Abstract
Aging is a risk factor for the development of multiple chronic diseases, including cardiovascular disease, cancer and dementia. Life expectancy has increased in certain countries but this phenomenon is associated with a reduction of years of healthy life. Aging is associated with a number of physical and functional changes, especially sarcopenia. Sarcopenia is a clinical condition associated with a decrease in skeletal muscle and muscle strength, however, sarcopenia is a reversible condition. On the basis of the current scientific literature, sarcopenia could more appropriately capture an individual’s vulnerability to negative health-related outcomes since it represents an early form of the chronic diseases. Recognition of this clinical condition can improve the management of older individuals in many different clinical settings. Despite the limitations of the indirect methods used to study body composition, the Italian College of the Academic Nutritionists ME/49 recommends that health authorities and health professionals around the world should make a greater effort to diagnose sarcopenia earlier and to manage it more effectively. In line with the development of cancer screening, the use of two diagnostic tools for sarcopenia (BIA and DXA) should be implemented.
1. Introduction
The ageing of societies around the world and the increase in life expectancy in certain countries is unfortunately also associated with a demographic and epidemiological transition towards a frail population [1,2,3]. Frail older adults are vulnerable to stress, which increases the risk of adverse outcomes including chronic noncommunicable diseases (NCDs), disability and mortality [4].
Italy is a striking example of this phenomenon and is second to Spain as the country in Europe with the highest average life expectancy at birth [2], which increased by 2.8 years between 2000 and 2015 [3]. Although at an international level, this increase is seen as a public health success story; in approximately the same period of time, there has been an increase in several NCDs, which include a significant proportion of cases of cancer, dementia, falls and cardiovascular, metabolic and respiratory diseases [5].
Furthermore, the recent COVID-19 pandemic has shown peculiar epidemiological traits with high rates of hospitalization and mortality among older adults in Italy [6,7], as well as Black, Asian and minority ethnic populations in both the United Kingdom and the United States [8,9,10]. This confirms that elderly people in countries like Italy are as frail as poorer ethnic populations in other countries [8,9,10]. Therefore, the dramatic increases in life expectancy, attributable to improved medical care, in reality often correspond to a reduction of years of healthy life.
A longer healthy life should thus be the objective of all health policies. Consequently, there is a need for a new paradigm in the identification of the risk factors for chronic illnesses and the management of social and health behaviours throughout life.
A common condition in elderly individuals is a suboptimal diet, which is characterized by inadequate calorie and protein intake. Malnutrition affects approximately one-third of older people, particularly those who are resident in institutionalized facilities [11], where malnutrition may substantially influence the need for hospitalization as well as the levels of mortality in the short and medium term [12]. A suboptimal diet accounts for one in every five deaths globally, and nonoptimal intake of three dietary factors (whole grains, fruit, and sodium) accounts for more than 50% of deaths and 66% of disability attributable to diet [13].
Interestingly, it has been reported that many elderly patients/individuals experience a loss of muscle mass before a weight loss, which is often stable due to the fat mass increase [14]. When a reduced loss of muscle mass is associated with a reduced muscle strength, then an individual is affected by sarcopenia. Sarcopenia is the most prevalent syndrome among hospitalized elderly individuals/older medical inpatients [14,15] compared to clinical conditions such as malnutrition, frailty, and cachexia [14], and is also reported in community-dwelling older adults [16,17]. Sarcopenia is often associated with decreases in energy intake [18], however, there are many factors are responsible for the decline in muscle mass and muscle strength associated with aging. Among these, physical inactivity or a decreased physical activity level in aged individuals is a part of the underlying mechanisms of sarcopenia [19]. Consequently, sarcopenia could more appropriately capture an individual’s vulnerability to negative health-related outcomes [20]. Regardless of the causes, sarcopenia identifies elderly people at risk of reducing their years of healthy life and of developing NCDs since it represents a subclinical or early form of these chronic diseases.
Older adults with severe levels of sarcopenia are approximately two to five times as likely to have a disability as older adults with optimal normal muscle mass [21,22,23]. Despite a positive association bidirectional relationship between NCDs and disability, this association is largely ignored by the global health community [24]. Thus, both the prevention of NCDs and NCD-related disability are important elements of any proposed strategy for healthy aging [24].
This document focuses on the importance in identifying elderly individuals who are apparently disease free but are actually affected by sarcopenia, a condition associated with subclinical NCDs, which predicts incident adverse events and disability. Furthermore, this document highlights the urgent need to use inexpensive, noninvasive, quick and accurate methods, which are already available to diagnose sarcopenia and its different phenotypes. The overall aim is thus to improve the diet as well as all other treatments that can help prevent the consequences of sarcopenia in the elderly.
2. Sarcopenia and Its Different Phenotypes as Predictors of Adverse Outcomes
2.1. Definition and Assessment of Sarcopenia
Sarcopenia is a progressive, persistent and generalized skeletal muscle disorder that is associated with an increased likelihood of adverse consequences such as including falls, fractures, physical disability and mortality [25]. Expert groups worldwide have published several definitions of sarcopenia [26,27,28]. Some researchers suggested the term “age-related sarcopenia,” whereas others, the term “myopenia” to indicate the presence of clinically relevant muscle wasting. Despite the lack of consensus on an exact definition, the term “sarcopenia” has created awareness for this condition and its management. In its 2018 definition, the European Working Group on Sarcopenia in Older People (EWGSOP)2 uses low muscle strength (a measure of muscle function) as the principal parameter of sarcopenia, which is confirmed by the presence of low muscle quantity or quality [25]. Since detection of low physical performance predicts adverse outcomes, it is used to identify the severity of sarcopenia [25].
Muscle quantity can be assessed as total body skeletal muscle mass (SMM), as appendicular skeletal muscle mass (ASM), or as the muscle cross-sectional area of definite muscle groups or body locations [25]. This body compartment can be measured accurately using computed tomography (CT) or magnetic resonance imaging (MRI). However, the costs, availability and, for CT, radiation exposure, prevent their use in clinical practice. Compared with these techniques, dual-energy X-ray absorptiometry (DXA) is a less invasive and less costly method for quantifying muscle mass [29]. DXA could be considered as a reference standard (but not a gold standard) for measuring muscle lean body mass [30].
However, since bioelectrical impedance analysis (BIA) has been cross-validated in several populations for the study of the body composition, the use of BIA in the study of human body composition has grown rapidly in the nutritional field in the past two decades. BIA is a simple, noninvasive, reliable, repeatable, rapid and inexpensive method for estimating muscle mass by prediction equations [25,31]. However, to ensure reliability, several factors (such as hydration status food intake, and physical exercise) must be taken into account controlled [32,33,34,35].
2.2. Epidemiology of Sarcopenia and Its Phenotypes
Sarcopenia affects is prevalent in up to 15% of apparently healthy older adults [15,36], up to 80% of acutely hospitalized older patients [37] and up to 70% of older postacute rehabilitation inpatients [38]. Sarcopenia is significantly higher in individuals with several NCDs with the highest prevalence in individuals with type 2 diabetes mellitus (T2DM) and cardiovascular disease (CVD) (14.7–78.6% for T2DM and 15.5–44.7% for CVD) compared to individuals with dementia and respiratory disease (12.6–33.3% for dementia and 21–23.9% for respiratory diseases) [16].
Furthermore, the loss of muscle mass seems strictly associated with the loss of bone mineral density (BMD) [39]. Several studies support the high prevalence of sarcopenia in elderly patients with fragility fractures [40,41,42]. The loss of muscle mass in osteoporotic patients affected by osteoporosis is significantly more common than in subjects with normal BMD [39]. Consequently, the term “osteosarcopenia” has been coined to describe a subgroup of older persons affected by both osteoporosis (osteopenia/osteoporosis) and sarcopenia. Among community-dwelling populations, the prevalence of osteosarcopenia is up to 60% in both genders ≥75 years [43,44].
Furthermore, sarcopenia might worsen the effects of obesity in older adults, resulting in a particular phenotype called “sarcopenic obesity.” Sarcopenic obesity is a condition of reduced lean body mass in the context of associated with increased excess adiposity. However, to date, a unifying definition of this syndrome does not exist [45], thus, its prevalence varies widely between studies, depending on population characteristics and different definitions.
Different amounts of adipose tissue and muscle mass may alter the bone biology [43]. In most but not all studies, fat mass was an additional independent contributor to reduced BMD, especially in postmenopausal women [39,43], resulting in a particular phenotype called “osteosarcopenic obesity.” The negative link between total fat mass and whole-body BMD may reflect the increased bone resorption associated with the synthesis of inflammatory cytokines in visceral fat [39], but only in women, whereas in men, FM and BMD are not associated, probably due to the different hormonal as well as physical activity pattern [39]. However, again, a unifying definition of this phenotype as well as its prevalence data does not exist.
2.3. Sarcopenia-Related Outcomes
While age-related sarcopenia is considered to be a primary sarcopenia, a number of disease states and metabolic conditions (such as hypercatabolism and cachexia) can lead to secondary sarcopenia [25]. Sarcopenia has thus been associated with an increased risk of hospitalization, it also requires long-term care and doubles the risk of dying over a period of 3–10 years [46,47,48,49].
Sarcopenia is associated with cardiovascular disease (CVD) [16,50], respiratory disease [16] and cognitive impairment [16,51]. In addition, sarcopenia is associated with greater cardiovascular mortality and all-cause mortality [20]. However, sarcopenic obese older men were found to have a higher risk of all-cause mortality than obese and sarcopenic individuals, suggesting a negative synergic role for the increased fat mass and reduced muscle mass in explaining the relationship between sarcopenic obesity and mortality [20,52]. It has also been reported that sarcopenic obesity is a key cause of long-term disability in older adults [53,54,55,56,57].
Osteopenia and osteoporosis have also been associated with an increased risk of mortality irrespectively of fragility fractures [58,59,60]. However, osteosarcopenia leads to significantly worsened outcomes than seen in either condition alone. A recent meta-analysis found that the odds of a fracture in those suffering from sarcopenia was approximately twice as high as a nonsarcopenic older person [61]. Osteosarcopenia is also associated with significantly increased mortality (1-year mortality rate of 15.1% in osteosarcopenic patients vs. 5.1% in osteoporotic and 10.3% in sarcopenic patients alone [62]. This implies a greater risk of frailty [63] and institutionalization as well as significant socioeconomic costs.
In the context of the risk of communicable disease (CDs), sarcopenia predicts the risk of infection after surgery, and among hospitalized patients, sarcopenia is associated with a twofold increase in the risk of developing nosocomial infections [64,65]. Sarcopenia also predicts the risk for community-acquired pneumonia in the elderly [66,67].
The burden of outcomes related to sarcopenia rests at both the individual and societal levels. In Europe, there will be an estimated 72% increase in the number of sarcopenic patients by 2045 [64]. Sarcopenia and its various phenotypes will thus become a public health concern in the future.
3. Potential Mechanisms Linking Sarcopenia to NCDs and Mortality
There is abundant evidence of the key crucial role of abnormal altered muscle metabolism in the genesis, and consequently prevention, of many common NCDs (Figure 1).
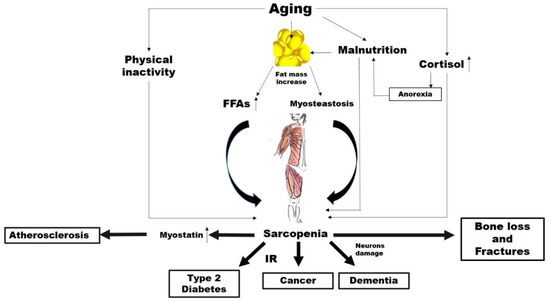
Figure 1.
Potential mechanisms linking aging, sarcopenia and noncommunicable diseases (NCDs). FFAs: free fatty acids, IR: insulin resistance..
Aging is characterized by an increase in the percentage of body fat which generally translates to is associated with an elevated availability concentration of free fatty acids (FFAs) leading, in turn, to insulin resistance (IR) [68,69].
However, increased accumulation of fat around and within organs that normally contain only small amounts of fat, such as in skeletal muscle, can impair the normal physiological function of those organs [70]. This phenomenon, known as myosteatosis, occurs before the onset of strength and functional abnormalities as well as before the metabolic alterations such as IR and T2D in the elderly [71,72]. Among older adults, myosteatosis is related to decreased muscle strength [73].
Irrespectively of all these mechanisms, a substudy carried out within the Look AHEAD clinical trial demonstrated that individuals with diabetes have a greater fat mass and smaller lean mass regardless of ethnicity compared to controls [74]. Individuals with hyperglycaemia or diabetes have less appendicular lean body mass and a lower muscle quality, which possibly reduces the surface area for glucose transport [75]. On the other hand, a higher percentage of total lean body mass has been associated with a lower likelihood of current diabetes, prediabetes and insulin resistance in cross-sectional studies [76]. As skeletal muscle is responsible for the majority of the body’s postprandial glucose disposal, IR in this body compartment results in substantial whole-body metabolic disorders.
Of course, it is possible that changes in lean body mass represent both a risk factor and a consequence of impaired glucose states, irrespectively of changes in total body fat. In addition, physical inactivity, which is a common feature of ageing, is associated with a decline in mitochondrial oxidative function in the muscle [77], which involves a reduced capacity to oxidize fatty acids leading, again, to IR [77]. It is well known that IR characterizes the metabolic syndrome, which is a condition preceding frank diabetes [78]. There is also a strong correlation between insulin resistance and the risk of developing CVD [79]. Furthermore, IR seems to be closely associated with the increased incidence of and/or mortality from a broad range of cancers such as breast [80], colorectal [81], pancreatic [82] and liver [83] cancer. A first mechanism linking sarcopenia to NCDs might thus involve IR.
The mechanical force applied to the bone, which is critical to bone health, could clarify another possible link between sarcopenia and NCDs. It is attributable to come from two primary sources: external gravitational loading (via ground reaction forces) and internal loading (via muscle contractions). These forces are reduced significantly with a sedentary lifestyle as in advanced age. Several researchers have postulated whether the loss of one tissue with ageing might predispose the loss of the other. Exposure to microgravity with spaceflight represents a valid model to understand the role of muscle on bone metabolism.
The accelerated loss of bone and muscle mass as a result of microgravity is well documented over the decades [84]. Interestingly, under this condition, muscle atrophy precedes the decline in bone mass [85]. In fact, muscle is a source of particular molecules, namely, myokines, which stimulate bone formation and also contribute to bone loss [86,87]. Most interestingly, in spinal cord injury (SCI), which is a clinical condition associated with both sarcopenia and osteoporosis, the primary cause of bone loss is the removal of muscle-induced mechanical stimuli on the bone [88]. The alteration in the skeletal muscle metabolism associated with a reduced mechanical loading might thus play a role in the association between sarcopenia and common NCDs, such as osteoporosis.
Other possible links specifically concern cardiovascular diseases. Myostatin is a member of the transforming growth factor-β (TGF-β) super family, which is predominantly produced in skeletal muscle in response to diverse stimuli, including oxidative stress, inflammation and others [89].
Myostatin increases in sarcopenia, by driving ubiquitin-proteasome-mediated protein degradation [90,91], while a myostatin deficiency has been shown to have beneficial effects on muscle size and mass [92], as well as adiposity and insulin sensitivity [93]. Myostatin has also been proposed as a putative new mediator of progressive atherosclerotic lesions, heart dysfunction, cardiac fibrosis, and cardiovascular cachexia [94,95].
The risk of neurodegenerative diseases may be another factor in the link between sarcopenia and NCDs. It has been reported that cortisol concentrations increase with age [96]. While ageing, the increase in cortisol levels can have various effects on body composition and multiple systems in older people [97]. The activity of 11β-hydroxysteroid dehydrogenase type 1, an enzyme which catalyses the synthesis of cortisol, is enhanced in various tissues, such as the central nervous system, skeletal muscles and bones [98]. As a catabolic hormone, higher cortisol levels are linked with weight loss, muscle mass reduction, bone loss as well as reduced physical performance and anorexia, leading to the fundamental characteristics of frailty [96,99,100]. High cortisol levels make neurons more vulnerable to several kinds of insults, and Alzheimer disease (AD) patients have mildly increased levels of cortisol compared to controls [101,102].
4. Now Is the Time to Screen for Sarcopenia
On the basis of all these data, a screening strategy is urgently needed that detects sarcopenia in its early stages. Importantly, the average cost of health care related to an individual affected by sarcopenia is three times higher than a nonsarcopenic individual [103].
Several factors need to be assessed when making a decision about disease screening, including how common, fatal or complicated by serious health impacts the condition to be screened is, and also whether treatment is available. Of course, screening programs are tailored toward those individuals who the disease is most likely to affect and where the most benefit can be gained. The benefit-to-harm ratio of screening for sarcopenia increases as an individual ages due to the increase in the prevalence of sarcopenia. We therefore believe that screening for sarcopenia, as with other types of screening, should be planned and promoted by health authorities and confined to a specific section of the population based on certain risk factors (i.e., elderly individuals).
An ideal screening test should be noninvasive, cost effective, easily performed and of course accurate. However, with reference to MRI, CT, DXA and BIA, several publications have concluded that currently, there are no practical diagnostic methods for sarcopenia [104,105,106]. Several proponents of screening tools have thus tried to exploit screening questionnaires, diagnostic grids, or prediction equations [107,108,109,110,111]. However, these screening tools provided contradictory results in many cases due to their scarce sensitivity but high specificity (in other words, only severe cases may be detected with a high rate of false negatives). Nevertheless, their predictive values in diagnosing sarcopenia are generally acceptable, especially for primary prevention [112,113]. Unfortunately, there is relatively limited evidence from experimental studies of their effectiveness and it is unclear which of these current tools is the most effective in screening for sarcopenia in the community. While these tools might be useful in primary prevention, in the secondary prevention of individuals who already have sarcopenia, these tools cannot be used for early diagnosis. On the other hand, BIA and DXA could have a primary role as they have most of the characteristics of an ideal tool.
In line with the development of cancer screening, especially for breast cancer, the use of these diagnostic tools for sarcopenia could be improved by addressing some of the uncertainties that some physicians have highlighted [114].
Briefly, breast cancer screening is the regular examination of asymptomatic, apparently healthy women. The aim is to identify breast cancer as early as possible, so that women can receive timely and effective treatment in order to prevent complications and mortality. In this sense, the screening for sarcopenia could be similar to that of breast cancer. Most national scientific groups recommend mammograms from the age of 40, however, international policies differ with respect to the target age group to be screened. In any case generally there is consensus on the use of mammography [114]. A mammogram exposes a woman to 0.4 mSv, which is approximately equivalent to the amount of radiation a person would expect to get from natural background exposure over 7 weeks. On the other hand, one chest X-ray exposes the patient to about 0.1 mSv [115], which is about the same amount of radiation people are exposed to naturally over the course of about 10 days [115]. Below 10 mSv, no direct epidemiological data support an increased cancer risk. However, this does not mean that this risk is not present. Theoretical concerns about radiation-induced breast cancer from exposure to repeated mammography have been raised, however, the potential benefits are thought to outweigh the risks. For example, the benefit-to-harm ratio is estimated to be 48.5 lives saved per 1 life lost due to radiation exposure [116]. There are no doubts, therefore, about the importance of this screening programme.
Yet curiously, there seems to be concern in relation to the radiation dose from DXA, if used for the screening of sarcopenia [105,106]. The potential risk for an individual associated with DXA techniques used for the assessment of body composition is very small because the radiation doses are low. Effective doses for whole-body DXA examinations were found to range between 0.0052 and 0.008 mSv [116], thus approximately 200 times lower than that from mammography [115]. It is clear that DXA is considerably safer than mammography. It is estimated that one in two women will have at least one false-positive mammogram result [117] and false positive results can provoke anxiety and increase costs [118].
Wide ranges were noted for the percentage of mammograms referred as judged to be atypical abnormal (1.2–15.0%), as well as for the positive predicted value (PPV) (3.4–48.7%), which represent the probability of presenting a breast cancer in the case of a positive screening test [119]. However, mammography is viewed as the most accurate technique for screening purposes. So, how accurate are the current instruments used for the body composition assessment?
There seems to be a strong correlation between hydrodensitometry (the reference method for the assessment of body composition in the past) and DXA lean mass assessment (correlation coefficients > 0.86) with only small differences in the mean body fat compartment levels [120,121], which enabled DXA technology to be validated. The accuracy of body-composition measurements in vivo by DXA was also assessed in pigs (because their body weights and compositions are close to those of human neonates) [122]. As coefficients of variation (CVs), a precision of <2%, <3% and 2% for fat mass, lean mass and percentage fat, respectively, has been reported [123]. However, CT and MRI, which are particularly accurate in assessing muscle and fat areas in cadaveric studies, are still considered as reference methods. Several studies have demonstrated that appendicular lean soft tissue mass measured by DXA is highly correlated with both MRI and CT measurements of skeletal muscle volume [124,125,126]. Although DXA tends to underestimate the extent of sarcopenia, overall, there is a good correlation between the measurements of skeletal muscle mass in the lower limbs using DXA and those using MRI and CT [126,127]. There is also a good agreement between DXA and CT for abdominal total tissue mass and fat mass [128]. MRI and CT are expensive, time consuming and, in the case of CT, requires excessive radiation doses and are thus impractical in clinical settings. Consequently, DXA is now preferred to assess body composition.
A study comparing the performance of five screening tools for the risk of sarcopenia, reported a PPV for the algorithm of the EWGSOP (in which the skeletal muscle index, SMI, was assessed using DXA) from 17.5% to 42.5%, with a sensitivity range of 33.3–70.6% and a specificity range of 88.5–91.1% [129]. These values are very similar to that of mammography.
One source of error in the assessment estimation of body compartments composition by DXA is the hydration status of the subject. However, it has been reported that the magnitude of error in fat or fat-free mass would not exceed 0.5 kg, even under conditions of extreme physiological variance in hydration status [130].
Bone mineral density (BMD) assessed by DXA is highly correlated with bone histomorphometry (r > 0.90) in patients with a number of metabolic bone diseases [131,132,133], as well as undergoing hip replacement [134]. Using the WHO cut-off values for the definition of osteoporosis, DXA can detect up to 88.2% of possible cases of osteoporosis (sensitivity) with a specificity of approximately 63% and a PPV of more than 80% [135], which is much higher than that of mammography. Typical in vivo BMD precision values reported for humans are 1–2% with a high accuracy (4–10%) [136]. Although there are differences in BMD values among the scanners, due to different calibration methods and different edge detection methods, nevertheless BMD values obtained on different machines were highly correlated (r > 0.99). BMD measurements do not fully account for changes in trabecular architecture, tissue properties and accumulation of microdamage [137,138]. In any case, BMD measurements are currently the standard method for diagnosing osteoporosis.
How can the overall performance of a screening tool be improved? Most studies have reported an increase in sensitivity by adding breast ultrasound to the mammogram. The PPV for ultrasound alone ranges from 6% to 11%, but is as high as 56% when combining mammography with ultrasound, which gives a sensitivity of approximately 80% [139,140,141,142,143,144,145,146]. However, hand-held breast ultrasound screening is operator dependent [147]. Other limitations in implementing widespread screening ultrasound include a lack of standardized scanning protocols [147].
In order to study body composition, one solution would be to combine a DXA scan with a BIA examination. In fact, both methods can be used to measure lean mass [25]. The advantages of DXA (precision, accuracy and assessment of BMD) fit well with those of BIA (noninvasiveness and low cost) jointly contributing to the diagnosis of sarcopenia, osteosarcopenia and sarcopenic obesity. A BIA can predict a reduced ASMM (with DXA as the gold standard) with a PPV of 73%, a sensitivity of 80% and a specificity of 91% in geriatric patients [148], better than breast ultrasonography alone and similar to the combination of mammography plus ultrasound. A recent study showed that compared to DXA, BIA is highly reliable in terms of assessing appendicular lean mass, with an intraclass correlation coefficient (ICC) of 0.89 (95% CI: 0.86–0.92), when performed by the same operator, and an ICC of 0.77 (95% CI: 0.72–0.82), when performed by two different operators [149]. All the potential systematic bias in the estimation of lean body mass measurements by BIA would be overcome by combining BIA with a more precise technique such as DXA.
In Italy, the average cost of breast cancer screening is around 95 euros, whereas sarcopenia screening would only cost around 76 euros [150].
5. Recommendations
It is time that health professionals made a greater effort to diagnose sarcopenia earlier and to manage it more effectively. Based on the available evidence supporting that sarcopenia as predictive biomarker of several NCDs and mortality, the Italian College of the Academic Nutritionists ME/49 (ICAN-49) recommends the following:
- Nutritionists are encouraged to screen for sarcopenia using a combination of DXA plus BIA as screening tools for secondary prevention.
- General practitioners (GPs) as well as other health care professionals should suggest a screening for sarcopenia in elderly individuals.
- Nutritionists as well as other health care professionals (as geriatrics, internists, neurologists and cardiologists) should ask National health authorities to plan screening for the early diagnosis of sarcopenia and its various phenotypes in the elderly.
- Nutritionists are encouraged to ask National health authorities for provide DXA and BIA equipment to the nutritional units and to reimburse the costs of all the treatments for those diagnosed with sarcopenia including where it is a secondary condition to chronic diseases.
- Nutritionists should collaborate with health care policy makers and health care providers regarding medical claims and common standards of screening technologies.
Specifically, we propose that adults older than 65 year undergo a measure of handgrip strength (prescreening), which is known to have a high specificity for identifying those at risk of dynapenia. Dynapenia, the age-associated loss of muscle strength, represents a clinical marker of mobility loss and deficiency in instrumental activities of daily living, better than the decline in muscle mass [151]. The handgrip strength test makes easier the strength measurement, and it is inexpensive. However, individuals who lose loss strength in addition to muscle mass are more predisposed to the loss of mobility than those who only lost muscle strength. Thus, in order to rule out the presence of sarcopenia and better dissect the contribution of the bone loss from muscle loss in the reduction of ASMM, the next step (I level exam) could be to perform a DXA scans in those who are positive for low grip strength (whole body as well as femoral and spinal scan). Since the evaluation of lean mass by DXA might underestimate the age-related decrease in muscle mass, due to the increase in total body water with ageing, a second step (II level exam) could be to perform BIA in order to confirm the reduction in ASMM.
To improve knowledge on this theme, ICAN-49 recommends the following:
- Promotion of centralized data collections in epidemiological studies on older adults.
- Development of RCTs on new treatments for sarcopenia in individuals with either primary or secondary sarcopenia.
- GPs should receive continuing medical training regarding sarcopenia.
- The government should fund information and prevention campaigns, in collaboration with patient associations and scientific research groups.
The aim of screening for sarcopenia is to counteract all the negative complications derived from sarcopenia i.e., falls, fractures, loss of independent living and, ultimately, NCDs and mortality. Since the aetiology of sarcopenia in the elderly is multifactorial, there need to be different treatment approaches. The scope of this document does not include a review of all the studies on the dietary and nondietary approaches to treat sarcopenia. However, here, we briefly summarize the cornerstones of sarcopenia treatment.
Although in some publications, the quality of evidence is not high, a number of systematic reviews and meta-analyses have shown several positive effects of nutritional and exercise interventions for treating sarcopenia in older people [152,153,154,155,156,157,158,159,160,161,162,163].
Skeletal muscle (SM) makes up approximately 40% of total body weight, consisting of 50–75% of total proteins. Inadequate dietary protein intake alters the whole-body protein balance inducing muscle catabolism to provide amino acids to allow for protein synthesis [164]. Consequently, there is a loss of muscle mass and bone, which then influences hormone synthesis, immune system response (and thus, susceptibility to infections) and autonomy [164]. A protein intake of between 1.2 and 2.0 g∙kg−1∙day−1 or higher for elderly adults has been recommended [165,166]. However, elderly adults typically eat less than younger adults do, and a lower protein intake has been reported [166,167]. It has been estimated that approximately 40% of adults of both genders have dietary protein intakes that are below the recommended dietary allowance (RDA) [167,168]. A recent study on the elderly in Italy and Brazil reported that the diet did not provide an adequate amount of protein to maintain functional status in older adults (only ~1.0 g∙kg−1∙day−1) [169], thus supporting the need to increase the protein intake for the elderly [170]. However, even with an appropriate supply of amino acids, reduced energy intake causes a reduction in protein synthesis with a consequent reduction in the size of myofibrils [171]. These findings indicate that to maintain muscle mass, an individual’s energy intake is crucial.
A higher intake of animal protein than plant protein was significantly associated with a 40% difference in the loss of lean body mass over time in elderly older adults, in favour of animal protein [172]. Notably, the ingestion of animal proteins (i.e., from milk) compared with the ingestion of vegetal proteins seems to stimulate muscle protein synthesis to a greater extent after resistance exercise [170,173,174]. Greater amounts of plant-based proteins are necessary to stimulate the anabolic mechanisms in the muscle mass than animal-based protein. The beneficial effects of the animal protein seem to be mainly attributable to the content of branched-chain amino acids (BCAAs) (especially leucine), which are potent stimulators of muscle protein synthesis via the activation of the mammalian target of rapamycin [175]. Indeed, lower systemic concentrations of BCAAs have been found in older adults/elderly individuals affected with sarcopenia than those that were nonsarcopenic [175].
A recent review [176], which summarized the results from 12 RCTs, concluded that the combination of whey protein with exercise improves muscle mass quantity, quality as well as physical performance [152,153,154,155,156,157,158,159,160,161,162,163,177]. However, it is difficult to establish from these studies what the exact contribution of whey is. Trials in older adults with whey supplementation alone have demonstrated that a daily dietary supplementation of 35 g of whey is likely to improve sarcopenic biomarkers in frail or sarcopenia individuals [163,178,179,180,181]. Although the greatest benefit for older sarcopenic individuals is resistance exercises [182], a meta-analysis concluded that dietary protein supplementation can be, if the usual protein intake is less than 1.6 g protein/kg/day, both sufficient and necessary to optimize resistance exercise training (RET)-induced gains in muscle mass and strength [183]. There appear to be no detrimental effects of increasing protein intake on bone health, [184]. It can thus be concluded that nutritional treatment plays a key role in the treatment of sarcopenia.
Although the number of publications is limited, there is also a growing literature pointing to the benefits of supplementing with high protein oral nutritional supplements enriched with β-hydroxy-β-methylbutyrate to address muscle-related problems that develop with aging and chronic disease [185]. Vitamin D and calcium supplementation also have a positive effect, especially among institutionalized older individuals with osteosarcopenia. Vitamin D and calcium supplementation help reduce the risk of hip fracture (by 16–33%) and any fracture (by 5–19%) [186]. Although vitamin D and calcium doses varied considerably among the studies, it is generally accepted that at least 800 IU/day should be added to a minimum of 500 mg/day of calcium supplementation (when an individual is unable to get adequate calcium through their diet) to have an effect on fracture risk [187]. Vitamin D supplementation of individuals presenting a 25(OH)D level less than 30 nmol/L also results in a significantly greater improvement in muscle strength, compared with those who present a 25(OH)D level of at least 30 nmol/L [188].
Several studies have also highlighted that RET is one of the most effective nonpharmacological strategies to increase BMD [189,190,191,192,193,194]. In fact, progressive resistance exercise is able to stimulate osteoblastogenesis [195].
Currently, there are no approved medications for treating sarcopenic obesity. Reviews seem to suggest that resistance exercise is essential in increasing muscle strength and physical performance parameters of people with sarcopenic obesity, whereas reducing calorie and fat intake positively affect body fat mass [196,197,198,199,200]. Overall, this evidence suggests that dietary interventions in older people should not focus only on increasing muscle strength, but also on optimizing muscle quality and improving muscle performance [72].
ICAN-49 recommends the following:
- A protein intake of 1.2–1.5 g∙kg−1∙day−1 or higher for elderly adults, according to the nutrient intake, renal function and severity of sarcopenia, with at least 20–35 g/daily of whey protein, in conjunction with resistance exercise for a person with sarcopenia. Higher doses of protein (up to 2 g/day) may be appropriate in persons with severe illness or a catabolic status
- Vitamin D, from dietary and supplemental sources, to any older person with vitamin D deficiency of insufficiency (at least 800 UI per day or more according to serum concentrations).
- Calcium, from dietary and supplemental sources, should be in adequate amounts (i.e., at least 1200 mg per day).
- Patients/older individuals at risk of or with established sarcopenia should be encouraged to be involved in regular physical activity.
These recommendations apply to both primary sarcopenia and secondary sarcopenia. In secondary sarcopenia, the diseases related to sarcopenia also need to be treated.
6. Conclusions
Despite the limitations of the indirect methods used to study body composition (particularly BIA and DXA) and the concerns related to standardizing protocols and treatments for sarcopenia, it is now strongly recommended that health authorities around the world should screen for sarcopenia. This treatable clinical condition has huge costs, precedes the appearance of diabetes, cancer, and dementia, and predicts mortality. Measures should be set up for the early identification of those individuals affected by sarcopenia and to treat them by combining nutritional interventions and exercise.
Cancer screening has several limitations, especially related to the ability of the technologies used to identify individuals who are really affected by cancer together with their invasiveness. However, these limitations have not prevented health authorities and the scientific community from implementing these screenings.
Accordingly, we strongly believe that nutritionists should start addressing this challenge and acquire diagnostic tools, rather than relying on alternative or surrogate tools. DXA has many advantages in terms of accuracy, simplicity, low cost, low radiation exposure, and low scanning time, and its role in sarcopenia diagnosis is thus emerging as the reference assessment technique in muscle mass evaluation. The assessment of body composition appears to be less reliant on underlying assumptions than most other methods. BIA is a popular, simple and portable technique for the estimation of body composition. It does not require skilled staff, is relatively inexpensive, and does not expose patients to radiation. Several studies have validated the use of BIA in determining appendicular muscle mass.
Treatment programs, including nutritional interventions associated with exercise training, may improve the quality and function of muscle mass. However, many older people have difficulties with swallowing, thus oral supplementations need to be prescribed.
If all the actions included in this document are implemented by nutritionists as well as other health care professionals, it should lead to a growing interest of national health authorities as well as the scientific community in this complex condition, and, as a consequence, a delay in the physical disability and mortality in older people.
Author Contributions
All authors contributed to the manuscript preparation. T.M., A.P. and A.D.L. conceived the manuscript. T.M. and A.P. were responsible for interpretation of data and wrote the paper. L.M.D., L.F., F.G., A.N., L.P., M.P., P.R., A.A.R., G.S., A.T., M.S. and D.R. substantively revised the manuscript and approved final manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of Interest
The authors have no competing financial interests in relation to the work described.
References
- World Health Organization. Noncommunicable Diseases Country Profiles 2018; License: CC BY-NC-SA 3.0 IGO; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Eurostat. Eurostat Database. Available online: https://ec.europa.eu/eurostat/en/web/products-eurostat-news/-/DDN-20181026-1 (accessed on 26 October 2018).
- OECD/European Observatory on Health Systems and Policies. Italy: Country Health Profile 2017, State of Health in the EU; OECD Publishing: Paris, France; European Observatory on Health Systems and Policies: Brussels, Belgium, 2017. [Google Scholar]
- GBD 2017 Italy Collaborators. Italy’s health performance, 1990–2017: Findings from the Global Burden of Disease Study 2017. Lancet Public Health 2019, 4, e645–e657. [Google Scholar] [CrossRef]
- Clegg, A.; Young, J.; Iliffe, S.; Rikkert, M.O.; Rockwood, K. Frailty in elderly people. Lancet 2013, 381, 752–762. [Google Scholar] [CrossRef]
- COVID-19 Surveillance Group. Characteristics of COVID-19 Patients Dying in Italy: Report Based on Available Data on July 9th, 2020. Instituto Superiore Di Sanità. Available online: https://www.epicentro.iss.it/en/coronavirus/bollettino/Report-COVID-2019_9_july_2020.pdf (accessed on 9 July 2020).
- Inciardi, R.M.; Adamo, M.; Lupi, L.; Cani, D.S.; Di Pasquale, M.; Tomasoni, D.; Italia, L.; Zaccone, G.; Tedino, C.; Fabbricatore, D.; et al. Characteristics and outcomes of patients hospitalized for COVID-19 and cardiac disease in Northern Italy. Eur. Heart J. 2020, 41, 1821–1829. [Google Scholar] [CrossRef] [PubMed]
- Office for National Statistics; National Records of Scotland; Northern Ireland Statistics and Research Agency. 2011 Census Aggregate Data; Edition: June 2016; UK Data Service: Colchester, UK, 2016. [Google Scholar]
- Yancy, C.W. COVID-19 and African Americans. JAMA 2020, 323, 1891–1892. [Google Scholar] [CrossRef]
- Garg, S.; Kim, L.; Whitaker, M.; O’Halloran, A.; Cummings, C.; Holstein, R.; Prill, M.; Chai, S.J.; Kirley, P.D.; Alden, N.B.; et al. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019—COVID-NET, 14 States, March 1–30, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 458–464. [Google Scholar] [CrossRef]
- Pauly, L.; Stehle, P.; Volkert, D. Nutritional situation of elderly nursing home residents. Z. Gerontol. Geriatr. 2007, 40, 3–12. [Google Scholar] [CrossRef]
- Valmorbida, E.; Trevisan, C.; Imoscopi, A.; Mazzochin, M.; Manzato, E.; Sergi, G. Malnutrition is associated with increased risk of hospital admission and death in the first 18 months of institutionalization. Clin. Nutr. 2020. [Google Scholar] [CrossRef]
- GBD 2017 Diet Collaborators. Health effects of dietary risks in 195 countries, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019, 393, 1958–1972. [Google Scholar]
- Gingrich, A.; Volkert, D.; Kiesswetter, E.; Thomanek, M.; Bach, S.; Sieber, C.C.; Zopf, Y. Prevalence and overlap of sarcopenia, frailty, cachexia and malnutrition in older medical inpatients. BMC Geriatr. 2019, 19, 120. [Google Scholar] [CrossRef]
- Pacifico, J.; Geerlings, M.A.; Reijnierse, E.M.; Phassouliotis, C.; Lim, W.K.; Maier, A.B. Prevalence of sarcopenia as a comorbid disease: A systematic review and meta-analysis. Exp. Gerontol. 2020, 131, 110801. [Google Scholar] [CrossRef]
- Mijnarends, D.M.; Schols, J.M.; Meijers, J.M.; Tan, F.E.; Verlaan, S.; Luiking, Y.C.; Morley, J.E.; Halfens, R.J. Instruments to assess sarcopenia and physical frailty in older people living in a community (care) setting: Similarities and discrepancies. J. Am. Med. Dir. Assoc. 2015, 16, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Reijnierse, E.M.; Trappenburg, M.C.; Blauw, G.J.; Verlaan, S.; de van der Schueren, M.A.; Meskers, C.G.; Maier, A.B. Common ground? The concordance of sarcopenia and frailty definitions. J. Am. Med. Dir. Assoc. 2016, 17, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Marty, E.; Liu, Y.; Samuel, A.; Or, O.; Lane, J. A review of sarcopenia: Enhancing awareness of an increasingly prevalent disease. Bone 2017, 105, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Montero-Fernandez, N.; Serra-Rexach, J. Role of exercise on sarcopenia in the elderly. Eur. J. Phys. Rehabil Med. 2013, 49, 131–143. [Google Scholar]
- Atkins, J.L.; Whincup, P.H.; Morris, R.W.; Lennon, L.T.; Papacosta, O.; Wannamethee, S.G. Sarcopenic obesity and risk of cardiovascular disease and mortality: A population-based cohort study of older men. J. Am. Geriatr. Soc. 2014, 62, 253–260. [Google Scholar] [CrossRef]
- Baumgartner, R.N.; Koehler, K.M.; Gallagher, D.; Romero, L.; Heymsfield, S.B.; Ross, R.R.; Garry, P.J.; Lindeman, R.D. Epidemiology of sarcopenia among the elderly in New Mexico. Am. J. Epidemiol. 1998, 147, 755–763. [Google Scholar] [CrossRef]
- Melton, L.J., 3rd; Khosla, S.; Crowson, C.S.; O’Connor, M.K.; O’Fallon, W.M.; Riggs, B.L. Epidemiology of sarcopenia. J. Am. Geriatr. Soc. 2000, 48, 625–630. [Google Scholar] [CrossRef]
- Janssen, I.; Heymsfield, S.B.; Ross, R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J. Am. Geriatr. Soc. 2002, 50, 889–896. [Google Scholar] [CrossRef]
- Prynn, J.E.; Kuper, H. Perspectives on Disability and Non-Communicable Diseases in Low-and Middle-Income Countries, with a Focus on Stroke and Dementia. Int. J. Environ. Res. Public Health 2019, 16, 3488. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Morley, J.E.; Abbatecola, A.M.; Argiles, J.M.; Baracos, V.; Bauer, J.; Bhasin, S.; Cederholm, T.; Coats, A.J.S.; Cummings, S.R.; Evans, W.J.; et al. Sarcopenia with limited mobility: An international consensus. J. Am. Med. Dir. Assoc. 2011, 12, 403–409. [Google Scholar] [CrossRef]
- Studenski, S.A.; Peters, K.W.; Alley, D.E.; Cawthon, P.M.; McLean, R.R.; Harris, T.B.; Ferrucci, L.; Guralnik, J.M.; Fragala, M.S.; Kenny, A.M.; et al. The FNIH sarcopenia project: Rationale, study description, conference recommendations, and final estimates. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2014, 69, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-K.; Liu, L.-K.; Woo, J.; Assantachai, P.; Auyeung, T.-W.; Bahyah, K.S.; Chou, M.-Y.; Chen, L.-Y.; Hsu, P.-S.; Krairit, O.; et al. Sarcopenia in Asia: Consensus report of the Asian Working Group for Sarcopenia. J. Am. Med. Dir. Assoc. 2014, 15, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Cooper, C.; Dere, W.; Evans, W.; Kanis, J.A.; Rizzoli, R.; Sayer, A.A.; Sieber, C.; Kaufman, J.-M.; Van Kan, G.A.; Boonen, S.; et al. Frailty and sarcopenia: Definitions and outcome parameters. Osteoporos. Int. 2012, 23, 1839–1848. [Google Scholar] [CrossRef] [PubMed]
- Buckinx, F.; Landi, F.; Cesari, M.; Fielding, R.A.; Visser, M.; Engelke, K.; Maggi, S.; Dennison, E.; Al-Daghri, N.M.; Allepaerts, S.; et al. Pitfalls in the measurement of muscle mass: A need for a reference standard. J. Cachexia Sarcopenia Muscle 2018, 9, 269–278. [Google Scholar] [CrossRef]
- Kyle, U.G.; Bosaeus, I.; De Lorenzo, A.D.; Deurenberg, P.; Elia, M.; Gómez, J.M.; Heitmann, B.L.; Kent-Smith, L.; Melchior, J.-C.; Pirlich, M.; et al. Bioelectrical impedance analysis—Part I: Review of principles and methods. Clin. Nutr. 2004, 23, 1226–1243. [Google Scholar] [CrossRef]
- Houtkooper, L.B.; Lohman, T.G.; Going, S.B.; Howell, W.H. Why bioelectrical impedance analysis should be used for estimating adiposity. Am. J. Clin. Nutr. 1996, 64, 436S–448S. [Google Scholar] [CrossRef]
- Beaudart, C.; Bruyère, O.; Geerinck, A.; Hajaoui, M.; Scafoglieri, A.; Perkisas, S.; Bautmans, I.; Gielen, E.; Reginster, J.-Y.; Buckinx, F. Equation models developed with bioelectric impedance analysis tools to assess muscle mass: A systematic review. Clin. Nutr. ESPEN 2020, 35, 47–62. [Google Scholar] [CrossRef]
- Sergi, G.; De Rui, M.; Stubbs, B.; Veronese, N.; Manzato, E. Measurement of lean body mass using bioelectrical impedance analysis: A consideration of the pros and cons. Aging Clin. Exp. Res. 2017, 29, 591–597. [Google Scholar] [CrossRef]
- Marra, M.; Sammarco, R.; De Lorenzo, A.; Iellamo, F.; Siervo, M.; Pietrobelli, A.; Donini, L.M.; Santarpia, L.; Cataldi, M.; Pasanisi, F.; et al. Assessment of body composition in health and disease using bioelectrical impedance analysis (BIA) and dual energy X-ray absorptiometry (DXA): A critical overview. Contrast Media Mol. Imaging 2019, 2019, 3548284. [Google Scholar] [CrossRef]
- Bianchi, L.; Abete, P.; Bellelli, G.; Bo, M.; Cherubini, A.; Corica, F.; Di Bari, M.; Maggio, M.; Manca, G.M.; Rizzo, M.R.; et al. Prevalence and clinical correlates of sarcopenia, identified according to the EWGSOP definition and diagnostic algorithm, in hospitalized older people: The GLISTEN study. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2017, 72, 1575–1581. [Google Scholar] [CrossRef] [PubMed]
- Reijnierse, E.M.; Trappenburg, M.C.; Leter, M.J.; Blauw, G.J.; Sipilä, S.; Sillanpää, E.; Narici, M.V.; Hogrel, J.-Y.; Butler-Browne, G.; McPhee, J.S.; et al. The impact of different diagnostic criteria on the prevalence of sarcopenia in healthy elderly participants and geriatric outpatients. Gerontology 2015, 61, 491–496. [Google Scholar] [CrossRef]
- Churilov, I.; Churilov, L.; MacIsaac, R.J.; Ekinci, E.I. Systematic review and meta-analysis of prevalence of sarcopenia in post acute inpatient rehabilitation. Osteoporos. Int. 2018, 29, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Verschueren, S.; Gielen, E.; O’neill, T.; Pye, S.; Adams, J.; Ward, K.; Wu, F.; Szulc, P.; Laurent, M.; Claessens, F.; et al. Sarcopenia and its relationship with bone mineral density in middle-aged and elderly European men. Osteoporos. Int. 2013, 24, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Fiatarone Singh, M.A.; Singh, N.A.; Hansen, R.D.; Finnegan, T.P.; Allen, B.J.; Diamond, T.H.; Diwan, A.D.; Lloyd, B.D.; Williamson, D.A.; Smith, E.U.; et al. Methodology and baseline characteristics for the Sarcopenia and Hip Fracture study: A 5-year prospective study. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2009, 64, 568–574. [Google Scholar] [CrossRef]
- Hida, T.; Ishiguro, N.; Shimokata, H.; Sakai, Y.; Matsui, Y.; Takemura, M.; Terabe, Y.; Harada, A. High prevalence of sarcopenia and reduced leg muscle mass in Japanese patients immediately after a hip fracture. Geriatr. Gerontol. Int. 2013, 13, 413–420. [Google Scholar] [CrossRef]
- Iolascon, G.; Giamattei, M.; Moretti, A.; Di Pietro, G.; Gimigliano, F.; Gimigliano, R. Sarcopenia in women with vertebral fragility fractures. Aging Clin. Exp. Res. 2013, 25, S129–S131. [Google Scholar] [CrossRef]
- Nielsen, B.R.; Abdulla, J.; Andersen, H.E.; Schwarz, P.; Suetta, C. Sarcopenia and osteoporosis in older people: A systematic review and meta-analysis. Eur. Geriatr. Med. 2018, 9, 419–434. [Google Scholar] [CrossRef]
- Fahimfar, N.; Tajrishi, F.Z.; Gharibzadeh, S.; Shafiee, G.; Tanha, K.; Heshmat, R.; Nabipour, I.; Raeisi, A.; Jalili, A.; Larijani, B.; et al. Prevalence of osteosarcopenia and its association with cardiovascular risk factors in Iranian older people: Bushehr elderly health (BEH) program. Calcif. Tissue Int. 2020, 106, 364–370. [Google Scholar] [CrossRef]
- Koliaki, C.; Liatis, S.; Dalamaga, M.; Kokkinos, A. Sarcopenic Obesity: Epidemiologic Evidence, Pathophysiology, and Therapeutic Perspectives. Curr. Obes. Rep. 2019, 8, 458–471. [Google Scholar] [CrossRef]
- Landi, F.; Calvani, R.; Tosato, M.; Martone, A.M.; Bernabei, R.; Onder, G.; Marzetti, E. Impact of physical function impairment and multimorbidity on mortality among community-living older persons with sarcopaenia: Results from the ilSIRENTE prospective cohort study. BMJ Open 2016, 6, e008281. [Google Scholar] [CrossRef] [PubMed]
- Arango-Lopera, V.; Arroyo, P.; Gutiérrez-Robledo, L.M.; Perez-Zepeda, M.; Cesari, M. Mortality as an adverse outcome of sarcopenia. J. Nutr. Health Aging 2013, 17, 259–262. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Landi, F.; Topinková, E.; Michel, J.P. Understanding sarcopenia as a geriatric syndrome. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Landi, F.; Liperoti, R.; Fusco, D.; Mastropaolo, S.; Quattrociocchi, D.; Proia, A.; Tosato, M.; Bernabei, R.; Onder, G. Sarcopenia and mortality among older nursing home residents. J. Am. Med. Dir. Assoc. 2012, 13, 121–126. [Google Scholar] [CrossRef]
- Bahat, G.; Ilhan, B. Sarcopenia and the cardiometabolic syndrome: A narrative review. Eur. Geriatr. Med. 2016, 7, 220–223. [Google Scholar] [CrossRef]
- Chang, K.V.; Hsu, T.H.; Wu, W.T.; Huang, K.C.; Han, D.S. Association between sarcopenia and cognitive impairment: A systematic review and meta-analysis. J. Am. Med. Dir. Assoc. 2016, 17, 1164–1167. [Google Scholar] [CrossRef]
- Tian, S.; Xu, Y. Association of sarcopenic obesity with the risk of all-cause mortality: A meta-analysis of prospective cohort studies. Geriatr. Gerontol. Int. 2016, 16, 155–166. [Google Scholar] [CrossRef]
- Newman, A.B.; Haggerty, C.L.; Goodpaster, B.; Harris, T.; Kritchevsky, S.; Nevitt, M.; Miles, T.P.; Visser, M. Strength and muscle quality in a well-functioning cohort of older adults: The Health, Aging and Body Composition Study. J. Am. Geriatr. Soc. 2003, 51, 323–330. [Google Scholar] [CrossRef]
- Kalinkovich, A.; Livshits, G. Sarcopenic obesity or obese sarcopenia: A cross talk between age-associated adipose tissue and skeletal muscle inflammation as a main mechanism of the pathogenesis. Ageing Res. Rev. 2017, 35, 200–221. [Google Scholar] [CrossRef]
- Stewart, S.T.; Cutler, D.M.; Rosen, A.B. Forecasting the effects of obesity and smoking on US life expectancy. N. Engl. J. Med. 2009, 361, 2252–2260. [Google Scholar] [CrossRef]
- Patterson, R.E.; Frank, L.L.; Kristal, A.R.; White, E. A comprehensive examination of health conditions associated with obesity in older adults. Am. J. Prev. Med. 2004, 27, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Barbat-Artigas, S.; Pion, C.H.; Leduc-Gaudet, J.-P.; Rolland, Y.; Aubertin-Leheudre, M. Exploring the role of muscle mass, obesity, and age in the relationship between muscle quality and physical function. J. Am. Med. Dir. Assoc. 2014, 15, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Nordström, A.; Eriksson, M.; Stegmayr, B.; Gustafson, Y.; Nordström, P. Low bone mineral density is an independent risk factor for stroke and death. Cerebrovasc. Dis. 2010, 29, 130–136. [Google Scholar] [CrossRef]
- Van Der Klift, M.; Pols, H.; Geleijnse, J.; Van der Kuip, D.; Hofman, A.; De Laet, C. Bone mineral density and mortality in elderly men and women: The Rotterdam Study. Bone 2002, 30, 643–648. [Google Scholar] [CrossRef]
- Trivedi, D.; Khaw, K. Bone mineral density at the hip predicts mortality in elderly men. Osteoporos. Int. 2001, 12, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Yeung, S.S.; Reijnierse, E.M.; Pham, V.K.; Trappenburg, M.C.; Lim, W.K.; Meskers, C.G.; Maier, A.B. Sarcopenia and its association with falls and fractures in older adults: A systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 2019, 10, 485–500. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.-I.; Kim, H.; Ha, Y.-C.; Kwon, H.-B.; Koo, K.-H. Osteosarcopenia in patients with hip fracture is related with high mortality. J. Korean Med. Sci. 2018, 33, e27. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Wang, Y.; Zhan, J.-K.; Tang, Z.-Y.; He, J.-Y.; Tan, P.; Deng, H.-Q.; Huang, W.; Liu, Y.-S. Sarco-osteoporosis: Prevalence and association with frailty in Chinese community-dwelling older adults. Int. J. Endocrinol. 2015, 2015, 482940. [Google Scholar] [CrossRef]
- Ethgen, O.; Beaudart, C.; Buckinx, F.; Bruyère, O.; Reginster, J.-Y. The future prevalence of sarcopenia in Europe: A claim for public health action. Calcif. Tissue Int. 2017, 100, 229–234. [Google Scholar] [CrossRef]
- Nakanishi, R.; Oki, E.; Sasaki, S.; Hirose, K.; Jogo, T.; Edahiro, K.; Korehisa, S.; Taniguchi, D.; Kudo, K.; Kurashige, J.; et al. Sarcopenia is an independent predictor of complications after colorectal cancer surgery. Surg. Today 2018, 48, 151–157. [Google Scholar] [CrossRef]
- Cosquéric, G.; Sebag, A.; Ducolombier, C.; Thomas, C.; Piette, F.; Weill-Engerer, S. Sarcopenia is predictive of nosocomial infection in care of the elderly. Br. J. Nutr. 2006, 96, 895–901. [Google Scholar] [CrossRef] [PubMed]
- Altuna-Venegas, S.; Aliaga-Vega, R.; Maguiña, J.L.; Parodi, J.F.; Runzer-Colmenares, F.M. Risk of community-acquired pneumonia in older adults with sarcopenia of a hospital from Callao, Peru 2010–2015. Arch. Gerontol. Geriatr. 2019, 82, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Randle, P.; Garland, P.; Hales, C.; Newsholme, E. The glucose fatty acid cycle; its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet 1963, 1, 785–789. [Google Scholar] [CrossRef]
- Ponti, F.; Santoro, A.; Mercatelli, D.; Gasperini, C.; Conte, M.; Martucci, M.; Sangiorgi, L.; Franceschi, C.; Bazzocchi, A. Aging and imaging assessment of body composition: From fat to facts. Front. Endocrinol. 2019, 10, 861. [Google Scholar] [CrossRef] [PubMed]
- Carobbio, S.; Rodriguez-Cuenca, S.; Vidal-Puig, A. Origins of metabolic complications in obesity: Ectopic fat accumulation. The importance of the qualitative aspect of lipotoxicity. Curr. Opin. Clin. Nutr. Metab. Care 2011, 14, 520–526. [Google Scholar] [CrossRef]
- Miljkovic, I.; Cauley, J.A.; Wang, P.Y.; Holton, K.F.; Lee, C.G.; Sheu, Y.; Barrett-Connor, E.; Hoffman, A.R.; Lewis, C.B.; Orwoll, E.S.; et al. Abdominal myosteatosis is independently associated with hyperinsulinemia and insulin resistance among older men without diabetes. Obesity 2013, 21, 2118–2125. [Google Scholar] [CrossRef]
- Zamboni, M.; Gattazzo, S.; Rossi, A.P. Myosteatosis: A relevant, yet poorly explored element of sarcopenia. Eur. Geriatr. Med. 2019, 10, 5–6. [Google Scholar] [CrossRef]
- Goodpaster, B.H.; Carlson, C.L.; Visser, M.; Kelley, D.E.; Scherzinger, A.; Harris, T.B.; Stamm, E.; Newman, A.B. Attenuation of skeletal muscle and strength in the elderly: The Health ABC Study. J. Appl. Physiol. 2001, 90, 2157–2165. [Google Scholar] [CrossRef]
- Heshka, S.; Ruggiero, A.; Bray, G.A.; Foreyt, J.; Kahn, S.E.; Lewis, C.E.; Saad, M.; Schwartz, A.V. Look AHEAD Research Group. Altered body composition in type 2 diabetes mellitus. Int. J. Obes. 2008, 32, 780–787. [Google Scholar] [CrossRef]
- Kalyani, R.R.; Corriere, M.; Ferrucci, L. Age-related and disease-related muscle loss: The effect of diabetes, obesity, and other diseases. Lancet Diabetes Endocrinol. 2014, 2, 819–829. [Google Scholar] [CrossRef]
- Srikanthan, P.; Karlamangla, A.S. Relative muscle mass is inversely associated with insulin resistance and prediabetes. Findings from the third National Health and Nutrition Examination Survey. J. Clin. Endocrinol. Metab. 2011, 96, 2898–2903. [Google Scholar] [CrossRef] [PubMed]
- Rimbert, V.; Boirie, Y.; Bedu, M.; Hocquette, J.-F.; Ritz, P.; Morio, B. Muscle fat oxidative capacity is not impaired by age but by physical inactivity: Association with insulin sensitivity. FASEB J. 2004, 18, 737–739. [Google Scholar] [CrossRef] [PubMed]
- Reaven, G.M. The insulin resistance syndrome: Definition and dietary approaches to treatment. Annu. Rev. Nutr. 2005, 25, 391–406. [Google Scholar] [CrossRef] [PubMed]
- Gast, K.B.; Tjeerdema, N.; Stijnen, T.; Smit, J.W.; Dekkers, O.M. Insulin resistance and risk of incident cardiovascular events in adults without diabetes: Meta-analysis. PLoS ONE 2012, 7, e52036. [Google Scholar] [CrossRef]
- Bjørge, T.; Lukanova, A.; Jonsson, H.; Tretli, S.; Ulmer, H.; Manjer, J.; Stocks, T.; Selmer, R.; Nagel, G.; Almquist, M.; et al. Metabolic syndrome and breast cancer in the me-can (metabolic syndrome and cancer) project. Cancer Epidemiol. Prev. Biomark. 2010, 19, 1737–1745. [Google Scholar] [CrossRef]
- Stocks, T.; Rapp, K.; Bjørge, T.; Manjer, J.; Ulmer, H.; Selmer, R.; Lukanova, A.; Johansen, D.; Concin, H.; Tretli, S. Blood glucose and risk of incident and fatal cancer in the metabolic syndrome and cancer project (me-can): Analysis of six prospective cohorts. PLoS Med. 2009, 6, e1000201. [Google Scholar] [CrossRef]
- Borena, W.; Strohmaier, S.; Lukanova, A.; Bjørge, T.; Lindkvist, B.; Hallmans, G.; Edlinger, M.; Stocks, T.; Nagel, G.; Manjer, J.; et al. Metabolic risk factors and primary liver cancer in a prospective study of 578,700 adults. Int. J. Cancer 2012, 131, 193–200. [Google Scholar] [CrossRef]
- Burger, E.; Klein-Nulend, J. Microgravity and bone cell mechanosensitivity. Bone 1998, 22, 127S–130S. [Google Scholar] [CrossRef]
- Lloyd, S.A.; Lang, C.H.; Zhang, Y.; Paul, E.M.; Laufenberg, L.J.; Lewis, G.S.; Donahue, H.J. Interdependence of muscle atrophy and bone loss induced by mechanical unloading. J. Bone Miner. Res. 2014, 29, 1118–1130. [Google Scholar] [CrossRef]
- Burr, D.B. Muscle strength, bone mass, and age-related bone loss. J. Bone Miner. Res. 1997, 12, 1547–1551. [Google Scholar] [CrossRef]
- Hamrick, M.W.; McNeil, P.L.; Patterson, S.L. Role of muscle-derived growth factors in bone formation. J. Musculoskelet. Neuronal Interact. 2010, 10, 64–70. [Google Scholar] [PubMed]
- Hamrick, M.W. A role for myokines in muscle-bone interactions. Exerc. Sport Sci. Rev. 2011, 39, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Bauman, W.A.; Spungen, A.M.; Wang, J.; Pierson, R.N., Jr.; Schwartz, E. Relationship of fat mass and serum estradiol with lower extremity bone in persons with chronic spinal cord injury. Am. J. Physiol. Endocrinol. Metab. 2006, 290, E1098–E1103. [Google Scholar] [CrossRef] [PubMed]
- Verzola, D.; Barisione, C.; Picciotto, D.; Garibotto, G.; Koppe, L. Emerging role of myostatin and its inhibition in the setting of chronic kidney disease. Kidney Int. 2019, 95, 506–517. [Google Scholar] [CrossRef]
- Léger, B.; Derave, W.; De Bock, K.; Hespel, P.; Russell, A.P. Human sarcopenia reveals an increase in SOCS-3 and myostatin and a reduced efficiency of Akt phosphorylation. Rejuvenation Res. 2008, 11, 163–175B. [Google Scholar] [CrossRef]
- McPherron, A.C.; Lawler, A.M.; Lee, S.-J. Regulation of skeletal muscle mass in mice by a new TGF-p superfamily member. Nature 1997, 387, 83–90. [Google Scholar] [CrossRef]
- Hill, E.W.; Gu, J.; Eivers, S.S.; Fonseca, R.G.; McGivney, B.A.; Govindarajan, P.; Orr, N.; Katz, L.M.; MacHugh, D. A sequence polymorphism in MSTN predicts sprinting ability and racing stamina in thoroughbred horses. PLoS ONE 2010, 5, e8645. [Google Scholar] [CrossRef]
- Brandt, C.; Hansen, R.H.; Hansen, J.B.; Olsen, C.H.; Galle, P.; Mandrup-Poulsen, T.; Gehl, J.; Pedersen, B.K.; Hojman, P. Over-expression of Follistatin-like 3 attenuates fat accumulation and improves insulin sensitivity in mice. Metabolism 2015, 64, 283–295. [Google Scholar] [CrossRef]
- Dschietzig, T.B. Myostatin—From the mighty mouse to cardiovascular disease and cachexia. Clin. Chim. Acta 2014, 433, 216–224. [Google Scholar] [CrossRef]
- Verzola, D.; Milanesi, S.; Bertolotto, M.; Garibaldi, S.; Villaggio, B.; Brunelli, C.; Balbi, M.; Ameri, P.; Montecucco, F.; Palombo, D. Myostatin mediates abdominal aortic atherosclerosis progression by inducing vascular smooth muscle cell dysfunction and monocyte recruitment. Sci. Rep. 2017, 7, 46362. [Google Scholar] [CrossRef]
- Yiallouris, A.; Agapidaki, E.; Ntourakis, D.; Tsioutis, C.; Zafeiri, M.; Johnson, E.O. Adrenal aging and its implications on stress responsiveness in humans. Front. Endocrinol. 2019, 10, 54. [Google Scholar] [CrossRef] [PubMed]
- Lamberts, S.W.; Van den Beld, A.W.; Van Der Lely, A.-J. The endocrinology of aging. Science 1997, 278, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Tiganescu, A.; Walker, E.A.; Hardy, R.S.; Mayes, A.E.; Stewart, P.M. Localization, age-and site-dependent expression, and regulation of 11β-hydroxysteroid dehydrogenase type 1 in skin. J. Investig. Dermatol. 2011, 131, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, M.; Häkkinen, K.; Antón, A.; Garrues, M.; Ibañez, J.; Ruesta, M.; Gorostiaga, E.M. Maximal strength and power, endurance performance, and serum hormones in middle-aged and elderly men. Med. Sci. Sports Exerc. 2001, 33, 1577–1587. [Google Scholar] [CrossRef]
- Peeters, G.; Van Schoor, N.; Visser, M.; Knol, D.; Eekhoff, E.; De Ronde, W.; Lips, P. Relationship between cortisol and physical performance in older persons. Clin. Endocrinol. 2007, 67, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Ennis, G.E.; An, Y.; Resnick, S.M.; Ferrucci, L.; O’Brien, R.J.; Moffat, S.D. Long-term cortisol measures predict Alzheimer disease risk. Neurology 2017, 88, 371–378. [Google Scholar] [CrossRef]
- Maeda, K.; Tanimoto, K.; Terada, T.; Shintani, T.; Kakigi, T. Elevated urinary free cortisol in patients with dementia. Neurobiol. Aging 1991, 12, 161–163. [Google Scholar] [CrossRef]
- Mijnarends, D.; Schols, J.; Halfens, R.; Meijers, J.; Luiking, Y.; Verlaan, S.; Evers, S. Burden-of-illness of Dutch community-dwelling older adults with sarcopenia: Health related outcomes and costs. Eur. Geriatr. Med. 2016, 7, 276–284. [Google Scholar] [CrossRef]
- Tang, T.; Wu, L.; Yang, L.; Jiang, J.; Hao, Q.; Dong, B.; Yang, M. A sarcopenia screening test predicts mortality in hospitalized older adults. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef]
- Yu, S.C.; Khow, K.S.; Jadczak, A.D.; Visvanathan, R. Clinical screening tools for sarcopenia and its management. Curr. Gerontol. Geriatr. Res. 2016, 2016, 5978523. [Google Scholar] [CrossRef]
- Nawi, S.N.M.; Yu, S.C. Screening Tools for Sarcopenia in Community-Dwellers: A Scoping Review. Ann. Acad Med. Singap. 2019, 48, 201–216. [Google Scholar]
- Malmstrom, T.K.; Morley, J.E. SARC-F: A simple questionnaire to rapidly diagnose sarcopenia. J. Am. Med. Dir. Assoc. 2013, 14, 531–532. [Google Scholar] [CrossRef] [PubMed]
- Goodman, M.J.; Ghate, S.R.; Mavros, P.; Sen, S.; Marcus, R.L.; Joy, E.; Brixner, D.I. Development of a practical screening tool to predict low muscle mass using NHANES 1999–2004. J. Cachexia Sarcopenia Muscle 2013, 4, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Visvanathan, R.; Yu, S.; Field, J.; Chapman, I.; Adams, R.; Wittert, G.; Visvanathan, T. Appendicular Skeletal Muscle Mass: Development and Validation of Anthropometric Prediction Equations. J. Frailty Aging 2012, 1, 147–151. [Google Scholar] [PubMed]
- Ishii, S.; Tanaka, T.; Shibasaki, K.; Ouchi, Y.; Kikutani, T.; Higashiguchi, T.; Obuchi, S.P.; Ishikawa-Takata, K.; Hirano, H.; Kawai, H. Development of a simple screening test for sarcopenia in older adults. Geriatr. Gerontol. Int. 2014, 14, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Appleton, S.; Chapman, I.; Adams, R.; Wittert, G.; Visvanathan, T.; Visvanathan, R. An anthropometric prediction equation for appendicular skeletal muscle mass in combination with a measure of muscle function to screen for sarcopenia in primary and aged care. J. Am. Med. Dir. Assoc. 2015, 16, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Krzymińska-Siemaszko, R.; Tobis, S.; Lewandowicz, M.; Wieczorowska-Tobis, K. Comparison of four sarcopenia screening questionnaires in community-dwelling older adults from Poland using six sets of international diagnostic criteria of sarcopenia. PLoS ONE 2020, 15, e0231847. [Google Scholar]
- Ida, S.; Kaneko, R.; Murata, K. SARC-F for screening of sarcopenia among older adults: A meta-analysis of screening test accuracy. J. Am. Med. Dir. Assoc. 2018, 19, 685–689. [Google Scholar] [CrossRef]
- Fletcher, S.W.; Elmore, J.G. Clinical practice. Mammographic screening for breast cancer. N. Engl. J. Med. 2003, 348, 1672–1680. [Google Scholar] [CrossRef]
- Damilakis, J.; Adams, J.E.; Guglielmi, G.; Link, T.M. Radiation exposure in X-ray-based imaging techniques used in osteoporosis. Eur. Radiol. 2010, 20, 2707–2714. [Google Scholar] [CrossRef]
- Blake, G.M.; Naeem, M.; Boutros, M. Comparison of effective dose to children and adults from dual X-ray absorptiometry examinations. Bone 2006, 38, 935–942. [Google Scholar] [CrossRef]
- Elmore, J.G.; Barton, M.B.; Moceri, V.M.; Polk, S.; Arena, P.J.; Fletcher, S.W. Ten-year risk of false positive screening mammograms and clinical breast examinations. N. Engl. J. Med. 1998, 338, 1089–1096. [Google Scholar] [CrossRef] [PubMed]
- Paskett, E.D.; Rimer, B.K. Psychosocial effects of abnormal Pap tests and mammograms: A review. J. Womens Health 1995, 4, 73–82. [Google Scholar] [CrossRef]
- Elmore, J.G.; Nakano, C.Y.; Koepsell, T.D.; Desnick, L.M.; D’orsi, C.J.; Ransohoff, D.F. International variation in screening mammography interpretations in community-based programs. J. Natl. Cancer Inst. 2003, 95, 1384–1393. [Google Scholar] [CrossRef] [PubMed]
- Hansen, N.J.; Lohman, T.G.; Going, S.B.; Hall, M.C.; Pamenter, R.W.; Bare, L.A.; Boyden, T.W.; Houtkooper, L.B. Prediction of body composition in premenopausal females from dual-energy X-ray absorptiometry. J. Appl. Physiol. 1993, 75, 1637–1641. [Google Scholar] [CrossRef]
- Wellens, R.; Chumlea, W.C.; Guo, S.; Roche, A.F.; Reo, N.V.; Siervogel, R.M. Body composition in white adults by dual-energy x-ray absorptiometry, densitometry, and total body water. Am. J. Clin. Nutr. 1994, 59, 547–555. [Google Scholar]
- Picaud, J.-C.; Rigo, J.; Nyamugabo, K.; Milet, J.; Senterre, J. Evaluation of dual-energy X-ray absorptiometry for body-composition assessment in piglets and term human neonates. Am. J. Clin. Nutr. 1996, 63, 157–163. [Google Scholar] [CrossRef]
- Svendsen, O.L.; Haarbo, J.; Hassager, C.; Christiansen, C. Accuracy of measurements of body composition by dual-energy x-ray absorptiometry in vivo. Am. J. Clin. Nutr. 1993, 57, 605–608. [Google Scholar] [CrossRef]
- Maden-Wilkinson, T.; Degens, H.; Jones, D.; McPhee, J. Comparison of MRI and DXA to measure muscle size and age-related atrophy in thigh muscles. J. Musculoskelet. Neuronal Interact. 2013, 13, 320–328. [Google Scholar]
- Bredella, M.A.; Ghomi, R.H.; Thomas, B.J.; Torriani, M.; Brick, D.J.; Gerweck, A.V.; Misra, M.; Klibanski, A.; Miller, K.K. Comparison of DXA and CT in the assessment of body composition in premenopausal women with obesity and anorexia nervosa. Obesity 2010, 18, 2227–2233. [Google Scholar] [CrossRef]
- Levine, J.A.; Abboud, L.; Barry, M.; Reed, J.E.; Sheedy, P.F.; Jensen, M.D. Measuring leg muscle and fat mass in humans: Comparison of CT and dual-energy X-ray absorptiometry. J. Appl. Physiol. 2000, 88, 452–456. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, Z.; Faith, M.S.; Kotler, D.; Shih, R.; Heymsfield, S.B. Regional skeletal muscle measurement: Evaluation of new dual-energy X-ray absorptiometry model. J. Appl. Physiol. 1999, 87, 1163–1171. [Google Scholar] [CrossRef] [PubMed]
- Glickman, S.G.; Marn, C.S.; Supiano, M.A.; Dengel, D.R. Validity and reliability of dual-energy X-ray absorptiometry for the assessment of abdominal adiposity. J. Appl. Physiol. 2004, 97, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Locquet, M.; Beaudart, C.; Reginster, J.-Y.; Petermans, J.; Bruyère, O. Comparison of the performance of five screening methods for sarcopenia. Clin. Epidemiol. 2018, 10, 71–82. [Google Scholar] [CrossRef]
- Kohrt, W.M. Dual-Energy X-ray Absorptiometry: Research Issues and Equipment; Carlson-Newberry, S.J., Costello, R.B., Eds.; National Academies Press (US): Washington, DC, USA, 1997; Volume 6, p. 151. [Google Scholar]
- Delmas, P.; Fontanges, E.; Duboeuf, F.; Boivin, G.; Chavassieux, P.; Meunier, P. Comparison of bone mass measured by histomorphometry on iliac biopsy and by dual photon absorptiometry of the lumbar spine. Bone 1988, 9, 209–213. [Google Scholar] [CrossRef]
- Wright, C.; Crawley, E.; Evans, W.; Garrahan, N.; Mellish, R.; Croucher, P.; Compston, J. The relationship between spinal trabecular bone mineral content and iliac crest trabecular bone volume. Calcif. Tissue Int. 1990, 46, 162–165. [Google Scholar] [CrossRef]
- Cosman, F.; Schnitzer, M.; McCann, P.; Parisien, M.; Dempster, D.; Lindsay, R. Relationships between quantitative histological measurements and noninvasive assessments of bone mass. Bone 1992, 13, 237–242. [Google Scholar] [CrossRef]
- Jindal, M.; Lakhwani, O.P.; Kaur, O.; Agarwal, S.; Garg, K. Bone Density versus Bone Quality as a Predictor of Bone Strength. Orthop. Rheumatol. Open Access J. 2018, 12, 555830. [Google Scholar] [CrossRef][Green Version]
- Humadi, A.; Alhadithi, R.H.; Alkudiari, S.I. Validity of the DEXA diagnosis of involutional osteoporosis in patients with femoral neck fractures. Indian J. Orthop. 2010, 44, 73–78. [Google Scholar] [CrossRef]
- Genant, H.K.; Engelke, K.; Fuerst, T.; Glüer, C.C.; Grampp, S.; Harris, S.T.; Jergas, M.; Lang, T.; Lu, Y.; Majumdar, S.; et al. Noninvasive assessment of bone mineral and structure: State of the art. J. Bone Miner. Res. 1996, 11, 707–730. [Google Scholar] [CrossRef]
- Kabel, J.; van Rietbergen, B.; Dalstra, M.; Odgaard, A.; Huiskes, R. The role of an effective isotropic tissue modulus in the elastic properties of cancellous bone. J. Biomech. 1999, 32, 673–680. [Google Scholar] [CrossRef]
- Homminga, J.; Mccreadie, B.R.; Weinans, H.; Huiskes, R. The dependence of the elastic properties of osteoporotic cancellous bone on volume fraction and fabric. J. Biomech. 2003, 36, 1461–1467. [Google Scholar] [CrossRef]
- Berg, W.A.; Blume, J.D.; Cormack, J.B.; Mendelson, E.B.; Lehrer, D.; Böhm-Vélez, M.; Pisano, E.D.; Jong, R.A.; Evans, W.P.; Morton, M.J.; et al. Combined screening with ultrasound and mammography vs mammography alone in women at elevated risk of breast cancer. JAMA 2008, 299, 2151–2163. [Google Scholar] [CrossRef] [PubMed]
- Health Quality Ontario. Ultrasound as an adjunct to mammography for breast cancer screening: A health technology assessment. Ont. Health Technol. Assess. Ser. 2016, 16, 1–71. [Google Scholar]
- Kolb, T.M.; Lichy, J.; Newhouse, J.H. Comparison of the performance of screening mammography, physical examination, and breast US and evaluation of factors that influence them: An analysis of 27,825 patient evaluations. Radiology 2002, 225, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Ciatto, S.; Houssami, N.; Ambrogetti, D.; Bonardi, R.; Collini, G.; Del Turco, M.R. Minority report–false negative breast assessment in women recalled for suspicious screening mammography: Imaging and pathological features, and associated delay in diagnosis. Breast Cancer Res. Treat. 2007, 105, 37–43. [Google Scholar] [CrossRef]
- Buchberger, W.; Niehoff, A.; Obrist, P.; DeKoekkoek-Doll, P.; Dünser, M. Clinically and mammographically occult breast lesions: Detection and classification with high-resolution sonography. Semin. Ultrasound CT MRI 2000, 21, 325–336. [Google Scholar] [CrossRef]
- Kaplan, S.S. Clinical utility of bilateral whole-breast US in the evaluation of women with dense breast tissue. Radiology 2001, 221, 641–649. [Google Scholar] [CrossRef]
- Weigert, J.; Steenbergen, S. The Connecticut experiment: The role of ultrasound in the screening of women with dense breasts. Breast J. 2012, 18, 517–522. [Google Scholar] [CrossRef]
- Berg, W.A.; Zhang, Z.; Lehrer, D.; Jong, R.A.; Pisano, E.D.; Barr, R.G.; Böhm-Vélez, M.; Mahoney, M.C.; Evans, W.P.; Larsen, L.H. Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. JAMA 2012, 307, 1394–1404. [Google Scholar]
- Berg, W.A.; Blume, J.D.; Cormack, J.B.; Mendelson, E.B. Operator dependence of physician-performed whole-breast US: Lesion detection and characterization. Radiology 2006, 241, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Deer, R.R.; Akhverdiyeva, L.; Kuo, Y.-F.; Volpi, E. Developing a screening tool for sarcopenia in hospitalized geriatric patients: Estimation of appendicular skeletal muscle mass using bioelectrical impedance. Clin. Nutr. 2019, 39, 2233–2237. [Google Scholar] [CrossRef] [PubMed]
- Buckinx, F.; Reginster, J.-Y.; Dardenne, N.; Croisiser, J.-L.; Kaux, J.-F.; Beaudart, C.; Slomian, J.; Bruyère, O. Concordance between muscle mass assessed by bioelectrical impedance analysis and by dual energy X-ray absorptiometry: A cross-sectional study. BMC Musculoskelet. Disord. 2015, 16, 60. [Google Scholar] [CrossRef] [PubMed]
- Regione del Veneto. Tariffario Unico Regionale. Available online: https://www.regione.veneto.it/web/sanita/tariffario-unico-regionale (accessed on 13 December 2011).
- Lauretani, F.; Russo, C.R.; Bandinelli, S.; Bartali, B.; Cavazzini, C.; Di Iorio, A.; Corsi, A.M.; Rantanen, T.; Guralnik, J.M.; Ferrucci, L. Age-associated changes in skeletal muscles and their effect on mobility: An operational diagnosis of sarcopenia. J. Appl. Physiol. 2003, 95, 1851–1860. [Google Scholar] [CrossRef] [PubMed]
- Nabuco, H.C.; Tomeleri, C.M.; Fernandes, R.R.; Sugihara Junior, P.; Venturini, D.; Barbosa, D.S.; Deminice, R.; Sardinha, L.B.; Cyrino, E.S. Effects of pre-or post-exercise whey protein supplementation on oxidative stress and antioxidant enzymes in older women. Scand. J. Med. Sci. Sports 2019, 29, 1101–1108. [Google Scholar] [CrossRef]
- Sugihara Junior, P.; Ribeiro, A.S.; Nabuco, H.C.; Fernandes, R.R.; Tomeleri, C.M.; Cunha, P.M.; Venturini, D.; Barbosa, D.S.; Schoenfeld, B.J.; Cyrino, E.S. Effects of whey protein supplementation associated with resistance training on muscular strength, hypertrophy, and muscle quality in preconditioned older women. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 528–535. [Google Scholar] [CrossRef]
- Mori, H.; Tokuda, Y. Effect of whey protein supplementation after resistance exercise on the muscle mass and physical function of healthy older women: A randomized controlled trial. Geriatr. Gerontol. Int. 2018, 18, 1398–1404. [Google Scholar] [CrossRef]
- Englund, D.A.; Kirn, D.R.; Koochek, A.; Zhu, H.; Travison, T.G.; Reid, K.F.; von Berens, Å.; Melin, M.; Cederholm, T.; Gustafsson, T. Nutritional supplementation with physical activity improves muscle composition in mobility-limited older adults, the VIVE2 study: A randomized, double-blind, placebo-controlled trial. J. Gerontol. Ser. A 2017, 73, 95–101. [Google Scholar] [CrossRef]
- Fielding, R.A.; Travison, T.G.; Kirn, D.R.; Koochek, A.; Reid, K.F.; von Berens, Å.; Zhu, H.; Folta, S.C.; Sacheck, J.M.; Nelson, M.E.; et al. Effect of structured physical activity and nutritional supplementation on physical function in mobility-limited older adults: Results from the VIVE2 randomized trial. J. Nutr. Health Aging 2017, 21, 936–942. [Google Scholar] [CrossRef]
- Chalé, A.; Cloutier, G.J.; Hau, C.; Phillips, E.M.; Dallal, G.E.; Fielding, R.A. Efficacy of whey protein supplementation on resistance exercise–induced changes in lean mass, muscle strength, and physical function in mobility-limited older adults. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2013, 68, 682–690. [Google Scholar] [CrossRef]
- Kirk, B.; Mooney, K.; Amirabdollahian, F.; Khaiyat, O. Exercise and Dietary-Protein as a Countermeasure to Skeletal Muscle Weakness Liverpool Hope University-Sarcopenia Ageing Trial (LHU-SAT). Front. Physiol. 2019, 10, 445. [Google Scholar] [CrossRef] [PubMed]
- Kirk, B.; Mooney, K.; Cousins, R.; Angell, P.; Jackson, M.; Pugh, J.N.; Coyles, G.; Amirabdollahian, F.; Khaiyat, O. Effects of exercise and whey protein on muscle mass, fat mass, myoelectrical muscle fatigue and health-related quality of life in older adults: A secondary analysis of the Liverpool Hope University—Sarcopenia Ageing Trial (LHU-SAT). Eur. J. Appl. Physiol. 2020, 120, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Niccoli, S.; Kolobov, A.; Bon, T.; Rafilovich, S.; Munro, H.; Tanner, K.; Pearson, T.; Lees, S.J. Whey protein supplementation improves rehabilitation outcomes in hospitalized geriatric patients: A double blinded, randomized controlled trial. J. Nutr. Gerontol. Geriatr. 2017, 36, 149–165. [Google Scholar] [CrossRef] [PubMed]
- Gade, J.; Beck, A.M.; Bitz, C.; Christensen, B.; Klausen, T.W.; Vinther, A.; Astrup, A. Protein-enriched, milk-based supplement to counteract sarcopenia in acutely ill geriatric patients offered resistance exercise training during and after hospitalisation: Study protocol for a randomised, double-blind, multicentre trial. BMJ Open 2018, 8, e019210. [Google Scholar] [CrossRef]
- Gade, J.; Beck, A.M.; Andersen, H.E.; Christensen, B.; Rønholt, F.; Klausen, T.W.; Vinther, A.; Astrup, A. Protein supplementation combined with low-intensity resistance training in geriatric medical patients during and after hospitalisation: A randomised, double-blind, multicentre trial. Br. J. Nutr. 2019, 122, 1006–1020. [Google Scholar] [CrossRef]
- Mancuso, M.; Orsucci, D.; LoGerfo, A.; Rocchi, A.; Petrozzi, L.; Nesti, C.; Galetta, F.; Santoro, G.; Murri, L.; Siciliano, G. Oxidative stress biomarkers in mitochondrial myopathies, basally and after cysteine donor supplementation. J. Neurol. 2010, 257, 774–781. [Google Scholar] [CrossRef]
- Carbone, J.W.; Pasiakos, S.M. Dietary Protein and Muscle Mass: Translating Science to Application and Health Benefit. Nutrients 2019, 11, 1136. [Google Scholar] [CrossRef]
- Wolfe, R.R.; Miller, S.L.; Miller, K.B. Optimal protein intake in the elderly. Clin. Nutr. 2008, 27, 675–684. [Google Scholar] [CrossRef]
- Volpi, E.; Campbell, W.W.; Dwyer, J.T.; Johnson, M.A.; Jensen, G.L.; Morley, J.E.; Wolfe, R.R. Is the optimal level of protein intake for older adults greater than the recommended dietary allowance? J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2013, 68, 677–681. [Google Scholar] [CrossRef]
- Fulgoni, V.L., III. Current protein intake in America: Analysis of the National Health and Nutrition Examination Survey, 2003–2004. Am. J. Clin. Nutr. 2008, 87, 1554S–1557S. [Google Scholar] [CrossRef]
- Pasiakos, S.M.; Agarwal, S.; Lieberman, H.R.; Fulgoni, V.L. Sources and amounts of animal, dairy, and plant protein intake of US adults in 2007–2010. Nutrients 2015, 7, 7058–7069. [Google Scholar] [CrossRef] [PubMed]
- Coelho-Junior, H.J.; Calvani, R.; Picca, A.; Gonçalves, I.O.; Landi, F.; Bernabei, R.; Cesari, M.; Uchida, M.C.; Marzetti, E. Association between Dietary Habits and Physical Function in Brazilian and Italian Older Women. Nutrients 2020, 12, 1635. [Google Scholar] [CrossRef] [PubMed]
- Houston, D.K.; Nicklas, B.J.; Ding, J.; Harris, T.B.; Tylavsky, F.A.; Newman, A.B.; Lee, J.S.; Sahyoun, N.R.; Visser, M.; Kritchevsky, S.B. Dietary protein intake is associated with lean mass change in older, community-dwelling adults: The Health, Aging, and Body Composition (Health ABC) Study. Am. J. Clin. Nutr. 2008, 87, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.X.; Liu, X.D.; Li, K.; Liu, W.Z.; Ren, Y.S.; Zhang, J.X. Different dietary energy intake affects skeletal muscle development through an Akt-dependent pathway in Dorper × small thin-tailed crossbred ewe lambs. Domest. Anim. Endocrinol. 2016, 57, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, C.J.; Della Gatta, P.A.; Petersen, A.C.; Cameron-Smith, D.; Markworth, J.F. Soy protein ingestion results in less prolonged p70S6 kinase phosphorylation compared to whey protein after resistance exercise in older men. J. Int. Soc. Sports Nutr. 2015, 12, 6. [Google Scholar] [CrossRef]
- Phillips, S.M.; Tang, J.E.; Moore, D.R. The role of milk-and soy-based protein in support of muscle protein synthesis and muscle protein accretion in young and elderly persons. J. Am. Coll. Nutr. 2009, 28, 343–354. [Google Scholar] [CrossRef]
- Tang, J.E.; Moore, D.R.; Kujbida, G.W.; Tarnopolsky, M.A.; Phillips, S.M. Ingestion of whey hydrolysate, casein, or soy protein isolate: Effects on mixed muscle protein synthesis at rest and following resistance exercise in young men. J. Appl. Physiol. 2009, 107, 987–992. [Google Scholar] [CrossRef]
- Mattick, J.S.; Kamisoglu, K.; Ierapetritou, M.G.; Androulakis, I.P.; Berthiaume, F. Branched-chain amino acid supplementation: Impact on signaling and relevance to critical illness. Wiley Interdiscip. Rev. Syst. Biol. Med. 2013, 5, 449–460. [Google Scholar] [CrossRef]
- Gilmartin, S.; O’Brien, N.; Giblin, L. Whey for Sarcopenia; Can Whey Peptides, Hydrolysates or Proteins Play a Beneficial Role? Foods 2020, 9, 750. [Google Scholar] [CrossRef]
- Nabuco, H.C.; Tomeleri, C.M.; Sugihara Junior, P.; Fernandes, R.R.; Cavalcante, E.F.; Antunes, M.; Ribeiro, A.S.; Teixeira, D.C.; Silva, A.M.; Sardinha, L.B. Effects of whey protein supplementation pre-or post-resistance training on muscle mass, muscular strength, and functional capacity in pre-conditioned older women: A randomized clinical trial. Nutrients 2018, 10, 563. [Google Scholar] [CrossRef]
- Chanet, A.; Verlaan, S.; Salles, J.; Giraudet, C.; Patrac, V.; Pidou, V.; Pouyet, C.; Hafnaoui, N.; Blot, A.; Cano, N.; et al. Supplementing breakfast with a vitamin D and leucine–enriched whey protein medical nutrition drink enhances postprandial muscle protein synthesis and muscle mass in healthy older men. J. Nutr. 2017, 147, 2262–2271. [Google Scholar] [CrossRef] [PubMed]
- Ascenzi, F.; Barberi, L.; Dobrowolny, G.; Villa Nova Bacurau, A.; Nicoletti, C.; Rizzuto, E.; Rosenthal, N.; Scicchitano, B.M.; Musarò, A. Effects of IGF-1 isoforms on muscle growth and sarcopenia. Aging Cell 2019, 18, e12954. [Google Scholar] [CrossRef] [PubMed]
- Rodondi, A.; Ammann, P.; Ghilardi-Beuret, S.; Rizzoli, R. Zinc increases the effects of essential amino acids-whey protein supplements in frail elderly. JNHA J. Nutr. Health Aging 2009, 13, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Nabuco, H.C.; Tomeleri, C.M.; Fernandes, R.R.; Junior, P.S.; Cavalcante, E.F.; Cunha, P.M.; Antunes, M.; Nunes, J.P.; Venturini, D.; Barbosa, D.S.; et al. Effect of whey protein supplementation combined with resistance training on body composition, muscular strength, functional capacity, and plasma-metabolism biomarkers in older women with sarcopenic obesity: A randomized, double-blind, placebo-controlled trial. Clin. Nutr. ESPEN 2019, 32, 88–95. [Google Scholar] [PubMed]
- Morton, R.W.; Murphy, K.T.; McKellar, S.R.; Schoenfeld, B.J.; Henselmans, M.; Helms, E.; Aragon, A.A.; Devries, M.C.; Banfield, L.; Krieger, J.W.; et al. A systematic review, meta-analysis and meta-regression of the effect of protein supplementation on resistance training-induced gains in muscle mass and strength in healthy adults. Br. J. Sports Med. 2018, 52, 376–384. [Google Scholar] [CrossRef]
- Darling, A.; Manders, R.; Sahni, S.; Zhu, K.; Hewitt, C.E.; Prince, R.L.; Millward, D.; Lanham-New, S. Dietary protein and bone health across the life-course: An updated systematic review and meta-analysis over 40 years. Osteoporos. Int. 2019, 30, 741–761. [Google Scholar] [CrossRef]
- Wallace, T.C.; Frankenfeld, C.L. Dietary protein intake above the current RDA and bone health: A systematic review and meta-analysis. J. Am. Coll. Nutr. 2017, 36, 481–496. [Google Scholar] [CrossRef]
- Oktaviana, J.; Zanker, J.; Vogrin, S.; Duque, G. The Effect of β-hydroxy-β-methylbutyrate (HMB) on Sarcopenia and Functional Frailty in Older Persons: A Systematic Review. J. Nutr. Health Aging 2019, 23, 145–150. [Google Scholar] [CrossRef]
- Chakhtoura, M.; Chamoun, N.; Rahme, M.; Fuleihan, G.E.-H. Impact of vitamin D supplementation on falls and fractures—A critical appraisal of the quality of the evidence and an overview of the available guidelines. Bone 2020, 131, 115112. [Google Scholar] [CrossRef]
- Bruyère, O.; Cavalier, E.; Reginster, J.-Y. Vitamin D and osteosarcopenia: An update from epidemiological studies. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 498–503. [Google Scholar] [CrossRef]
- Beaudart, C.; Buckinx, F.; Rabenda, V.; Gillain, S.; Cavalier, E.; Slomian, J.; Petermans, J.; Reginster, J.-Y.; Bruyère, O. The effects of vitamin D on skeletal muscle strength, muscle mass, and muscle power: A systematic review and meta-analysis of randomized controlled trials. J. Clin. Endocrinol. Metab. 2014, 99, 4336–4345. [Google Scholar] [CrossRef] [PubMed]
- Kelley, G.A.; Kelley, K.S.; Tran, Z.V. Resistance training and bone mineral density in women: A meta-analysis of controlled trials. Am. J. Phys. Med. Rehabil. 2001, 80, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Layne, J.E.; Nelson, M.E. The effects of progressive resistance training on bone density: A review. Med. Sci. Sports Exerc. 1999, 31, 25–30. [Google Scholar] [CrossRef]
- Martyn-St James, M.; Carroll, S. High-intensity resistance training and postmenopausal bone loss: A meta-analysis. Osteoporos. Int. 2006, 17, 1225–1240. [Google Scholar] [CrossRef] [PubMed]
- Senderovich, H.; Kosmopoulos, A. An insight into the effect of exercises on the prevention of osteoporosis and associated fractures in high-risk individuals. Rambam Maimonides Med. J. 2018, 9, e0005. [Google Scholar] [CrossRef]
- Nelson, M.E.; Fiatarone, M.A.; Morganti, C.M.; Trice, I.; Greenberg, R.A.; Evans, W.J. Effects of high-intensity strength training on multiple risk factors for osteoporotic fractures: A randomized controlled trial. JAMA 1994, 272, 1909–1914. [Google Scholar] [CrossRef]
- Souza, D.; Barbalho, M.; Ramirez-Campillo, R.; Martins, W.; Gentil, P. High and low-load resistance training produce similar effects on bone mineral density of middle-aged and older people: A systematic review with meta-analysis of randomized clinical trials. Exp. Gerontol. 2020, 138, 110973. [Google Scholar] [CrossRef]
- Daly, R.M.; Gianoudis, J.; Kersh, M.E.; Bailey, C.A.; Ebeling, P.R.; Krug, R.; Nowson, C.A.; Hill, K.; Sanders, K.M. Effects of a 12-month supervised, community-based, multimodal exercise program followed by a 6-month research-to-practice transition on bone mineral density, trabecular microarchitecture, and physical function in older adults: A randomized controlled trial. J. Bone Miner. Res. 2020, 35, 419–429. [Google Scholar] [CrossRef]
- Hita-Contreras, F.; Bueno-Notivol, J.; Martínez-Amat, A.; Cruz-Díaz, D.; Hernandez, A.V.; Pérez-López, F.R. Effect of exercise alone or combined with dietary supplements on anthropometric and physical performance measures in community-dwelling elderly people with sarcopenic obesity: A meta-analysis of randomized controlled trials. Maturitas 2018, 116, 24–35. [Google Scholar] [CrossRef]
- Martínez-Amat, A.; Aibar-Almazán, A.; Fábrega-Cuadros, R.; Cruz-Díaz, D.; Jiménez-García, J.D.; Pérez-López, F.R.; Achalandabaso, A.; Barranco-Zafra, R.; Hita-Contreras, F. Exercise alone or combined with dietary supplements for sarcopenic obesity in community-dwelling older people: A systematic review of randomized controlled trials. Maturitas 2018, 110, 92–103. [Google Scholar] [CrossRef]
- Yin, Y.-H.; Liu, J.Y.W.; Välimäki, M. Effectiveness of non-pharmacological interventions on the management of sarcopenic obesity: A systematic review and meta-analysis. Exp. Gerontol. 2020, 135, 110937. [Google Scholar] [CrossRef] [PubMed]
- Hsu, K.J.; Liao, C.D.; Tsai, M.W.; Chen, C.N. Effects of Exercise and Nutritional Intervention on Body Composition, Metabolic Health, and Physical Performance in Adults with Sarcopenic Obesity: A Meta-Analysis. Nutrients 2019, 11, 2163. [Google Scholar] [CrossRef] [PubMed]
- Muscariello, E.; Nasti, G.; Siervo, M.; Di Maro, M.; Lapi, D.; D’Addio, G.; Colantuoni, A. Dietary protein intake in sarcopenic obese older women. Clin. Interv. Aging 2016, 11, 133–140. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).