org/10.3390/nu12092720





