Vitamin K and Kidney Transplantation
Abstract
1. Introduction
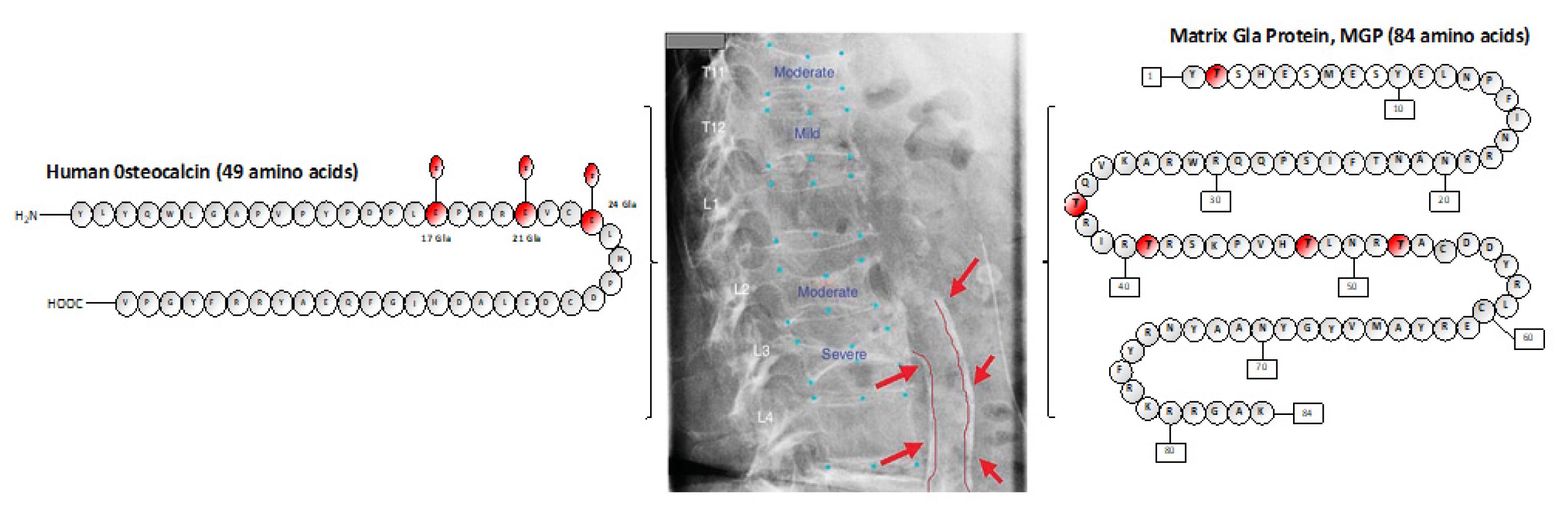
2. Vitamin K, a Family of Vitamers: Types, Status, and Vitamin-K-Dependent Proteins (VKDPs)
2.1. Vitamin K and Cardiovascular Disease in Kidney Transplantation
2.2. Vitamin K and Bone Fractures in Kidney Transplantation
2.3. Vitamin K and Cancer in Kidney Transplantation
2.4. Growth Arrest-Specific Protein 6 (Gas6)
2.5. Periostin
2.6. Vitamin K Administration in Cancer Prevention and Treatment
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lorent, M.; Foucher, Y.; Kerleau, K.; Brouard, S.; Baayen, C.; Lebouter, S.; Naesens, M.; Bestard Matamoros, O.; Åsberg, A.; Giral, M. The EKiTE network (epidemiology in kidney transplantation—A European validated database): An initiative epidemiological and translational European collaborative research. BMC Nephrol. 2019, 20, 365. [Google Scholar] [CrossRef] [PubMed]
- Savoj, J.; Becerra, B.; Kim, J.K.; Fusaro, M.; Gallieni, M.; Lombardo, D.; Lau, W.L. Utility of cardiac biomarkers in the setting of kidney disease. Nephron 2019, 141, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.-F.; Trenson, S.; Thijs, L.; Huang, Q.-F.; Zhang, Z.-Y.; Yang, W.; Moliterno, P.; Allegaert, K.; Boggia, J.; Janssens, S.; et al. Desphospho-uncarboxylated matrix Gla protein is a novel circulating biomarker predicting deterioration of renal function in the general population. Nephrol. Dial. Transplant. 2018, 33, 1122–1128. [Google Scholar] [CrossRef] [PubMed]
- Van Ballegooijen, A.J.; Beulens, J.W.J.; Keyzer, C.A.; Navis, G.J.; Berger, S.P.; De Borst, M.H.; Vervloet, M.G.; Bakker, S.J.L. Joint association of vitamins D and K status with long-term outcomes in stable kidney transplant recipients. Nephrol. Dial. Transplant. 2020, 35, 706–714. [Google Scholar] [CrossRef]
- Keyzer, C.A.; Vermeer, C.; Joosten, M.M.; Knapen, M.H.; Drummen, N.E.; Navis, G.; Bakker, S.J.; De Borst, M.H. Vitamin K status and mortality after kidney transplantation: A cohort study. Am. J. Kidney Dis. 2015, 65, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Ball, A.M.; Gillen, D.L.; Sherrard, D.; Weiss, N.S.; Emerson, S.S.; Seliger, S.L.; Kestenbaum, B.R.; Stehman-Breen, C. Risk of hip fracture among dialysis and renal transplant recipients. JAMA 2002, 288, 3014–3018. [Google Scholar] [CrossRef]
- Evenepoel, P.; Claes, K.; Meijers, B.; Laurent, M.R.; Bammens, B.; Naesens, M.; Sprangers, B.; Pottel, H.; Cavalier, E.; Kuypers, D. Poor vitamin K Status is associated with low bone mineral density and increased fracture risk in end-stage renal disease. J. Bone Miner. Res. 2019, 34, 262–269. [Google Scholar] [CrossRef]
- Shearer, M.J.; Newman, P. Metabolism and cell biology of vitamin K. Thromb. Haemost. 2008, 100, 530–547. [Google Scholar] [CrossRef]
- Fusaro, M.; Gallieni, M.; Rizzo, M.A.; Stucchi, A.; Delanaye, P.; Cavalier, E.; Moyses, R.M.; Jorgetti, V.; Iervasi, G.; Giannini, S.; et al. Vitamin K plasma levels determination in human health. Clin. Chem. Lab. Med. 2017, 55, 789–799. [Google Scholar] [CrossRef]
- Booth, S.L. Vitamin K: Food composition and dietary intakes. Food Nutr. Res. 2012, 56, 5505. [Google Scholar] [CrossRef]
- Società Italiana di Nutrizione Umana—SINU. Livelli di Assunzione di Riferimento di Nutrienti ed Energia per la Popolazione Italiana, (LARN) Revisione 2014; SICS: Roma, Italy, 2014. [Google Scholar]
- Conseil Supérieur de la Santé. Recommandations nutritionnelles pour la Belgique—CSS n° 9285; Conseil Supérieur de la Santé: Brussels, Belgium, 2016; pp. 160–164. Available online: https://www.health.belgium.be/sites/default/files/uploads/fields/fpshealth_theme_file/9285_avis_rec_nutr_corr_170105_0.pdf (accessed on 3 September 2020).
- Fusaro, M.; Gallieni, M.; Porta, C.; Nickolas, T.L.; Khairallah, P. Vitamin K effects in human health: New insights beyond bone and cardiovascular health. J. Nephrol. 2020, 33, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Silaghi, C.N.; Ilyés, T.; Filip, V.P.; Farcaș, M.; Van Ballegooijen, A.J.; Crăciun, A.M. Vitamin K dependent proteins in kidney disease. Int. J. Mol. Sci. 2019, 20, 1571. [Google Scholar] [CrossRef] [PubMed]
- Fusaro, M.; Mereu, M.C.; Aghi, A.; Iervasi, G.; Gallieni, M. Vitamin K and bone. Clin. Cases Miner. Bone Metab. 2017, 14, 200–206. [Google Scholar] [CrossRef]
- Azuma, K.; Casey, S.C.; Urano, T.; Horie-Inoue, K.; Ouchi, Y.; Blumberg, B.; Inoue, S. Pregnane X receptor knockout mice display aging-dependent wearing of articular cartilage. PLoS ONE 2015, 10, e0119177. [Google Scholar] [CrossRef] [PubMed]
- Sadowski, J.A.; Hood, S.J.; Dallal, G.E.; Garry, P.J. Phylloquinone in plasma from elderly and young adults: Factors influencing its concentration. Am. J. Clin. Nutr. 1989, 50, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Pilkey, R.M.; Morton, A.R.; Boffa, M.B.; Noordhof, C.; Day, A.G.; Su, Y.; Miller, L.M.; Koschinsky, M.L.; Booth, S.L. Subclinical vitamin K deficiency in hemodialysis patients. Am. J. Kidney Dis. 2007, 49, 432–439. [Google Scholar] [CrossRef] [PubMed]
- McCabe, K.M.; Adams, M.A.; Holden, R.M. Vitamin K status in chronic kidney disease. Nutrients 2013, 5, 4390–4398. [Google Scholar] [CrossRef] [PubMed]
- Kuwabara, A.; Tanaka, K.; Tsugawa, N.; Nakase, H.; Tsuji, H.; Shide, K.; Kamao, M.; Chiba, T.; Inagaki, N.; Okano, T.; et al. High prevalence of vitamin K and D deficiency and decreased BMD in inflammatory bowel disease. Osteoporos. Int. 2009, 20, 935–942. [Google Scholar] [CrossRef] [PubMed]
- Riphagen, I.J.; van der Molen, J.C.; Van Faassen, M.H.J.R.; Navis, G.; De Borst, M.H.; Muskiet, F.A.J.; de Jong, W.H.; Bakker, S.J.; Kema, I.P. Measurement of plasma vitamin K1 (phylloquinone) and K2 (menaquinones-4 and -7) using HPLC-tandem mass spectrometry. Clin. Chem. Lab. Med. 2016, 54, 1201–1210. [Google Scholar] [CrossRef]
- Fusaro, M.; D’Alessandro, C.; Noale, M.; Tripepi, G.; Plebani, M.; Veronese, N.; Iervasi, G.; Giannini, S.; Rossini, M.; Tarroni, G.; et al. Low vitamin K1 intake in haemodialysis patients. Clin. Nutr. 2017, 36, 601–607. [Google Scholar] [CrossRef]
- Cranenburg, E.C.M.; Schurgers, L.J.; Uiterwijk, H.H.; Beulens, J.W.; Dalmeijer, G.W.; Westerhuis, R.; Magdeleyns, E.J.; Herfs, M.; Vermeer, C.; Laverman, G.D. Vitamin K intake and status are low in hemodialysis patients. Kidney Int. 2012, 82, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Jansz, T.T.; Neradova, A.; van Ballegooijen, A.J.; Verhaar, M.C.; Vervloet, M.G.; Schurgers, L.J.; van Jaarsveld, B.C. The role of kidney transplantation and phosphate binder use in vitamin K status. PLoS ONE 2018, 13, e0203157. [Google Scholar] [CrossRef] [PubMed]
- Fusaro, M.; Noale, M.; Viola, V.; Galli, F.; Tripepi, G.; Vajente, N.; Plebani, M.; Zaninotto, M.; Guglielmi, G.; Miotto, D.; et al. Vitamin K, vertebral fractures, vascular calcifications, and mortality: VItamin K Italian (VIKI) dialysis study. J. Bone Miner. Res. 2012, 27, 2271–2278. [Google Scholar] [CrossRef] [PubMed]
- Fusaro, M.; Aghi, A.; Khairallah, P.; Gallieni, M.; Cozzolino, M.G.; Russo, D.; Mereu, M.C.; Ravera, M.; Tripepi, G.L.; Nickolas, T. Sevelamer use is associated with decreased vitamin K levels in hemodialysis patients: Results from the Vitamin K Italian (VIKI) study. Abstract FR-PO146. J. Am. Soc. Nephrol. 2019, 30, 471. [Google Scholar]
- Weijs, B.; Blaauw, Y.; Rennenberg, R.J.; Schurgers, L.J.; Timmermans, C.C.; Pison, L.; Nieuwlaat, R.; Hofstra, L.; Kroon, A.A.; Wildberger, J.; et al. Patients using vitamin K antagonists show increased levels of coronary calcification: An observational study in low-risk atrial fibrillation patients. Eur. Hear. J. 2011, 32, 2555–2562. [Google Scholar] [CrossRef]
- Gage, B.F.; Birman-Deych, E.; Radford, M.J.; Nilasena, D.S.; Binder, E.F. Risk of osteoporotic fracture in elderly patients taking warfarin. Arch. Intern. Med. 2006, 166, 241–246. [Google Scholar] [CrossRef]
- Fusaro, M.; Tripepi, G.; Noale, M.; Plebani, M.; Zaninotto, M.; Piccoli, A.; Naso, A.; Miozzo, D.; Giannini, S.; Avolio, M.; et al. Prevalence of vertebral fractures, vascular calcifications, and mortality in warfarin treated hemodialysis patients. Curr. Vasc. Pharmacol. 2015, 13, 248–258. [Google Scholar] [CrossRef]
- Lin, M.C.; Streja, E.; SooHoo, M.; Hanna, M.; Savoj, J.; Kalantar-Zadeh, K.; Lau, W.L. Warfarin use and increased mortality in end-stage renal disease. Am. J. Nephrol. 2017, 46, 249–256. [Google Scholar] [CrossRef]
- Fusaro, M.; Giannini, S.; Gallieni, M.; Noale, M.; Tripepi, G.; Rossini, M.; Messa, P.; Rigotti, P.; Pati, T.; Barbisoni, F.; et al. Calcimimetic and vitamin D analog use in hemodialyzed patients is associated with increased levels of vitamin K dependent proteins. Endocrine 2016, 51, 333–341. [Google Scholar] [CrossRef]
- Nguyen, P.T.; Coche, E.; Goffin, E.; Beguin, C.; Vlassenbroek, A.; Devuyst, O.; Robert, A.; Jadoul, M. Prevalence and determinants of coronary and aortic calcifications assessed by chest CT in renal transplant recipients. Am. J. Nephrol. 2007, 27, 329–335. [Google Scholar] [CrossRef]
- Fusaro, M.; Khairallah, P.; Aghi, A.; Plebani, M.; Zaninotto, M.; Cosma, C.; Farias, M.A.A.; Cortez, N.E.; Tripepi, G.L.; Nickolas, T. Vitamin K-dependent proteins after kidney transplantation: Results from a prospective study. Abstract FR-PO167. J. Am. Soc. Nephrol. 2019, 30, 477. [Google Scholar]
- Lees, J.S.; Chapman, F.A.; Witham, M.D.; Jardine, A.G.; Mark, P.B. Vitamin K status, supplementation and vascular disease: A systematic review and meta-analysis. Heart 2018, 105, 938–945. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-G.; Sheng, L.-T.; Zhang, Y.-B.; Cao, A.-L.; Lai, Y.-W.; Kunutsor, S.K.; Jiang, L.; Pan, A. Association of vitamin K with cardiovascular events and all-cause mortality: A systematic review and meta-analysis. Eur. J. Nutr. 2019, 58, 2191–2205. [Google Scholar] [CrossRef] [PubMed]
- Mansour, A.; Hariri, E.; Daaboul, Y.; Korjian, S.; El Alam, A.; Protogerou, A.D.; Kilany, H.; Karam, A.; Stephan, A.; Bahous, S.A. Vitamin K2 supplementation and arterial stiffness among renal transplant recipients—A single-arm, single-center clinical trial. J. Am. Soc. Hypertens. 2017, 11, 589–597. [Google Scholar] [CrossRef]
- Keyzer, C.A. Placebo-controlled double-blind randomized controlled trial investigating vitamin K supplementation on vascular calcification propensity in vitamin K deficient renal transplant recipients. EudraCT Number: 2019-004906-88. Available online: https://www.clinicaltrialsregister.eu/ctr-search/trial/2019-004906-88/NL/ (accessed on 3 September 2020).
- Boxma, P.Y.; Berg, E.V.D.; Geleijnse, J.M.; Laverman, G.D.; Schurgers, L.J.; Vermeer, C.; Kema, I.P.; Muskiet, F.A.; Navis, G.; Bakker, S.J.L.; et al. Vitamin K intake and plasma desphospho-uncarboxylated matrix Gla-protein levels in kidney transplant recipients. PLoS ONE 2012, 7, e47991. [Google Scholar] [CrossRef]
- Witham, M.D.; Lees, J.S.; White, M.; Band, M.; Bell, S.; Chantler, D.J.; Ford, I.; Fulton, R.L.; Kennedy, G.; Littleford, R.C.; et al. Vitamin K supplementation to improve vascular stiffness in CKD: The K4Kidneys randomized controlled trial. J. Am. Soc. Nephrol. 2020. [Google Scholar] [CrossRef]
- Førli, L.; Bollerslev, J.; Simonsen, S.; Isaksen, G.A.; Kvamsdal, K.E.; Godang, K.; Gadeholt, G.; Pripp, A.H.; Bjortuft, O. Dietary vitamin K2 supplement improves bone status after lung and heart transplantation. Transplantation 2010, 89, 458–464. [Google Scholar] [CrossRef]
- Alem, A.M.; Sherrard, D.J.; Gillen, D.L.; Weiss, N.S.; Beresford, S.A.; Heckbert, S.R.; Wong, C.; Stehman-Breen, C. Increased risk of hip fracture among patients with end-stage renal disease. Kidney Int. 2000, 58, 396–399. [Google Scholar] [CrossRef]
- Jadoul, M.; Albert, J.M.; Akiba, T.; Akizawa, T.; Arab, L.; Bragg-Gresham, J.; Mason, N.; Prutz, K.-G.; Young, E.; Pisoni, R. Incidence and risk factors for hip or other bone fractures among hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study. Kidney Int. 2006, 70, 1358–1366. [Google Scholar] [CrossRef]
- Nagata, Y.; Inaba, M.; Imanishi, Y.; Okazaki, H.; Yamada, S.; Mori, K.; Shoji, S.; Koyama, H.; Okuno, S. Increased undercarboxylated osteocalcin/intact osteocalcin ratio in patients undergoing hemodialysis. Osteoporos. Int. 2015, 26, 1053–1061. [Google Scholar] [CrossRef]
- Ducy, P.; Desbois, C.; Boyce, B.; Pinero, G.; Story, B.; Dunstan, C.; Smith, E.; Bonadio, J.; Goldstein, S.; Gundberg, C.; et al. Increased bone formation in osteocalcin-deficient mice. Nature 1996, 382, 448–452. [Google Scholar] [CrossRef] [PubMed]
- Hirota, Y.; Suhara, Y. New aspects of vitamin K research with synthetic ligands: Transcriptional activity via SXR and neural differentiation Activity. Int. J. Mol. Sci. 2019, 20, 3006. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhao, L.; Wang, N.; Li, J.; He, F.; Li, X.; Wu, S. Unexpected role of matrix Gla protein in osteoclasts: Inhibiting osteoclast differentiation and bone resorption. Mol. Cell. Boil. 2019, 39, e00012-19. [Google Scholar] [CrossRef] [PubMed]
- Vassalle, C.; Mazzone, A. Bone loss and vascular calcification: A bi-directional interplay? Vasc. Pharmacol. 2016, 86, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Gao, B.; Yasui, T.; Li, Y.; Liu, T.; Mao, X.; Hirose, M.; Wu, Y.; Yu, D.; Zhu, Q.; et al. Matrix Gla protein is involved in crystal formation in kidney of hyperoxaluric rats. Kidney Blood Press. Res. 2013, 37, 15–23. [Google Scholar] [CrossRef]
- Cheung, A.M.; Tile, L.; Lee, Y.; Tomlinson, G.; Hawker, G.; Scher, J.; Hu, H.; Vieth, R.; Thompson, L.; Jamal, S.; et al. Vitamin K supplementation in postmenopausal women with osteopenia (ECKO Trial): A randomized controlled trial. PLoS Med. 2008, 5, e196. [Google Scholar] [CrossRef]
- Mott, A.; Bradley, T.; Wright, K.; Cockayne, E.S.; Shearer, M.J.; Adamson, J.; Lanham-New, S.A.; Torgerson, D.J. Effect of vitamin K on bone mineral density and fractures in adults: An updated systematic review and meta-analysis of randomised controlled trials. Osteoporos. Int. 2019, 30, 1543–1559. [Google Scholar] [CrossRef]
- Cohen-Bucay, A.; Gordon, C.E.; Francis, J.M. Non-immunological complications following kidney transplantation. F1000Research 2019, 8. [Google Scholar] [CrossRef]
- Engels, E.A.; Pfeiffer, R.M.; Fraumeni, J.F.; Kasiske, B.L.; Israni, A.K.; Snyder, J.J.; Wolfe, R.A.; Goodrich, N.P.; Bayakly, A.R.; Clarke, C.A.; et al. Spectrum of cancer risk among US solid organ transplant recipients. JAMA 2011, 306, 1891–1901. [Google Scholar] [CrossRef]
- Sasaki, T.; Knyazev, P.G.; Clout, N.J.; Cheburkin, Y.; Göhring, W.; Ullrich, A.; Timpl, R.; Hohenester, E. Structural basis for Gas6–Axl signalling. EMBO J. 2006, 25, 80–87. [Google Scholar] [CrossRef]
- Buehler, M.; Tse, B.W.-C.; Leboucq, A.; Jacob, F.; Caduff, R.; Fink, D.; Goldstein, D.R.; Heinzelmann-Schwarz, V. Meta-analysis of microarray data identifies GAS6 expression as an independent predictor of poor survival in ovarian cancer. BioMed Res. Int. 2013, 2013, 238284. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Shiozawa, Y.; Wang, J.; McGregor, N.; Dai, J.; Park, S.I.; Berry, J.E.; Havens, A.M.; Joseph, J.; Kim, J.K.; et al. Prevalence of prostate cancer metastases after intravenous inoculation provides clues into the molecular basis of dormancy in the bone marrow microenvironment. Neoplasia 2012, 14, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Sainaghi, P.P.; Castello, L.; Bergamasco, L.; Galletti, M.; Bellosta, P.; Avanzi, G.C. Gas6 induces proliferation in prostate carcinoma cell lines expressing the Axl receptor. J. Cell. Physiol. 2005, 204, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Mao, S.; Wu, Y.; Wang, R.; Guo, Y.; Bi, D.; Ma, W.; Zhang, W.; Zhang, J.; Yan, Y.; Yao, X. Overexpression of GAS6 promotes cell proliferation and invasion in bladder cancer by activation of the PI3K/AKT pathway. OncoTargets Ther. 2020, 13, 4813–4824. [Google Scholar] [CrossRef]
- Norris, R.A.; Damon, B.; Mironov, V.; Kasyanov, V.; Ramamurthi, A.; Moreno-Rodriguez, R.; Trusk, T.; Potts, J.D.; Goodwin, R.L.; Davis, J.; et al. Periostin regulates collagen fibrillogenesis and the biomechanical properties of connective tissues. J. Cell. Biochem. 2007, 101, 695–711. [Google Scholar] [CrossRef]
- González-González, L.; Alonso, J. Periostin: A matricellular protein with multiple functions in cancer development and progression. Front. Oncol. 2018, 8, 225. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, Z.; Cui, D.; Ouyang, G. The multiaspect functions of periostin in tumor progression. Adv. Exp. Med. Biol. 2019, 1132, 125–136. [Google Scholar] [CrossRef]
- Ratajczak-Wielgomas, K.; Grzegrzolka, J.; Piotrowska, A.; Matkowski, R.; Wojnar, A.; Rys, J.; Ugorski, M.; Dziegiel, P. Expression of periostin in breast cancer cells. Int. J. Oncol. 2017, 51, 1300–1310. [Google Scholar] [CrossRef]
- Kim, G.-E.; Lee, J.S.; Park, M.H.; Yoon, J.H. Epithelial periostin expression is correlated with poor survival in patients with invasive breast carcinoma. PLoS ONE 2017, 12, e0187635. [Google Scholar] [CrossRef]
- Lambert, A.W.; Wong, C.K.; Ozturk, S.; Papageorgis, P.; Raghunathan, R.; Alekseyev, Y.O.; Gower, A.C.; Reinhard, B.M.; Abdolmaleky, H.M.; Thiagalingam, S. Tumor cell-derived periostin regulates cytokines that maintain breast cancer stem cells. Mol. Cancer Res. 2016, 14, 103–113. [Google Scholar] [CrossRef]
- Li, C.; Xu, J.; Wang, Q.; Geng, S.; Yan, Z.; You, J.; Li, Z.; Zou, X. Prognostic value of periostin in early-stage breast cancer treated with conserving surgery and radiotherapy. Oncol. Lett. 2018, 15, 8072–8078. [Google Scholar] [CrossRef] [PubMed]
- Dong, N.; Zhang, L.; Jia, L.; Ji, W.; Wang, Z.; Ren, L.; Niu, R.; Zhou, Y. Identification of serum periostin as a potential diagnostic and prognostic marker for colorectal cancer. Clin. Lab. 2018, 64, 973–981. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.Y.; Park, S.Y.; Lee, H.W.; Choi, Y.-K.; Park, K.-G.; Yoon, G.S.; Tak, W.Y.; Kweon, Y.O.; Hur, K.; Lee, W.K. The combination of periostin overexpression and microvascular invasion is related to a poor prognosis for hepatocellular carcinoma. Gut Liver 2016, 10, 948–954. [Google Scholar] [CrossRef] [PubMed]
- Cattrini, C.; Rubagotti, A.; Nuzzo, P.V.; Zinoli, L.; Salvi, S.; Boccardo, S.; Perachino, M.; Cerbone, L.; Vallome, G.; Latocca, M.M.; et al. Overexpression of periostin in tumor biopsy samples is associated with prostate cancer phenotype and clinical outcome. Clin. Genitourin. Cancer 2018, 16, e1257–e1265. [Google Scholar] [CrossRef] [PubMed]
- Hong, L.-Z.; Wei, X.-W.; Chen, J.-F.; Shi, Y. Overexpression of periostin predicts poor prognosis in non-small cell lung cancer. Oncol. Lett. 2013, 6, 1595–1603. [Google Scholar] [CrossRef]
- Oh, H.J.; Bae, J.M.; Wen, X.-Y.; Cho, N.-Y.; Kim, J.H.; Kang, G.H. Overexpression of POSTN in Tumor Stroma Is a Poor Prognostic Indicator of Colorectal Cancer. J. Pathol. Transl. Med. 2017, 51, 306–313. [Google Scholar] [CrossRef]
- Silvers, C.R.; Liu, Y.-R.; Wu, C.-H.; Miyamoto, H.; Messing, E.M.; Lee, Y.-F. Identification of extracellular vesicle-borne periostin as a feature of muscle-invasive bladder cancer. Oncotarget 2016, 7, 23335–23345. [Google Scholar] [CrossRef]
- Riener, M.-O.; Fritzsche, F.R.; Soll, C.; Pestalozzi, B.C.; Probst-Hensch, N.; Clavien, P.-A.; Jochum, W.; Soltermann, A.; Moch, H.; Kristiansen, G. Expression of the extracellular matrix protein periostin in liver tumours and bile duct carcinomas. Histopathology 2010, 56, 600–606. [Google Scholar] [CrossRef]
- Kiely, M.; Hodgins, S.J.; Merrigan, B.A.; Tormey, S.; Kiely, P.A.; O’Connor, E.M. Real-time cell analysis of the inhibitory effect of vitamin K2 on adhesion and proliferation of breast cancer cells. Nutr. Res. 2015, 35, 736–743. [Google Scholar] [CrossRef]
- Refolo, M.G.; D’Alessandro, R.; Lippolis, C.; Carella, N.; Cavallini, A.; Messa, C.; Carr, B.I. IGF-1R tyrosine kinase inhibitors and Vitamin K1 enhance the antitumor effects of Regorafenib in HCC cell lines. Oncotarget 2017, 8, 103465–103476. [Google Scholar] [CrossRef]
- Dahlberg, S.; Ede, J.; Schött, U. Vitamin K and cancer. Scand. J. Clin. Lab. Investig. 2017, 77, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Nimptsch, K.; Rohrmann, S.; Kaaks, R.; Linseisen, J. Dietary vitamin K intake in relation to cancer incidence and mortality: Results from the Heidelberg cohort of the European Prospective Investigation into Cancer and Nutrition (EPIC-Heidelberg). Am. J. Clin. Nutr. 2010, 91, 1348–1358. [Google Scholar] [CrossRef] [PubMed]
- Juanola-Falgarona, M.; Salas-Salvadó, J.; Martínez-González, M.A.; Corella, D.; Estruch, R.; Ros, E.; Fitó, M.; Arós, F.; Gomez-Gracia, E.; Fiol, M.; et al. Dietary intake of vitamin k is inversely associated with mortality risk. J. Nutr. 2014, 144, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Shiratori, Y.; Kudo, M.; Shiina, S.; Mizuta, T.; Kojiro, M.; Yamamoto, K.; Koike, Y.; Saito, K.; Koyanagi, N.; et al. Effect of vitamin K2 on the recurrence of hepatocellular carcinoma. Hepatology 2011, 54, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Bin Riaz, I.; Riaz, H.; Riaz, T.; Rahman, S.; Amir, M.; Badshah, M.B.; Kazi, A.N. Role of vitamin K2 in preventing the recurrence of hepatocellular carcinoma after curative treatment: A meta-analysis of randomized controlled trials. BMC Gastroenterol. 2012, 12, 170. [Google Scholar] [CrossRef]
- Habu, D.; Shiomi, S.; Tamori, A.; Takeda, T.; Tanaka, T.; Kubo, S.; Nishiguchi, S. Role of Vitamin K2 in the Development of hepatocellular carcinoma in women with viral cirrhosis of the liver. JAMA 2004, 292, 358–361. [Google Scholar] [CrossRef]
| Molecule | Direct Measurement | Indirect Measurement |
|---|---|---|
| PK | General population: <0.3 nmol/L [17] CKD patients <0.4 nmol/L [18] | / |
| MKs | Uncertain | / |
| PIVKA | / | >2 nmol/L [19] |
| ucBGP | / | >20% or ≥4.5 ng/mL [19,20] |
| dp-ucMGP | / | >500 pmol/L [21] |
| Increase in VKDPs Activity | Decrease in VKDPs Activity |
|---|---|
| Calcimimetics use [31] | Poor vitamin K intake [22] |
| Vitamin D analogs use [31] | Dysbiosis due to the uremic condition [7] |
| MMF use [5,32] | Hemodialysis treatment [23] |
| Kidney transplantation [33] | Sevelamer use [24,26] |
| Warfarin use [25] |
| Author, Year | Patient Number | % of Patients with Vitamin K Deficiency | VKDP Measured |
|---|---|---|---|
| Boxma, 2012 [38] | 60 | 80% | dp-ucMGP |
| Keyzer, 2015 [5] | 518 | 91% | dp-ucMGP |
| Mansour, 2017 [36] | 60 | 53.3% | dp-ucMGP |
| Jansz, 2018 [24] | 32 | 62%. | dp-ucMGP |
| Evenepoel, 2019 [7] | 468 | 90% | dp-ucMGP |
| van Ballegooijen, 2020 [4] | 461 | 50% | dp-ucMGP |
| Site of Expression | Cancer | Cancer Outcome |
|---|---|---|
| Stroma | Prostate cancer [67] Lung cancer [68] Colorectal cancer [69] Breast cancer [62] Bladder cancer [70] Hepatocellular cancer [71] Pancreatic cancer [59] Ovarian cancer [59] | Poor prognosis Reduced OS Reduced PFS Advanced stage and metastasis |
| Cancer epithelial cells | Colorectal cancer [69] Breast cancer [63] Hepatocellular cancer [71] Pancreatic cancer [59] Ovarian cancer [59] | Reduced OS Reduced PFS Tumor grade (poor prognosis) Increased microvascular invasion (poor prognosis) Advanced stages and cancer recurrence |
| Cancer-associated fibroblast | Breast cancer [62] | Reduced OS Reduced PFS |
| Extracellular vesicles | Bladder Cancer [70] | Tumor stage (poor prognosis) |
| Tumor | Osteosarcoma [59] | Reduced OS Reduced PFS |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fusaro, M.; Cosmai, L.; Evenepoel, P.; Nickolas, T.L.; Cheung, A.M.; Aghi, A.; Tripepi, G.; Plebani, M.; Iervasi, G.; Vettor, R.; et al. Vitamin K and Kidney Transplantation. Nutrients 2020, 12, 2717. https://doi.org/10.3390/nu12092717
Fusaro M, Cosmai L, Evenepoel P, Nickolas TL, Cheung AM, Aghi A, Tripepi G, Plebani M, Iervasi G, Vettor R, et al. Vitamin K and Kidney Transplantation. Nutrients. 2020; 12(9):2717. https://doi.org/10.3390/nu12092717
Chicago/Turabian StyleFusaro, Maria, Laura Cosmai, Pieter Evenepoel, Thomas L. Nickolas, Angela M. Cheung, Andrea Aghi, Giovanni Tripepi, Mario Plebani, Giorgio Iervasi, Roberto Vettor, and et al. 2020. "Vitamin K and Kidney Transplantation" Nutrients 12, no. 9: 2717. https://doi.org/10.3390/nu12092717
APA StyleFusaro, M., Cosmai, L., Evenepoel, P., Nickolas, T. L., Cheung, A. M., Aghi, A., Tripepi, G., Plebani, M., Iervasi, G., Vettor, R., Zaninotto, M., Ravera, M., Foramitti, M., Giannini, S., Sella, S., & Gallieni, M. (2020). Vitamin K and Kidney Transplantation. Nutrients, 12(9), 2717. https://doi.org/10.3390/nu12092717







