Vitamin B6, Inflammation, and Cardiovascular Outcome in a Population-Based Cohort: The Prevention of Renal and Vascular End-Stage Disease (PREVEND) Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Data Collection, Laboratory Measurements, and Definitions
2.3. Follow-Up and Ascertainment of Cardiovascular (CV) Events
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Roth, G.A.; Johnson, C.; Abajobir, A.; Abd-Allah, F.; Abera, S.F.; Abyu, G.; Ahmed, M.; Aksut, B.; Alam, T.; Alam, K.; et al. Global, Regional, and National Burden of Cardiovascular Diseases for 10 Causes, 1990 to 2015. J. Am. Coll. Cardiol. 2017, 70, 1–25. [Google Scholar] [CrossRef]
- Roth, G.A.; Forouzanfar, M.H.; Moran, A.E.; Barber, R.; Nguyen, G.; Feigin, V.L.; Naghavi, M.; Mensah, G.A.; Murray, C.J.L. Demographic and epidemiologic drivers of global cardiovascular mortality. N. Engl. J. Med. 2015, 372, 1333–1341. [Google Scholar] [CrossRef]
- Folsom, A.R.; Nieto, F.J.; McGovern, P.G.; Tsai, M.Y.; Malinow, M.R.; Eckfeldt, J.H.; Hess, D.L.; Davis, C.E. Prospective Study of Coronary Heart Disease Incidence in Relation to Fasting Total Homocysteine, Related Genetic Polymorphisms, and B Vitamins The Atherosclerosis Risk in Communities (ARIC) Study. Circulation 1998, 98, 204–210. [Google Scholar] [CrossRef]
- Kelly, P.J.; Shih, V.E.; Kistler, J.P.; Barron, M.; Lee, H.; Mandell, R.; Furie, K.L. Low vitamin B6 but not homocyst(e)ine is associated with increased risk of stroke and transient ischemic attack in the era of folic acid grain fortification. Stroke 2003, 34, e51–e54. [Google Scholar] [CrossRef] [PubMed]
- Dierkes, J.; Hoffmann, K.; Klipstein-Grobusch, K.; Weikert, C.; Boeing, H.; Zyriax, B.C.; Windler, E.; Kratzsch, J. Low plasma pyridoxal-5’phosphate and cardiovascular disease risk in women: Results from the Coronary Risk Factors for Atherosclerosis in Women Study. Am. J. Clin. Nutr. 2005, 81, 725. [Google Scholar] [CrossRef] [PubMed]
- Page, J.H.; Ma, J.; Chiuve, S.E.; Stampfer, M.J.; Selhub, J.; Manson, J.E.; Rimm, E.B. Plasma vitamin B6 and risk of myocardial infarction in women. Circulation 2009, 120, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Ueland, P.M.; Ulvik, A.; Rios-Avila, L.; Midttun, O.; Gregory, J.F. Direct and Functional Biomarkers of Vitamin B6 Status. Annu. Rev. Nutr. 2015, 35, 33–70. [Google Scholar] [CrossRef] [PubMed]
- Leklem, J.E. Vitamin B-6: A status report. J. Nutr. 1990, 120, 1503–1507. [Google Scholar] [CrossRef]
- Friso, S.; Girelli, D.; Martinelli, N.; Olivieri, O.; Lotto, V.; Bozzini, C.; Pizzolo, F.; Faccini, G.; Beltrame, F.; Corrocher, R. Low plasma vitamin B-6 concentrations and modulation of coronary artery disease risk. Am. J. Clin. Nutr. 2004, 79, 992–998. [Google Scholar] [CrossRef]
- Lin, P.T.; Cheng, C.H.; Liaw, Y.P.; Lee, B.J.; Lee, T.W.; Huang, Y.C. Low pyridoxal 5′-phosphate is associated with increased risk of coronary artery disease. Nutrition 2006, 22, 1146–1151. [Google Scholar] [CrossRef]
- Cheng, C.H.; Lin, P.T.; Liaw, Y.P.; Ho, C.C.; Tsai, T.P.; Chou, M.C.; Huang, Y.C. Plasma pyridoxal 5′-phosphate and high-sensitivity C-reactive protein are independently associated with an increased risk of coronary artery disease. Nutrition 2008, 24, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Dierkes, J.; Weikert, C.; Klipstein-Grobusch, K.; Westphal, S.; Luley, C.; Möhlig, M.; Spranger, J.; Boeing, H. Plasma pyridoxal-5-phosphate and future risk of myocardial infarction in the European Prospective Investigation into Cancer and Nutrition Potsdam cohort. Am. J. Clin. Nutr. 2007, 86, 214–220. [Google Scholar] [PubMed]
- Otvos, J.D.; Shalaurova, I.; Wolak-Dinsmore, J.; Connelly, M.A.; Mackey, R.H.; Stein, J.H.; Tracy, R.P. GlycA: A composite nuclear magnetic resonance biomarker of systemic inflammation. Clin. Chem. 2015, 61, 714–723. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, S.C.; Würtz, P.; Nath, A.P.; Abraham, G.; Havulinna, A.S.; Fearnley, L.G.; Sarin, A.P.; Kangas, A.J.; Soininen, P.; Aalto, K.; et al. The Biomarker GlycA is Associated with Chronic Inflammation and Predicts Long-Term Risk of Severe Infection. Cell Syst. 2015, 1, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Muhlestein, J.B.; May, H.T.; Galenko, O.; Knowlton, K.U.; Otvos, J.D.; Connelly, M.A.; Lappe, D.L.; Anderson, J.L. GlycA and hsCRP are independent and additive predictors of future cardiovascular events among patients undergoing angiography: The intermountain heart collaborative study. Am. Heart J. 2018, 202, 27–32. [Google Scholar] [CrossRef]
- McGarrah, R.W.; Kelly, J.P.; Craig, D.M.; Haynes, C.; Jessee, R.C.; Huffman, K.M.; Kraus, W.E.; Shah, S.H. A novel protein glycan-derived inflammation biomarker independently predicts cardiovascular disease and modifies the association of HDL subclasses with mortality. Clin. Chem. 2017, 63, 288–296. [Google Scholar] [CrossRef]
- Akinkuolie, A.O.; Glynn, R.J.; Padmanabhan, L.; Ridker, P.M.; Mora, S. Circulating N-Linked Glycoprotein Side-Chain Biomarker, Rosuvastatin Therapy, and Incident Cardiovascular Disease: An Analysis From the JUPITER Trial. J. Am. Heart Assoc. 2016, 5, e003822. [Google Scholar] [CrossRef]
- Akinkuolie, A.O.; Buring, J.E.; Ridker, P.M.; Mora, S. A novel protein glycan biomarker and future cardiovascular disease events. J. Am. Heart Assoc. 2014, 3, e001221. [Google Scholar] [CrossRef]
- Duprez, D.A.; Otvos, J.; Sanchez, O.A.; Mackey, R.H.; Tracy, R.; Jacobs, D.R. Comparison of the predictive value of GlycA and other biomarkers of inflammation for total death, incident cardiovascular events, noncardiovascular and noncancer inflammatory-related events, and total cancer events. Clin. Chem. 2016, 62, 1020–1031. [Google Scholar] [CrossRef]
- Gruppen, E.G.; Riphagen, I.J.; Connelly, M.A.; Otvos, J.D.; Bakker, S.J.L.; Dullaart, R.P.F. GlycA, a pro-inflammatory glycoprotein biomarker, and incident cardiovascular disease: Relationship with C-reactive protein and renal function. PLoS ONE 2015, 10, e0139057. [Google Scholar] [CrossRef]
- Connelly, M.A.; Otvos, J.D.; Shalaurova, I.; Playford, M.P.; Mehta, N.N. GlycA, a novel biomarker of systemic inflammation and cardiovascular disease risk. J. Transl. Med. 2017, 15, 219. [Google Scholar] [CrossRef] [PubMed]
- Halbesma, N.; Jansen, D.F.; Heymans, M.W.; Stolk, R.P.; de Jong, P.E.; Gansevoort, R.T. Development and validation of a general population renal risk score. Clin. J. Am. Soc. Nephrol. 2011, 6, 1731–1738. [Google Scholar] [CrossRef] [PubMed]
- Hillege, H.L.; Janssen, W.M.T.; Bak, A.A.A.; Diercks, G.F.H.; Grobbee, D.E.; Van Gilst, W.H.; De Zeeuw, D.; De Jong, P.E. Microalbuminuria is common, also in a nondiabetic, nonhypertensive population, and an independent indicator of cardiovascular risk factors and cardiovascular morbidity. J. Intern. Med. 2001, 249, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Visser, S.T.; Schuiling-Veninga, C.C.; Bos, J.H.; de Jong-van den Berg, L.T.; Postma, M.J. The population-based prescription database IADB.nl: Its development, usefulness in outcomes research and challenges. Expert Rev. Pharm. Outcomes Res. 2013, 13, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Talwar, D.; Quasim, T.; McMillan, D.C.; Kinsella, J.; Williamson, C.; O’Reilly, D.S.J. Optimisation and validation of a sensitive high-performance liquid chromatography assay for routine measurement of pyridoxal 5-phosphate in human plasma and red cells using pre-column semicarbazide derivatisation. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2003, 792, 333–343. [Google Scholar] [CrossRef]
- Stover, P.J.; Field, M.S. Vitamin B-61. Adv. Nutr. 2015, 6, 132–133. [Google Scholar] [CrossRef]
- Joshi, A.A.; Lerman, J.B.; Aberra, T.M.; Afshar, M.; Teague, H.L.; Rodante, J.A.; Krishnamoorthy, P.; Ng, Q.; Aridi, T.Z.; Salahuddin, T.; et al. GlycA Is a Novel Biomarker of Inflammation and Subclinical Cardiovascular Disease in Psoriasis. Circ. Res. 2016, 119, 1242–1253. [Google Scholar] [CrossRef]
- Committee for Medicinal Products for Human Use. Guideline on Bioanalytical Method Validation; European Medicines Agency: London, UK, 2011. [Google Scholar]
- Dullaart, R.P.F.; Perton, F.; van der Klauw, M.M.; Hillege, H.L.; Sluiter, W.J. High plasma lecithin:cholesterol acyltransferase activity does not predict low incidence of cardiovascular events: Possible attenuation of cardioprotection associated with high HDL cholesterol. Atherosclerosis 2010, 208, 537–542. [Google Scholar] [CrossRef]
- Corsetti, J.P.; Gansevoort, R.T.; Bakker, S.J.L.; Sparks, C.E.; Vart, P.; Dullaart, R.P.F. Apolipoprotein B attenuates albuminuria-associated cardiovascular disease in Prevention of Renal and Vascular Endstage Disease (PREVEND) participants. J. Am. Soc. Nephrol. 2014, 25, 2906–2915. [Google Scholar] [CrossRef]
- Borggreve, S.E.; Hillege, H.L.; Dallinga-Thie, G.M.; De Jong, P.E.; Wolffenbuttel, B.H.R.; Grobbee, D.E.; Van Tol, A.; Dullaart, R.P.F. High plasma cholesteryl ester transfer protein levels may favour reduced incidence of cardiovascular events in men with low triglycerides. Eur. Heart J. 2007, 28, 1012–1018. [Google Scholar] [CrossRef]
- Inker, L.A.; Schmid, C.H.; Tighiouart, H.; Eckfeldt, J.H.; Feldman, H.I.; Greene, T.; Kusek, J.W.; Manzi, J.; Van Lente, F.; Zhang, Y.L.; et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N. Engl. J. Med. 2012, 367, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Abraira, V.; Muriel, A.; Emparanza, J.I.; Pijoan, J.I.; Royuela, A.; Plana, M.N.; Cano, A.; Urreta, I.; Zamora, J. Reporting quality of survival analyses in medical journals still needs improvement. A minimal requirements proposal. J. Clin. Epidemiol. 2013, 66, 1340–1346.e5. [Google Scholar] [CrossRef] [PubMed]
- Harel, O.; Zhou, X.H. Multiple imputation: Review of theory, implementation and software. Stat. Med. 2007, 26, 3057–3077. [Google Scholar] [CrossRef] [PubMed]
- Oterdoom, L.H.; de Vries, A.P.; van Ree, R.M.; Gansevoort, R.T.; van Son, W.J.; van der Heide, J.J.H.; Navis, G.; de Jong, P.E.; Gans, R.O.; Bakker, S.J. N-Terminal Pro-B-Type Natriuretic Peptide and Mortality in Renal Transplant Recipients Versus the General Population. Transplantation 2009, 87, 1562–1570. [Google Scholar] [CrossRef]
- Heeringa, S.G.; West, B.T.; Berglund, P.A. Applied Survey Data Analysis, 2nd ed.; Chapman and Hall/CRC: Cleveland, OH, USA, 2017. [Google Scholar]
- Selvin, S. Statistical Analysis of Epidemiological Data, 3rd ed.; Oxford University Press Inc.: Oxford, UK, 2004. [Google Scholar]
- Fleiss, J.L. Analysis of Data from Multiclinic Trials. Control. Clin. Trials 1986, 7, 267–275. [Google Scholar] [CrossRef]
- Ulvik, A.; Pedersen, E.R.; Svingen, G.F.T.; McCann, A.; Midttun, Ø.; Nygård, O.; Ueland, P.M. Vitamin B-6 catabolism and long-term mortality risk in patients with coronary artery disease. Am. J. Clin. Nutr. 2016, 103, 1417–1425. [Google Scholar] [CrossRef]
- Weikert, C.; Dierkes, J.; Hoffmann, K.; Berger, K.; Drogan, D.; Klipstein-Grobusch, K.; Spranger, J.; Möhlig, M.; Luley, C.; Boeing, H. B vitamin plasma levels and the risk of ischemic stroke and transient ischemic attack in a German cohort. Stroke 2007, 38, 2912–2918. [Google Scholar] [CrossRef]
- Van der Gaag, M.S.; Ubbink, J.B.; Sillanaukee, P.; Nikkari, S.; Hendriks, H.F.J. Effect of consumption of red wine, spirits, and beer on serum homocysteine. Lancet 2000, 355, 1522. [Google Scholar] [CrossRef]
- Ueland, P.M.; McCann, A.; Midttun, Ø.; Ulvik, A. Inflammation, vitamin B6 and related pathways. Mol. Asp. Med. 2017, 53, 10–27. [Google Scholar] [CrossRef]
- Bleie, Ø.; Semb, A.G.; Grundt, H.; Nordrehaug, J.E.; Vollset, S.E.; Ueland, P.M.; Nilsen, D.W.T.; Bakken, A.M.; Refsum, H.; Nygård, O.K. Homocysteine-lowering therapy does not affect inflammatory markers of atherosclerosis in patients with stable coronary artery disease. J. Intern. Med. 2007, 262, 244–253. [Google Scholar] [CrossRef]
- Davis, S.R.; Quinlivan, E.P.; Stacpoole, P.W.; Gregory, J.F. Nutrient Physiology, Metabolism, and Nutrient-Nutrient Interactions Plasma Glutathione and Cystathionine Concentrations Are Elevated but Cysteine Flux Is Unchanged by Dietary Vitamin B-6 Restriction in Young Men and Women. J. Nutr. 2006, 136, 373–378. [Google Scholar] [CrossRef] [PubMed][Green Version]
- National Center for Environmental Health. Second National Report on Biochemical Indicators of Diet and Nutrition in the U.S. Population; National Center for Environmental Health: Atlanta, GA, USA, 2012. [Google Scholar]
- Krumsiek, J.; Mittelstrass, K.; Do, K.T.; Stückler, F.; Ried, J.; Adamski, J.; Peters, A.; Illig, T.; Kronenberg, F.; Friedrich, N.; et al. Gender-specific pathway differences in the human serum metabolome. Metabolomics 2015, 11, 1815–1833. [Google Scholar] [CrossRef] [PubMed]
- Shue, G.M. Interrelation of Vitamin B6 and Sex on Response of Rats to Hypercholesterolemic Diets. J. Nutr. 1965, 85, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Chen, Y.; Yang, B.; Yang, J.; Wahlqvist, M.L.; Li, D. Meta-analysis of B vitamin supplementation on plasma homocysteine, cardiovascular and all-cause mortality. Clin. Nutr. 2012, 31, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Tan, S.; Xu, Y.; Chandra, A.; Shi, C.; Song, B.; Qin, J.; Gao, Y. Vitamin B supplementation, homocysteine levels, and the risk of cerebrovascular disease: A meta-analysis. Neurology 2013, 81, 1298–1307. [Google Scholar] [CrossRef]
- Albert, C.M.; Nancy Cook, M.R.; Michael Gaziano, S.J.; Elaine Zaharris, M.; Jean MacFadyen, B.; Eleanor Danielson, B.; Julie Buring, M.E.; JoAnn Manson, S.E.; Zaharris, M.; Medicine Albert, C.; et al. Effect of Folic Acid and B Vitamins on Risk of Cardiovascular Events and Total Mortality Among Women at High Risk for Cardiovascular Disease A Randomized Trial. JAMA 2008, 299, 2027–2036. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Pi, F.; Ding, Z.; Chen, W.; Pang, S.; Dong, W.; Zhang, Q. Efficacy of supplementation with B vitamins for stroke prevention: A network meta-analysis of randomized controlled trials. PLoS ONE 2015, 10, e0137533. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, Z.Y.; Qin, Y.Y.; Yu, F.F.; Zhou, Y.H. Association between B vitamins supplementation and risk of cardiovascular outcomes: A cumulative meta-analysis of randomized controlled trials. PLoS ONE 2014, 9, e0107060. [Google Scholar] [CrossRef]
- Dusitanond, P.; Eikelboom, J.W.; Hankey, G.J.; Thom, J.; Gilmore, G.; Loh, K.; Yi, Q.; Klijn, C.J.M.; Langton, P.; Van Bockxmeer, F.M.; et al. Homocysteine-lowering treatment with folic acid, cobalamin, and pyridoxine does not reduce blood markers of inflammation, endothelial dysfunction, or hypercoagulability in patients with previous transient ischemic attack or stroke: A randomized substudy of the VITATOPS trial. Stroke 2005, 36, 144–146. [Google Scholar]
- Christen, W.G.; Cook, N.R.; Van Denburgh, M.; Zaharris, E.; Albert, C.M.; Manson, J.A.E. Effect of combined treatment with Folic Acid, vitamin B6, and vitamin B12 on plasma biomarkers of inflammation and endothelial dysfunction in women. J. Am. Heart Assoc. 2018, 7, e008517. [Google Scholar] [CrossRef]
- Wang, X.; Demirtas, H.; Xu, X. Homocysteine, B vitamins, and cardiovascular disease. N. Engl. J. Med. 2006, 355, 207–211. [Google Scholar] [PubMed]
- Quinlivan, E.P.; Gregory, J.F., 3rd. Homocysteine, B vitamins, and cardiovascular disease. N. Engl. J. Med. 2006, 355, 206–211. [Google Scholar] [PubMed]
- Haarhaus, M.; Brandenburg, V.; Kalantar-Zadeh, K.; Stenvinkel, P.; Magnusson, P. Alkaline phosphatase: A novel treatment target for cardiovascular disease in CKD. Nat. Rev. Nephrol. 2017, 13, 429–442. [Google Scholar] [CrossRef] [PubMed]
- Midttun, Ø.; Townsend, M.K.; Nygård, O.; Tworoger, S.S.; Brennan, P.; Johansson, M.; Ueland, P.M. Most blood biomarkers related to vitamin status, one-carbon metabolism, and the kynurenine pathway show adequate preanalytical stability and within-person reproducibility to allow assessment of exposure or nutritional status in healthy women and cardiovascular patients. J. Nutr. 2014, 144, 784–790. [Google Scholar] [PubMed]
- Johansson, M.; Relton, C.; Magne Ueland, P.; Emil Vollset, S.; Midttun, Ø.; Nygård, O.; Slimani, N.; Boffetta, P.; Jenab, M.; Clavel-Chapelon, F.; et al. Serum B Vitamin Levels and Risk of Lung Cancer. JAMA 2010, 303, 2377–2385. [Google Scholar] [CrossRef] [PubMed]
- Fanidi, A.; Muller, D.C.; Yuan, J.M.; Stevens, V.L.; Weinstein, S.J.; Albanes, D.; Prentice, R.; Thomsen, C.A.; Pettinger, M.; Cai, Q.; et al. Circulating Folate, Vitamin B6, and Methionine in Relation to Lung Cancer Risk in the Lung Cancer Cohort Consortium (LC3). J. Natl. Cancer Inst. 2018, 110, 57–67. [Google Scholar]
- Gylling, B.; Myte, R.; Schneede, J.; Hallmans, G.; Häggström, J.; Johansson, I.; Ulvik, A.; Ueland, P.M.; Van Guelpen, B.; Palmqvist, R. Vitamin B-6 and colorectal cancer risk: A prospective population-based study using 3 distinct plasma markers of Vitamin B-6 status. Am. J. Clin. Nutr. 2017, 105, 897–904. [Google Scholar] [CrossRef]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
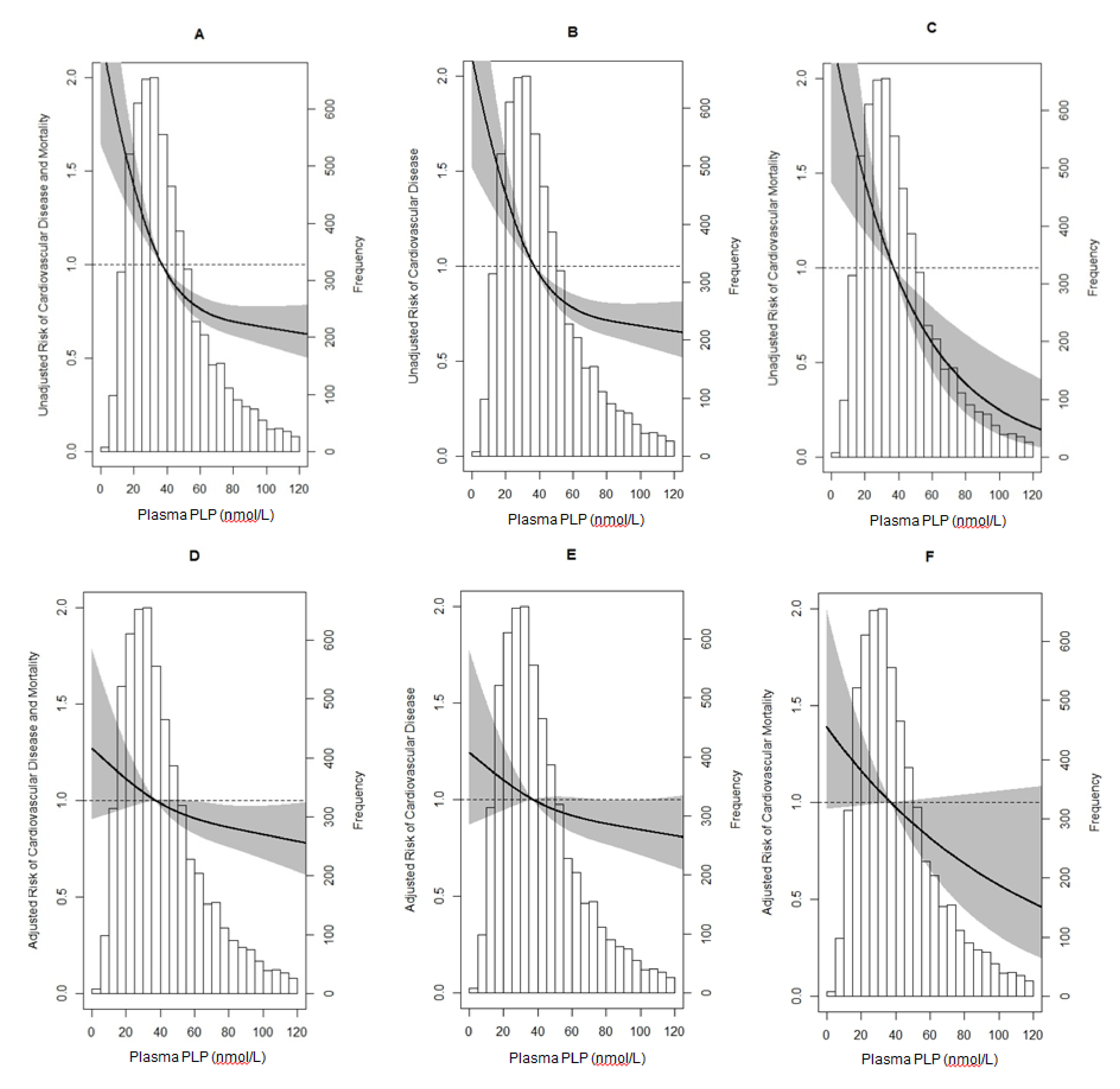
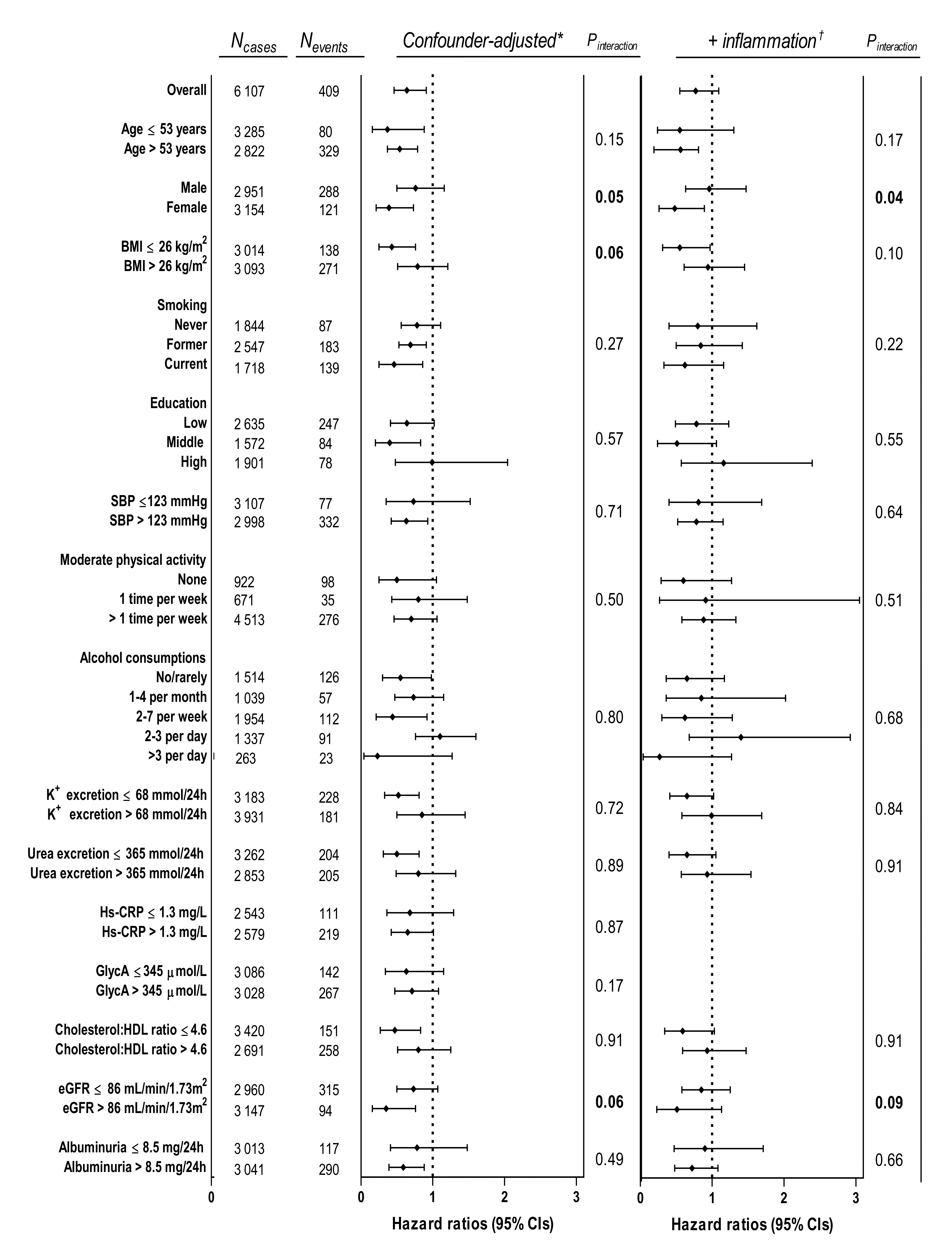
| Linear Regression Models | ||||||||
|---|---|---|---|---|---|---|---|---|
| Vitamin B6 Status According to Plasma PLP Concentration | Univariable | Age- and Sex-Adjusted | ||||||
| Total Study Population | Deficient (<20 nmol/L) | Insufficient (20–30 nmol/L) | Sufficient (>30 nmol/L) | Stand. β | P for Trend | Stand. β | P for Trend | |
| N (% of total study population) | 6249 (100.0) | 902 (14.4) | 1261 (20.2) | 4086 (65.4) | ||||
| Plasma PLP, nmol/L | 37.2 (25.1–57.0) | 15.4 (12.6–18.0) | 25.0 (22.4–27.3) | 49.3 (38.0–71.7) | ||||
| Demographics | ||||||||
| Age, years | 53.0 ± 11.9 | 56.1 ± 12.3 | 53.6 ± 12.0 | 52.2 ± 11.6 | 0.10 | <0.001 | ||
| Male gender, n (%) | 3018 (48.3) | 394 (43.7) | 558 (44.3) | 2066 (50.6) | 0.02 | 0.21 | ||
| BMI, kg/m2 | 26.1 (23.6–29.0) | 26.6 (23.9–29.6) | 26.3 (23.8–29.4) | 25.9 (23.6–28.7) | 0.08 | <0.001 | 0.06 | <0.001 |
| Vitamin B6 supplementation, n (%) | 12 (0.2) | 0 (0) | 0 (0) | 12 (0.2) | 0.14 | <0.001 | 0.14 | <0.001 |
| Smoking, n (%) | ||||||||
| Never | 1857 (29.7) | 204 (22.6) | 339 (26.9) | 1314 (32.2) | Ref. | Ref. | . | |
| Former | 2572 (41.2) | 309 (34.4) | 496 (39.3) | 1767 (43.2) | 0.03 | 0.04 | 0.02 | 0.29 |
| Current | 1740 (28.2) | 377 (42.2) | 412 (33.0) | 951 (23.6) | 0.15 | <0.001 | 0.16 | <0.001 |
| Cotinine excretion, µg/24 h | 0 (0–493) | 10 (0–1399) | 0 (0–842) | 0 (0–69) | 0.20 | <0.001 | 0.22 | <0.001 |
| Education, n (%) | ||||||||
| Low | 2690 (43.0) | 523 (58.0) | 593 (47.0) | 1574 (38.5) | Ref. | Ref. | ||
| Middle | 1605 (25.7) | 199 (22.1) | 334 (26.7) | 1069 (26.2) | 0.08 | <0.001 | 0.07 | <0.001 |
| High | 1954 (31.3) | 180 (20.0) | 331 (26.2) | 1443 (35.3) | 0.16 | <0.001 | 0.14 | <0.001 |
| SBP, mmHg | 123 (112–136) | 127 (114–143) | 124 (112–138) | 122 (112–135) | 0.08 | <0.001 | 0.05 | 0.002 |
| DBP, mmHg | 73 (67–79) | 74 (68–80) | 73 (67–79) | 72 (67–79) | 0.05 | <0.001 | 0.03 | 0.06 |
| Creatinine excretion, mmol/24 h | 12.0 (9.9–14.6) | 11.5 (9.6–13.9) | 11.8(9.8–14.3) | 12.1 (10.0–14.9) | 0.02 | 0.15 | 0.01 | 0.58 |
| Moderate physical activity, n (%) | ||||||||
| None | 942 (15.2) | 210 (23.5) | 223 (17.9) | 509 (12.6) | Ref. | Ref. | ||
| 1 time per week | 677 (10.9) | 94 (10.5) | 141 (11.3) | 442 (10.9) | 0.07 | <0.001 | 0.06 | 0.001 |
| >1 time per week | 4564 (73.8) | 589 (66.0) | 885 (70.9) | 3090 (76.5) | 0.12 | <0.001 | 0.11 | <0.001 |
| Dietary intake | ||||||||
| Coffee consumer, n (%) | 5831 (94.2) | 847 (94.6) | 1181 (94.6) | 3803 (94.0) | 0.04 | 0.004 | 0.03 | 0.03 |
| Alcohol consumptions, n (%) | ||||||||
| No/rarely | 1541 (24.9) | 353 (39.4) | 338 (27.1) | 850 (21.0) | Ref. | Ref. | ||
| 1–4 per month | 1054 (17.0) | 181 (20.2) | 240 (19.2) | 633 (15.7) | 0.04 | 0.02 | 0.03 | 0.05 |
| 2–7 per week | 1973 (31.9) | 239 (26.7) | 418 (33.5) | 1315 (32.5) | 0.10 | <0.001 | 0.09 | <0.001 |
| 2–3 per day | 1355 (21.9) | 107 (11.9) | 217 (17.4) | 1031 (25.5) | 0.15 | <0.001 | 0.15 | <0.001 |
| >3 per day | 266 (4.3) | 16 (1.8) | 35 (2.8) | 215 (5.3) | 0.10 | <0.001 | 0.10 | <0.001 |
| Ethylglucuronide excretion, µg/24 h | 144 (0–3751) | 15 (0–860) | 60 (0–1898) | 404 (3–4692) | 0.08 | <0.001 | 0.09 | <0.001 |
| Potassium excretion, mmol/24 h | 68.6 ± 21.9 | 61.8 ± 21.0 | 65.60 ± 20.4 | 70.9 ± 22.1 | 0.13 | <0.001 | 0.13 | <0.001 |
| Urea excretion, mmol/24 h | 365 ± 114 | 341 ± 114 | 359 ± 107 | 372 ± 115 | 0.06 | <0.001 | 0.06 | <0.001 |
| Inflammation | ||||||||
| Hs-CRP, mg/L | 1.3 (0.6–3.0) | 2.4 (1.0–5.4) | 1.5 (0.8–3.4) | 1.1 (0.5–2.5) | 0.21 | <0.001 | 0.20 | <0.001 |
| GlycA, µmol/L | 345 (308–388) | 376 (333–426) | 355 (317–393) | 336 (302–377) | 0.22 | <0.001 | 0.21 | <0.001 |
| Glucose homeostasis | ||||||||
| Diabetes, n (%) | 353 (5.7) | 79 (8.8) | 78 (6.2) | 196 (4.8) | 0.05 | <0.001 | 0.03 | 0.02 |
| Glucose, mmol/L | 4.8 (4.4–5.3) | 4.8 (4.4–5.4) | 4.8 (4.4–5.3) | 4.8 (4.4–5.3) | 0.06 | <0.001 | 0.04 | 0.01 |
| Lipids | ||||||||
| Total cholesterol, mmol/L | 5.5 ± 1.0 | 5.4 ± 1.1 | 5.4 ± 1.0 | 5.5 ± 1.0 | 0.03 | 0.06 | 0.05 | <0.001 |
| HDL-cholesterol, mmol/L | 1.2 (1.0–1.7) | 1.2 (1.0–1.4) | 1.2 (1.0–1.4) | 1.3 (1.1–1.5) | 0.05 | <0.001 | 0.05 | <0.001 |
| LDL-cholesterol, mmol/L | 3.6 ± 0.9 | 3.6 ± 1.0 | 3.6 ± 0.9 | 3.6 ± 0.9 | 0.01 | 0.83 | 0.02 | 0.18 |
| Triglycerides, mmol/L | 1.1 (0.8–1.6) | 1.2 (0.9–1.7) | 1.1 (0.8–1.6) | 1.1 (0.8–1.6) | 0.01 | 0.47 | 0.01 | 0.80 |
| total cholesterol:HDL cholesterol ratio | 4.6 ± 1.4 | 4.8 ± 1.4 | 4.6 ± 1.3 | 4.5 ± 1.4 | 0.09 | <0.001 | 0.09 | <0.001 |
| Kidney function | ||||||||
| Serum creatinine, µmol/L | 72.3 ± 18.3 | 71.1 ± 15.8 | 71.5 ± 16.1 | 72.9 ± 19.4 | 0.03 | 0.08 | 0.04 | 0.01 |
| Cystatin C, mg/L | 0.90 ± 0.20 | 0.96 ± 0.22 | 0.92 ± 0.20 | 0.88 ± 0.19 | 0.13 | <0.001 | 0.12 | <0.001 |
| eGFR, mL/min/1,73 m2 | 86.1 ± 17.4 | 82.7 ± 18.1 | 85.5 ± 18.1 | 87.1 ± 16.7 | 0.08 | <0.001 | 0.05 | 0.008 |
| Albumin excretion, mg/24 h | 8.53 (6.04–15.13) | 9.8 (6.3–22.0) | 8.7 (6.0–17.3) | 8.3 (6.0–13.9) | 0.09 | <0.001 | 0.08 | <0.001 |
| Use of drugs, n (%) | ||||||||
| Antihypertensives | 1054 (19.4) | 192 (23.6) | 255 (23.1) | 607 (17.2) | 0.05 | <0.001 | 0.02 | 0.21 |
| Antidiabetics | 184 (3.4) | 49 (6.0) | 39 (3.5) | 96 (2.7) | 0.05 | 0.002 | 0.03 | 0.03 |
| Statins | 339 (6.2) | 66 (8.1) | 77 (7.0) | 196 (5.6) | 0.04 | 0.02 | 0.02 | 0.18 |
| Per Increment of Log Transformed Plasma PLP | Vitamin B6 Status According to Plasma PLP Concentration | |||
|---|---|---|---|---|
| Deficient (<20 nmol/L) | Insufficient (20–30 nmol/L) | Sufficient (>30 nmol/L) | ||
| Composite Cardiovascular Outcome | ||||
| Cases | 6205 | 3868 | 1163 | 806 |
| Person-years | 48,466 | 32,068 | 9636 | 6762 |
| Events | 409 | 217 | 97 | 95 |
| Crude model | 0.35 (0.25–0.49) | 2.09 (1.64–2.66) | 1.49 (1.18–2.66) | 1.00 (ref) |
| Model 1 * | 0.53 (0.38–0.74) | 1.58 (1.24–2.02) | 1.38 (1.09–1.76) | 1.00 (ref) |
| Model 2 † | 0.60 (0.42–0.84) | 1.44 (1.12–1.85) | 1.32 (1.04–1.68) | 1.00 (ref) |
| Model 3 § | 0.66 (0.47–0.93) | 1.31 (1.02–1.68) | 1.25 (0.98–1.59) | 1.00 (ref) |
| Cardiovascular disease | ||||
| Cases | 6205 | 3868 | 1163 | 806 |
| Person-years | 48,466 | 32,068 | 9636 | 6762 |
| Events | 379 | 203 | 92 | 84 |
| Crude model | 0.38 (0.27–0.55) | 1.96 (1.52–2.53) | 1.51 (1.18–1.93) | 1.00 (ref) |
| Model 1 | 0.56 (0.40–0.80) | 1.51 (1.17–1.96) | 1.41 (1.10–1.80) | 1.00 (ref) |
| Model 2 | 0.63 (0.40–0.91) | 1.38 (1.06–1.79) | 1.34 (1.04–1.72) | 1.00 (ref) |
| Model 3 | 0.70 (0.49–1.01) | 1.25 (0.96–1.63) | 1.27 (0.99–1.63) | 1.00 (ref) |
| Cardiovascular mortality | ||||
| Cases | 6184 | 4057 | 1248 | 902 |
| Person-years | 49,911 | 32,846 | 9987 | 7078 |
| Events | 77 | 35 | 18 | 24 |
| Crude model | 0.15 (0.07–0.32) | 3.18 (1.89–5.35) | 1.70 (0.96–3.00) | 1.00 (ref) |
| Model 1 | 0.33 (0.16–0.71) | 1.91 (1.14–3.23) | 1.41 (0.80–2.49) | 1.00 (ref) |
| Model 2 | 0.36 (0.17–0.79) | 1.77 (1.04–3.02) | 1.34 (0.76–2.36) | 1.00 (ref) |
| Model 3 | 0.39 (0.18–0.85) | 1.59 (0.93–2.72) | 1.22 (0.69–2.17) | 1.00 (ref) |
| Per increment of Log Transformed Plasma PLP | Percentage of Association Explained | Vitamin B6 Status According to Plasma PLP Concentration | |||
|---|---|---|---|---|---|
| Deficient (<20 nmol/L) | Insufficient (20–30 nmol/L) | Sufficient (>30 nmol/L) | |||
| Composite cardiovascular outcome | |||||
| Covariate-adjusted * | 0.66 (0.47–0.93) | 1.31 (1.02–1.68) | 1.25 (0.98–1.59) | 1.00 (ref) | |
| + hs-CRP † | 0.72 (0.51–1.01) | 9 | 1.23 (0.96–1.59) | 1.21 (0.95–1.54) | 1.00 (ref) |
| + GlycA ‡ | 0.74 (0.53–1.05) | 12 | 1.19 (0.92–1.54) | 1.21 (0.95–1.53) | 1.00 (ref) |
| Fully adjusted § | 0.75 (0.53–1.07) | 14 | 1.18 (0.91–1.53) | 1.20 (0.94–1.53) | 1.00 (ref) |
| Cardiovascular disease | |||||
| Covariate-adjusted | 0.70 (0.49–1.01) | 1.25 (0.96–1.63) | 1.27 (0.99–1.63) | 1.00 (ref) | |
| + hs-CRP | 0.76 (0.53–1.10) | 9 | 1.18 [0.90–1.55) | 1.23 (0.96–1.58) | 1.00 (ref) |
| + GlycA | 0.79 (0.55–1.13) | 13 | 1.14 (0.86–1.50) | 1.22 (0.95–1.57) | 1.00 (ref) |
| Fully adjusted | 0.80 (0.56–1.15) | 14 | 1.13 (0.86–1.49) | 1.22 (0.95–1.56) | 1.00 (ref) |
| Cardiovascular mortality | |||||
| Covariate-adjusted | 0.39 (0.18–0.85) | 1.59 (0.93–2.72) | 1.22 (0.69–2.17) | 1.00 (ref) | |
| + hs-CRP | 0.46 (0.21–0.99) | 18 | 1.43 (0.83–2.48) | 1.17 (0.66–2.08) | 1.00 (ref) |
| + GlycA | 0.47 (0.21–1.02) | 21 | 1.39 (0.80–2.43) | 1.19 (0.67–2.12) | 1.00 (ref) |
| Fully adjusted | 0.48 (0.22–1.05) | 23 | 1.37 (0.78–2.39) | 1.17 (0.66–2.08) | 1.00 (ref) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minović, I.; Kieneker, L.M.; Gansevoort, R.T.; Eggersdorfer, M.; Touw, D.J.; Voerman, A.-J.; Connelly, M.A.; Boer, R.A.d.; Hak, E.; Bos, J.; et al. Vitamin B6, Inflammation, and Cardiovascular Outcome in a Population-Based Cohort: The Prevention of Renal and Vascular End-Stage Disease (PREVEND) Study. Nutrients 2020, 12, 2711. https://doi.org/10.3390/nu12092711
Minović I, Kieneker LM, Gansevoort RT, Eggersdorfer M, Touw DJ, Voerman A-J, Connelly MA, Boer RAd, Hak E, Bos J, et al. Vitamin B6, Inflammation, and Cardiovascular Outcome in a Population-Based Cohort: The Prevention of Renal and Vascular End-Stage Disease (PREVEND) Study. Nutrients. 2020; 12(9):2711. https://doi.org/10.3390/nu12092711
Chicago/Turabian StyleMinović, Isidor, Lyanne M. Kieneker, Ron T. Gansevoort, Manfred Eggersdorfer, Daan J. Touw, Albert-Jan Voerman, Margery A. Connelly, Rudolf A. de Boer, Eelko Hak, Jens Bos, and et al. 2020. "Vitamin B6, Inflammation, and Cardiovascular Outcome in a Population-Based Cohort: The Prevention of Renal and Vascular End-Stage Disease (PREVEND) Study" Nutrients 12, no. 9: 2711. https://doi.org/10.3390/nu12092711
APA StyleMinović, I., Kieneker, L. M., Gansevoort, R. T., Eggersdorfer, M., Touw, D. J., Voerman, A.-J., Connelly, M. A., Boer, R. A. d., Hak, E., Bos, J., Dullaart, R. P. F., Kema, I. P., & Bakker, S. J. L. (2020). Vitamin B6, Inflammation, and Cardiovascular Outcome in a Population-Based Cohort: The Prevention of Renal and Vascular End-Stage Disease (PREVEND) Study. Nutrients, 12(9), 2711. https://doi.org/10.3390/nu12092711








