Backstage of Eating Disorder—About the Biological Mechanisms behind the Symptoms of Anorexia Nervosa
Abstract
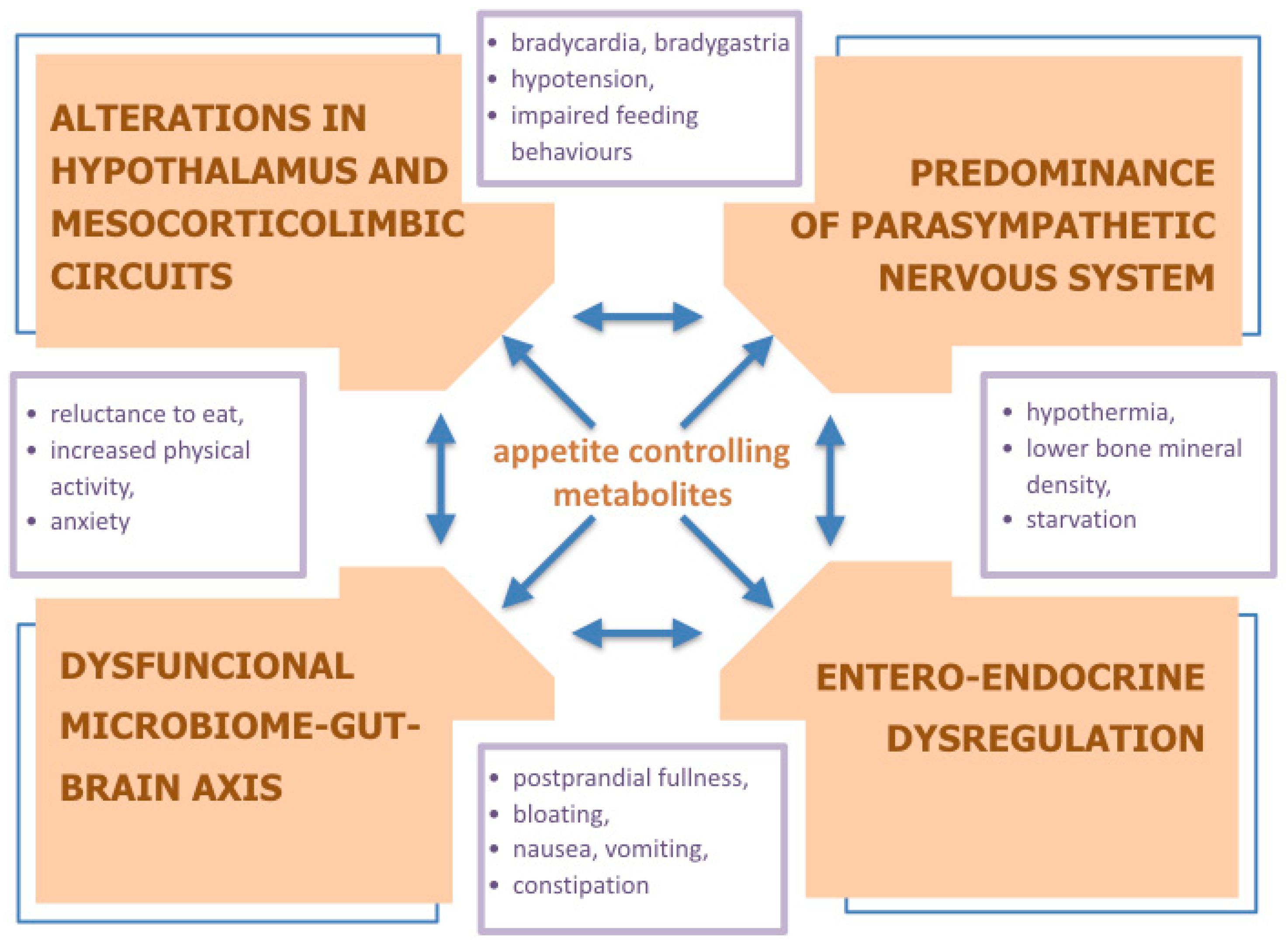
1. Introduction
2. Neurobiological Determinants of Anorexia
2.1. Opioids as Key Regulators of Dopaminergic Activity
2.2. Hypothalamic Regulation
2.3. Neuroinflammation
3. Autonomic Nervous System
4. Gut–Brain Axis Dysregulation
4.1. Microbiome Dysbiosis
4.2. Microbial Metabolites
4.3. Entero-Endocrine Alterations
4.4. Gastrointestinal Malfunction
5. Endocrine Dysregulation
6. Psychopathology
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ACTH | adrenocorticotropic hormone |
| AgRP | agouti-related peptide |
| a-MSH | alpha-melanocyte-stimulating hormone |
| AN | anorexia nervosa |
| ANBP | anorexia nervosa purging type |
| ANR | anorexia nervosa restricting type |
| ANS | autonomic nervous system |
| BAT | brown adipose tissue |
| BCFA | branched-chain fatty acids |
| β-EP | β-endorphin |
| β-LP | β-lipotropin |
| BMI | body mass index |
| CART | cocaine- and amphetamine-regulated transcript |
| CCK | cholecystokinin |
| ClpB | caseinolytic protease B |
| CRH | corticotropin-releasing hormone |
| CSF | cerebrospinal fluid |
| D2 | dopamine D2 receptor |
| D3 | dopamine D3 receptor |
| DSM | Diagnostic and Statistical Manual of Mental Disorders |
| E. Coli | Escherichia coli |
| FGF21 | fibroblast growth factor 21 |
| fMRI | functional magnetic resonance imaging |
| gAN | mice with the gut microbiota of patients with AN |
| GF | germ-free |
| GH | growth hormone |
| gHC | mice with the gut microbiota of healthy controls |
| GHSR | growth hormone secretagogue receptor |
| GIP | glucose-dependent insulinotropic peptide |
| GLP-1 | glucagon-like peptide 1 |
| GLP-2 | glucagon-like peptide 2 |
| GnRH | gonadotropin-releasing hormone |
| GUS | β-glucuronidase |
| HPA | hypothalamic–pituitary–adrenal (axis) |
| IBS | irritable bowel syndrome |
| IGF-1 | insulin-like growth factor 1 |
| IL-15 | interleukin 15 |
| IL-6 | interleukin 6 |
| KISS1R | kisspeptin receptor |
| LH | luteinizing hormone |
| MOR | Mu-opioid receptor |
| NA | noradrenaline |
| NPY | neuropeptide Y |
| OPRD | delta-opioid receptor |
| PNS | parasympathetic nervous system |
| POMC | pro-opiomelanocortin |
| PYY | peptide YY |
| SCFA | short-chain fatty acid |
| SNS | sympathetic nervous system |
| SPF | specific pathogen-free |
| STAT5 | signal transducer and activator of transcription 5 |
| T3 | triiodothyronine |
| T4 | thyroxine |
| TLR | toll-like receptors |
| TNF-α | tumor necrosis factor-alfa |
| TSH | thyroid-stimulating hormone |
| VTA | ventral tegmental area |
References
- Micali, N.; Hagberg, K.W.; Petersen, I.; Treasure, J.L. The incidence of eating disorders in the UK in 2000–2009: Findings from the General Practice Research Database. BMJ Open 2013. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, C.B.; Mors, O.; Bertelsen, A.; LindumWaltoft, B.; Agerbo, E.; McGrath, J.J.; Mortensen, P.B.; Eaton, W. A comprehensive nationwide study of the incidence rate and lifetime risk for treated mental disorders. JAMA Psychiatry 2014. [Google Scholar] [CrossRef] [PubMed]
- Hudson, J.I.; Hiripi, E.; Pope, H.G.; Kessler, R.C. The Prevalence and Correlates of Eating Disorders in the National Comorbidity Survey Replication. Biol. Psychiatry 2007. [Google Scholar] [CrossRef]
- Swanson, S.A. Prevalence and Correlates of Eating Disorders in Adolescents. Arch. Gen. Psychiatry 2011. [Google Scholar] [CrossRef] [PubMed]
- Herpertz-Dahlmann, B. Adolescent Eating Disorders: Update on Definitions, Symptomatology, Epidemiology, and Comorbidity. Child Adolesc. Psychiatr. Clin. N. Am. 2015, 24, 177–196. [Google Scholar] [CrossRef]
- Smink, F.R.E.; Van Hoeken, D.; Donker, G.A.; Susser, E.S.; Oldehinkel, A.J.; Hoek, H.W. Three decades of eating disorders in Dutch primary care: Decreasing incidence of bulimia nervosa but not of anorexia nervosa. Psychol. Med. 2016. [Google Scholar] [CrossRef]
- Javaras, K.N.; Runfola, C.D.; Thornton, L.M.; Agerbo, E.; Birgegård, A.; Norring, C.; Yao, S.; Råstam, M.; Larsson, H.; Lichtenstein, P.; et al. Sex- and age-specific incidence of healthcare-register-recorded eating disorders in the complete swedish 1979–2001 birth cohort. Int. J. Eat. Disord. 2015. [Google Scholar] [CrossRef]
- Seitz, J.; Belheouane, M.; Schulz, N.; Dempfle, A.; Baines, J.F.; Herpertz-Dahlmann, B. The impact of starvation on the microbiome and gut-brain interaction in anorexia nervosa. Front. Endocrinol. 2019. [Google Scholar] [CrossRef]
- Holland, J.; Hall, N.; Yeates, D.G.R.; Goldacre, M. Trends in hospital admission rates for anorexia nervosa in Oxford (1968–2011) and England (1990–2011): Database studies. J. R. Soc. Med. 2016. [Google Scholar] [CrossRef]
- Steinhausen, H.C.; Jensen, C.M. Time trends in lifetime incidence rates of first-time diagnosed anorexia nervosa and bulimia nervosa across 16 years in a danish nationwide psychiatric registry study. Int. J. Eat. Disord. 2015. [Google Scholar] [CrossRef]
- Diagnostic and Statistical Manual of Mental Disorders, 4th ed.; Text Revision (DSM-IV-TR); American Psychological Association: Washington, DC, USA, 2000.
- Attia, E.; Becker, A.E.; Bryant-Waugh, R.; Hoek, H.W.; Kreipe, R.E.; Marcus, M.D.; Mitchell, J.E.; Striegel, R.H.; Timothy Walsh, B.; Wilson, G.T.; et al. Feeding and eating disorders in DSM-5. Am. J. Psychiatry 2013, 170, 1237–1239. [Google Scholar] [CrossRef] [PubMed]
- Call, C.; Walsh, B.T.; Attia, E. From DSM-IV to DSM-5: Changes to eating disorder diagnoses. Curr. Opin. Psychiatry 2013, 26, 532–536. [Google Scholar] [CrossRef] [PubMed]
- Westmoreland, P.; Krantz, M.J.; Mehler, P.S. Medical Complications of Anorexia Nervosa and Bulimia. Am. J. Med. 2016, 129, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Kaye, W. Neurobiology of Anorexia and Bulimia Nervosa Purdue Ingestive Behavior Research Center Symposium Influences on Eating and Body Weight over the Lifespan: Children and Adolescents. Physiol. Behav. 2008. [Google Scholar] [CrossRef]
- Claudino, A.M.; Silva de Lima, M.; Hay, P.P.; Bacaltchuk, J.; Schmidt, U.U.; Treasure, J. Antidepressants for anorexia nervosa. Cochrane Database Syst. Rev. 2006. [Google Scholar] [CrossRef]
- Casper, R.C. Behavioral activation and lack of concern, core symptoms of anorexia nervosa? Int. J. Eat. Disord. 1998. [Google Scholar] [CrossRef]
- Hebebrand, J.; Exner, C.; Hebebrand, K.; Holtkamp, C.; Casper, R.C.; Remschmidt, H.; Herpertz-Dahlmann, B.; Klingenspor, M. Hyperactivity in patients with anorexia nervosa and in semistarved rats: Evidence for a pivotal role of hypoleptinemia. Physiol. Behav. 2003, 79, 25–37. [Google Scholar] [CrossRef]
- Carrera, O.; Adan, R.A.H.; Gutierrez, E.; Danner, U.N.; Hoek, H.W.; van Elburg, A.A.; Kas, M.J.H. Hyperactivity in anorexia nervosa: Warming up not just burning-off calories. PLoS ONE 2012, 7, e41851. [Google Scholar] [CrossRef]
- Gull, W.W. Anorexia nervosa (apepsia hysterica, anorexia hysterica). 1868. Obes. Res. 1997. [Google Scholar] [CrossRef]
- Lasegue, C.; Falret, J. La folie a deux ou folie communiquee. Ann. Med. Psychol. 1877, 18, 321–355. [Google Scholar]
- Holtkamp, K.; Herpertz-Dahlmann, B.; Mika, C.; Heer, M.; Heussen, N.; Fichter, M.; Herpertz, S.; Senf, W.; Blum, W.F.; Schweiger, U.; et al. Elevated Physical Activity and Low Leptin Levels Co-occur in Patients with Anorexia Nervosa. J. Clin. Endocrinol. Metab. 2003. [Google Scholar] [CrossRef] [PubMed]
- Van Elburg, A.A.; Kas, M.J.H.; Hillebrand, J.J.G.; Eijkemans, R.J.C.; Van Engeland, H. The impact of hyperactivity and leptin on recovery from anorexia nervosa. J. Neural Transm. 2007. [Google Scholar] [CrossRef] [PubMed]
- Bergh, C.; Södersten, P. Anorexia nervosa, self-starvation and the reward of stress. Nat. Med. 1996, 2, 21–22. [Google Scholar] [CrossRef]
- Seitz, J.; Bühren, K.; von Polier, G.G.; Heussen, N.; Herpertz-Dahlmann, B.; Konrad, K. Morphological changes in the brain of acutely ill and weight-recovered patients with anorexia nervosa. A meta-analysis and qualitative review. Z. Kinder. Jugendpsychiatr. Psychother. 2014, 42, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Frintrop, L.; Trinh, S.; Liesbrock, J.; Leunissen, C.; Kempermann, J.; Etdöger, S.; Kas, M.J.; Tolba, R.; Heussen, N.; Neulen, J.; et al. The reduction of astrocytes and brain volume loss in anorexia nervosa—The impact of starvation and refeeding in a rodent model. Transl. Psychiatry 2019, 9, 159. [Google Scholar] [CrossRef] [PubMed]
- Yeomans, M.R.; Gray, R.W. Opioid peptides and the control of human ingestive behaviour. Neurosci. Biobehav. Rev. 2002, 26, 713–728. [Google Scholar] [CrossRef]
- Jalabert, M.; Bourdy, R.; Courtin, J.; Veinante, P.; Manzoni, O.J.; Barrot, M.; Georges, F. Neuronal circuits underlying acute morphine action on dopamine neurons. Proc. Natl. Acad. Sci. USA 2011, 108, 16446–16450. [Google Scholar] [CrossRef]
- Kaye, W.H.; Pickar, D.; Naber, D.; Ebert, M.H. Cerebrospinal fluid opioid activity in anorexia nervosa. Am. J. Psychiatry 1982, 139, 643–645. [Google Scholar] [CrossRef]
- Kaye, W.H. Neuropeptide abnormalities in anorexia nervosa. Psychiatry Res. 1996, 62, 65–74. [Google Scholar] [CrossRef]
- Lesem, M.D.; Berrettini, W.H.; Kaye, W.H.; Jimerson, D.C. Measurement of CSF dynorphin a 1–8 immunoreactivity in anorexia nervosa and normal-weight bulimia. Biol. Psychiatry 1991, 29, 244–252. [Google Scholar] [CrossRef]
- Mendez, I.A.; Ostlund, S.B.; Maidment, N.T.; Murphy, N.P. Involvement of Endogenous Enkephalins and β-Endorphin in Feeding and Diet-Induced Obesity. Neuropsychopharmacology 2015, 40, 2103–2112. [Google Scholar] [CrossRef] [PubMed]
- Gerner, R.H.; Sharp, B. CSF β-endorphin-immunoreactivity in normal, schiziphrenic, depressed, manic and anorexic subjects. Brain Res. 1982, 237, 244–247. [Google Scholar] [CrossRef]
- Kaye, W.H.; Berrettini, W.H.; Gwirtsman, H.E.; Chretien, M.; Gold, P.W.; George, D.T.; Jimerson, D.C.; Ebert, M.H. Reduced cerebrospinal fluid levels of immunoreactive pro-opiomelanocortin related peptides (including beta-endorphin) in anorexia nervosa. Life Sci. 1987. [Google Scholar] [CrossRef]
- Brambilla, F.; Cavagnini, F.; Invitti, C.; Poterzio, F.; Lampertico, M.; Sali, L.; Maggioni, M.; Candolfi, C.; Panerai, A.E.; Müdler, E.E. Neuroendocrine and psychopathological measures in anorexia nervosa: Resemblances to primary affective disorders. Psychiatry Res. 1985. [Google Scholar] [CrossRef]
- Melchior, J.C.; Rigaud, D.; Colas-Linhart, N.; Rozen, R.; Fantino, M.; Apfelbaum, M. Negative allesthesia and decreased endogenous opiate system activity in anorexia nervosa. Pharmacol. Biochem. Behav. 1990, 35, 885–888. [Google Scholar] [CrossRef]
- Tepper, R.; Weizman, A.; Apter, A.; Tyano, S.; Beyth, Y. Elevated plasma immunoreactive β-endorphin in anorexia nervosa. Clin. Neuropharmacol. 1992, 15, 387–391. [Google Scholar] [CrossRef]
- Baranowska, B. Are disturbances in opioid and adrenergic systems involved in the hormonal dysfunction of anorexia nervosa? Psychoneuroendocrinology 1990, 15, 371–379. [Google Scholar] [CrossRef]
- Brambilla, F.; Ferrari, E.; Petraglia, F.; Facchinetti, F.; Catalano, M.; Genazzani, A.R. Peripheral opioid secretory pattern in anorexia nervosa. Psychiatry Res. 1991, 39, 115–127. [Google Scholar] [CrossRef]
- Brambilla, F.; Brunetta, M.; Peirone, A.; Perna, G.; Sacerdote, P.; Manfredi, B.; Panerai, A.E. T-lymphocyte cholecystokinin-8 and beta-endorphin concentrations in eating disorders: I. Anorexia nervosa. Psychiatry Res. 1995, 59, 43–50. [Google Scholar] [CrossRef]
- Marrazzi, M.A.; Luby, E.D.; Kinzie, J.; Munjal, I.D.; Spector, S. Endogenous codeine and morphine in anorexia and bulimia nervosa. Life Sci. 1997, 60, 1741–1747. [Google Scholar] [CrossRef]
- Rask-Andersen, M.; Olszewski, P.K.; Levine, A.S.; Schiöth, H.B. Molecular mechanisms underlying anorexia nervosa: Focus on human gene association studies and systems controlling food intake. Brain Res. Rev. 2010, 62, 147–164. [Google Scholar] [CrossRef] [PubMed]
- O’Hara, C.B.; Campbell, I.C.; Schmidt, U. A reward-centred model of anorexia nervosa: A focussed narrative review of the neurological and psychophysiological literature. Neurosci. Biobehav. Rev. 2015, 52, 131–152. [Google Scholar] [CrossRef] [PubMed]
- Frank, G.K.; Bailer, U.F.; Henry, S.E.; Drevets, W.; Meltzer, C.C.; Price, J.C.; Mathis, C.A.; Wagner, A.; Hoge, J.; Ziolko, S.; et al. Increased dopamine D2/D3 receptor binding after recovery from anorexia nervosa measured by positron emission tomography and [11C]raclopride. Biol. Psychiatry 2005. [Google Scholar] [CrossRef]
- Kaye, W.H.; Frank, G.K.W.; McConaha, C. Altered dopamine activity after recovery from restricting-type anorexia nervosa. Neuropsychopharmacology 1999. [Google Scholar] [CrossRef]
- Kaye, W.H.; Wierenga, C.E.; Bischoff-Grethe, A.; Berner, L.A.; Ely, A.V.; Bailer, U.F.; Paulus, M.P.; Fudge, J.L. Neural Insensitivity to the Effects of Hunger in Women Remitted From Anorexia Nervosa. Am. J. Psychiatry 2020. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.; Aizenstein, H.; Mazurkewicz, L.; Fudge, J.; Frank, G.K.; Putnam, K.; Bailer, U.F.; Fischer, L.; Kaye, W.H. Altered insula response to taste stimuli in individuals recovered from restricting-type anorexia nervosa. Neuropsychopharmacology 2008. [Google Scholar] [CrossRef]
- Oberndorfer, T.; Simmons, A.; McCurdy, D.; Strigo, I.; Matthews, S.; Yang, T.; Irvine, Z.; Kaye, W. Greater anterior insula activation during anticipation of food images in women recovered from anorexia nervosa versus controls. Psychiatry Res. Neuroimaging 2013. [Google Scholar] [CrossRef]
- DeGuzman, M.; Shott, M.E.; Yang, T.T.; Riederer, J.; Frank, G.K.W. Association of elevated reward prediction error response with weight gain in adolescent anorexia nervosa. Am. J. Psychiatry 2017. [Google Scholar] [CrossRef]
- Frank, G.K.W.; Deguzman, M.C.; Shott, M.E.; Laudenslager, M.L.; Rossi, B.; Pryor, T. Association of Brain Reward Learning Response with Harm Avoidance, Weight Gain, and Hypothalamic Effective Connectivity in Adolescent Anorexia Nervosa. JAMA Psychiatry 2018. [Google Scholar] [CrossRef]
- Goldzak-Kunik, G.; Friedman, R.; Spitz, M.; Sandler, L.; Leshem, M. Intact sensory function in anorexia nervosa. Am. J. Clin. Nutr. 2012. [Google Scholar] [CrossRef]
- Frank, G.K.W.; Reynolds, J.R.; Shott, M.E.; Jappe, L.; Yang, T.T.; Tregellas, J.R.; O’Reilly, R.C. Anorexia nervosa and obesity are associated with opposite brain reward response. Neuropsychopharmacology 2012. [Google Scholar] [CrossRef] [PubMed]
- Jacquemot, A.M.M.C.; Park, R. The Role of Interoception in the Pathogenesis and Treatment of Anorexia Nervosa: A Narrative Review. Front. Psychiatry 2020. [Google Scholar] [CrossRef] [PubMed]
- Simon, J.J.; Stopyra, M.A.; Mönning, E.; Sailer, S.C.A.M.; Lavandier, N.; Kihm, L.; Bendszus, M.; Preissl, H.; Herzog, W.; Friederich, H.-C. Neuroimaging of hypothalamic mechanisms related to glucose metabolism in anorexia nervosa and obesity. J. Clin. Investig. 2020. [Google Scholar] [CrossRef] [PubMed]
- Smeets, P.A.M.; De Graaf, C.; Stafleu, A.; Van Osch, M.J.P.; Van Der Grond, J. Functional MRI of human hypothalamic responses following glucose ingestion. Neuroimage 2005. [Google Scholar] [CrossRef]
- Frank, G.K.W.; Kaye, W.H. Current status of functional imaging in eating disorders. Int. J. Eat. Disord. 2012. [Google Scholar] [CrossRef]
- Castro, D.C.; Cole, S.L.; Berridge, K.C. Lateral hypothalamus, nucleus accumbens, and ventral pallidum roles in eating and hunger: Interactions between homeostatic and reward circuitry. Front. Syst. Neurosci. 2015, 9, 90. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Furukawa, T.A.; Tsigkaropoulou, E.; Karavia, A.; Gournellis, R.; Soureti, A.; Bellos, I.; Douzenis, A.; Michopoulos, I. Adipokines in anorexia nervosa: A systematic review and meta-analysis. Psychoneuroendocrinology 2020, 112, 104485. [Google Scholar] [CrossRef]
- Eddy, K.T.; Lawson, E.A.; Meade, C.; Meenaghan, E.; Horton, S.E.; Misra, M.; Klibanski, A.; Miller, K.K. Appetite regulatory hormones in women with anorexia nervosa: Binge-eating/purging versus restricting type. J. Clin. Psychiatry 2015, 76, 19–24. [Google Scholar] [CrossRef]
- Padilla, S.L.; Qiu, J.; Soden, M.E.; Sanz, E.; Nestor, C.C.; Barker, F.D.; Quintana, A.; Zweifel, L.S.; Rønnekleiv, O.K.; Kelly, M.J.; et al. Agouti-related peptide neural circuits mediate adaptive behaviors in the starved state. Nat. Neurosci. 2016, 19, 734–741. [Google Scholar] [CrossRef]
- Li, C.; Hou, Y.; Zhang, J.; Sui, G.; Du, X.; Licinio, J.; Wong, M.L.; Yang, Y. AGRP neurons modulate fasting-induced anxiolytic effects. Transl. Psychiatry 2019. [Google Scholar] [CrossRef]
- Dardennes, R.M.; Zizzari, P.; Tolle, V.; Foulon, C.; Kipman, A.; Romo, L.; Iancu-Gontard, D.; Boni, C.; Sinet, P.M.; Thérèse Bluet, M.; et al. Family trios analysis of common polymorphisms in the obestatin/ghrelin, BDNF and AGRP genes in patients with Anorexia nervosa: Association with subtype, body-mass index, severity and age of onset. Psychoneuroendocrinology 2007. [Google Scholar] [CrossRef] [PubMed]
- Luo, N.; Marcelin, G.; Liu, S.M.; Schwartz, G.; Chua, S. Neuropeptide Y and agouti-related peptide mediate complementary functions of hyperphagia and reduced energy expenditure in leptin receptor deficiency. Endocrinology 2011. [Google Scholar] [CrossRef] [PubMed]
- Baranowska, B.; Wasilewska-Dziubińska, E.; Radzikowska, M.; Płonowski, A.; Roguski, K. Neuropeptide Y, galanin, and leptin release in obese women and in women with anorexia nervosa. Metabolism 1997. [Google Scholar] [CrossRef]
- Baranowska, B.; Wolinska-Witort, E.; Wasilewska-Dziubinska, E.; Roguski, K.; Chmielowska, M. Plasma leptin, neuropeptide Y (NPY) and galanin concentrations in bulimia nervosa and in anorexia nervosa. Neuroendocrinol. Lett. 2001, 22, 356–358. [Google Scholar]
- Sedlácková, D.; Kopečková, J.; Papežová, H.; Vybíral, S.; Kvasničková, H.; Hill, M.; Nedvídková, J. Changes of plasma obestatin, ghrelin and NPY in anorexia and bulimia nervosa patients before and after a high-carbohydrate breakfast. Physiol. Res. 2011. [Google Scholar] [CrossRef] [PubMed]
- Galusca, B.; Prévost, G.; Germain, N.; Dubuc, I.; Ling, Y.; Anouar, Y.; Estour, B.; Chartrel, N. Neuropeptide Y and α-MSH circadian levels in two populations with low body weight: Anorexia nervosa and constitutional thinness. PLoS ONE 2015. [Google Scholar] [CrossRef]
- Mercer, R.E.; Chee, M.J.S.; Colmers, W.F. The role of NPY in hypothalamic mediated food intake. Front. Neuroendocrinol. 2011, 32, 398–415. [Google Scholar] [CrossRef]
- Galusca, B.; Jeandel, L.; Germain, N.; Alexandre, D.; Leprince, J.; Anouar, Y.; Estour, B.; Chartrel, N. Orexigenic neuropeptide 26RFa: New evidence for an adaptive profile of appetite regulation in anorexia nervosa. J. Clin. Endocrinol. Metab. 2012. [Google Scholar] [CrossRef]
- Teske, J.A.; Mavanji, V. Energy Expenditure. Role of Orexin. In Vitamins and Hormones; Elsevier: Amsterdam, The Netherlands, 2012; Volume 89, pp. 91–109. [Google Scholar]
- D’agostino, G.; Diano, S. Alpha-melanocyte stimulating hormone: Production and degradation. J. Mol. Med. 2010, 88, 1195–1201. [Google Scholar] [CrossRef]
- Fan, W.; Ellacott, K.L.J.; Halatchev, I.G.; Takahashi, K.; Yu, P.; Cone, R.D. Cholecystokinin-mediated suppression of feeding involves the brainstem melanocortin system. Nat. Neurosci. 2004. [Google Scholar] [CrossRef]
- Candler, T.; Kühnen, P.; Prentice, A.M.; Silver, M. Epigenetic regulation of POMC; implications for nutritional programming, obesity and metabolic disease. Front. Neuroendocrinol. 2019, 54, 100773. [Google Scholar] [CrossRef] [PubMed]
- De Bond, J.-A.P.; Tolson, K.P.; Nasamran, C.; Kauffman, A.S.; Smith, J.T. Unaltered Hypothalamic Metabolic Gene Expression in Kiss1r Knockout Mice Despite Obesity and Reduced Energy Expenditure. J. Neuroendocrinol. 2016, 28. [Google Scholar] [CrossRef] [PubMed]
- Bacopoulou, F.; Lambrou, G.I.; Rodanaki, M.-E.; Stergioti, E.; Efthymiou, V.; Deligeoroglou, E.; Markantonis, S.L. Serum kisspeptin concentrations are negatively correlated with body mass index in adolescents with anorexia nervosa and amenorrhea. Hormones 2017, 16, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, T.; Elbelt, U.; Haas, V.; Ahnis, A.; Klapp, B.F.; Rose, M.; Stengel, A. Plasma kisspeptin and ghrelin levels are independently correlated with physical activity in patients with anorexia nervosa. Appetite 2017, 108, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Pałasz, A.; Tyszkiewicz-Nwafor, M.; Suszka-Świtek, A.; Bacopoulou, F.; Dmitrzak-Węglarz, M.; Dutkiewicz, A.; Słopień, A.; Janas-Kozik, M.; Wilczyński, K.M.; Filipczyk, Ł.; et al. Longitudinal study on novel neuropeptides phoenixin, spexin and kisspeptin in adolescent inpatients with anorexia nervosa–association with psychiatric symptoms. Nutr. Neurosci. 2019. [Google Scholar] [CrossRef] [PubMed]
- Bacopoulou, F.; Rodanaki, M.E.; Markantonis, S.; Stergioti, E.; Andreadou, I.; Efthymiou, V.; Deligeoroglou, E.; Chrousos, G. Kisspeptin-1 serum levels in adolescents with Anorexia Nervosa and other specified eating disorders. Endocr. Rev. 2014, 35, 3. [Google Scholar]
- Skowron, K.; Jasiński, K.; Kurnik-Łucka, M.; Stach, P.; Kalita, K.; Węglarz, W.P.; Gil, K. Hypothalamic and brain stem neurochemical profile in anorectic rats after peripheral administration of kisspeptin-10 using 1H-nmr spectroscopy in vivo. NMR Biomed. 2020. [Google Scholar] [CrossRef]
- Prinz, P.; Scharner, S.; Friedrich, T.; Schalla, M.; Goebel-Stengel, M.; Rose, M.; Stengel, A. Central and peripheral expression sites of phoenixin-14 immunoreactivity in rats. Biochem. Biophys. Res. Commun. 2017. [Google Scholar] [CrossRef]
- Yosten, G.L.C.; Lyu, R.-M.; Hsueh, A.J.W.; Avsian-Kretchmer, O.; Chang, J.-K.; Tullock, C.W.; Dun, S.L.; Dun, N.; Samson, W.K. A novel reproductive peptide, phoenixin. J. Neuroendocrinol. 2013, 25, 206–215. [Google Scholar] [CrossRef]
- Schalla, M.; Prinz, P.; Friedrich, T.; Scharner, S.; Kobelt, P.; Goebel-Stengel, M.; Rose, M.; Stengel, A. Phoenixin-14 injected intracerebroventricularly but not intraperitoneally stimulates food intake in rats. Peptides 2017, 96, 53–60. [Google Scholar] [CrossRef]
- Wang, M.; Deng, S.P.; Chen, H.P.; Jiang, D.; Tian, C.X.; Yang, W.; Wu, T.L.; Zhu, C.H.; Zhang, Y.; Li, G.L. Phoenixin participated in regulation of food intake and growth in spotted scat, Scatophagus argus. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, T.; Ahnis, A.; Elbelt, U.; Rose, M.; Klapp, B.F.; Stengel, A. NUCB2/nesfatin-1 is associated with elevated levels of anxiety in anorexia nervosa. PLoS ONE 2015. [Google Scholar] [CrossRef] [PubMed]
- Ogiso, K.; Asakawa, A.; Amitani, H.; Nakahara, T.; Ushikai, M.; Haruta, I.; Koyama, K.I.; Amitani, M.; Harada, T.; Yasuhara, D.; et al. Plasma nesfatin-1 concentrations in restricting-type anorexia nervosa. Peptides 2011. [Google Scholar] [CrossRef] [PubMed]
- Watson, H.J.; Yilmaz, Z.; Thornton, L.M.; Hübel, C.; Coleman, J.R.I.; Gaspar, H.A.; Bryois, J.; Hinney, A.; Leppä, V.M.; Mattheisen, M.; et al. Genome-wide association study identifies eight risk loci and implicates metabo-psychiatric origins for anorexia nervosa. Nat. Genet. 2019. [Google Scholar] [CrossRef]
- Misra, M.; Miller, K.K.; Kuo, K.; Griffin, K.; Stewart, V.; Hunter, E.; Herzog, D.B.; Klibanski, A. Secretory dynamics of ghrelin in adolescent girls with anorexia nervosa and healthy adolescents. Am. J. Physiol. Endocrinol. Metab. 2005. [Google Scholar] [CrossRef] [PubMed]
- Nova, E.; Samartín, S.; Gómez, S.; Morandé, G.; Marcos, A. The adaptive response of the immune system to the particular malnutrition of eating disorders. Eur. J. Clin. Nutr. 2002. [Google Scholar] [CrossRef] [PubMed]
- Solmi, M.; Veronese, N.; Favaro, A.; Santonastaso, P.; Manzato, E.; Sergi, G.; Correll, C.U. Inflammatory cytokines and anorexia nervosa: A meta-analysis of cross-sectional and longitudinal studies. Psychoneuroendocrinology 2015, 51, 237–252. [Google Scholar] [CrossRef]
- Dalton, B.; Bartholdy, S.; Robinson, L.; Solmi, M.; Ibrahim, M.A.A.; Breen, G.; Schmidt, U.; Himmerich, H. A meta-analysis of cytokine concentrations in eating disorders. J. Psychiatr. Res. 2018, 103, 252–264. [Google Scholar] [CrossRef]
- McCarthy, D.O. Tumor necrosis factor alpha and interleukin-6 have differential effects on food intake and gastric emptying in fasted rats. Res. Nurs. Health 2000. [Google Scholar] [CrossRef]
- Scarlett, J.M.; Zhu, X.; Enriori, P.J.; Bowe, D.D.; Batra, A.K.; Levasseur, P.R.; Grant, W.F.; Meguid, M.M.; Cowley, M.A.; Marks, D.L. Regulation of agouti-related protein messenger ribonucleic acid transcription and peptide secretion by acute and chronic inflammation. Endocrinology 2008. [Google Scholar] [CrossRef]
- Asakawa, A.; Inui, A.; Kaga, T.; Yuzuriha, H.; Nagata, T.; Fujimiya, M.; Katsuura, G.; Makino, S.; Fujino, M.A.; Kasuga, M. A role of ghrelin in neuroendocrine and behavioral responses to stress in mice. Neuroendocrinology 2001. [Google Scholar] [CrossRef]
- Inui, A. Eating behavior in anorexia nervosa—An excess of both orexigenic and anorexigenic signalling? Mol. Psychiatry 2001. [Google Scholar] [CrossRef] [PubMed]
- Holtmann, G.; Talley, N.J. The stomach-brain axis. Best Pract. Res. Clin. Gastroenterol. 2014, 28, 967–979. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, I.A.K. The ANX/ANX mouse—A valuable resource in anorexia nervosa research. Front. Neurosci. 2019, 13. [Google Scholar] [CrossRef] [PubMed]
- Rantala, M.J.; Luoto, S.; Krama, T.; Krams, I. Eating disorders: An evolutionary psychoneuroimmunological approach. Front. Psychol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Lonigro, A.; Pallini, S.; Zanna, V.; Marech, L.; Rosa, M.; Criscuolo, M.; Chianello, I.; Laghi, F. Autonomic response to the Adult Attachment Projective in anorexia nervosa. Eat. Weight Disord. 2019. [Google Scholar] [CrossRef] [PubMed]
- Sidiropoulos, M. Anorexia nervosa: The physiological consequences of starvation and the need for primary prevention efforts. McGill J. Med. 2007, 10, 20–25. [Google Scholar]
- Simões-Capela, N.; Schiavone, G.; De Raedt, W.; Vrieze, E.; Van Hoof, C. Toward Quantifying the Psychopathology of Eating Disorders From the Autonomic Nervous System Perspective: A Methodological Approach. Front. Neurosci. 2019, 13, 606. [Google Scholar] [CrossRef]
- Takimoto, Y.; Yoshiuchi, K.; Ishizawa, T.; Yamamoto, Y.; Akabayashi, A. Autonomic dysfunction responses to head-up tilt in anorexia nervosa. Clin. Auton. Res. 2014. [Google Scholar] [CrossRef]
- Pirke, K.M. Central and peripheral noradrenalin regulation in eating disorders. Psychiatry Res. 1996, 62, 43–49. [Google Scholar] [CrossRef]
- Nudel, D.B.; Gootman, N.; Nussbaum, M.P.; Shenker, I.R. Altered exercise performance and abnormal sympathetic responses to exercise in patients with anorexia nervosa. J. Pediatr. 1984. [Google Scholar] [CrossRef]
- Billeci, L.; Tonacci, A.; Brunori, E.; Raso, R.; Calderoni, S.; Maestro, S.; Morales, M.A. Autonomic nervous system response during light physical activity in adolescents with anorexia nervosa measured by wearable devices. Sensors 2019, 19, 2820. [Google Scholar] [CrossRef] [PubMed]
- Lechin, F.; van der Dijs, B. Central Nervous System Circuitries Underlying Two Types of Peripheral Autonomic Nervous System Disorders~!2008-04-24~!2008-09-26~!2008-12-05~! Open Neurosci. J. 2008, 2, 41–50. [Google Scholar] [CrossRef][Green Version]
- Lechin, F.; Van Der Dijs, B.; Pardey-Maldonado, B.; Rivera, J.E.; Baez, S.; Lechin, M.E. Anorexia nervosa depends on adrenal sympathetic hyperactivity: Opposite neuroautonomic profile of hyperinsulinism syndrome. Diabetes Metab. Syndr. Obes. Targets Ther. 2010, 3, 311–317. [Google Scholar] [CrossRef]
- Paeger, L.; Karakasilioti, I.; Altmüller, J.; Frommolt, P.; Brüning, J.; Kloppenburg, P. Antagonistic modulation of NPY/AgRP and POMC neurons in the arcuate nucleus by noradrenalin. eLife 2017. [Google Scholar] [CrossRef]
- Furness, J.B. The enteric nervous system: Normal functions and enteric neuropathies. Neurogastroenterol. Motil. 2008, 20, 32–38. [Google Scholar] [CrossRef]
- Schwartz, G.J. The role of gastrointestinal vagal afferents in the control of food intake: Current prospects. Nutrition 2000, 16, 866–873. [Google Scholar] [CrossRef]
- Furness, J.B.; Rivera, L.R.; Cho, H.J.; Bravo, D.M.; Callaghan, B. The gut as a sensory organ. Nat. Rev. Gastroenterol. Hepatol. 2013. [Google Scholar] [CrossRef]
- Iovino, P.; Azpiroz, F.; Domingo, E.; Malagelada, J.R. The sympathetic nervous system modulates perception and reflex responses to gut distention in humans. Gastroenterology 1995. [Google Scholar] [CrossRef]
- Brookes, S.J.H.; Spencer, N.J.; Costa, M.; Zagorodnyuk, V.P. Extrinsic primary afferent signalling in the gut. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 286–296. [Google Scholar] [CrossRef]
- Wang, G.J.; Tomasi, D.; Backus, W.; Wang, R.; Telang, F.; Geliebter, A.; Korner, J.; Bauman, A.; Fowler, J.S.; Thanos, P.K.; et al. Gastric distention activates satiety circuitry in the human brain. Neuroimage 2008. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M. Integrated Upper Gastrointestinal Response to Food Intake. Gastroenterology 2006, 131, 640–658. [Google Scholar] [CrossRef] [PubMed]
- Santonicola, A.; Gagliardi, M.; Guarino, M.P.L.; Siniscalchi, M.; Ciacci, C.; Iovino, P. Eating disorders and gastrointestinal diseases. Nutrients 2019, 11, 3038. [Google Scholar] [CrossRef] [PubMed]
- Emmanuel, A.V.; Stern, J.; Treasure, J.; Forbes, A.; Kamm, M.A. Anorexia nervosa in gastrointestinal practice. Eur. J. Gastroenterol. Hepatol. 2004. [Google Scholar] [CrossRef]
- Sudo, N. Microbiome, HPA axis and production of endocrine hormones in the gut. Adv. Exp. Med. Biol. 2014. [Google Scholar] [CrossRef]
- Zmora, N.; Zilberman-Schapira, G.; Suez, J.; Mor, U.; Dori-Bachash, M.; Bashiardes, S.; Kotler, E.; Zur, M.; Regev-Lehavi, D.; Brik, R.B.Z.; et al. Personalized Gut Mucosal Colonization Resistance to Empiric Probiotics Is Associated with Unique Host and Microbiome Features. Cell 2018. [Google Scholar] [CrossRef]
- Casper, R.C. The Pathophysiology of Anorexia Nervosa and Bulimia Nervosa. Annu. Rev. Nutr. 1986. [Google Scholar] [CrossRef]
- Breton, J.; Déchelotte, P.; Ribet, D. Intestinal microbiota and Anorexia Nervosa. Clin. Nutr. Exp. 2019, 28, 11–21. [Google Scholar] [CrossRef]
- Smith, M.I.; Yatsunenko, T.; Manary, M.J.; Trehan, I.; Mkakosya, R.; Cheng, J.; Kau, A.L.; Rich, S.S.; Concannon, P.; Mychaleckyj, J.C.; et al. Gut microbiomes of Malawian twin pairs discordant for kwashiorkor. Science 2013. [Google Scholar] [CrossRef]
- Hata, T.; Miyata, N.; Takakura, S.; Yoshihara, K.; Asano, Y.; Kimura-Todani, T.; Yamashita, M.; Zhang, X.T.; Watanabe, N.; Mikami, K.; et al. The Gut Microbiome Derived from Anorexia Nervosa Patients Impairs Weight Gain and Behavioral Performance in Female Mice. Endocrinology 2019. [Google Scholar] [CrossRef]
- Lippert, K.; Kedenko, L.; Antonielli, L.; Kedenko, I.; Gemeier, C.; Leitner, M.; Kautzky-Willer, A.; Paulweber, B.; Hackl, E. Gut microbiota dysbiosis associated with glucose metabolism disorders and the metabolic syndrome in older adults. Benef. Microbes 2017. [Google Scholar] [CrossRef] [PubMed]
- Armougom, F.; Henry, M.; Vialettes, B.; Raccah, D.; Raoult, D. Monitoring bacterial community of human gut microbiota reveals an increase in Lactobacillus in obese patients and Methanogens in anorexic patients. PLoS ONE 2009. [Google Scholar] [CrossRef] [PubMed]
- Million, M.; Angelakis, E.; Maraninchi, M.; Henry, M.; Giorgi, R.; Valero, R.; Vialettes, B.; Raoult, D. Correlation between body mass index and gut concentrations of Lactobacillus reuteri, Bifidobacterium animalis, Methanobrevibacter smithii and Escherichia coli. Int. J. Obes. 2013. [Google Scholar] [CrossRef] [PubMed]
- Kleiman, S.C.; Watson, H.J.; Bulik-Sullivan, E.C.; Huh, E.Y.; Tarantino, L.M.; Bulik, C.M.; Carroll, I.M. The Intestinal Microbiota in Acute Anorexia Nervosa and during Renourishment: Relationship to Depression, Anxiety, and Eating Disorder Psychopathology. Psychosom. Med. 2015. [Google Scholar] [CrossRef] [PubMed]
- Morita, C.; Tsuji, H.; Hata, T.; Gondo, M.; Takakura, S.; Kawai, K.; Yoshihara, K.; Ogata, K.; Nomoto, K.; Miyazaki, K.; et al. Gut Dysbiosis in Patients with Anorexia Nervosa. PLoS ONE 2015. [Google Scholar] [CrossRef]
- Mack, I.; Cuntz, U.; Grmer, C.; Niedermaier, S.; Pohl, C.; Schwiertz, A.; Zimmermann, K.; Zipfel, S.; Enck, P.; Penders, J. Weight gain in anorexia nervosa does not ameliorate the faecal microbiota, branched chain fatty acid profiles, and gastrointestinal complaints. Sci. Rep. 2016. [Google Scholar] [CrossRef]
- Borgo, F.; Riva, A.; Benetti, A.; Casiraghi, M.C.; Bertelli, S.; Garbossa, S.; Anselmetti, S.; Scarone, S.; Pontiroli, A.E.; Morace, G.; et al. Microbiota in anorexia nervosa: The triangle between bacterial species, metabolites and psychological tests. PLoS ONE 2017. [Google Scholar] [CrossRef]
- Mörkl, S.; Lackner, S.; Müller, W.; Gorkiewicz, G.; Kashofer, K.; Oberascher, A.; Painold, A.; Holl, A.; Holzer, P.; Meinitzer, A.; et al. Gut microbiota and body composition in anorexia nervosa inpatients in comparison to athletes, overweight, obese, and normal weight controls. Int. J. Eat. Disord. 2017. [Google Scholar] [CrossRef]
- Mörkl, S.; Lackner, S.; Meinitzer, A.; Mangge, H.; Lehofer, M.; Halwachs, B.; Gorkiewicz, G.; Kashofer, K.; Painold, A.; Holl, A.K.; et al. Gut microbiota, dietary intakes and intestinal permeability reflected by serum zonulin in women. Eur. J. Nutr. 2018. [Google Scholar] [CrossRef]
- Hanachi, M.; Manichanh, C.; Schoenenberger, A.; Pascal, V.; Levenez, F.; Cournède, N.; Doré, J.; Melchior, J.C. Altered host-gut microbes symbiosis in severely malnourished anorexia nervosa (AN) patients undergoing enteral nutrition: An explicative factor of functional intestinal disorders? Clin. Nutr. 2019. [Google Scholar] [CrossRef]
- Fava, F.; Gitau, R.; Griffin, B.A.; Gibson, G.R.; Tuohy, K.M.; Lovegrove, J.A. The type and quantity of dietary fat and carbohydrate alter faecal microbiome and short-chain fatty acid excretion in a metabolic syndrome “at-risk” population. Int. J. Obes. 2013. [Google Scholar] [CrossRef] [PubMed]
- Ghoshal, U.; Shukla, R.; Srivastava, D.; Ghoshal, U.C. Irritable bowel syndrome, particularly the constipation-predominant form, involves an increase in Methanobrevibacter smithii, which is associated with higher methane production. Gut Liver 2016. [Google Scholar] [CrossRef] [PubMed]
- Michalovich, D.; Rodriguez-Perez, N.; Smolinska, S.; Pirozynski, M.; Mayhew, D.; Uddin, S.; Van Horn, S.; Sokolowska, M.; Altunbulakli, C.; Eljaszewicz, A.; et al. Obesity and disease severity magnify disturbed microbiome-immune interactions in asthma patients. Nat. Commun. 2019. [Google Scholar] [CrossRef]
- Isokpehi, R.D.; Simmons, S.S.; Johnson, M.O.; Payton, M. Genomic evidence for bacterial determinants influencing obesity development. Int. J. Environ. Res. Public Health 2017, 14, 345. [Google Scholar] [CrossRef]
- Derrien, M.; Vaughan, E.E.; Plugge, C.M.; de Vos, W.M. Akkermansia municiphila gen. nov., sp. nov., a human intestinal mucin-degrading bacterium. Int. J. Syst. Evol. Microbiol. 2004. [Google Scholar] [CrossRef] [PubMed]
- Marcobal, A.; Southwick, A.M.; Earle, K.A.; Sonnenburg, J.L. A refined palate: Bacterial consumption of host glycans in the gut. Glycobiology 2013, 23, 1038–1046. [Google Scholar] [CrossRef] [PubMed]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G.; Delzenne, N.M.; et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. USA 2013. [Google Scholar] [CrossRef] [PubMed]
- Tilg, H.; Danese, S. Roseburia hominis: A novel guilty player in ulcerative colitis pathogenesis? Gut 2013, 63, 1204–1205. [Google Scholar] [CrossRef] [PubMed]
- Imhann, F.; Vich Vila, A.; Bonder, M.J.; Fu, J.; Gevers, D.; Visschedijk, M.C.; Spekhorst, L.M.; Alberts, R.; Franke, L.; Van Dullemen, H.M.; et al. Interplay of host genetics and gut microbiota underlying the onset and clinical presentation of inflammatory bowel disease. Gut 2018. [Google Scholar] [CrossRef]
- Adan, R.A.H.; Vink, T. Drug target discovery by pharmacogenetics: Mutations in the melanocortin system and eating disorders. Eur. Neuropsychopharmacol. 2001. [Google Scholar] [CrossRef]
- Breton, J.; Tennoune, N.; Lucas, N.; Francois, M.; Legrand, R.; Jacquemot, J.; Goichon, A.; Guérin, C.; Peltier, J.; Pestel-Caron, M.; et al. Gut commensal E. coli proteins activate host satiety pathways following nutrient-induced bacterial growth. Cell Metab. 2016. [Google Scholar] [CrossRef] [PubMed]
- Breton, J.; Legrand, R.; Akkermann, K.; Järv, A.; Harro, J.; Déchelotte, P.; Fetissov, S.O. Elevated plasma concentrations of bacterial ClpB protein in patients with eating disorders. Int. J. Eat. Disord. 2016. [Google Scholar] [CrossRef] [PubMed]
- Monda, V.; Villano, I.; Messina, A.; Valenzano, A.; Esposito, T.; Moscatelli, F.; Viggiano, A.; Cibelli, G.; Chieffi, S.; Monda, M.; et al. Exercise modifies the gut microbiota with positive health effects. Oxid. Med. Cell. Longev. 2017, 2017, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Achamrah, N.; Coëeffier, M.; Déchelotte, P. Physical activity in patients with anorexia nervosa. Nutr. Rev. 2016. [Google Scholar] [CrossRef]
- Sudo, N. Biogenic Amines: Signals Between Commensal Microbiota and Gut Physiology. Front. Endocrinol. 2019. [Google Scholar] [CrossRef]
- Barrett, E.; Ross, R.P.; O’Toole, P.W.; Fitzgerald, G.F.; Stanton, C. γ-Aminobutyric acid production by culturable bacteria from the human intestine. J. Appl. Microbiol. 2012. [Google Scholar] [CrossRef]
- Clarke, G.; Stilling, R.M.; Kennedy, P.J.; Stanton, C.; Cryan, J.F.; Dinan, T.G. Minireview: Gut microbiota: The neglected endocrine organ. Mol. Endocrinol. 2014, 28, 1221–1238. [Google Scholar] [CrossRef]
- Bienenstock, J.; Forsythe, P.; Karimi, K.; Kunze, W. Neuroimmune aspects of food intake. Int. Dairy J. 2010, 20, 253–258. [Google Scholar] [CrossRef]
- Asano, Y.; Hiramoto, T.; Nishino, R.; Aiba, Y.; Kimura, T.; Yoshihara, K.; Koga, Y.; Sudo, N. Critical role of gut microbiota in the production of biologically active, free catecholamines in the gut lumen of mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2012. [Google Scholar] [CrossRef]
- Singh, S.K.; Yamashita, A.; Gouaux, E. Antidepressant binding site in a bacterial homologue of neurotransmitter transporters. Nature 2007. [Google Scholar] [CrossRef]
- Mathias, M. Autointoxication and historical precursors of the microbiome–gut–brain axis. Microb. Ecol. Health Dis. 2018. [Google Scholar] [CrossRef] [PubMed]
- Glenny, E.M.; Bulik-Sullivan, E.C.; Tang, Q.; Bulik, C.M.; Carroll, I.M. Eating Disorders and the Intestinal Microbiota: Mechanisms of Energy Homeostasis and Behavioral Influence. Curr. Psychiatry Rep. 2017, 19, 51. [Google Scholar] [CrossRef] [PubMed]
- Alcock, J.; Maley, C.C.; Aktipis, C.A. Is eating behavior manipulated by the gastrointestinal microbiota? Evolutionary pressures and potential mechanisms. BioEssays 2014, 36, 940–949. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, G.T.; Gibson, G.R.; Cummings, J.H. Comparison of fermentation reactions in different regions of the human colon. J. Appl. Bacteriol. 1992. [Google Scholar] [CrossRef]
- Ruusunen, A.; Rocks, T.; Jacka, F.; Loughman, A. The gut microbiome in anorexia nervosa: Relevance for nutritional rehabilitation. Psychopharmacology 2019, 236, 1545–1558. [Google Scholar] [CrossRef]
- Begg, D.P.; Woods, S.C. The endocrinology of food intake. Nat. Rev. Endocrinol. 2013, 9, 584–597. [Google Scholar] [CrossRef]
- Worthington, J.J. The intestinal immunoendocrine axis: Novel cross-talk between enteroendocrine cells and the immune system during infection and inflammatory disease. Biochem. Soc. Trans. 2015. [Google Scholar] [CrossRef]
- Lamers, C.B.; Lieverse, R.J.; Masclee, A.A.; Jansen, J.B. The role of cholecystokinin in appetite control. Br. J. Hosp. Med. 1995, 53, 113. [Google Scholar]
- Philipp, E.; Pirke, K.M.; Kellner, M.B.; Krieg, J.C. Disturbed cholecystokinin secretion in patients with eating disorders. Life Sci. 1991. [Google Scholar] [CrossRef]
- Tamai, H.; Takemura, J.; Kobayashi, N.; Matsubayashi, S.; Matsukura, S.; Nakagawa, T. Changes in plasma cholecystokinin concentrations after oral glucose tolerance test in anorexia nervosa before and after therapy. Metabolism 1993. [Google Scholar] [CrossRef]
- MacIntosh, C.G.; Morley, J.E.; Wishart, J.; Morris, H.; Jansen, J.B.M.J.; Horowitz, M.; Chapman, I.M. Effect of exogenous cholecystokinin (CCK)-8 on food intake and plasma CCK, leptin, and insulin concentrations in older and young adults: Evidence for increased CCK activity as a cause of the anorexia of aging. J. Clin. Endocrinol. Metab. 2001. [Google Scholar] [CrossRef] [PubMed]
- Cuntz, U.; Enck, P.; Frühauf, E.; Lehnert, P.; Riepl, R.L.; Fichter, M.M.; Otto, B. Cholecystokinin Revisited: CCK and the Hunger Trap in Anorexia Nervosa. PLoS ONE 2013. [Google Scholar] [CrossRef] [PubMed]
- Holzer, P.; Reichmann, F.; Farzi, A. Neuropeptide Y, peptide YY and pancreatic polypeptide in the gut-brain axis. Neuropeptides 2012, 46, 261–274. [Google Scholar] [CrossRef] [PubMed]
- Batterham, R.L.; Ffytche, D.H.; Rosenthal, J.M.; Zelaya, F.O.; Barker, G.J.; Withers, D.J.; Williams, S.C.R. PYY modulation of cortical and hypothalamic brain areas predicts feeding behaviour in humans. Nature 2007. [Google Scholar] [CrossRef] [PubMed]
- Nakahara, T.; Kojima, S.; Tanaka, M.; Yasuhara, D.; Harada, T.; Sagiyama, K.I.; Muranaga, T.; Nagai, N.; Nakazato, M.; Nozoe, S.I.; et al. Incomplete restoration of the secretion of ghrelin and PYY compared to insulin after food ingestion following weight gain in anorexia nervosa. J. Psychiatr. Res. 2007. [Google Scholar] [CrossRef]
- Misra, M.; Miller, K.K.; Tsai, P.; Gallagher, K.; Lin, A.; Lee, N.; Herzog, D.B.; Klibanski, A. Elevated peptide YY levels in adolescent girls with anorexia nervosa. J. Clin. Endocrinol. Metab. 2006. [Google Scholar] [CrossRef]
- Lawson, E.A.; Eddy, K.T.; Donoho, D.; Misra, M.; Miller, K.K.; Meenaghan, E.; Lydecker, J.; Herzog, D.; Klibanski, A. Appetite-regulating hormones cortisol and peptide YY are associated with disordered eating psychopathology, independent of body mass index. Eur. J. Endocrinol. 2011. [Google Scholar] [CrossRef]
- Germain, N.; Galusca, B.; Grouselle, D.; Frere, D.; Billard, S.; Epelbaum, J.; Estour, B. Ghrelin and obestatin circadian levels differentiate bingeing-purging from restrictive anorexia nervosa. J. Clin. Endocrinol. Metab. 2010. [Google Scholar] [CrossRef]
- Tam, F.I.; Seidel, M.; Boehm, I.; Ritschel, F.; Bahnsen, K.; Biemann, R.; Weidner, K.; Roessner, V.; Ehrlich, S. Peptide YY3–36 concentration in acute- and long-term recovered anorexia nervosa. Eur. J. Nutr. 2020. [Google Scholar] [CrossRef]
- Schalla, M.A.; Stengel, A. The role of ghrelin in anorexia nervosa. Int. J. Mol. Sci. 2018, 19, 2117. [Google Scholar] [CrossRef]
- Berthoud, H.R. Vagal and hormonal gut-brain communication: From satiation to satisfaction. Neurogastroenterol. Motil. 2008, 20, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Otto, B.; Cuntz, U.; Fruehauf, E.; Wawarta, R.; Folwaczny, C.; Riepl, R.L.; Heiman, M.L.; Lehnert, P.; Fichter, M.; Tschöp, M. Weight gain decreases elevated plasma ghrelin concentrations of patients with anorexia nervosa. Eur. J. Endocrinol. 2001. [Google Scholar] [CrossRef]
- Tolle, V.; Kadem, M.; Bluet-Pajot, M.T.; Frere, D.; Foulon, C.; Bossu, C.; Dardennes, R.; Mounier, C.; Zizzari, P.; Lang, F.; et al. Balance in Ghrelin and leptin plasma levels in anorexia nervosa patients and constitutionally thin women. J. Clin. Endocrinol. Metab. 2003. [Google Scholar] [CrossRef] [PubMed]
- Blauwhoff-Buskermolen, S.; Langius, J.A.E.; Heijboer, A.C.; Becker, A.; de van der Schueren, M.A.E.; Verheul, H.M.W. Plasma ghrelin levels are associated with anorexia but not cachexia in patients with NSCLC. Front. Physiol. 2017. [Google Scholar] [CrossRef]
- Holtkamp, K.; Mogharrebi, R.; Hanisch, C.; Schumpelick, V.; Herpertz-Dahlmann, B. Gastric dilatation in a girl with former obesity and atypical anorexia nervosa. Int. J. Eat. Disord. 2002. [Google Scholar] [CrossRef]
- McCallum, R.W.; Grill, B.B.; Lange, R.; Planky, M.; Glass, E.E.; Greenfeld, D.G. Definition of a gastric emptying abnormality in patients with anorexia nervosa. Dig. Dis. Sci. 1985. [Google Scholar] [CrossRef]
- Kamal, N.; Chami, T.; Andersen, A.; Rosell, F.A.; Schuster, M.M.; Whitehead, W.E. Delayed gastrointestinal transit times in anorexia nervosa and bulimia nervosa. Gastroenterology 1991. [Google Scholar] [CrossRef]
- Hutson, W.R.; Wald, A. Gastric emptying in patients with bulimia nervosa and anorexia nervosa. Am. J. Gastroenterol. 1990, 85, 41–46. [Google Scholar]
- Rigaud, D.; Bedig, G.; Merrouche, M.; Vulpillat, M.; Bonfils, S.; Apfelbaum, M. Delayed gastric emptying in anorexia nervosa is improved by completion of a renutrition program. Dig. Dis. Sci. 1988. [Google Scholar] [CrossRef]
- Hill, L.D.; Kozarek, R.A.; Kraemer, S.J.M.; Aye, R.W.; Mercer, C.D.; Low, D.E.; Pope, C.E. The gastroesophageal flap valve: In vitro and in vivo observations. Gastrointest. Endosc. 1996. [Google Scholar] [CrossRef]
- Abell, T.L.; Malagelada, J.R.; Lucas, A.R.; Brown, M.L.; Camilleri, M.; Go, V.L.W.; Azpiroz, F.; CallaWay, C.W.; Kao, P.C.; Zinsmeister, A.R.; et al. Gastric electromechanical and neurohormonal function in anorexia nervosa. Gastroenterology 1987. [Google Scholar] [CrossRef]
- Boyd, C.; Abraham, S.; Kellow, J. Psychological features are important predictors of functional gastrointestinal disorders in patients with eating disorders. Scand. J. Gastroenterol. 2005. [Google Scholar] [CrossRef] [PubMed]
- Passananti, V.; Siniscalchi, M.; Zingone, F.; Bucci, C.; Tortora, R.; Iovino, P.; Ciacci, C. Prevalence of eating disorders in adults with celiac disease. Gastroenterol. Res. Pract. 2013. [Google Scholar] [CrossRef]
- Johansson, A.K.; Norring, C.; Unell, L.; Johansson, A. Eating disorders and oral health: A matched case-control study. Eur. J. Oral Sci. 2012. [Google Scholar] [CrossRef]
- Paszyńska, E.; Słopień, A.; Ślebioda, Z.; Dyszkiewicz-Konwińska, M.; Wȩglarz, M.; Rajewski, A. Ocena makroskopowa błony śluzowej jamy ustnej i analiza pH śliny u pacjentów z jadłowstrȩtem psychicznym. Psychiatr. Pol. 2014, 48, 453–464. [Google Scholar]
- Malczyk, Ż.; Oświęcimska, J. Gastrointestinal complications and refeeding guidelines in patients with anorexia nervosa. Psychiatr. Pol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Luscombe, G.M.; Boyd, C.; Kellow, J.; Abraham, S. Functional gastrointestinal disorders in eating disorder patients: Altered distribution and predictors using ROME III compared to ROME II criteria. World J. Gastroenterol. 2014. [Google Scholar] [CrossRef] [PubMed]
- Pacciardi, B.; Cargioli, C.; Mauri, M. Barrett’s esophagus in anorexia nervosa: A case report. Int. J. Eat. Disord. 2015. [Google Scholar] [CrossRef]
- Bluemel, S.; Menne, D.; Milos, G.; Goetze, O.; Fried, M.; Schwizer, W.; Fox, M.; Steingoetter, A. Relationship of body weight with gastrointestinal motor and sensory function: Studies in anorexia nervosa and obesity. BMC Gastroenterol. 2017. [Google Scholar] [CrossRef]
- Lee, S.; Lee, A.M.; Ngai, E.; Lee, D.T.S.; Wing, Y.K. Rationales for food refusal in Chinese patients with anorexia nervosa. Int. J. Eat. Disord. 2001. [Google Scholar] [CrossRef]
- Holt, S.; Ford, M.J.; Grant, S.; Heading, R.C. Abnormal gastric emptying in primary anorexia nervosa. Br. J. Psychiatry 1981. [Google Scholar] [CrossRef] [PubMed]
- Robinson, P.H. Perceptivity and paraceptivity during measurement of gastric emptying in anorexia and bulimia nervosa. Br. J. Psychiatry 1989. [Google Scholar] [CrossRef] [PubMed]
- Benini, L.; Todesco, T.; Dalle Grave, R.; Deiorio, F.; Salandini, L.; Vantini, I. Gastric emptying in patients with restricting and binge/purging subtypes of anorexia nervosa. Am. J. Gastroenterol. 2004. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Fukudo, S. Gastrointestinal symptoms and disorders in patients with eating disorders. Clin. J. Gastroenterol. 2015, 8, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Szmukler, G.I.; Young, G.P.; Lichtenstein, M.; Andrews, J.T. A serial study of gastric emptying in anorexia nervosa and bulimia. Aust. N. Z. J. Med. 1990. [Google Scholar] [CrossRef]
- Henningsen, P.; Zimmermann, T.; Sattel, H. Medically unexplained physical symptoms, anxiety, and depression: A meta-analytic review. Psychosom. Med. 2003, 65, 528–533. [Google Scholar] [CrossRef]
- Harer, K.N. Irritable bowel syndrome, disordered eating, and eating disorders. Gastroenterol. Hepatol. 2019, 15, 280–282. [Google Scholar]
- Reed-Knight, B.; Squires, M.; Chitkara, D.K.; van Tilburg, M.A.L. Adolescents with irritable bowel syndrome report increased eating-associated symptoms, changes in dietary composition, and altered eating behaviors: A pilot comparison study to healthy adolescents. Neurogastroenterol. Motil. 2016. [Google Scholar] [CrossRef]
- Satherley, R.; Howard, R.; Higgs, S. Disordered eating practices in gastrointestinal disorders. Appetite 2015, 84, 240–250. [Google Scholar] [CrossRef]
- Vanheel, H.; Farré, R. Changes in gastrointestinal tract function and structure in functional dyspepsia. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 142–149. [Google Scholar] [CrossRef]
- Stanghellini, V.; Chan, F.K.L.; Hasler, W.L.; Malagelada, J.R.; Suzuki, H.; Tack, J.; Talley, N.J. Gastroduodenal disorders. Gastroenterology 2016. [Google Scholar] [CrossRef] [PubMed]
- Schalla, M.A.; Stengel, A. Gastrointestinal alterations in anorexia nervosa—A systematic review. Eur. Eat. Disord. Rev. 2019, 27, 447–461. [Google Scholar] [CrossRef] [PubMed]
- Hausteiner-Wiehle, C.; Henningsen, P. Irritable bowel syndrome: Relations with functional, mental, and somatoform disorders. World J. Gastroenterol. 2014. [Google Scholar] [CrossRef] [PubMed]
- Carpinelli, L.; Bucci, C.; Santonicola, A.; Zingone, F.; Ciacci, C.; Iovino, P. Anhedonia in irritable bowel syndrome and in inflammatory bowel diseases and its relationship with abdominal pain. Neurogastroenterol. Motil. 2019. [Google Scholar] [CrossRef] [PubMed]
- Bellini, M.; Usai-Satta, P.; Bove, A.; Bocchini, R.; Galeazzi, F.; Battaglia, E.; Alduini, P.; Buscarini, E.; Bassotti, G.; Balzano, A.; et al. Chronic constipation diagnosis and treatment evaluation: The “CHRO.CO.DI.T.E.” study. BMC Gastroenterol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Zipfel, S.; Sammet, I.; Rapps, N.; Herzog, W.; Herpertz, S.; Martens, U. Gastrointestinal disturbances in eating disorders: Clinical and neurobiological aspects. Auton. Neurosci. Basic Clin. 2006. [Google Scholar] [CrossRef]
- Chun, A.B.; Sokol, M.S.; Kaye, W.H.; Hutson, W.R.; Wald, A. Colonic and anorectal function in constipated patients with anorexia nervosa. Am. J. Gastroenterol. 1997, 92, 1879–1883. [Google Scholar]
- Chiarioni, G.; Bassotti, G.; Monsignori, A.; Menegotti, M.; Salandini, L.; Matteo, G.D.I.; Vantini, I.; Whitehead, W.E. Anorectal dysfunction in constipated women with anorexia nervosa. Mayo Clin. Proc. 2000. [Google Scholar] [CrossRef]
- Støving, R.K. Anorexia nervosa and endocrinology: A clinical update. Eur. J. Endocrinol. 2019, 180, R9–R27. [Google Scholar] [CrossRef]
- Campos, G.V.; de Noronha, S.R.; de Souza, A.A.; Lima, P.M.; Abreu, A.R.; Chianca, D., Jr.; de Menezes, R.C. Estrogen receptor β activation within dorsal raphe nucleus reverses anxiety-like behavior induced by food restriction in female rats. Behav. Brain Res. 2019. [Google Scholar] [CrossRef]
- Fazeli, P.K.; Klibanski, A. Bone metabolism in anorexia nervosa. Curr. Osteoporos. Rep. 2014, 12, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Robinson, L.; Micali, N.; Misra, M. Eating disorders and bone metabolism in women. Curr. Opin. Pediatr. 2017, 29, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Mikhail, M.E.; Fowler, N.; Culbert, K.M.; Klump, K.L. The Role of Puberty and Ovarian Hormones in the Genetic Diathesis of Eating Disorders in Females. Child Adolesc. Psychiatr. Clin. N. Am. 2019, 28, 617–628. [Google Scholar] [CrossRef] [PubMed]
- Inui, A. Ghrelin: An orexigenic and somatotrophic signal from the stomach. Nat. Rev. Neurosci. 2001, 2, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Grinspoon, S.; Gulick, T.; Askari, H.; Landt, M.; Lee, K.; Anderson, E.; Ma, Z.; Vignati, L.; Bowsher, R.; Herzog, D.; et al. Serum leptin levels in women with anorexia nervosa. J. Clin. Endocrinol. Metab. 1996. [Google Scholar] [CrossRef]
- Ferron, F.; Considine, R.V.; Peino, R.; Lado, I.G.; Dieguez, C.; Casanueva, F.F. Serum leptin concentrations in patients with anorexia nervosa, bulimia nervosa and non-specific eating disorders correlate with the body mass index but are independent of the respective disease. Clin. Endocrinol. 1997. [Google Scholar] [CrossRef]
- Hebebrand, J.; Blum, W.F.; Barth, N.; Coners, H.; Englaro, P.; Juul, A.; Ziegler, A.; Warnke, A.; Rascher, W.; Remschmidt, H. Leptin levels in patients with anorexia nervosa are reduced in the acute stage and elevated upon short-term weight restoration. Mol. Psychiatry 1997. [Google Scholar] [CrossRef]
- Haas, V.; Onur, S.; Paul, T.; Nutzinger, D.O.; Bosy-Westphal, A.; Hauer, M.; Brabant, G.; Klein, H.; Müller, M.J. Leptin and body weight regulation in patients with anorexia nervosa before and during weight recovery. Am. J. Clin. Nutr. 2005. [Google Scholar] [CrossRef]
- Miller, K.K.; Parulekar, M.S.; Schoenfeld, E.; Anderson, E.; Hubbard, J.; Klibanski, A.; Grinspoon, S.K. Decreased leptin levels in normal weight women with hypothalamic amenorrhea: The effects of body composition and nutritional intake. J. Clin. Endocrinol. Metab. 1998. [Google Scholar] [CrossRef]
- Biver, E.; Salliot, C.; Combescure, C.; Gossec, L.; Hardouin, P.; Legroux-Gerot, I.; Cortet, B. Influence of adipokines and ghrelin on bone mineral density and fracture risk: A systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 2011, 96, 2703–2713. [Google Scholar] [CrossRef]
- Misra, M.; Miller, K.K.; Cord, J.; Prabhakaran, R.; Herzog, D.B.; Goldstein, M.; Katzman, D.K.; Klibanski, A. Relationships between serum adipokines, insulin levels, and bone density in girls with anorexia nervosa. J. Clin. Endocrinol. Metab. 2007. [Google Scholar] [CrossRef] [PubMed]
- Bredella, M.A.; Fazeli, P.K.; Freedman, L.M.; Calder, G.; Lee, H.; Rosen, C.J.; Klibanski, A. Young women with cold-activated brown adipose tissue have higher bone mineral density and lower Pref-1 than women without brown adipose tissue: A study in women with anorexia nervosa, women recovered from anorexia nervosa, and normal-weight women. J. Clin. Endocrinol. Metab. 2012. [Google Scholar] [CrossRef] [PubMed]
- Misra, M.; Miller, K.K.; Herzog, D.B.; Ramaswamy, K.; Aggarwal, A.; Almazan, C.; Neubauer, G.; Breu, J.; Klibanski, A. Growth hormone and ghrelin responses to an oral glucose load in adolescent girls with anorexia nervosa and controls. J. Clin. Endocrinol. Metab. 2004. [Google Scholar] [CrossRef] [PubMed]
- Støving, R.K.; Veldhuis, J.D.; Flyvbjerg, A.; Vinten, J.; Hangaard, J.; Koldkjær, O.G.; Kristiansen, J.; Hagen, C. Jointly amplified basal and pulsatile growth hormone (GH) secretion and increased process irregularity in women with anorexia nervosa: Indirect evidence for disruption of feedback regulation within the GH-insulin-like growth factor I axis. J. Clin. Endocrinol. Metab. 1999. [Google Scholar] [CrossRef]
- Støving, R.K.; Chen, J.W.; Glintborg, D.; Brixen, K.; Flyvbjerg, A.; Hørder, K.; Frystyk, J. Bioactive Insulin-like Growth Factor (IGF) I and IGF-binding protein-1 in anorexia nervosa. J. Clin. Endocrinol. Metab. 2007. [Google Scholar] [CrossRef] [PubMed]
- Counts, D.R.; Gwirtsman, H.; Carlsson, L.M.S.; Lesem, M. The effect of anorexia nervosa and refeeding on growth hormone-binding protein, the insulin-like growth factors (IGFs), and the IGF-binding proteins. J. Clin. Endocrinol. Metab. 1992. [Google Scholar] [CrossRef]
- Inagaki, T.; Lin, V.Y.; Goetz, R.; Mohammadi, M.; Mangelsdorf, D.J.; Kliewer, S.A. Inhibition of Growth Hormone Signaling by the Fasting-Induced Hormone FGF21. Cell Metab. 2008. [Google Scholar] [CrossRef]
- Fazeli, P.K.; Misra, M.; Goldstein, M.; Miller, K.K.; Klibanski, A. Fibroblast growth factor-21 may mediate growth hormone resistance in anorexia nervosa. J. Clin. Endocrinol. Metab. 2010. [Google Scholar] [CrossRef]
- Brambilla, F.; Santonastaso, P.; Caregaro, L.; Favaro, A. Growth hormone and insulin-like growth factor 1 secretions in eating disorders: Correlations with psychopathological aspects of the disorders. Psychiatry Res. 2018. [Google Scholar] [CrossRef]
- Grilo, C.M.; Sanislow, C.A.; Skodol, A.E.; Gunderson, J.G.; Stout, R.L.; Tracie Shea, M.; Zanarini, M.C.; Bender, D.S.; Morey, L.C.; Dyck, I.R.; et al. Do eating disorders co-occur with personality disorders? Comparison groups matter. Int. J. Eat. Disord. 2003. [Google Scholar] [CrossRef]
- Garner, D.M. Eating Disorder Inventory-3 (EDI-3): Professional Manual; Psychological Assessment Resources, Inc.: Lutz, FL, USA, 2004. [Google Scholar]
- Agüera, Z.; Paslakis, G.; Munguía, L.; Sánchez, I.; Granero, R.; Sánchez-González, J.; Steward, T.; Jiménez-Murcia, S.; Fernández-Aranda, F. Gender-Related Patterns of Emotion Regulation among Patients with Eating Disorders. J. Clin. Med. 2019, 8, 161. [Google Scholar] [CrossRef] [PubMed]
- Keski-Rahkonen, A.; Mustelin, L. Epidemiology of eating disorders in Europe: Prevalence, incidence, comorbidity, course, consequences, and risk factors. Curr. Opin. Psychiatry 2016, 29, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Holland, G.; Tiggemann, M. A systematic review of the impact of the use of social networking sites on body image and disordered eating outcomes. Body Image 2016, 17, 100–110. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, P.H.B.; dos Alvarenga, M.S.; Ferreira, M.E.C. An etiological model of disordered eating behaviors among Brazilian women. Appetite 2017. [Google Scholar] [CrossRef] [PubMed]
- Izydorczyk, B.; Khanh, H.T.T.; Lizińczyk, S.; Sitnik-Warchulska, K.; Lipowska, M.; Gulbicka, A. Body dissatisfaction, restrictive, and bulimic behaviours among young women: A Polish–Japanese comparison. Nutrients 2020, 12, 666. [Google Scholar] [CrossRef]
| Author | Opioid or Metabolite | Location | Observation |
|---|---|---|---|
| Kaye et al., 1982 [29] | Overall opioid act.(MOR) | CSF | Increased level |
| Gerner et al., 1982 [33] | β-endorphin | CSF | Normal level |
| Kaye et al., 1987b [34] | β-endorphin | CSF | Reduced level |
| Lesem et al., 1991 [31] | Dynorphin | CSF | Normal level |
| Brambilla et al., 1985 [35]; Melchior et al. 1990 [36]; Tepper et al., 1992 [37] | β-endorphin | Plasma | Increased level |
| Baranowska, 1990 [38] | β-endorphin | Plasma | Reduced level |
| Brambilla et al., 1991 [39] | β-endorphinβ-lipotropin | Plasma | Loss of circadian rhythm (increased level) * |
| Brambilla et al., 1995 [40] | β-endorphin | T-lymphocytes | Increased level |
| Marrazzi et al., 1997 [41] | Codeine | Plasma | Increased level |
| Metabolite | Concentration Shift |
|---|---|
| Neuropeptide Y, AgRP | Mixed reports |
| Insulin | Mixed reports |
| Peptide YY | Mixed reports * |
| Leptin [58] | Decrease |
| Adiponectin [58] | Increase |
| Nesfatin-1 | Decrease |
| Kisspeptin | No change |
| Phoenixin | Decrease |
| Ghrelin | Increase |
| Orexins | Increase |
| 26RFa | Increase |
| HPA axis (CRH, ACTH, cortisol) | Increase |
| Gonadal hormones (estrogen, testosterone) | Decrease |
| References | Number and Sex of Participants | AN Patients Characteristics | Exclusion Criteria | Microbiota Diversity | Microbial Metabolites |
|---|---|---|---|---|---|
| Armougom et al., 2009 [124] | AN patients (n = 9), normal weight (n = 20) and obese (n = 20) controls, all female | 19 to 36 years-old, BMI 12.73 ± 1.6 at enrollment, meeting the DSM IV-TR criteria | Use of probiotics prior to the study | Firmicutes, Bacteroidetes and Lactobacillus levels in AN patients were reported to be similar to normal weight controls. | - |
| Million et al., 2013 [125] | AN patients (n = 14 F +1 M), lean (n = 36 F + 40 M, overweight (n = 6 F + 32 M), obese (69 F + 65 M) controls | Age = 27.3 ± 10.8, BMI 13.5 at enrollment, meeting the DSM IV-TR criteria | A history of colon cancer, the presence of an inflammatory bowel disease, an acute or a chronic diarrhea 4 weeks and an antibiotic administration 6 months prior to the study | Firmicutes was found in almost all of the individuals (>98.5%), whereas Bacteroidetes was detected in 67%. Bacteroides animalis was the rarest of species (11%), and Methanobrevibacter smithii (64%) was more prevalent than E. coli (51%). A lower concentration of E. coli was found in obese vs. anorexic, lean and overweight participants, and a higher concentration of E. coli was associated with a lower BMI. | - |
| Kleiman et al., 2015 [126] | AN patients (n = 16; only 10 patients provided samples after weight restoration) and age-matched, healthy (n = 12) controls, all female | Age = 28.0 ± 11.7, BMI 16.2 ± 1.5 at enrollment, meeting the DSM IV-TR criteria, presented with less than 75% of ideal body weight | A history of gastrointestinal tract surgery (other than appendectomy or cholecystectomy), inflammatory bowel disease, irritable bowel syndrome, celiac disease or any other diagnosis that could explain chronic or recurring bowel symptoms; use of antibiotics, NSAID, steroids or probiotics 2 months prior to the study | Alpha diversity was lower in AN both before and after inpatient renourishment, however after hospital-based renourishment, intestinal microbiota diversity showed a trend toward a healthier state. Greater levels of depression were negatively correlated with the number of bacterial species. | - |
| Morita et al., 2015 [127] | ANR (n = 14) and ANBP (n = 11) patients and age-matched, healthy (n = 21) controls, all female | Age = 30.0 ± 10.2, BMI = 12.8 ± 1.3 at enrollment, meeting the DSM IV-TR criteria | Severe physical (renal failure) and infectious diseases and a history of antibiotics use or a regular intake of yoghurt or probiotics 3 months prior to the study | AN patients exhibited lower amounts of total bacteria—Clostridium coccoides group, Clostridium leptum subgroup, Bacteroides fragilis and Streptococcus in comparison to age-matched healthy women. | SCFA (acetate and propionate) levels were found to be reduced in AN patients in comparison to normal-weight participants. |
| Mack et al., 2016 [128] | AN patients (n = 55, only 44 provided samples after weight restoration), both ANR (n = 14) and ANBP (n = 11), and age-matched, healthy (n = 55) controls, all female | Age = 23.8 ± 6.8, BMI = 15.3 ± 1.4 at enrollment | A use of antibiotics 8 weeks prior to the study, or severe diseases including renal failure and liver dysfunction, or limited German verbal skills, or unable to understand the instructions and perform stool sampling | Alpha diversity was reduced in AN patients both before and after inpatient renourishment. | SCFA levels (excluding lowered butyrate levels) were comparable between AN patients and normal-weight participants, BCFA levels (especially valerate and isobutyrate) were increased in AN patients at the time of hospital admission and after weight gain. |
| Borgo et al., 2017 [129] | AN patients (n = 15) and age-matched, healthy (n = 15) controls, all female | Age not reported, BMI = 13.9 ± 2.1 at enrollment, meeting the DSM V-TR criteria | Use of antibiotics or probiotics a month prior to the study, celiac disease, irritable bowel syndrome, history of colorectal cancer, diabetes mellitus, binge eating or purging behavior, recent enteral/parenteral nutrition | An unbalanced Gram positive/Gram negative relative abundance as well as Bacteroidetes enrichment and Firmicutes depletion was characteristic for AN. | SCFA (in particular butyrate and propionate levels) levels were found to be reduced in AN patients in comparison to normal-weight participants. |
| Mörkl et al., 2017 [130] | AN patients, (n = 18) athletes (n = 20), normal weight (n = 26), overweight (n = 22) and obese(n = 20) controls, all female | Age = 22.44 ± 3.2, BMI = 15.29 ± 1.28 at enrollment, meeting ICD-10 criteria | Antibiotic or antifungal treatment 2 months prior to the study, daily or irregular intake of prebiotics or probiotics 2 months prior to the study (yoghurt and dairy products were permitted), acute or chronic diseases, or infections (including upper respiratory tract infections, fever, chronic inflammatory disorders, autoimmune disorders) 2 months prior to the study, alcohol- or drug abuse, major cognitive deficits, life-threatening conditions, history of digestive diseases, such as inflammatory bowel disease, and irritable bowel syndrome, history of gastrointestinal surgery (other than appendectomy), pregnancy, and period of breastfeeding | Microbial richness was reduced in obese woman and AN patients compared to athletes. Coriobacteriaceae was the only enriched phylotype in AN compared to other participants. Alpha-diversity was negatively correlated with depression scores. | - |
| Mörkl et al., 2018 [131] | AN patients (n = 17, including six with ANR type) and normal weight athletes (n = 20), normal weight (n = 25), overweight (n = 21) and obese(n = 19) controls, all female | Age = 21.79 ± 3.62, BMI = 15.22 ± 1.27 at enrollment, meeting ICD-10 criteria | Antibiotic or antifungal treatment 2 months prior to the study, daily or irregular intake of prebiotics or probiotics two months 2 months prior to the study (yoghurt and dairy products were permitted), regular intake of medication (except for AN patients), acute or chronic diseases or infections (including upper respiratory tract infections, fever, chronic inflammatory disorders, autoimmune disorders) 2 months prior to the study, alcohol- or drug abuse, major cognitive deficits, life-threatening conditions, history of digestive diseases, such as inflammatory bowel disease, and irritable bowel syndrome, history of gastrointestinal surgery (other than appendectomy), pregnancy, and period of breastfeeding | Zonulin levels were comparable when participants were divided according to their BMI. No difference on phylum level of gut microbiota between the high and low-zonulin group was reported. Ruminococcaceae and Faecalibacterium were more abundant in the low-zonulin. Increased levels of inflammatory markers (CRP and IL-6) were reported in the high-zonulin group. | - |
| Hanachi et al., 2019 [132] | ANR (n = 22) and ANBP (n = 11) patients and age-matched, healthy (n = 22) controls, all female | Age = 32 ± 12, BMI = 11.7 ± 1.5 at enrollment; meeting the DSM IV-TR criteria | Use of antibiotics 2 months prior hospitalization, diabetes, digestive pathology, metabolic disease, a history of obesity, inflammatory and/or autoimmune disease before the onset of AN | AN patients showed a reduced alpha-diversity compared to controls. The severity of malnutrition was negatively correlated with the Verrucomicrobiaceae and Ruminococcacea families and positively with the Clostridiales order, Turicibacteraceae and Eubacteriaceae families. | - |
| Hata et al., 2019 [122] | ANR patients (n = 10) and age-matched, healthy (n= 10) controls, all female | Age = 23.0 ± 3.4, BMI = 13.7 ± 0.1 at enrollment; meeting the DSM IV-TR criteria | A history of digestive diseases such as inflammatory bowel disease and irritable bowel syndrome and severe conditions (renal failure) and infectious diseases, and/or a history of antibiotic use or regular intake of yogurt or probiotics 3 months prior to the study | A lower relative abundance of Bacteroidetes was observed in AN in comparison to age-matched healthy women. | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skowron, K.; Kurnik-Łucka, M.; Dadański, E.; Bętkowska-Korpała, B.; Gil, K. Backstage of Eating Disorder—About the Biological Mechanisms behind the Symptoms of Anorexia Nervosa. Nutrients 2020, 12, 2604. https://doi.org/10.3390/nu12092604
Skowron K, Kurnik-Łucka M, Dadański E, Bętkowska-Korpała B, Gil K. Backstage of Eating Disorder—About the Biological Mechanisms behind the Symptoms of Anorexia Nervosa. Nutrients. 2020; 12(9):2604. https://doi.org/10.3390/nu12092604
Chicago/Turabian StyleSkowron, Kamil, Magdalena Kurnik-Łucka, Emil Dadański, Barbara Bętkowska-Korpała, and Krzysztof Gil. 2020. "Backstage of Eating Disorder—About the Biological Mechanisms behind the Symptoms of Anorexia Nervosa" Nutrients 12, no. 9: 2604. https://doi.org/10.3390/nu12092604
APA StyleSkowron, K., Kurnik-Łucka, M., Dadański, E., Bętkowska-Korpała, B., & Gil, K. (2020). Backstage of Eating Disorder—About the Biological Mechanisms behind the Symptoms of Anorexia Nervosa. Nutrients, 12(9), 2604. https://doi.org/10.3390/nu12092604





