Modification of In Vitro and In Vivo Antioxidant Activity by Consumption of Cooked Chickpea in a Colon Cancer Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Chickpea (Cicer arietinum L.) Seed
2.2. Characterization of Nutritional and Non-Nutritional Compounds of Chickpea Seed
2.2.1. Nutritional Composition
2.2.2. Non-Nutritional Composition
2.2.3. In Vitro Antioxidant Properties
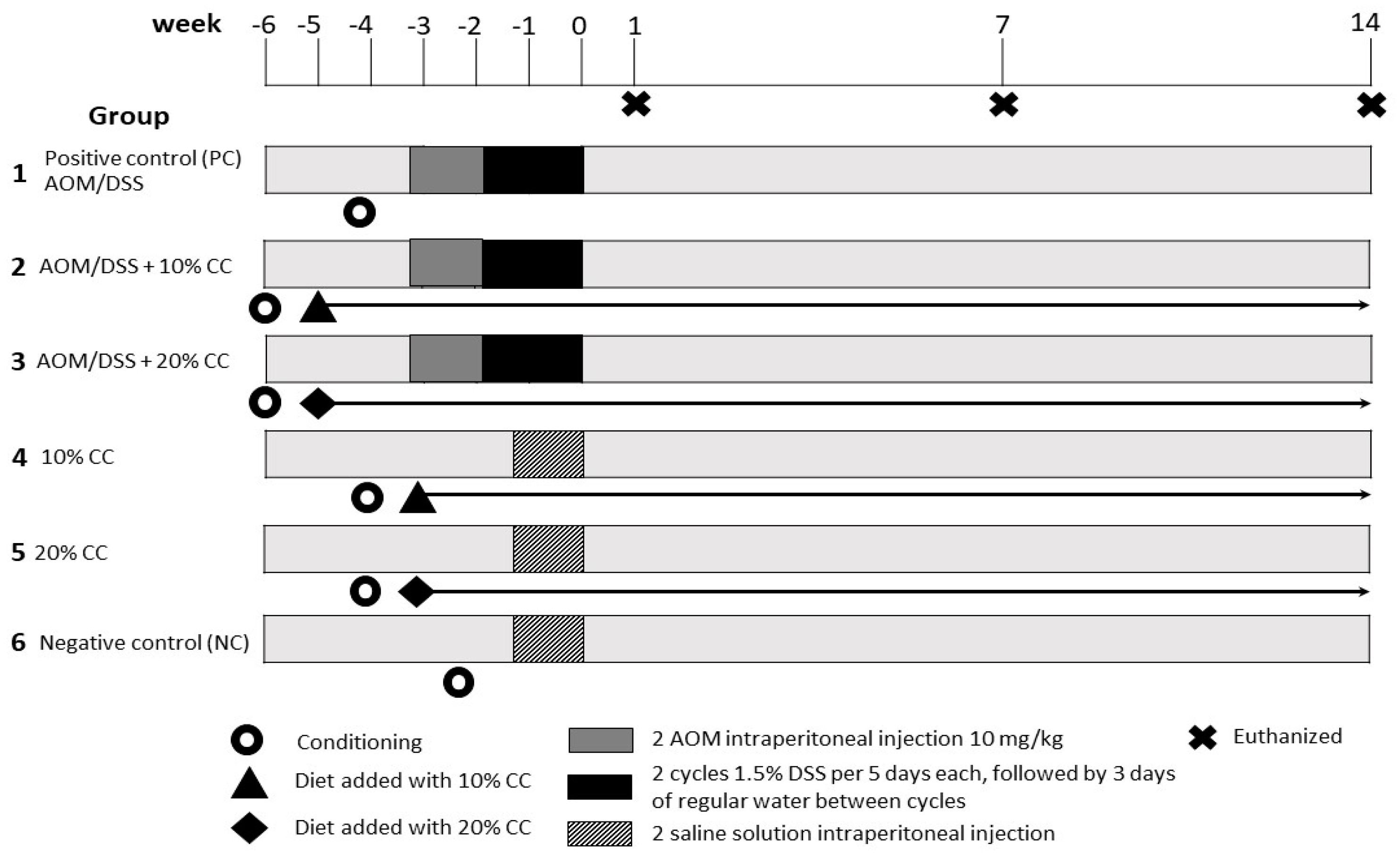
2.3. In Vivo Antioxidant Activity
2.3.1. Ethical Approval
2.3.2. Animals
2.3.3. Cooked Chickpea Diets
2.3.4. Colon Cancer Induction
2.3.5. Experimental Protocol
2.3.6. Determination of Nitric Oxide
2.3.7. Determination of Oxidized Proteins
2.3.8. Determination of Lipid Peroxidation
2.3.9. Immunohistochemistry of 4-HNE
2.4. Statistical Analyses
3. Results
3.1. Nutritional and Non-Nutritional Composition of RC and CC
3.2. In Vitro Antioxidant Properties of RC and CC
3.3. In Vivo Antioxidant Activity of CC
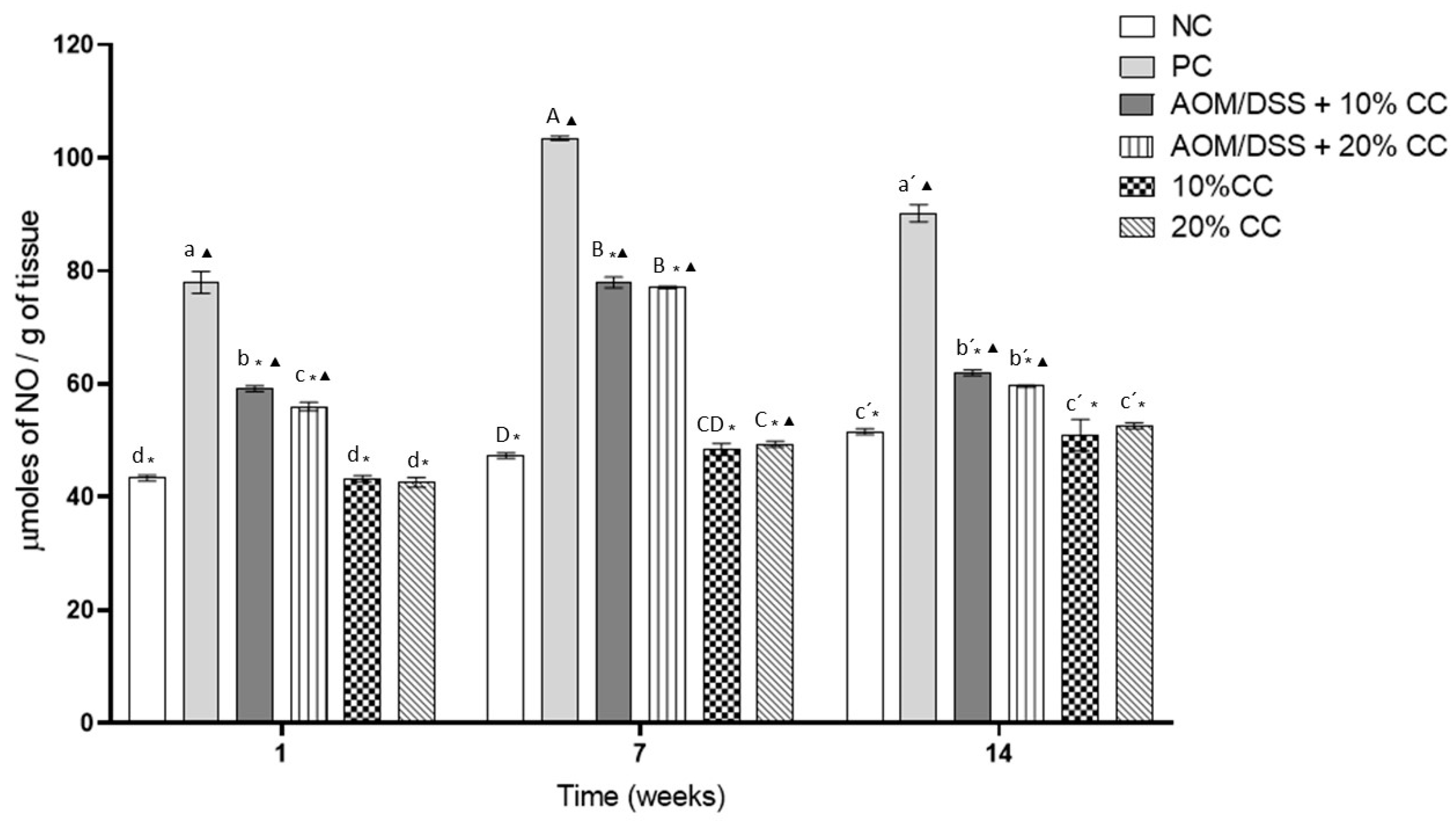
3.3.1. Nitric Oxide (NO) Concentration in Colon Homogenates
3.3.2. Quantification Oxidized Carbonyl Groups (CO) from Proteins in Colon Homogenates
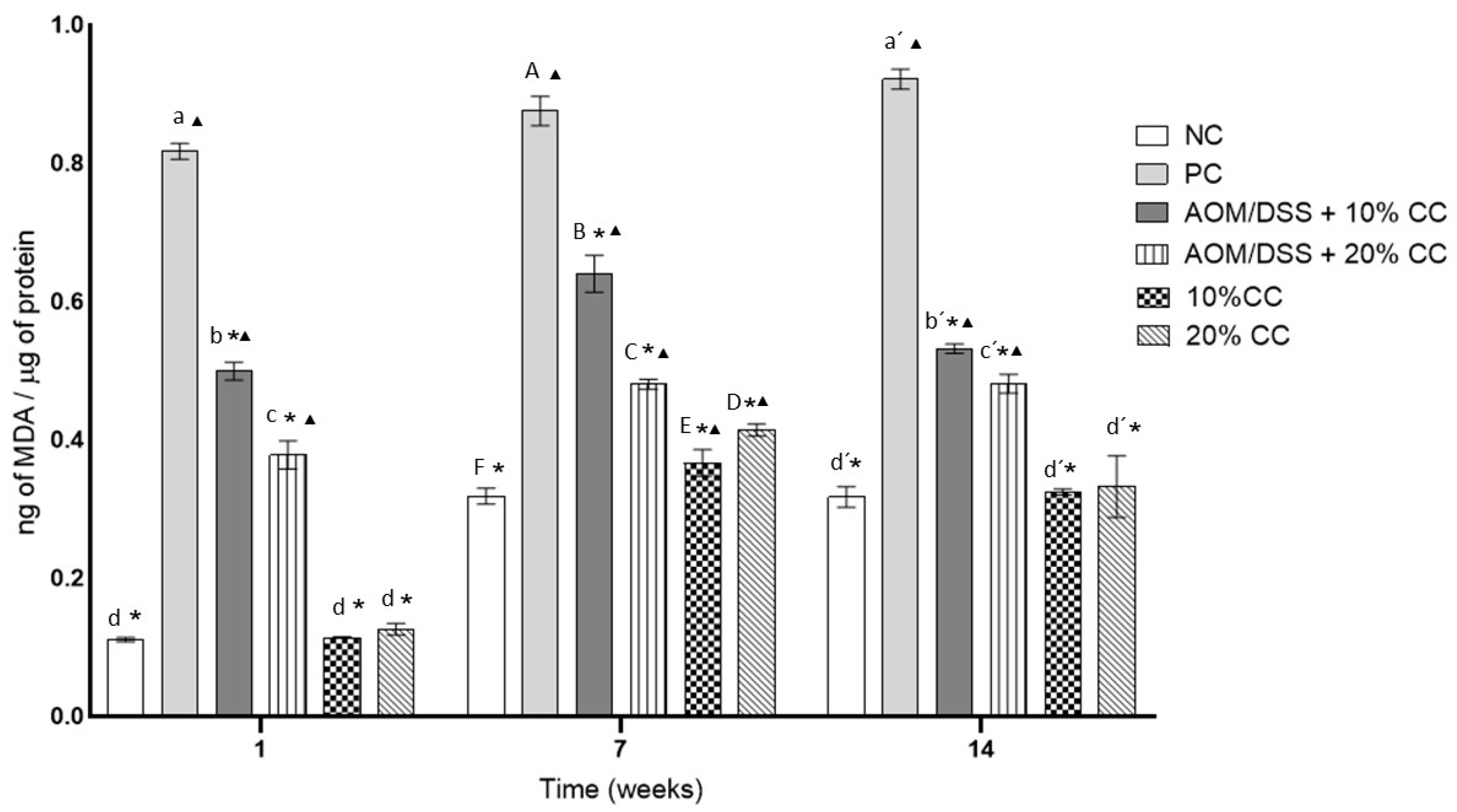
3.3.3. Concentration of MDA from Oxidized Lipids in Colon Homogenates
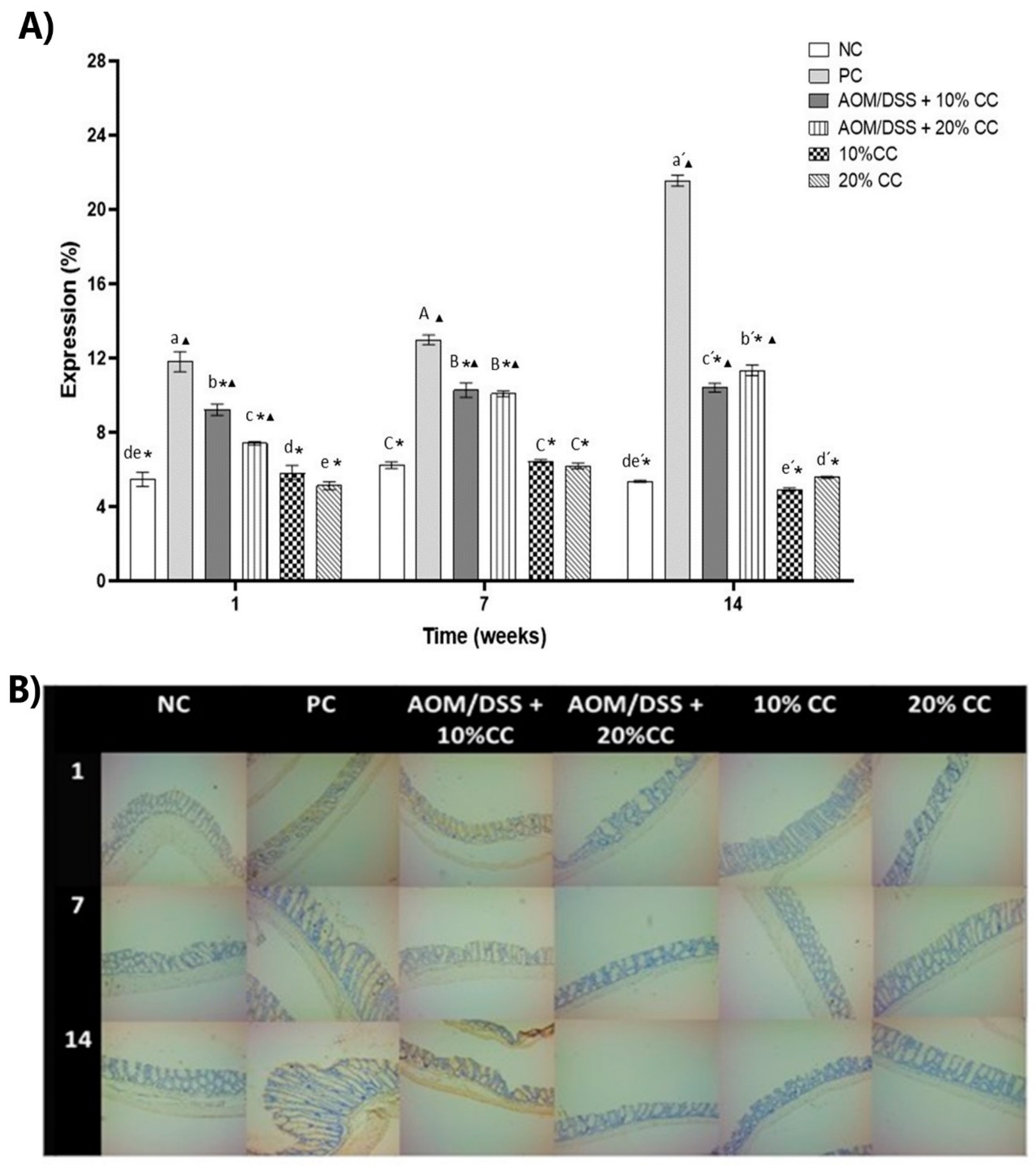
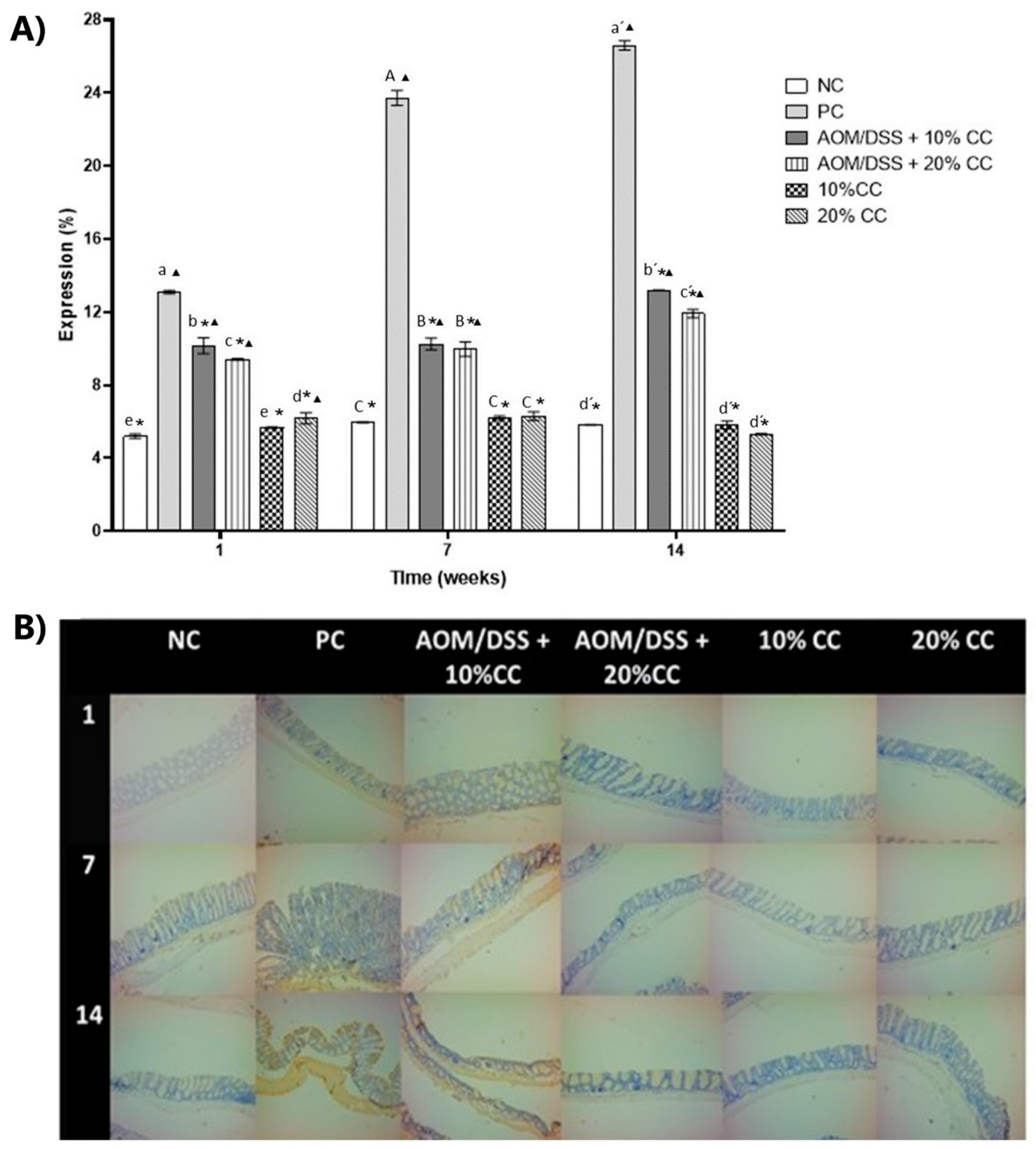
3.3.4. Expression of 4-HNE on Colon
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Real Hernandez, L.M.; Gonzalez de Mejia, E. Enzymatic Production, Bioactivity, and Bitterness of Chickpea (Cicer arietinum) Peptides. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1913–1946. [Google Scholar] [CrossRef]
- Fabbri, A.D.T.; Crosby, G.A. A review of the impact of preparation and cooking on the nutritional quality of vegetables and legumes. Int. J. Gastron. Food Sci. 2016, 3, 2–11. [Google Scholar] [CrossRef]
- Avola, G.; Patanè, C.; Barbagallo, R.N. Effect of water cooking on proximate composition of grain in three Sicilian chickpeas (Cicer arietinum L.). LWT Food Sci. Technol. 2012, 49, 217–220. [Google Scholar] [CrossRef]
- Margier, M.; Georgé, S.; Hafnaoui, N.; Remond, D.; Nowicki, M.; Du Chaffaut, L.; Amiot, M.-J.; Reboul, E. Nutritional Composition and Bioactive Content of Legumes: Characterization of Pulses Frequently Consumed in France and Effect of the Cooking Method. Nutrients 2018, 10, 1668. [Google Scholar] [CrossRef] [PubMed]
- Frias, J.; Vidal-Valverde, C.; Sotomayor, C.; Diaz-Pollan, C.; Urbano, G. Influence of processing on available carbohydrate content and antinutritional factors of chickpeas. Eur. Food Res. Technol. 2000, 210, 340–345. [Google Scholar] [CrossRef]
- Olika, E.; Abera, S.; Fikre, A. Physicochemical Properties and Effect of Processing Methods on Mineral Composition and Antinutritional Factors of Improved Chickpea (Cicer arietinum L.) Varieties Grown in Ethiopia. Int. J. Food Sci. 2019, 7. [Google Scholar] [CrossRef]
- Bulbula, D.D.; Urga, K. Study on the effect of traditional processing methods on nutritional composition and anti nutritional factors in chickpea (Cicer arietinum). Cogent. Food Agric. 2018, 4, 1422370. [Google Scholar] [CrossRef]
- Giusti, F.; Capuano, E.; Sagratini, G.; Pellegrini, N. A comprehensive investigation of the behaviour of phenolic compounds in legumes during domestic cooking and in vitro digestion. Food Chem. 2019, 285, 458–467. [Google Scholar] [CrossRef]
- Muzquiz, M.; Varela, A.; Burbano, C.; Cuadrado, C.; Guillamón, E.; Pedrosa, M.M. Bioactive compounds in legumes: Pronutritive and antinutritive actions. Implications for nutrition and health. Phytochem. Rev. 2012, 11, 227–244. [Google Scholar] [CrossRef]
- Gupta, R.K.; Gupta, K.; Sharma, A.; Das, M.; Ansari, I.A.; Dwivedi, P.D. Health risks and benefits of chickpea (Cicer arietinum) consumption. J. Agric. Food Chem. 2016, 65, 6–22. [Google Scholar] [CrossRef]
- Juárez-Chairez, M.F.; Cid-Gallegos, M.S.; Meza-Márquez, O.G.; Jiménez-Martínez, C. Biological Activities of Chickpea in Human Health (Cicer arietinum L.). A Review. Plant. Food Hum. Nutr. 2020, 75, 142–153. [Google Scholar] [CrossRef]
- Kim, S.J.; De Souza, R.J.; Choo, V.L.; Ha, V.; Cozma, A.I.; Chiavaroli, L.; Mirrahimi, A.; Blanco Mejia, S.; Di Buono, M.; Bernstein, A.M. Effects of dietary pulse consumption on body weight: A systematic review and meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2016, 103, 1213–1223. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Chino, X.M.; Jiménez-Martínez, C.; Vásquez-Garzón, V.R.; Álvarez-González, I.; Villa-Treviño, S.; Madrigal-Bujaidar, E.; Dávila-Ortiz, G.; Baltiérrez-Hoyos, R. Cooked chickpea consumption inhibits colon carcinogenesis in mice induced with azoxymethane and dextran sulfate sodium. J. Am. Coll. Nutr. 2017, 36, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Chino, X.M.; Jiménez Martínez, C.; León-Espinosa, E.B.; Garduño-Siciliano, L.; Álvarez-González, I.; Madrigal-Bujaidar, E.; Vásquez-Garzón, V.R.; Baltiérrez-Hoyos, R.; Dávila-Ortiz, G. Protective effect of chickpea protein hydrolysates on colon carcinogenesis associated with a hypercaloric diet. J. Am. Coll. Nutr. 2018, 38, 162–170. [Google Scholar] [CrossRef]
- Bhagyawant, S.S.; Narvekar, D.T.; Gupta, N.; Bhadkaria, A.; Gautam, A.K.; Srivastava, N. Chickpea (Cicer arietinum L.) Lectin Exhibit Inhibition of ACE-I, α-amylase and α-glucosidase Activity. Protein Pept. Lett. 2019, 26, 494–501. [Google Scholar] [CrossRef]
- Bruno, J.A.; Feldman, C.H.; Konas, D.W.; Kerrihard, A.L.; Matthews, E.L. Incorporating sprouted chickpea flour in pasta increases brachial artery flow-mediated dilation. Physiol. Int. 2019, 106, 207–212. [Google Scholar] [CrossRef]
- Murillo, G.; Choi, U.K.; Pan, O.; Constantinou, A.I.; Mehta, R.G. Efficacy of garbanzo and soybean flour in suppression of aberrant crypt foci in the colons of CF-1 mice. Anticancer Res. 2004, 24, 3049–3056. [Google Scholar]
- Macarulla, M.T.; Medina, C.; De Diego, M.A.; Chavarri, M.; Zulet, M.Á.; Martínez, J.A.; Nöel-Suberville, C.; Higueret, P.; Portillo, M.P. Effects of the whole seed and a protein isolate of faba bean (Vicia faba) on the cholesterol metabolism of hypercholesterolaemic rats. Br. J. Nutr. 2001, 85, 607–614. [Google Scholar] [CrossRef]
- Hutchins, A.M.; Winham, D.M.; Thompson, S.V. Phaseolus beans: Impact on glycaemic response and chronic disease risk in human subjects. Br. J. Nutr. 2012, 108, S52–S65. [Google Scholar] [CrossRef]
- Ďuračková, Z. Some current insights into oxidative stress. Physiol. Res. 2010, 59, 459–469. [Google Scholar]
- Gill, J.G.; Piskounova, E.; Morrison, S.J. Cancer, oxidative stress, and metastasis. Cold Spring Harb. Perspect. Med. 2016, 81, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Klaunig, J.E. Oxidative stress and cancer. Curr. Pharm. Des. 2018, 24, 4771–4778. [Google Scholar] [CrossRef] [PubMed]
- Klaunig, J.E.; Wang, Z. Oxidative stress in carcinogenesis. Curr. Opin. Toxicol. 2018, 7, 116–121. [Google Scholar] [CrossRef]
- Itzkowitz, S.H.; Yio, X. Inflammation and cancer IV. Colorectal cancer in inflammatory bowel disease: The role of inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 287, G7–G17. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Zhang, H. The role of proinflammatory pathways in the pathogenesis of colitis-associated colorectal cancer. Mediat. Inflamm. 2017, 28. [Google Scholar] [CrossRef]
- Glasauer, A.; Chandel, N.S. Targeting antioxidants for cancer therapy. Biochem. Pharmacol. 2014, 92, 90–101. [Google Scholar] [CrossRef]
- Lima, H.R.S.; da Silva, J.S.; de Oliveira Farias, E.A.; Teixeira, P.R.S.; Eiras, C.; Nunes, L.C.C. Electrochemical sensors and biosensors for the analysis of antineoplastic drugs. Biosens. Bioelectron. 2018, 108, 27–37. [Google Scholar] [CrossRef]
- Fernando, W.; Rupasinghe, H.P.V.; Hoskin, D.W. Dietary phytochemicals with anti-oxidant and pro-oxidant activities: A double-edged sword in relation to adjuvant chemotherapy and radiotherapy? Cancer Lett. 2019, 452, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Phan, M.A.T.; Paterson, J.; Bucknall, M.; Arcot, J. Interactions between phytochemicals from fruits and vegetables: Effects on bioactivities and bioavailability. Crit. Rev. Food Sci. Nutr. 2018, 58, 1310–1329. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Leo, E.E.; Altamirano, T.V.; Segura-Campos, M.R. Chapter 14—Functional Foods and Chemoprevention in Cancer. In Therapeutic Foods, 1st ed.; Holban, A.M., Grumezescu, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2018; Volume 8, pp. 431–448. [Google Scholar]
- Faris, M.A.-I.E.; Takruri, H.R.; Shomaf, M.S.; Bustanji, Y.K. Chemopreventive effect of raw and cooked lentils (Lens culinaris L.) and soybeans (Glycine max) against azoxymethane-induced aberrant crypt foci. Nutr. Res. 2009, 29, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Monk, J.M.; Lu, J.T.; Zarepoor, L.; Wu, W.; Liu, R.; Pauls, K.P.; Wood, G.A.; Robinson, L.; Tsao, R. Cooked navy and black bean diets improve biomarkers of colon health and reduce inflammation during colitis. Br. J. Nutr. 2014, 111, 1549–1563. [Google Scholar] [CrossRef] [PubMed]
- Hamer, H.M.; Jonkers, D.; Venema, K.; Vanhoutvin, S.; Troost, F.J.; Brummer, R.J. The role of butyrate on colonic function. Aliment. Pharm. Ther. 2008, 27, 104–119. [Google Scholar] [CrossRef] [PubMed]
- Leonel, A.J.; Alvarez-Leite, J.I. Butyrate: Implications for intestinal function. Curr. Opin. Clin. Nutr. 2012, 15, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Campos-Vega, R.; Oomah, B.D.; Loarca-Piña, G.; Vergara-Castañeda, H.A. Common Beans and Their Non-Digestible Fraction: Cancer Inhibitory Activity—An Overview. Foods 2013, 2, 374–392. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis of AOAC (2019), 21st ed.; Horwitz, W., Latimer, G.W., Eds.; International Gaithersburg: Gaithersburg, MD, USA, 2019. [Google Scholar]
- Luo, J.; Cai, W.; Wu, T.; Xu, B. Phytochemical distribution in hull and cotyledon of adzuki bean (Vigna angularis L.) and mung bean (Vigna radiate L.), and their contribution to antioxidant, anti-inflammatory and anti-diabetic activities. Food Chem. 2016, 201, 350–360. [Google Scholar] [CrossRef]
- Corzo-Ríos, L.J.; Sánchez-Chino, X.M.; Cardador-Martínez, A.; Martínez-Herrera, J.; Jiménez-Martínez, C. Effect of cooking on nutritional and non-nutritional compounds in two species of Phaseolus (P. vulgaris and P. coccineus) cultivated in Mexico. Int. J. Gastron. Food Sci. 2020, 20, 100206. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, J.; Shen, J.; Silva, A.; Dennis, D.A.; Barrow, C.J. A simple 96-well microplate method for estimation of total polyphenol content in seaweeds. J. Appl. Phycol. 2006, 18, 445–450. [Google Scholar] [CrossRef]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef]
- Held, P. Performing Oxygen Radical Absorbance Capacity Assays with Synergy HT. ORAC Antioxidant Tests. Biotek Application Note. Available online: https://www.biotek.com/resources/docs/ORAC_Assay_Application_Note.pdf (accessed on 7 August 2020).
- de Avellar, I.G.J.; Magalhães, M.M.M.; Silva, A.B.; Souza, L.L.; Leitão, A.C.; Hermes-Lima, M. Reevaluating the role of 1,10-phenanthroline in oxidative reactions involving ferrous ions and DNA damage. BBA GEN Subj. 2004, 1675, 46–53. [Google Scholar] [CrossRef]
- Pavithra, K.; Vadivukkarasi, S. Evaluation of free radical scavenging activity of various extracts of leaves from Kedrostis foetidissima (Jacq.) Cogn. Food Sci. Hum. Wellness 2015, 4, 42–46. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, B.; Zhang, T.; Mu, W.; Liu, J. Antioxidant and free radical-scavenging activities of chickpea protein hydrolysate (CPH). Food Chem. 2008, 106, 444–450. [Google Scholar] [CrossRef]
- Monk, J.M.; Lepp, D.; Wu, W.; Graf, D.; McGillis, L.H.; Hussain, A.; Carey, C.; Robinson, L.E.; Liu, R.; Tsao, R.; et al. Chickpea-supplemented diet alters the gut microbiome and enhances gut barrier integrity in C57Bl/6 male mice. J. Funct. Foods 2017, 38, 663–674. [Google Scholar] [CrossRef]
- Tanaka, T. Development of an inflammation-associated colorectal cancer model and its application for research on carcinogenesis and chemoprevention. Int. J. Inflamm. 2012, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Camacho-Cantera, S. Genotoxic and Oxidative Effect of Duloxetine In Vivo—“Efecto Genotóxico y Oxidativo de la Duloxetina In Vivo”. Master’s Thesis, Instituto Politécnico Nacional, Ciudad de Mexico, Mexico, June 2017. [Google Scholar]
- Ouazib, M.; Garzon, R.; Zaidi, F.; Rosell, C.M. Germinated, toasted and cooked chickpea as ingredients for breadmaking. J. Food Sci. Technol. 2016, 53, 2664–2672. [Google Scholar] [CrossRef]
- Wang, N.; Hatcher, D.W.; Tyler, R.T.; Toews, R.; Gawalko, E.J. Effect of cooking on the composition of beans (Phaseolus vulgaris L.) and chickpeas (Cicer arietinum L.). Food Res. Int. 2010, 43, 589–594. [Google Scholar] [CrossRef]
- Byun, S.-Y.; Kim, D.-B.; Kim, E. Curcumin ameliorates the tumor-enhancing effects of a high-protein diet in an azoxymethane-induced mouse model of colon carcinogenesis. Nutr. Res. 2015, 35, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Elimrani, I.; Koenekoop, J.; Dionne, S.; Marcil, V.; Delvin, E.; Levy, E.; Seidman, E.G. Vitamin D reduces colitis-and inflammation-associated colorectal cancer in mice independent of NOD2. Nutr. Cancer 2017, 69, 276–288. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J.; Choi, Y.J.; Kim, N.; Nam, R.H.; Lee, S.; Lee, H.S.; Lee, H.-N.; Surh, Y.-J.; Lee, D.H. Acai berries inhibit colon tumorigenesis in azoxymethane/dextran sulfate sodium-treated mice. Gut Liver 2017, 11, 243. [Google Scholar] [CrossRef]
- Drulyte, D.; Orlien, V. The effect of processing on digestion of legume proteins. Foods 2019, 8, 224. [Google Scholar] [CrossRef]
- Rehman, Z.; Shah, W.H. Thermal heat processing effects on antinutrients, protein and starch digestibility of food legumes. Food Chem. 2005, 91, 327–331. [Google Scholar] [CrossRef]
- Villa, C.; Moura, M.B.M.V.; Costa, J.; Mafra, I. Immunoreactivity of Lupine and Soybean Allergens in Foods as Affected by Thermal Processing. Foods 2020, 9, 254. [Google Scholar] [CrossRef] [PubMed]
- Carrasco-Castilla, J.; Hernández-Álvarez, A.J.; Jiménez-Martínez, C.; Jacinto-Hernández, C.; Alaiz, M.; Girón-Calle, J.; Vioque, J.; Dávila-Ortiz, G. Antioxidant and metal chelating activities of peptide fractions from phaseolin and bean protein hydrolysates. Food Chem. 2012, 135, 1789–1795. [Google Scholar] [CrossRef] [PubMed]
- Chinedum, E.; Sanni, S.; Theressa, N.; Ebere, A. Effect of domestic cooking on the starch digestibility, predicted glycemic indices, polyphenol contents and alpha amylase inhibitory properties of beans (Phaseolis vulgaris) and breadfruit (Treculia africana). Int. J. Biol. Macromol. 2018, 106, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Stea, T.H.; Johansson, M.; Jägerstad, M.; Frølich, W. Retention of folates in cooked, stored and reheated peas, broccoli and potatoes for use in modern large-scale service systems. Food Chem. 2007, 101, 1095–1107. [Google Scholar] [CrossRef]
- Carvalho, L.M.J.; Corrêa, M.M.; Pereira, E.J.; Nutti, M.R.; Carvalho, J.L.V.; Ribeiro, E.M.G.; Freitas, S.C. Iron and zinc retention in common beans (Phaseolus vulgaris L.) after home cooking. Food Nutr. Res. 2012, 56. [Google Scholar] [CrossRef]
- Ferreira, A.S.T.; Naozuka, J.; Kelmer, G.A.R.; Oliveira, P.V. Effects of the domestic cooking on elemental chemical composition of beans species (Phaseolus vulgaris L.). J. Food Process. 2014, 1–6. [Google Scholar] [CrossRef]
- Jukanti, A.K.; Gaur, P.M.; Gowda, C.L.L.; Chibbar, R.N. Nutritional quality and health benefits of chickpea (Cicer arietinum L.): A review. Br. J. Nutr. 2012, 108, S11–S26. [Google Scholar] [CrossRef]
- Shi, J.; Xue, S.J.; Ma, Y.; Li, D.; Kakuda, Y.; Lan, Y. Kinetic study of saponins B stability in navy beans under different processing conditions. J. Food Eng. 2009, 93, 59–65. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic composition and antioxidant potential of grain legume seeds: A review. Food Res. Int. 2017, 101, 1–16. [Google Scholar] [CrossRef]
- Udensi, E.A.; Ekwu, F.C.; Isinguzo, J.N. Antinutrient factors of vegetable cowpea (Sesquipedalis) seeds during thermal processing. Pak. J. Nutr. 2007, 6, 194–197. [Google Scholar]
- Urbano, G.; López-Jurado, M.; Aranda, P.; Vidal-Valverde, C.; Tenorio, E.; Porres, J. The role of phytic acid in legumes: Antinutrient or beneficial function? J. Physiol. Biochem. 2000, 56, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Chino, X.M.; Jiménez-Martínez, C.; Dávila-Ortiz, G.; Álvarez-González, I.; Madrigal-Bujaidar, E. Nutrient and nonnutrient components of legumes, and its chemopreventive activity: A review. Nutr. Cancer 2015, 67, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Sinha, A.K.; Makkar, H.P.S.; Becker, K. Dietary roles of phytate and phytase in human nutrition: A review. Food Chem. 2010, 120, 945–959. [Google Scholar] [CrossRef]
- Chan, Y.S.; Zhang, Y.; Ng, T.B. Brown Kidney Bean Bowman—Birk Trypsin Inhibitor is Heat and pH Stable and Exhibits Anti-proliferative Activity. Appl. Biochem. Biotechnol. 2013, 169, 1306–1314. [Google Scholar] [CrossRef] [PubMed]
- Luna-Vital, D.A.; Mojica, L.; González de Mejía, E.; Mendoza, S.; Loarca-Piña, G. Biological potential of protein hydrolysates and peptides from common bean (Phaseolus vulgaris L.): A review. Food Res. Int. 2015, 76, 39–50. [Google Scholar] [CrossRef]
- Perron, N.R.; Brumaghim, J.L. A review of the antioxidant mechanisms of polyphenol compounds related to iron binding. Cell Biochem. Biophys. 2009, 53, 75–100. [Google Scholar] [CrossRef]
- Karam, J.; Bibiloni, M.; Tur, J.A. Polyphenol estimated intake and dietary sources among older adults from Mallorca Island. PLoS ONE 2018, 13, e0191573. [Google Scholar] [CrossRef]
- Wang, S.; Moustaid-Moussa, N.; Chen, L.; Mo, H.; Shastri, A.; Su, R.; Bapat, P.; Kwun, I.; Shen, C.-L. Novel insights of dietary polyphenols and obesity. J. Nutr. Biochem. 2014, 25, 1–18. [Google Scholar] [CrossRef]
- Megías, C.; Pedroche, J.; Yust, M.M.; Girón-Calle, J.; Alaiz, M.; Millán, F.; Vioque, J. Affinity Purification of Copper Chelating Peptides from Chickpea Protein Hydrolysates. J. Agric. Food Chem. 2007, 55, 3949–3954. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, T.; Jiang, B.; Miao, M.; Mu, W. The effects of an antioxidative pentapeptide derived from chickpea protein hydrolysates on oxidative stress in Caco-2 and HT-29 cell lines. J. Funct. Foods 2014, 7, 719–726. [Google Scholar] [CrossRef]
- Fratianni, F.; Cardinale, F.; Cozzolino, A.; Granese, T.; Albanese, D.; Di Matteo, M.; Zaccardelli, M.; Coppola, R.; Nazzaro, F. Polyphenol composition and antioxidant activity of different grass pea (Lathyrus sativus), lentils (Lens culinaris), and chickpea (Cicer arietinum) ecotypes of the Campania region (Southern Italy). J. Funct. Foods 2014, 7, 551–557. [Google Scholar] [CrossRef]
- Xiao, Y.; Xing, G.; Rui, X.; Li, W.; Chen, X.; Jiang, M.; Dong, M. Enhancement of the antioxidant capacity of chickpeas by solid state fermentation with Cordyceps militaris SN-18. J. Funct. Foods 2014, 10, 210–222. [Google Scholar] [CrossRef]
- Mecha, E.; Leitão, S.T.; Carbas, B.; Serra, A.T.; Moreira, P.M.; Veloso, M.M.; Gomes, R.; Figueira, M.E.; Brites, C.; Vaz Patto, M.C.; et al. Characterization of Soaking Process’ Impact in Common Beans Phenolic Composition: Contribute from the Unexplored Portuguese Germplasm. Foods 2019, 8, 296. [Google Scholar] [CrossRef] [PubMed]
- Ombra, M.N.; d’Acierno, A.; Nazzaro, F.; Riccardi, R.; Spigno, P.; Zaccardelli, M.; Pane, C.; Maione, M.; Fratianni, F. Phenolic composition and antioxidant and antiproliferative activities of the extracts of twelve common bean (Phaseolus vulgaris L.) endemic ecotypes of southern Italy before and after cooking. Oxid. Med. Cell Longev. 2016, 1398298. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Chang, S.K.C. Effect of soaking, boiling, and steaming on total phenolic contentand antioxidant activities of cool season food legumes. Food Chem. 2008, 110, 1–13. [Google Scholar] [CrossRef]
- Siah, S.; Konczak, I.; Wood, J.A.; Agboola, S.; Blanchard, C.L. Effects of roasting on phenolic composition and in vitro antioxidant capacity of Australian grown faba beans (Vicia faba L.). Plant. Foods Hum. Nutr. 2014, 69, 85–91. [Google Scholar] [CrossRef]
- Siah, S.; Wood, J.A.; Agboola, S.; Konczak, I.; Blanchard, C.L. Effects of soaking, boiling and autoclaving on the phenolic contents and antioxidant activities of faba beans (Vicia faba L.) differing in seed coat colours. Food Chem. 2014, 142, 461–468. [Google Scholar] [CrossRef]
- Summo, C.; De Angelis, D.; Rochette, I.; Mouquet-Rivier, C.; Pasqualone, A. Influence of the preparation process on the chemical composition and nutritional value of canned purée of kabuli and Apulian black chickpeas. Heliyon 2019, 5, e01361. [Google Scholar] [CrossRef]
- Kapral, M.; Wawszczyk, J.; Jesse, K.; Paul-Samojedny, M.; Kuśmierz, D.; Węglarz, L. Inositol Hexaphosphate Inhibits Proliferation and Induces Apoptosis of Colon Cancer Cells by Suppressing the AKT/mTOR Signaling Pathway. Molecules 2017, 22, 1657. [Google Scholar] [CrossRef]
- Torres-Fuentes, C.; Contreras, M.M.; Recio, I.; Alaiz, M.; Vioque, J. Identification and characterization of antioxidant peptides from chickpea protein hydrolysates. Food Chem. 2015, 180, 194–202. [Google Scholar] [CrossRef]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.T.; Yang, C.C.; Shyur, L.F. Phytomedicine—Modulating oxidative stress and the tumor microenvironment for cancer therapy. Pharmacol. Res. 2016, 114, 128–143. [Google Scholar] [CrossRef] [PubMed]
- Scibior-Bentkowska, D.; Czeczot, H. Cancer cells and oxidative stress. Postep. Hig. Med. Dosw. 2009, 63, 58. [Google Scholar]
- Zharkov, D.O. Mechanisms of Genome Protection and Repair. In DNA Repair and Mutagenesis in Vertebrate Mitochondria: Evidence for Asymmetric DNA Strand Inheritance, 1st ed.; Matkarimov, B.T., Saparbaev, M.K., Eds.; Springer: Cham, Switzerland, 2020; Volume 1241, pp. 82–84. [Google Scholar]
- Song, C.-H.; Kim, N.; Lee, S.M.; Nam, R.H.; Choi, S.I.; Kang, S.R.; Shin, E.; Lee, D.H.; Lee, H.-N.; Surh, Y.-J. Effects of 17β-estradiol on colorectal cancer development after azoxymethane/dextran sulfate sodium treatment of ovariectomized mice. Biochem. Pharmacol. 2019, 164, 139–151. [Google Scholar] [CrossRef]
- Terasaki, M.; Iida, T.; Kikuchi, F.; Tamura, K.; Endo, T.; Kuramitsu, Y.; Tanaka, T.; Maeda, H.; Miyashita, K.; Mutoh, M. Fucoxanthin potentiates anoikis in colon mucosa and prevents carcinogenesis in AOM/DSS model mice. J. Nutr. Biochem. 2019, 64, 198–205. [Google Scholar] [CrossRef]
- Terasaki, M.; Kuramitsu, Y.; Kojoma, M.; Kim, S.-Y.; Tanaka, T.; Maeda, H.; Miyashita, K.; Kawagoe, C.; Kohno, S.; Mutoh, M. High fucoxanthin wakame (Undaria pinnatifida) prevents tumor microenvironment formation in an AOM/DSS mouse carcinogenic model. J. Funct. Foods 2020, 64, 103709. [Google Scholar] [CrossRef]
- Lamattina, L.; García-Mata, C.; Graziano, M.; Pagnussat, G. Nitric oxide: The versatility of an extensive signal molecule. Ann. Rev. Plant. Biol. 2003, 54, 109–136. [Google Scholar] [CrossRef]
- Durnin, L.; Lees, A.; Manzoor, S.; Sasse, K.C.; Sanders, K.M.; Mutafova-Yambolieva, V.N. Loss of nitric oxide-mediated inhibition of purine neurotransmitter release in the colon in the absence of interstitial cells of Cajal. Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 313, G419–G433. [Google Scholar] [CrossRef]
- Walker, M.Y.; Pratap, S.; Southerland, J.H.; Farmer-Dixon, C.M.; Lakshmyya, K.; Gangula, P.R. Role of oral and gut microbiome in nitric oxide-mediated colon motility. Nitric Oxide 2018, 73, 81–88. [Google Scholar] [CrossRef]
- Caria, C.R.P.; Moscato, C.H.; Tomé, R.B.G.; Pedrazzoli, J.; Ribeiro, M.; Gambero, A. Nitric oxide interferes with hypoxia signaling during colonic inflammation. Arq. Gastroenterol. 2014, 51, 302–308. [Google Scholar] [CrossRef][Green Version]
- Milán-Noris, A.K.; Gutiérrez-Uribe, J.A.; Santacruz, A.; Serna-Saldívar, S.O.; Martínez-Villaluenga, C. Peptides and isoflavones in gastrointestinal digests contribute to the anti-inflammatory potential of cooked or germinated desi and kabuli chickpea (Cicer arietinum L.). Food Chem. 2018, 268, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Masroor, D.; Baig, S.G.; Ahmed, S.; Ahmad, S.M.; Hasan, M.M. Analgesic, anti-inflammatory and diuretic activities of Cicer arietinum L. Pak. J. Pharm. Sci. 2018, 31, 553–558. [Google Scholar] [PubMed]
- Liu, R.H. Potential synergy of phytochemicals in cancer prevention: Mechanism of action. J. Nutr. 2004, 134, 3479s–3485s. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Yao, Y.; Shi, Z.; Everaert, N.; Ren, G. Synergistic Effect of Bioactive Anticarcinogens from Soybean on Anti-Proliferative Activity in MDA-MB-231 and MCF-7 Human Breast Cancer Cells In Vitro. Molecules 2018, 23, 1557. [Google Scholar] [CrossRef] [PubMed]
- Bouchenak, M.; Lamri-Senhadji, M. Nutritional quality of legumes, and their role in cardiometabolic risk prevention: A review. J. Med. Food 2013, 16, 185–198. [Google Scholar] [CrossRef]
- Hecker, M.; Wagner, A.H. Role of protein carbonylation in diabetes. J. Inherit. Metab. Dis. 2018, 41, 29–38. [Google Scholar] [CrossRef]
- Díaz-Acosta, A.E.; Membrillo-Hernández, J. Consecuencias fisiológicas de la oxidación de proteínas por carbonilación en diversos sistemas biológicos. TIP 2006, 9, 34–44. Available online: http://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S1405-888X2006000100034&lng=en&nrm=iso> (accessed on 7 July 2020).
- Luna-Vital, D.A.; González-de Mejía, E.; Loarca-Piña, G. Dietary peptides from Phaseolus vulgaris L. reduced AOM/DSS-induced colitis-associated colon carcinogenesis in Balb/c mice. Plant. Food Hum. Nutr. 2017, 72, 445–447. [Google Scholar] [CrossRef]
- Guardado-Félix, D.; Antunes-Ricardo, M.; Rocha-Pizaña, M.R.; Martínez-Torres, A.-C.; Gutiérrez-Uribe, J.A.; Serna Saldivar, S.O. Chickpea (Cicer arietinum L.) sprouts containing supranutritional levels of selenium decrease tumor growth of colon cancer cells xenografted in immune-suppressed mice. J. Funct. Foods 2019, 53, 76–84. [Google Scholar] [CrossRef]
- Zhou, Y.-H.; Yu, J.-P.; Liu, Y.-F.; Teng, X.-J.; Ming, M.; Lv, P.; An, P.; Liu, S.-Q.; Yu, H.-G. Effects of Ginkgo biloba extract on inflammatory mediators (SOD, MDA, TNF-α, NF-κBp65, IL-6) in TNBS-induced colitis in rats. Mediat. Inflamm. 2006, 92642. [Google Scholar] [CrossRef]
- Guéraud, F.; Taché, S.; Steghens, J.-P.; Milkovic, L.; Borovic-Sunjic, S.; Zarkovic, N.; Gaultier, E.; Naud, N.; Héliès-Toussaint, C.; Pierre, F.; et al. Dietary polyunsaturated fatty acids and heme iron induce oxidative stress biomarkers and a cancer promoting environment in the colon of rats. Free Radic. Biol. Med. 2015, 83, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Rehman, M.U.; Khan, R.; Lateef, M.A.; Ali, F. Amelioration of 1, 2 Dimethylhydrazine (DMH) Induced Colon Oxidative Stress, Inflammation and Tumor Promotion. Asian Pac. J. Cancer Prev. 2012, 13, 4393. [Google Scholar] [CrossRef]
- Larrosa, M.; Luceri, C.; Vivoli, E.; Pagliuca, C.; Lodovici, M.; Moneti, G.; Dolara, P. Polyphenol metabolites from colonic microbiota exert anti-inflammatory activity on different inflammation models. Mol. Nutr. Food Res. 2009, 53, 1044–1054. [Google Scholar] [CrossRef] [PubMed]
- Guan, F.; Li, G.; Liu, A.B.; Lee, M.-J.; Yang, Z.; Chen, Y.-K.; Lin, Y.; Shih, W.; Yang, C.S. δ-and γ-tocopherols, but not α-tocopherol, inhibit colon carcinogenesis in azoxymethane-treated F344 rats. Cancer Prev. Res. 2012, 5, 644–654. [Google Scholar] [CrossRef] [PubMed]
- Monk, J.M.; Wu, W.; McGillis, L.H.; Wellings, H.R.; Hutchinson, A.L.; Liddle, D.M.; Graf, D.; Robinson, L.E.; Power, K.A. Chickpea supplementation prior to colitis onset reduces inflammation in dextran sodium sulfate-treated C57Bl/6 male mice. Appl. Physiol. Nutr. Metab. 2018, 43, 893–901. [Google Scholar] [CrossRef]

| RC | CC | |
|---|---|---|
| Moisture * | 7.8 ± 0.30 a | 2.9 ± 0.09 b |
| Ash * | 2.83 ± 0.06 a | 1.22 ± 0.28 b |
| Lipid * | 7.11 ± 0.28 b | 9.76 ± 0.10 a |
| Protein (NX5.8) * | 25.17 ± 1.65 a | 27.32 ± 1.78 a |
| Fiber * | 1.71 ± 0.4 a | 1.41 ± 0.5 a |
| Carbohydrates * | 63.07 ± 1.65 a | 60.28 ± 1.7 a |
| Saponins 1 | 1.78 ± 0.00 a | 1.25 ± 0.01 b |
| Phytates 2 | 249.33 ± 10.1 a | 202.33 ± 6.5 b |
| Trypsin Inhibitors 3 | 12.11 ± 0.02 a | 1.88 ± 0.03 b |
| TPC 1 | Antioxidant Activity | |||
|---|---|---|---|---|
| ORAC 2 | OH− Radical 3 | Superoxide Radical 4 | ||
| RC | 60.09 ± 4.17 a | 52.73 ± 0.96 a | 56.36 ± 1.54 a | 57.05 ± 1.92 a |
| CC | 45.44 ± 2.32 b | 27.32 ± 1.22 b | 38.42 ± 2.01 b | 35.03 ± 1.76 b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cid-Gallegos, M.S.; Sánchez-Chino, X.M.; Álvarez-González, I.; Madrigal-Bujaidar, E.; Vásquez-Garzón, V.R.; Baltiérrez-Hoyos, R.; Villa-Treviño, S.; Dávila-Ortíz, G.; Jiménez-Martínez, C. Modification of In Vitro and In Vivo Antioxidant Activity by Consumption of Cooked Chickpea in a Colon Cancer Model. Nutrients 2020, 12, 2572. https://doi.org/10.3390/nu12092572
Cid-Gallegos MS, Sánchez-Chino XM, Álvarez-González I, Madrigal-Bujaidar E, Vásquez-Garzón VR, Baltiérrez-Hoyos R, Villa-Treviño S, Dávila-Ortíz G, Jiménez-Martínez C. Modification of In Vitro and In Vivo Antioxidant Activity by Consumption of Cooked Chickpea in a Colon Cancer Model. Nutrients. 2020; 12(9):2572. https://doi.org/10.3390/nu12092572
Chicago/Turabian StyleCid-Gallegos, María S., Xariss M. Sánchez-Chino, Isela Álvarez-González, Eduardo Madrigal-Bujaidar, Verónica R. Vásquez-Garzón, Rafael Baltiérrez-Hoyos, Saúl Villa-Treviño, Gloria Dávila-Ortíz, and Cristian Jiménez-Martínez. 2020. "Modification of In Vitro and In Vivo Antioxidant Activity by Consumption of Cooked Chickpea in a Colon Cancer Model" Nutrients 12, no. 9: 2572. https://doi.org/10.3390/nu12092572
APA StyleCid-Gallegos, M. S., Sánchez-Chino, X. M., Álvarez-González, I., Madrigal-Bujaidar, E., Vásquez-Garzón, V. R., Baltiérrez-Hoyos, R., Villa-Treviño, S., Dávila-Ortíz, G., & Jiménez-Martínez, C. (2020). Modification of In Vitro and In Vivo Antioxidant Activity by Consumption of Cooked Chickpea in a Colon Cancer Model. Nutrients, 12(9), 2572. https://doi.org/10.3390/nu12092572





