Long-Term Effects of Ketoanalogues on Mortality and Renal Outcomes in Advanced Chronic Kidney Disease Patients Receiving a Low-Protein Diet
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection
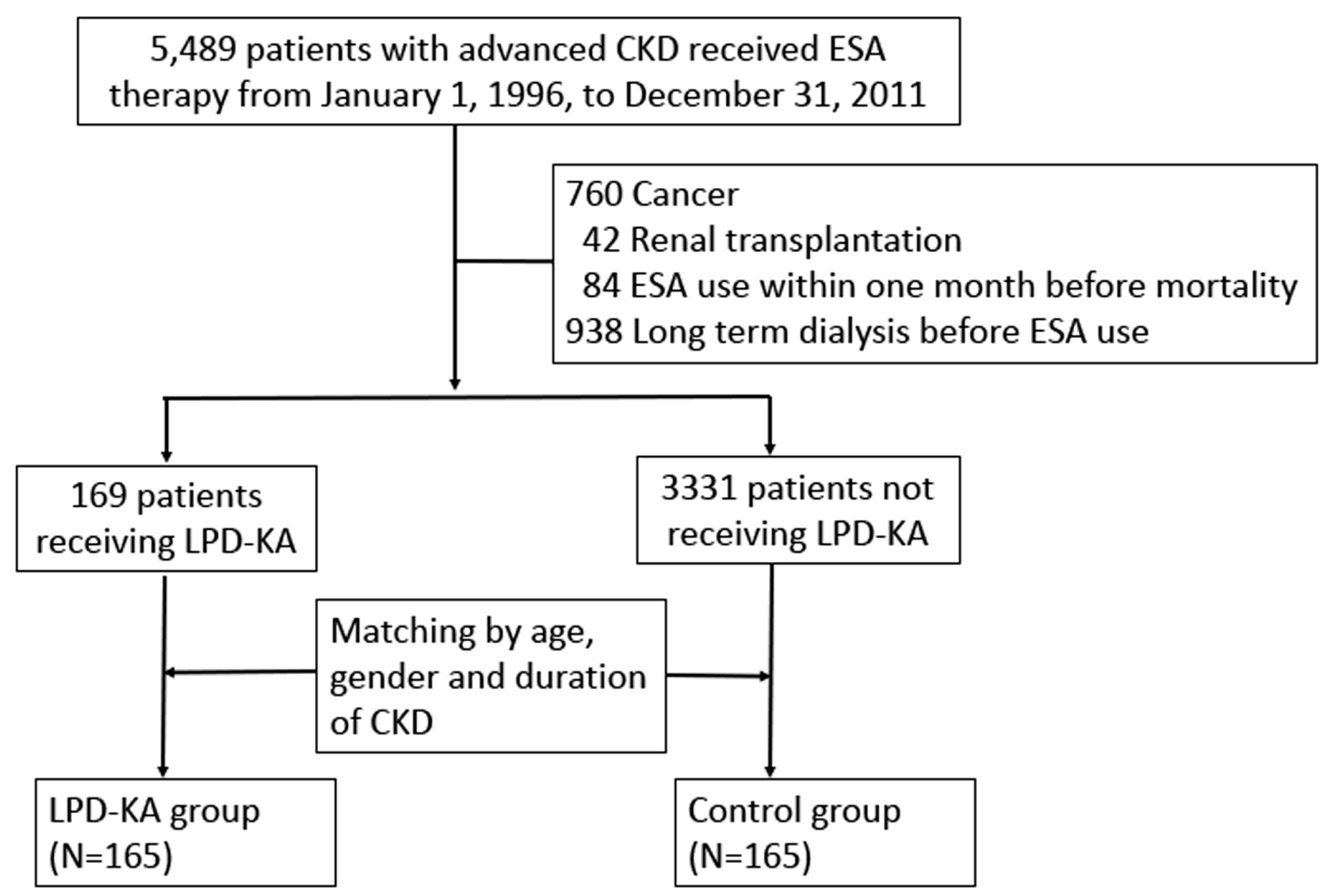
2.2. Study Population
2.3. Covariates
2.4. Outcome Measurements and Definitions
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shah, B.V.; Patel, Z.M. Role of low protein diet in management of different stages of chronic kidney disease-practical aspects. BMC Nephrol. 2016, 17, 156. [Google Scholar] [CrossRef]
- Pedrini, M.T.; Levey, A.S.; Lau, J.; Chalmers, T.C.; Wang, P.H. The effect of dietary protein restriction on the progression of diabetic and nondiabetic renal diseases: A meta-analysis. Ann. Intern. Med. 1996, 124, 627–632. [Google Scholar] [CrossRef] [PubMed]
- Fouque, D.; Laville, M. Low protein diets for chronic kidney disease in non diabetic adults. Cochrane Database Syst. Rev. 2009, CD001892. [Google Scholar] [CrossRef] [PubMed]
- Mitch, W.E.; Remuzzi, G. Diets for patients with chronic kidney disease, still worth prescribing. J. Am. Soc. Nephrol. 2004, 15, 234–237. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Su, X.; Xu, B.; Qiao, X.; Wang, L. Effect of diet protein restriction on progression of chronic kidney disease: A systematic review and meta-analysis. PLoS ONE 2018, 13, e0206134. [Google Scholar] [CrossRef]
- Hahn, D.; Hodson, E.M.; Fouque, D. Low protein diets for non-diabetic adults with chronic kidney disease. Cochrane Database Syst. Rev. 2018, 10, CD001892. [Google Scholar] [CrossRef]
- Garneata, L.; Stancu, A.; Dragomir, D.; Stefan, G.; Mircescu, G. Ketoanalogue-Supplemented Vegetarian Very Low-Protein Diet and CKD Progression. J. Am. Soc. Nephrol. 2016, 27, 2164–2176. [Google Scholar] [CrossRef]
- Cheng, B.C.; Kao, T.W.; Lee, Y.T.; Kuo, L.C.; Chen, J.B. Progression of chronic renal failure: A comparison of low dose versus standard dose keto acid analogues. Acta Nephrolgoica 2016, 30, 87–95. [Google Scholar]
- Wu, C.H.; Yang, Y.W.; Hung, S.C.; Kuo, K.L.; Wu, K.D.; Wu, V.C.; Hsieh, T.C.; National Taiwan University Study Group on Acute Renal Failure. Ketoanalogues supplementation decreases dialysis and mortality risk in patients with anemic advanced chronic kidney disease. PLoS ONE 2017, 12, e0176847. [Google Scholar] [CrossRef]
- Li, A.; Lee, H.Y.; Lin, Y.C. The Effect of Ketoanalogues on Chronic Kidney Disease Deterioration: A Meta-Analysis. Nutrients 2019, 11, 957. [Google Scholar] [CrossRef]
- Levey, A.S.; Adler, S.; Caggiula, A.W.; England, B.K.; Greene, T.; Hunsicker, L.G.; Kusek, J.W.; Rogers, N.L.; Teschan, P.E. Effects of dietary protein restriction on the progression of advanced renal disease in the Modification of Diet in Renal Disease Study. Am. J. Kidney Dis. 1996, 27, 652–663. [Google Scholar] [CrossRef]
- Menon, V.; Kopple, J.D.; Wang, X.; Beck, G.J.; Collins, A.J.; Kusek, J.W.; Greene, T.; Levey, A.S.; Sarnak, M.J. Effect of a very low-protein diet on outcomes: Long-term follow-up of the Modification of Diet in Renal Disease (MDRD) Study. Am. J. Kidney Dis. 2009, 53, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Kasiske, B.L.; Lakatua, J.D.; Ma, J.Z.; Louis, T.A. A meta-analysis of the effects of dietary protein restriction on the rate of decline in renal function. Am. J. Kidney Dis. 1998, 31, 954–961. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.H.; Kim, D.K.; Park, J.T.; Kang, E.W.; Yoo, T.H.; Kim, B.S.; Choi, K.H.; Lee, H.Y.; Han, D.S.; Shin, S.K. Influence of ketoanalogs supplementation on the progression in chronic kidney disease patients who had training on low-protein diet. Nephrology (Carlton) 2009, 14, 750–757. [Google Scholar] [CrossRef]
- Thilly, N. Low-protein diet in chronic kidney disease: From questions of effectiveness to those of feasibility. Nephrol. Dial. Transplant. 2013, 28, 2203–2205. [Google Scholar] [CrossRef]
- Klahr, S.; Levey, A.S.; Beck, G.J.; Caggiula, A.W.; Hunsicker, L.; Kusek, J.W.; Striker, G. The effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease. Modification of Diet in Renal Disease Study Group. N. Engl. J. Med. 1994, 330, 877–884. [Google Scholar] [CrossRef]
- Johnson, D.W. Dietary protein restriction as a treatment for slowing chronic kidney disease progression: The case against. Nephrology (Carlton) 2006, 11, 58–62. [Google Scholar] [CrossRef]
- Friedman, A.N. New evidence for an old strategy to help delay the need for dialysis. Am. J. Kidney Dis. 2007, 49, 563–565. [Google Scholar] [CrossRef]
- Lin, H.F.; Li, Y.H.; Wang, C.H.; Chou, C.L.; Kuo, D.J.; Fang, T.C. Increased risk of cancer in chronic dialysis patients: A population-based cohort study in Taiwan. Nephrol. Dial. Transplant. 2012, 27, 1585–1590. [Google Scholar] [CrossRef]
- Chou, C.L.; Hsieh, T.C.; Wang, C.H.; Hung, T.H.; Lai, Y.H.; Chen, Y.Y.; Lin, Y.L.; Kuo, C.H.; Wu, Y.J.; Fang, T.C. Long-term outcomes of dialysis patients after coronary revascularization: A population-based cohort study in Taiwan. Arch. Med. Res. 2014, 45, 188–194. [Google Scholar] [CrossRef]
- Kuo, C.H.; Hsieh, T.C.; Wang, C.H.; Chou, C.L.; Lai, Y.H.; Chen, Y.Y.; Lin, Y.L.; Wu, S.T.; Fang, T.C. Increased risks of mortality and atherosclerotic complications in incident hemodialysis patients subsequently with bone fractures: A nationwide case-matched cohort study. PLoS ONE 2015, 10, e0121705. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.C.; Hsieh, T.C.; Chou, C.L.; Wu, J.L.; Fang, T.C. Risks of Adverse Events Following Coprescription of Statins and Calcium Channel Blockers: A Nationwide Population-Based Study. Medicine (Baltimore) 2016, 95, e2487. [Google Scholar] [CrossRef]
- Hsieh, T.C.; Chou, C.L.; Chen, J.S.; Kuo, C.H.; Wang, Y.C.; Lai, Y.H.; Lin, Y.L.; Wang, C.H.; Fang, T.C. Risk of Mortality and of Atherosclerotic Events Among Patients Who Underwent Hemodialysis and Subsequently Developed Retinal Vascular Occlusion: A Taiwanese Retrospective Cohort Study. JAMA Ophthalmol. 2016, 134, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Chien, L.N.; Chou, C.L.; Chen, H.H.; Kao, C.C.; Lin, Y.C.; Wu, Y.L.; Chen, J.S.; Chen, L.Y.; Fang, T.C. Association Between Stroke Risk and Metformin Use in Hemodialysis Patients with Diabetes Mellitus: A Nested Case-Control Study. J. Am. Heart Assoc. 2017, 6, e007611. [Google Scholar] [CrossRef] [PubMed]
- Chou, C.L.; Hsieh, T.C.; Chen, J.S.; Fang, T.C. Sudden Sensorineural Hearing Loss in Hemodialysis Patients Could be a Marker of Pathogenic Progression in the Mortality and Atherosclerotic Events: A National Cohort Study. Otol. Neurotol. 2018, 39, 1241–1249. [Google Scholar] [CrossRef]
- Chou, C.L.; Hsieh, T.C.; Chen, J.S.; Fang, T.C. Risks of all-cause mortality and major kidney events in patients with new-onset primary open-angle glaucoma: A nationwide long-term cohort study in Taiwan. BMJ Open 2018, 8, e021270. [Google Scholar] [CrossRef]
- Hung, T.H.; Chou, C.L.; Fang, T.C. Impact of renal dysfunction in cirrhotic patients with bacterial infections other than spontaneous bacterial peritonitis. Hepatol. Res. 2014, 44, 863–870. [Google Scholar] [CrossRef]
- Huang, M.C.; Chen, M.E.; Hung, H.C.; Chen, H.C.; Chang, W.T.; Lee, C.H.; Wu, Y.Y.; Chiang, H.C.; Hwang, S.J. Inadequate energy and excess protein intakes may be associated with worsening renal function in chronic kidney disease. J. Renal Nutr. 2008, 18, 187–194. [Google Scholar] [CrossRef]
- Kovesdy, C.P.; Sharma, K.; Kalantar-Zadeh, K. Glycemic control in diabetic CKD patients: Where do we stand? Am. J. Kidney Dis. 2008, 52, 766–777. [Google Scholar] [CrossRef]
- Piccoli, G.B.; Vigotti, F.N.; Leone, F.; Capizzi, I.; Daidola, G.; Cabiddu, G.; Avagnina, P. Low-protein diets in CKD: How can we achieve them? A narrative, pragmatic review. Clin. Kidney J. 2015, 8, 61–70. [Google Scholar] [CrossRef]
| Comorbidity/Medication | ICD-9-CM Disease Codes/ATC Codes |
|---|---|
| Congestive heart failure | 428–428.9 |
| Peripheral vascular disease | 443.9, 441–441.9, 785.4, V43.4 |
| Cerebrovascular Disease | 430–437 |
| Dementia | 290–290.9 |
| COPD | 490–496, 505, 506.4 |
| Rheumatic disease | 710.0, 710.1, 710.4, 714.0–714.2, 714.81, 725 |
| Peptic Ulcer Disease | 531–534.9 |
| DM | 250–250.3, 250.7 |
| Hemiplegia | 342, 438 |
| Moderate or severe liver disease | 456–456.21, 572.2–572.8 |
| ACEI/ARB | C09 |
| Advanced CKD | Advanced CKD with DM | Advanced CKD without DM | |||||||
|---|---|---|---|---|---|---|---|---|---|
| KA User (n = 165) | KA Nonuser (n = 165) | p | KA User (n = 67) | KA Nonuser (n = 107) | p | KA User (n = 98) | KA Nonuser (n = 58) | p | |
| Age (years) | 57.8 ± 12.1 | 57.8 ± 12.1 | 0.99 | 62.0 ± 8.3 | 59.3 ± 10.2 | 0.07 | 55.0 ± 13.5 | 55.0 ± 14.8 | 0.99 |
| Gender (male/female) | 74/91 (44.9/55.1) | 74/91 (44.9/55.1) | - | 38/29 (56.7/43.3) | 51/56 (47.7/52.3) | 0.28 | 36/62 (36.7/63.3) | 23/35 (39.7/60.3) | 0.74 |
| Charlson score | 6.4 ± 3.0 | 7.6 ± 3.1 | <0.01 | 4.8 ± 2.0 | 5.3 ± 2.7 | 0.13 | 8.8 ± 2.6 | 8.9 ± 2.7 | 0.97 |
| Congestive heart failure | 20 (12.1%) | 31 (18.8%) | 0.09 | 15 (22.4%) | 27 (25.2%) | 0.70 | 5 (5.1%) | 4 (6.9%) | 0.73 |
| Peripheral vascular disease | 4 (2.4%) | 14 (8.5%) | <0.01 | 3 (4.5%) | 11 (10.3%) | 0.17 | 1 (1.0%) | 3(5.2%) | 0.15 |
| Cerebrovascular Disease | 23 (13.9%) | 41 (24.9%) | 0.01 | 17 (25.4%) | 35 (32.7%) | 0.30 | 6 (6.1%) | 6 (10.3%) | 0.36 |
| Dementia | 2 (1.2%) | 3 (1.8%) | 0.31 | 1 (1.5%) | 3 (2.8%) | 0.66 | 1 (1%) | 0 (0%) | 1.00 |
| COPD | 36 (21.8%) | 50 (30.3%) | 0.08 | 21 (31.3%) | 34 (31.8%) | 0.95 | 15 (15.3) | 16 (27.6%) | 0.06 |
| Rheumatic disease | 8 (4.8%) | 16 (9.7%) | 0.09 | 3 (4.5%) | 8 (7.5%) | 0.53 | 5 (5.1%) | 8 (13.8%) | 0.07 |
| Peptic Ulcer Disease | 66 (40.0%) | 79 (47.9%) | 0.15 | 31 (46.3%) | 58 (54.2%) | 0.31 | 35 (35.7%) | 21 (36.2%) | 0.95 |
| DM | 67 (40.6%) | 107 (64.8%) | <0.01 | - | - | ||||
| Hemiplegia | 7 (4.2%) | 4 (2.4%) | 0.36 | 5 (7.5%) | 4 (3.7%) | 0.31 | 2 (2.0%) | 0(0) | 0.53 |
| Moderate or severe liver disease | 0 (0) | 1 (0.61%) | - | 0 (0%) | 0 (0%) | - | 0 (0%) | 1 (1.7%) | - |
| ACEI/ARB | 156 (94.6) | 152 (92.1) | 0.38 | 67 (100.0) | 102 (95.3) | 0.16 | 89 (90.8) | 50 (86.2) | 0.37 |
| Advanced CKD | Advanced CKD with DM | Advanced CKD without DM | |||||
|---|---|---|---|---|---|---|---|
| KA User (n = 165) | KA Nonuser (n = 165) | KA User (n = 67) | KA Nonuser (n = 107) | KA User (n = 98) | KA Nonuser (n = 58) | ||
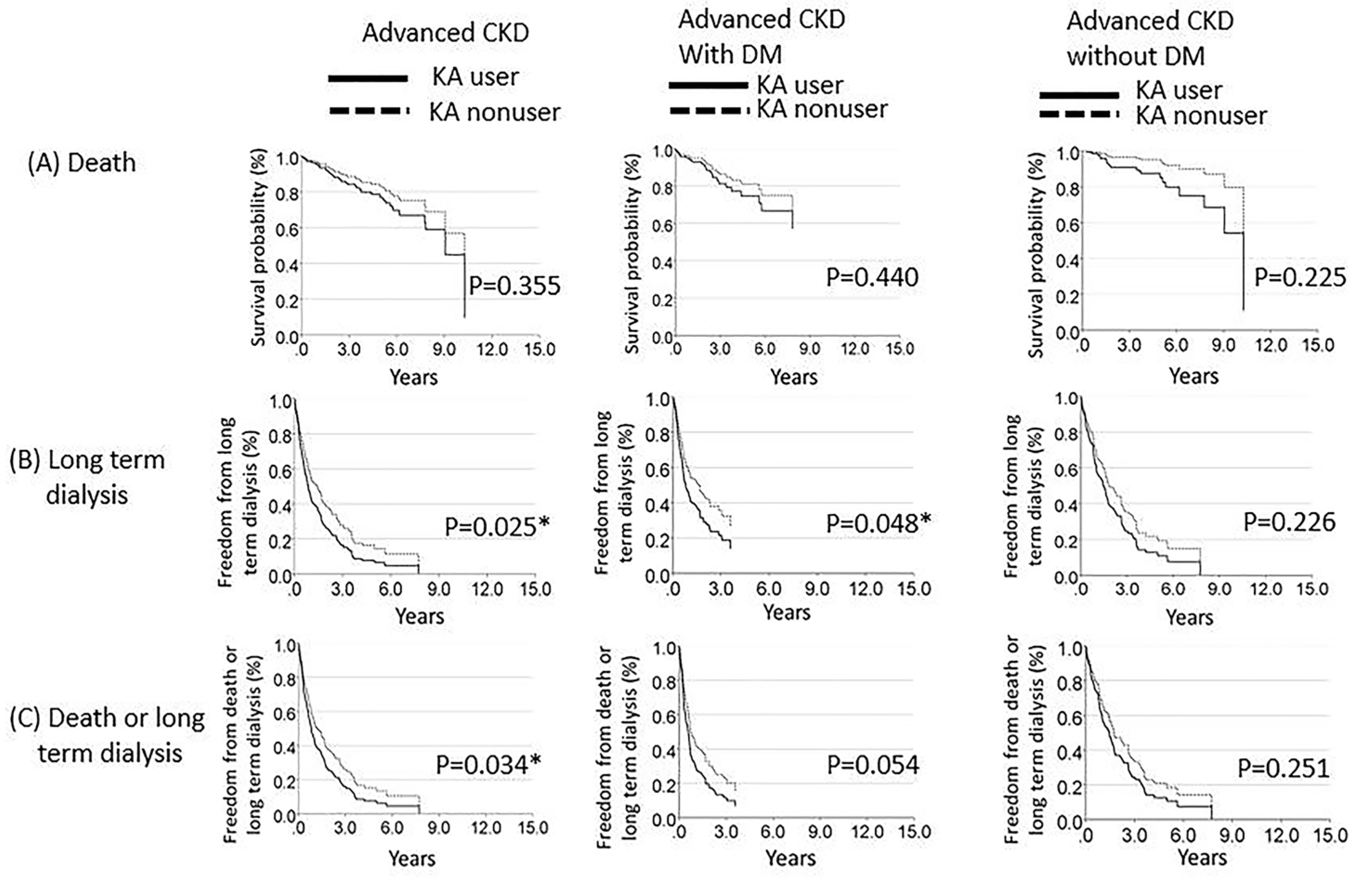
| Death | Number (%) | 34 (20.6%) | 12 (7.3%) | 17 (25.4%) | 10 (9.3%) | 17 (17.3%) | 2 (3.4%) |
| Incidence rate per 1000 patient-years | 58.96 | 44.64 | 89.55 | 63.71 | 43.95 | 17.88 | |
| Adjusted HR (95% CI) (KA user vs. KA nonuser) | 1.41 (0.68—2.93), p = 0.355 | 1.46 (0.59—3.33), p = 0.440 | 2.69 (0.54—13.31), p = 0.225 | ||||
| Long-term dialysis | Number (%) | 124 (75.2%) | 83 (50.3%) | 49 (73.1%) | 57 (53.3%) | 75 (76.5%) | 26 (44.8%) |
| Incidence rate per 1000 patient-years | 696.97 | 486.49 | 977.67 | 601.30 | 586.88 | 342.95 | |
| Adjusted HR (95% CI) (KA user vs. KA nonuser) | 1.41 (1.04—1.90), p = 0.025 * | 1.49 (1.00—2.20), p = 0.048 * | 1.35 (0.83—2.20), p = 0.226 | ||||
| Death or long-term dialysis | Number (%) | 128 (77.6%) | 88 (53.3%) | 52 (77.6%) | 61 (57.0%) | 76 (77.6%) | 27 (46.6%) |
| Incidence Rate per 1000 patient-years | 721.14 | 521.17 | 1043.46 | 652.77 | 595.32 | 358.23 | |
| Adjusted HR (95% CI) (KA user vs. KA nonuser) | 1.37 (1.02—1.83), p = 0.034 * | 1.45 (0.99—2.13), p = 0.054 | 1.32 (0.82—2.14), p = 0.251 | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.-C.; Juan, S.-H.; Chou, C.-L.; Hsieh, T.-C.; Wu, J.-L.; Fang, T.-C. Long-Term Effects of Ketoanalogues on Mortality and Renal Outcomes in Advanced Chronic Kidney Disease Patients Receiving a Low-Protein Diet. Nutrients 2020, 12, 2708. https://doi.org/10.3390/nu12092708
Wang Y-C, Juan S-H, Chou C-L, Hsieh T-C, Wu J-L, Fang T-C. Long-Term Effects of Ketoanalogues on Mortality and Renal Outcomes in Advanced Chronic Kidney Disease Patients Receiving a Low-Protein Diet. Nutrients. 2020; 12(9):2708. https://doi.org/10.3390/nu12092708
Chicago/Turabian StyleWang, Yi-Chun, Shu-Hui Juan, Chu-Lin Chou, Tsung-Cheng Hsieh, Jung-Lun Wu, and Te-Chao Fang. 2020. "Long-Term Effects of Ketoanalogues on Mortality and Renal Outcomes in Advanced Chronic Kidney Disease Patients Receiving a Low-Protein Diet" Nutrients 12, no. 9: 2708. https://doi.org/10.3390/nu12092708
APA StyleWang, Y.-C., Juan, S.-H., Chou, C.-L., Hsieh, T.-C., Wu, J.-L., & Fang, T.-C. (2020). Long-Term Effects of Ketoanalogues on Mortality and Renal Outcomes in Advanced Chronic Kidney Disease Patients Receiving a Low-Protein Diet. Nutrients, 12(9), 2708. https://doi.org/10.3390/nu12092708





