Moderate Wine Consumption Reduces Faecal Water Cytotoxicity in Healthy Volunteers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Phenolic Compounds
2.2. Red Wine
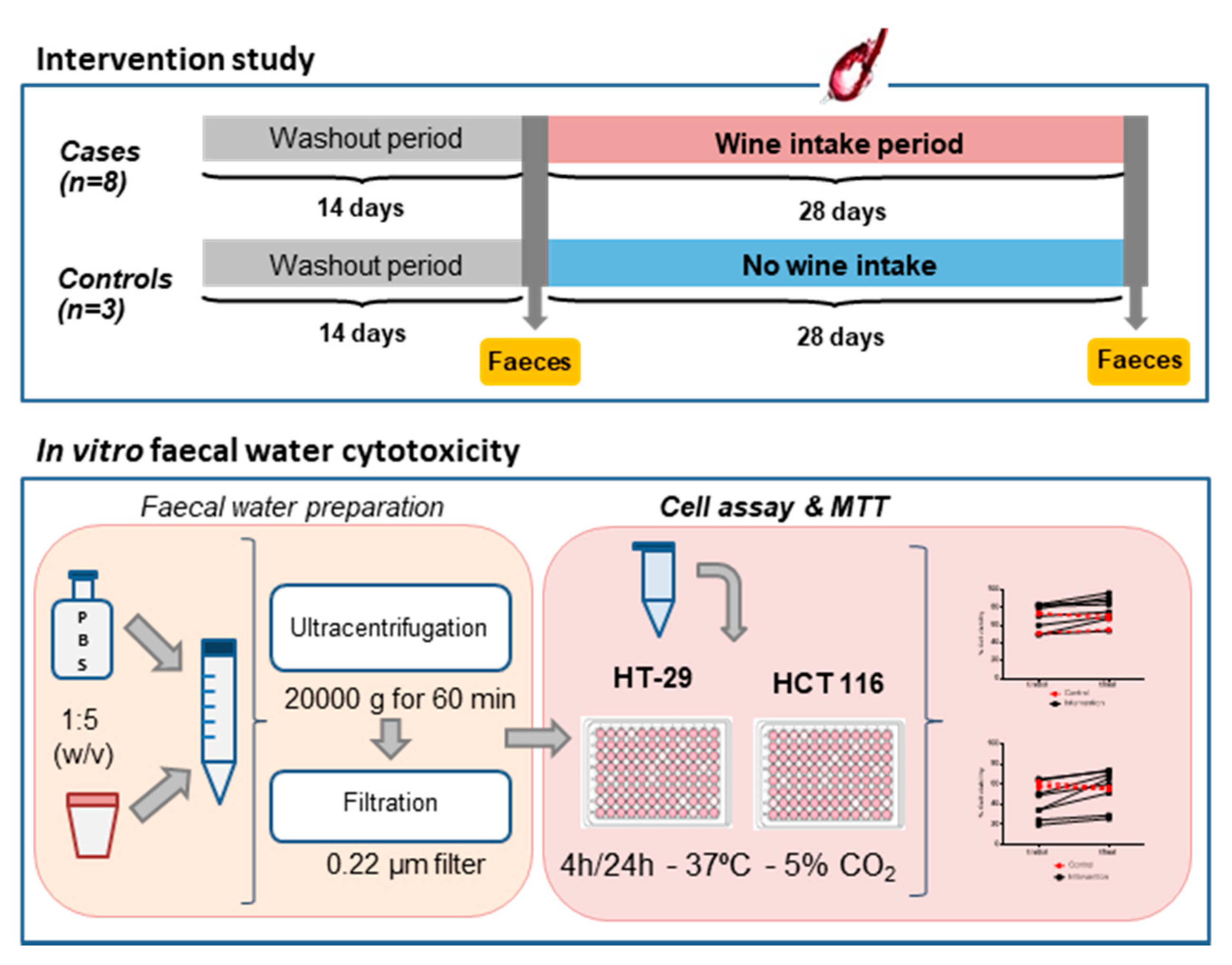
2.3. Wine Intervention Study
2.4. Faecal Water Preparation
2.5. Cell Cytotoxicity Assay
2.6. Phenolic Acids Analysis
2.7. Short-Chain Fatty Acids Analysis
2.8. Statistical Analysis
3. Results and Discussion
3.1. Assessment of the FW Cytotoxicity Assays
3.2. Evaluation of FW Cytotoxicity before and after Moderate Red Wine Consumption
3.3. Correlation Between Cytotoxicity and Some Targeted Metabolites in FW
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cueva, C.; Gil-Sánchez, I.; Ayuda-Durán, B.; González-Manzano, S.; González-Paramás, A.M.; Santos-Buelga, C.; Bartolomé, B.; Moreno-Arribas, M.V. An integrated view of the effects of wine polyphenols and their relevant metabolites on gut and host health. Molecules 2017, 22, 99. [Google Scholar] [CrossRef]
- Oliphant, K.; Allen-Vercoe, E. Macronutrient metabolism by the human gut microbiome: Major fermentation by-products and their impact on host health. Microbiome 2019, 7, 91. [Google Scholar] [CrossRef] [PubMed]
- Woting, A.; Blaut, M. The intestinal microbiota in metabolic disease. Nutrients 2016, 8, 202. [Google Scholar] [CrossRef] [PubMed]
- Portune, K.; Del Pulgar, E.M.G.; Cerrudo, V.; Sanz, Y.; Benítez-Páez, A. Gut microbiota, diet, and obesity-related disorders-The good, the bad, and the future challenges. Mol. Nutr. Food Res. 2017, 61, 1600252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biesalski, H.K. Nutrition meets the microbiome: Micronutrients and the microbiota. Ann. N. Y. Acad. Sci. 2016, 1372, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Klinder, A.; Karlsson, P.C.; Clune, Y.; Hughes, R.; Glei, M.; Rafter, J.J.; Rowland, I.; Collins, J.K.; Pool-Zobel, B.L. Fecal water as a non-invasive biomarker in nutritional intervention: Comparison of preparation methods and refinement of different endpoints. Nutr. Cancer 2007, 57, 158–167. [Google Scholar] [CrossRef]
- Gill, C.I.; Heavey, P.; McConville, E.; Bradbury, I.; Fässler, C.; Mueller, S.; Cresci, A.; Dore, J.; Norin, E.; Rowland, I. Effect of fecal water on an in vitro model of colonic mucosal barrier function. Nutr. Cancer 2007, 57, 59–65. [Google Scholar] [CrossRef]
- Pearson, J.R.; Gill, C.I.; Rowland, I.R. Diet, fecal water, and colon cancer—Development of a biomarker. Nutr. Rev. 2009, 67, 509–526. [Google Scholar] [CrossRef]
- Mundi, M.; Mikal, K.M.; Ahmed, O.H.; Sarbini, S.R. A review on the effects of prebiotics on cell toxicity and integrity. Int. J. Food Prop. 2017, 20, S1045–S1052. [Google Scholar] [CrossRef] [Green Version]
- Beaumont, M.; Portune, K.J.; Steuer, N.; Cerrudo, V.; Dumont, F.; Mancano, G.; Khodorova, N.; Andriamihaja, M.; Airinei, G.; Benamouzig, R.; et al. Quantity and source of dietary protein influence metabolite production by gut microbiota and rectal mucosa gene expression: A randomized, parallel, double-blind trial in overweight humans. Am. J. Clin. Nutr. 2017, 106, 1005–1019. [Google Scholar] [CrossRef] [Green Version]
- Ijssennagger, N.; Derrien, M.; Van Doorn, G.M.; Rijnierse, A.; Bogert, B.V.D.; Muller, M.; Dekker, J.; Kleerebezem, M.; Van Der Meer, R. Dietary heme alters microbiota and mucosa of mouse colon without functional changes in host-microbe cross-Talk. PLoS ONE 2012, 7, e49868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Federici, E.; Prete, R.; Lazzi, C.; Pellegrini, N.; Moretti, M.; Corsetti, A.; Cenci, G. Bacterial composition, genotoxicity, and cytotoxicity of fecal samples from individuals consuming omnivorous or vegetarian diets. Front. Microbiol. 2017, 8, 300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oßwald, K.; Becker, T.W.; Grimm, M.; Jahreis, G.; Pool-Zobel, B.L. Inter- and intra-individual variation of faecal water—Genotoxicity in human colon cells. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2000, 472, 59–70. [Google Scholar] [CrossRef]
- Muñoz-González, I.; Jiménez-Girón, A.; Martín-Álvarez, P.J.; Bartolomé, B.; Moreno-Arribas, M.V. Profiling of microbial-derived phenolic metabolites in human feces after moderate red wine intake. J. Agric. Food Chem. 2013, 61, 9470–9479. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Patán, F.; Monagas, M.; Moreno-Arribas, M.V.; Bartolomé, B. Determination of microbial phenolic acids in human faeces by UPLC-ESI-TQ MS. J. Agric. Food Chem. 2011, 59, 2241–2247. [Google Scholar] [CrossRef] [PubMed]
- Cueva, C.; Jiménez-Girón, A.; Muñoz-González, I.; Esteban-Fernández, A.; Gil-Sánchez, I.; Dueñas, M.; Martin-Alvarez, P.; Pozo-Bayón, M.; Bartolomé, B.; Moreno-Arribas, M.V. Application of a new Dynamic Gastrointestinal Simulator (SIMGI) to study the impact of red wine in colonic metabolism. Food Res. Int. 2015, 72, 149–159. [Google Scholar] [CrossRef]
- King, R.A.; May, B.; Davies, D.A.; Bird, A.R. Measurement of phenol and p-cresol in urine and feces using vacuum microdistillation and high-performance liquid chromatography. Anal. Biochem. 2009, 384, 27–33. [Google Scholar] [CrossRef]
- Diether, N.E.; Willing, B.P. Microbial fermentation of dietary protein: An important factor in diet–Microbe–Host interaction. Microorganism 2019, 7, 19. [Google Scholar] [CrossRef] [Green Version]
- Andriamihaja, M.; Lan, A.; Beaumont, M.; Audebert, M.; Wong, X.; Yamada, K.; Yin, J.; Tomé, D.; Carrasco, C.; Gotteland, M.; et al. The deleterious metabolic and genotoxic effects of the bacterial metabolite p-cresol on colonic epithelial cells. Free. Radic. Biol. Med. 2015, 85, 219–227. [Google Scholar] [CrossRef]
- Al Hinai, E.A.; Kullamethee, P.; Rowland, I.R.; Swann, J.; Walton, G.; Commane, D.M. Modelling the role of microbial p-cresol in colorectal genotoxicity. Gut Microbes 2019, 10, 398–411. [Google Scholar] [CrossRef] [Green Version]
- Pedersen, G.; Brynskov, J.; Saermark, T. Phenol toxicity and conjugation in human colonic epithelial cells. Scand. J. Gastroenterol. 2002, 37, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Schnäbele, K.; Briviba, K.; Bub, A.; Roser, S.; Pool-Zobel, B.L.; Rechkemmer, G. Effects of carrot and tomato juice consumption on faecal markers relevant to colon carcinogenesis in humans. Br. J. Nutr. 2008, 99, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Bastide, N.M.; Naud, N.; Nassy, G.; Vendeuvre, J.-L.; Taché, S.; Guéraud, F.; Hobbs, D.; Kuhnle, G.G.; Corpet, D.; Pierre, F.H.F. Red wine and pomegranate extracts suppress cured meat promotion of colonic mucin-depleted foci in carcinogen-induced rats. Nutr. Cancer 2017, 69, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Adebola, O.; Corcoran, O.; Morgan, W. Protective effects of prebiotics inulin and lactulose from cytotoxicity and genotoxicity in human colon adenocarcinoma cells. Food Res. Int. 2013, 52, 269–274. [Google Scholar] [CrossRef]
- Windey, K.; François, I.; Broekaert, W.; De Preter, V.; Delcour, J.A.; Louat, T.; Herman, J.; Verbeke, K. High dose of prebiotics reduces fecal water cytotoxicity in healthy subjects. Mol. Nutr. Food Res. 2014, 58, 2206–2218. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.T.; Cheng, H.C.; Chen, H.L. Ameliorative effects of konjac glucomannan on human faecal beta-glucuronidase activity, secondary bile acid levels and faecal water toxicity towards Caco-2 cells. Br. J. Nutr. 2011, 105, 593–600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scholtens, P.A.; Alles, M.S.; Willemsen, L.E.; Van Den Braak, C.; Bindels, J.G.; Boehm, G.; Govers, M.J. Dietary fructo-oligosaccharides in healthy adults do not negatively affect faecal cytotoxicity: A randomised, double-blind, placebo-controlled crossover trial. Br. J. Nutr. 2006, 95, 1143–1149. [Google Scholar] [CrossRef] [Green Version]
- Klewicka, E.; Nowak, A.; Zduńczyk, Z.; Cukrowska, B.; Blasiak, J. Protective effect of lactofermented beetroot juice against aberrant crypt foci formation and genotoxicity of fecal water in rats. Exp. Toxicol. Pathol. 2012, 64, 599–604. [Google Scholar] [CrossRef]
- Slizewska, K.; Nowak, A.; Smulikowska, S. Probiotic preparation reduces faecal water genotoxicity and cytotoxicity in chickens fed ochratoxin A contaminated feed (in vivo study). Acta Biochim. Pol. 2016, 63, 281–286. [Google Scholar] [CrossRef]
- Oberreuther-Moschner, D.L.; Jahreis, G.; Rechkemmer, G.; Pool-Zobel, B.L. Dietary intervention with the probiotics Lactobacillus acidophilus 145 and Bifidobacterium longum 913 modulates the potential of human faecal water to induce damage in HT29clone19A cells. Br. J. Nutr. 2004, 91, 925–932. [Google Scholar] [CrossRef] [Green Version]
- Burns, A.J.; Rowland, I.R. Antigenotoxicity of probiotics and prebiotics on faecal water-induced DNA damage in human colon adenocarcinoma cells. Mutat. Res. 2004, 551, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Oliva, A.; Miele, M.C.; De Angelis, M.; Costantini, S.; Mascellino, M.T.; Mastroianni, C.; Vullo, V.; D’Ettorre, G. Antibacterial effectiveness of fecal water and in vitro activity of a multi-strain probiotic formulation against multi-drug resistant microorganisms. Microorganism 2020, 8, 332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blachier, F.; Beaumont, M.; Portune, K.J.; Steuer, N.; Lan, A.; Audebert, M.; Khodorova, N.; Andriamihaja, M.; Airinei, G.; Benamouzig, R.; et al. High-protein diets for weight management: Interactions with the intestinal microbiota and consequences for gut health. A position paper by the my new gut study group. Clin. Nutr. 2019, 38, 1012–1022. [Google Scholar] [CrossRef]
- Daniela, E.; Soldi, S.; Malavolti, M.; Giovanni, A.; Meynier, A.; Sophie, V.; Cristina, C.M.; Casiraghi, M.C. Fecal water genotoxicity in healthy free-living young Italian people. Food Chem. Toxicol. 2014, 64, 104–109. [Google Scholar] [CrossRef]
- Jiménez-Girón, A.; Ibáñez, C.; Cifuentes, A.; Simó, C.; Muñoz-González, I.; Martín-Álvarez, P.J.; Bartolomé, B.; Moreno-Arribas, M.V. Faecal metabolomic fingerprint after moderate consumption of red wine by healthy subjects. J. Proteome Res. 2015, 14, 897–905. [Google Scholar] [CrossRef]
- Esteban-Fernández, A.; Ibañez, C.; Simó, C.; Bartolomé, B.; Moreno-Arribas, M.V. An UHPLC-TOF MS metabolomic approach to study the impact of moderate red wine consumption on urinary metabolome. J. Proteome Res. 2018, 17, 1624–1635. [Google Scholar] [CrossRef]
- Rowland, I.; Gibson, G.R.; Heinken, A.; Scott, K.P.; Swann, J.; Thiele, I.; Tuohy, K.M. Gut microbiota functions: Metabolism of nutrients and other food components. Eur. J. Nutr. 2018, 57, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Niccolai, E.; Baldi, S.; Ricci, F.; Russo, E.; Nannini, G.; Menicatti, M.; Poli, G.; Taddei, A.; Bartolucci, G.; Calabrò, A.S.; et al. Evaluation and comparison of short chain fatty acids composition in gut diseases. World J. Gastroenterol. 2019, 25, 5543–5558. [Google Scholar] [CrossRef]

| Phenol | p-Cresol | |||
|---|---|---|---|---|
| Time | HT-29 | HCT 116 | HT-29 | HCT 116 |
| 4 h | 15.8 | 23.84 | 5.40 | 5.64 |
| 24 h | 6.16 | 11.41 | 2.13 | 4.16 |
| Control Group (n = 3) | Intervention Group (n = 8) | ||||||
|---|---|---|---|---|---|---|---|
| tinitial | tfinal | p Value * | tinitial | tfinal | p Value * | ||
| Time | Dilution | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | ||
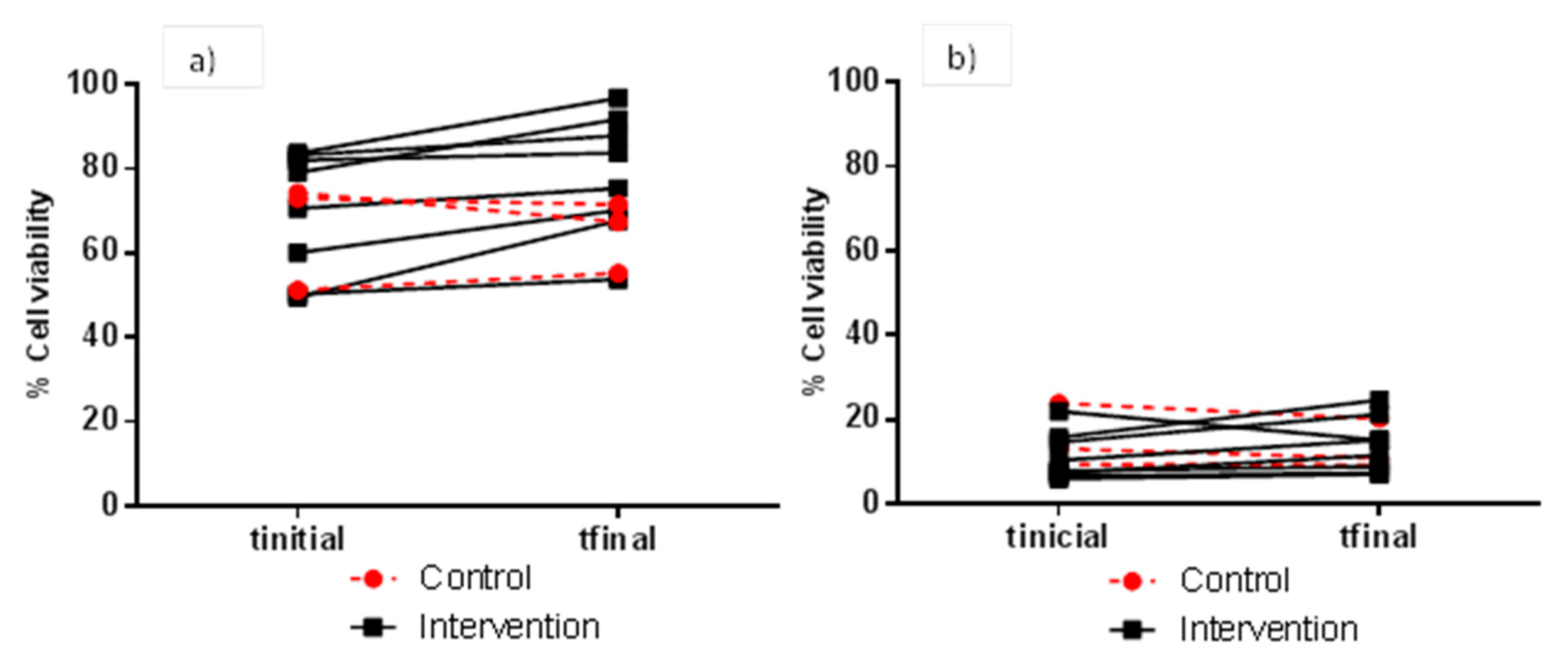
| 4 h | 1:1 | 13.9 ± 2.57 | 16.3 ± 7.39 | 0.556 | 21.0 ± 22.0 | 23.3 ± 19.8 | 0.081 |
| 1:2 | 66.2 ± 12.9 | 64.7 ± 8.46 | 0.272 | 69.8 ± 14.6 | 78.4 ± 14.3 | 0.004 * | |
| 1:5 | 82.7 ± 8.17 | 81.2 ± 4.09 | 0.808 | 88.7 ± 6.31 | 91.2 ± 6.03 | 0.047 * | |
| 1:10 | 91.4 ± 2.55 | 89.7 ± 4.91 | 0.568 | 93.1 ± 3.96 | 96.7 ± 2.83 | 0.020 * | |
| 24 h | 1:1 | 6.29 ± 1.88 | 11.3 ± 6.37 | 0.193 | 5.51 ± 0.63 | 6.39 ± 0.80 | 0.005 * |
| 1:2 | 15.5 ± 7.54 | 15.2 ± 5.5 | 0.194 | 11.4 ± 5.66 | 14.0 ± 6.47 | 0.169 | |
| 1:5 | 36.2 ± 16.5 | 41.8 ± 24.7 | 0.358 | 26.4 ± 22.8 | 32.4 ± 21.1 | 0.114 | |
| 1:10 | 60.7 ± 21.7 | 64.1 ± 28.4 | 0.530 | 48.2 ± 23.9 | 54.0 ± 21.6 | 0.072 | |
| Control Group (n = 3) | Intervention Group (n = 8) | ||||||
|---|---|---|---|---|---|---|---|
| tinitial | tfinal | p Value * | tinitial | tfinal | p Value * | ||
| Time | Dilution | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | ||
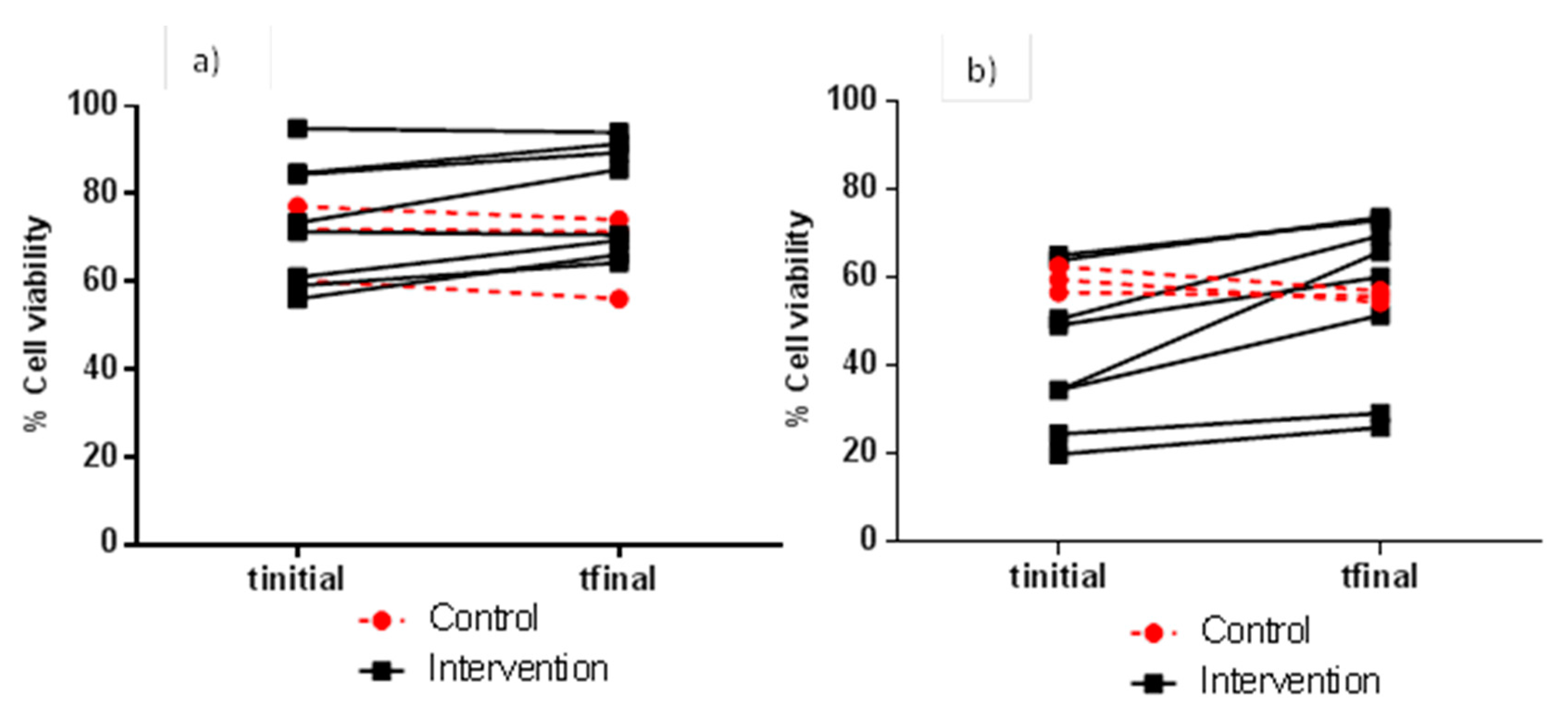
| 4 h | 1:1 | 26.9 ± 3.62 | 27.1 ± 7.1 | 0.962 | 27.2 ± 17.1 | 27.9 ± 16.8 | 0.806 |
| 1:2 | 62.5 ± 7.52 | 58.6 ± 5.1 | 0.343 | 59.0 ± 19.4 | 62.4 ± 14.6 | 0.514 | |
| 1:5 | 69.9 ± 8.62 | 67.3 ± 9.7 | 0.143 | 73.2 ± 14.0 | 78.9 ± 12.4 | 0.011 * | |
| 1:10 | 76.6 ± 3.04 | 73.9 ± 8.1 | 0.563 | 81.0 ± 11.8 | 84.0 ± 13.0 | 0.079 | |
| 24 h | 1:1 | 11.9 ± 5.00 | 12.6 ± 5.6 | 0.234 | 8.92 ± 2.51 | 10.0 ± 2.73 | 0.088 |
| 1:2 | 35.1 ± 17.0 | 33.9 ± 14.7 | 0.654 | 23.9 ± 13.5 | 34.1 ± 15.2 | 0.000 * | |
| 1:5 | 59.6 ± 2.98 | 55.8 ± 1.3 | 0.126 | 42.7 ± 17.1 | 56.1 ± 19.1 | 0.004 * | |
| 1:10 | 71.4 ± 4.765 | 66.1 ± 3.7 | 0.148 | 66.9 ± 18.7 | 79.0 ± 15.2 | 0.004 * | |
| HT-29 (1:2) | HCT 116 (1:5) | ||||
|---|---|---|---|---|---|
| Time (h) | R2 | p Value * | R2 | p Value * | |
| SCFA | |||||
| Acetic acid | 4 | 0.059 | 0.366 | 0.121 | 0.186 |
| 24 | 0.022 | 0.585 | 0.005 | 0.791 | |
| Propionic acid | 4 | 0.020 | 0.597 | 0.279 | 0.035 * |
| 24 | 0.006 | 0.781 | 0.049 | 0.408 | |
| Butyric acid | 4 | 0.010 | 0.711 | 0.163 | 0.121 |
| 24 | 0.005 | 0.788 | 0.001 | 0.910 | |
| Isobutyric acid | 4 | 0.026 | 0.511 | 0.499 | 0.002 * |
| 24 | 0.069 | 0.327 | 0.281 | 0.035 * | |
| Isovaleric acid | 4 | 0.016 | 0.643 | 0.337 | 0.018 * |
| 24 | 0.092 | 0.255 | 0.348 | 0.016 * | |
| Phenolic acids | |||||
| Phenylacetic acid | 4 | 0.100 | 0.234 | 0.517 | 0.002 * |
| 24 | 0.143 | 0.149 | 0.494 | 0.002 * | |
| Phenylpropionic acid | 4 | 0.060 | 0.246 | 0.357 | 0.015 * |
| 24 | 0.059 | 0.244 | 0.048 | 0.414 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zorraquín-Peña, I.; González de Llano, D.; Tamargo, A.; Moreno-Arribas, M.V.; Bartolomé, B. Moderate Wine Consumption Reduces Faecal Water Cytotoxicity in Healthy Volunteers. Nutrients 2020, 12, 2716. https://doi.org/10.3390/nu12092716
Zorraquín-Peña I, González de Llano D, Tamargo A, Moreno-Arribas MV, Bartolomé B. Moderate Wine Consumption Reduces Faecal Water Cytotoxicity in Healthy Volunteers. Nutrients. 2020; 12(9):2716. https://doi.org/10.3390/nu12092716
Chicago/Turabian StyleZorraquín-Peña, Irene, Dolores González de Llano, Alba Tamargo, M. Victoria Moreno-Arribas, and Begoña Bartolomé. 2020. "Moderate Wine Consumption Reduces Faecal Water Cytotoxicity in Healthy Volunteers" Nutrients 12, no. 9: 2716. https://doi.org/10.3390/nu12092716
APA StyleZorraquín-Peña, I., González de Llano, D., Tamargo, A., Moreno-Arribas, M. V., & Bartolomé, B. (2020). Moderate Wine Consumption Reduces Faecal Water Cytotoxicity in Healthy Volunteers. Nutrients, 12(9), 2716. https://doi.org/10.3390/nu12092716






