Dietary Patterns and Interventions to Alleviate Chronic Pain
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Caloric Restriction and Fasting
3.2. Enriched Polyunsaturated Fatty Acid Diets
3.3. Low-Fat Plant-Based Diet
3.4. High-Protein Diet
3.5. Elimination Diets
3.6. Antioxidant Vitamins and Minerals
3.7. Fruits and Fibers
3.8. Prebiotics and Probiotics
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- European Pain Federation. Available online: https://europeanpainfederation.eu/ (accessed on 8 June 2020).
- Bennett, M.I.; Kaasa, S.; Barke, A.; Korwisi, B.; Rief, W.; Treede, R.D. The IASP classification of chronic pain for ICD-11: Chronic cancer-related pain. Pain 2019, 160, 38–44. [Google Scholar] [CrossRef]
- Bannister, K.; Sachau, J.; Baron, R.; Dickenson, A.H. Neuropathic Pain: Mechanism-Based Therapeutics. Annu. Rev. Pharm. Toxicol. 2020, 60, 257–274. [Google Scholar] [CrossRef]
- Olesen, A.E.; Farmer, A.D.; Olesen, S.S.; Aziz, Q.; Drewes, A.M. Management of chronic visceral pain. Pain Manag. 2016, 6, 469–486. [Google Scholar] [CrossRef]
- Yamani, N.; Olesen, J. New daily persistent headache: A systematic review on an enigmatic disorder. J. Headache Pain 2019, 20, 80. [Google Scholar] [CrossRef]
- Feher, G.; Nemeskeri, Z.; Pusch, G.; Zadori, I.; Bank, G.; Gurdan, Z.; Meszaros, J.; Mak, K.; Tibold, A.; Komoly, S. Chronic orofacial pain. Orv. Hetil. 2019, 160, 1047–1056. [Google Scholar]
- Nijs, J.; Van Houdenhove, B.; Oostendorp, R.A. Recognition of central sensitization in patients with musculoskeletal pain: Application of pain neurophysiology in manual therapy practice. Management 2010, 15, 135–141. [Google Scholar] [CrossRef]
- Nicholas, M.; Vlaeyen, J.W.S.; Rief, W.; Barke, A.; Aziz, Q.; Benoliel, R.; Cohen, M.; Evers, S.; Giamberardino, M.A.; Goebel, A.; et al. The IASP classification of chronic pain for ICD-11: Chronic primary pain. Pain 2019, 160, 28–37. [Google Scholar] [CrossRef]
- Vos, T.A.; Arora, M. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1211–1259. [Google Scholar] [CrossRef]
- Mills, S.E.E.; Nicolson, K.P.; Smith, B.H. Chronic pain: A review of its epidemiology and associated factors in population-based studies. Br. J. Anaesth. 2019, 123, e273–e283. [Google Scholar] [CrossRef]
- Azevedo, L.F.; Costa-Pereira, A.; Mendonca, L.; Dias, C.C.; Castro-Lopes, J.M. Epidemiology of chronic pain: A population-based nationwide study on its prevalence, characteristics and associated disability in Portugal. J. Pain 2012, 13, 773–783. [Google Scholar] [CrossRef]
- Dean, E.; Soderlund, A. What is the role of lifestyle behaviour change associated with non-communicable disease risk in managing musculoskeletal health conditions with special reference to chronic pain? BMC Musculoskelet. Disord. 2015, 16, 87. [Google Scholar] [CrossRef]
- Philpot, U.; Johnson, M.I. Diet therapy in the management of chronic pain: Better diet less pain? Pain Manag. 2019, 9, 335–338. [Google Scholar] [CrossRef]
- Lean, M.E.J. Principles of human nutrition. Medicine 2011, 39, 1–5. [Google Scholar] [CrossRef]
- Casas, R.; Estruch, R.S. Dietary Patterns, Foods, Nutrients and Chronic Inflammatory Disorders. Immun. Res. 2016, 12, 1–10. [Google Scholar] [CrossRef]
- Bowen, K.J.; Sullivan, V.K.; Kris-Etherton, P.M.; Petersen, K.S. Nutrition and Cardiovascular Disease-an Update. Curr. Atheroscler. Rep. 2018, 20, 8. [Google Scholar] [CrossRef]
- Seaman, D.R. The diet-induced proinflammatory state: A cause of chronic pain and other degenerative diseases? J. Manip. Physiol. 2002, 25, 168–179. [Google Scholar] [CrossRef]
- Perrot, S.; Cohen, M.; Barke, A.; Korwisi, B.; Rief, W.; Treede, R.D. The IASP classification of chronic pain for ICD-11: Chronic secondary musculoskeletal pain. Pain 2019, 160, 77–82. [Google Scholar] [CrossRef]
- Cordain, L.; Eaton, S.B.; Sebastian, A.; Mann, N.; Lindeberg, S.; Watkins, B.A.; O’Keefe, J.H.; Brand-Miller, J. Origins and evolution of the Western diet: Health implications for the 21st century. Am. J. Clin. Nutr. 2005, 81, 341–354. [Google Scholar] [CrossRef]
- Sabia M., K.J. Nutrition and its effects on inflammation and chronic pain. J. Pub Health Cat. 2018, 1, 2. [Google Scholar] [CrossRef]
- Shivappa, N.; Hébert, J.R.; Rietzschel, E.R.; De Buyzere, M.L.; Langlois, M.; Debruyne, E.; Marcos, A.; Huybrechts, I. Associations between dietary inflammatory index and inflammatory markers in the Asklepios Study. Br. J. Nutr. 2015, 113, 665–671. [Google Scholar] [CrossRef]
- Sesti, F.; Capozzolo, T.; Pietropolli, A.; Collalti, M.; Bollea, M.R.; Piccione, E. Dietary therapy: A new strategy for management of chronic pelvic pain. Nutr. Res. Rev. 2011, 24, 31–38. [Google Scholar] [CrossRef]
- De Gregori, M.; Muscoli, C.; Schatman, M.E.; Stallone, T.; Intelligente, F.; Rondanelli, M.; Franceschi, F.; Arranz, L.I.; Lorente-Cebrian, S.; Salamone, M.; et al. Combining pain therapy with lifestyle: The role of personalized nutrition and nutritional supplements according to the SIMPAR Feed Your Destiny approach. J. Pain Res. 2016, 9, 1179–1189. [Google Scholar] [CrossRef]
- Sun, C.; Zhao, C.; Guven, E.C.; Paoli, P.; Simal-Gandara, J.; Ramkumar, K.M.; Wang, S.; Buleu, F.; Pah, A.; Turi, V.; et al. Dietary polyphenols as antidiabetic agents: Advances and opportunities. Food Front. 2020, 1, 18–44. [Google Scholar] [CrossRef]
- Naseri, R.; Farzaei, F.; Fakhri, S.; El-Senduny, F.F.; Altouhamy, M.; Bahramsoltani, R.; Ebrahimi, F.; Rahimi, R.; Farzaei, M.H. Polyphenols for diabetes associated neuropathy: Pharmacological targets and clinical perspective. Daru 2019, 27, 781–798. [Google Scholar] [CrossRef]
- Rosillo, M.A.; Alarcón-de-la-Lastra, C.; Sánchez-Hidalgo, M. An update on dietary phenolic compounds in the prevention and management of rheumatoid arthritis. Food Funct. 2016, 7, 2943–2969. [Google Scholar] [CrossRef]
- Costa de Miranda, R.; Paiva, E.S.; Suter Correia Cadena, S.M.; Brandt, A.P.; Vilela, R.M. Polyphenol-Rich Foods Alleviate Pain and Ameliorate Quality of Life in Fibromyalgic Women. Int. J. Vitam Nutr Res. 2017, 87, 66–74. [Google Scholar] [CrossRef]
- Bhardwaj, P.; Garg, P.K.; Maulik, S.K.; Saraya, A.; Tandon, R.K.; Acharya, S.K. A randomized controlled trial of antioxidant supplementation for pain relief in patients with chronic pancreatitis. Gastroenterology 2009, 136, 149–159.e2. [Google Scholar] [CrossRef]
- Elma, Ö.; Yilmaz, S.T.; Deliens, T.; Coppieters, I.; Clarys, P.; Nijs, J.; Malfliet, A. Do Nutritional Factors Interact with Chronic Musculoskeletal Pain? A Systematic Review. J. Clin. Med. 2020, 9, 702. [Google Scholar] [CrossRef]
- Mauro, G.L.; Martorana, U.; Cataldo, P.; Brancato, G.; Letizia, G. Vitamin B12 in low back pain: A randomised, double-blind, placebo-controlled study. Eur. Rev. Med. Pharm. Sci. 2000, 4, 53–58. [Google Scholar]
- Martin, K.R.; Reid, D.M. Is there role for vitamin D in the treatment of chronic pain? Adv. Musculoskelet. Dis. 2017, 9, 131–135. [Google Scholar] [CrossRef]
- Straube, S.; Derry, S.; Straube, C.; Moore, R.A. Vitamin D for the treatment of chronic painful conditions in adults. Cochrane Database Syst. Rev. 2015. [Google Scholar] [CrossRef] [PubMed]
- Shaik, M.M.; Gan, S.H. Vitamin supplementation as possible prophylactic treatment against migraine with aura and menstrual migraine. Biomed. Res. Int. 2015, 2015, 469529. [Google Scholar] [CrossRef] [PubMed]
- Brain, K.; Burrows, T.L.; Rollo, M.E.; Chai, L.K.; Clarke, E.D.; Hayes, C.; Hodson, F.J.; Collins, C.E. A systematic review and meta-analysis of nutrition interventions for chronic noncancer pain. J. Hum. Nutr. Diet. 2019, 32, 198–225. [Google Scholar] [CrossRef] [PubMed]
- Park, R.; Ho, A.M.H.; Pickering, G.; Arendt-Nielsen, L.; Mohiuddin, M.; Gilron, I. Magnesium for the Management of Chronic Noncancer Pain in Adults: Protocol for a Systematic Review. Jmir Res. Protoc. 2019, 8, e11654. [Google Scholar] [CrossRef]
- Mariqueo, T.A.; Zúñiga-Hernández, J. Omega-3 derivatives, specialized pro-resolving mediators: Promising therapeutic tools for the treatment of pain in chronic liver disease. Prostaglandins Leukot. Essent. Fat. Acids 2020, 158, 102095. [Google Scholar] [CrossRef]
- Kim, T.H.; Kang, J.W.; Lee, T.H. Therapeutic options for aromatase inhibitor-associated arthralgia in breast cancer survivors: A systematic review of systematic reviews, evidence mapping, and network meta-analysis. Maturitas 2018, 118, 29–37. [Google Scholar] [CrossRef]
- Distrutti, E.; Monaldi, L.; Ricci, P.; Fiorucci, S. Gut microbiota role in irritable bowel syndrome: New therapeutic strategies. World J. Gastroenterol. 2016, 22, 2219–2241. [Google Scholar] [CrossRef]
- Georgescu, D.; Reisz, D.; Gurban, C.V.; Georgescu, L.A.; Ionita, I.; Ancusa, O.E.; Lighezan, D. Migraine in young females with irritable bowel syndrome: Still a challenge. Neuropsychiatr. Dis. Treat. 2018, 14, 21–28. [Google Scholar] [CrossRef]
- Pusceddu, M.M.; Gareau, M.G. Visceral pain: Gut microbiota, a new hope? J. Biomed. Sci 2018, 25, 73. [Google Scholar] [CrossRef]
- Asha, M.Z.; Khalil, S.F.H. Efficacy and Safety of Probiotics, Prebiotics and Synbiotics in the Treatment of Irritable Bowel Syndrome: A systematic review and meta-analysis. Sultan Qaboos Univ. Med. J. 2020, 20, e13–e24. [Google Scholar] [CrossRef]
- Geir, B.; Salvatore, C.; Maryam, D.; Joeri, J.P.; Monica Daniela, D.; Lyudmila, P.; Yulia, S.; Jan, A. Insights on Nutrients as Analgesics in Chronic Pain. Curr. Med. Chem. 2019, 26, 1–16. [Google Scholar]
- Di Lorenzo, C.; Coppola, G.; Sirianni, G.; Di Lorenzo, G.; Bracaglia, M.; Di Lenola, D.; Siracusano, A.; Rossi, P.; Pierelli, F. Migraine improvement during short lasting ketogenesis: A proof-of-concept study. Eur. J. Neurol. 2015, 22, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Messier, S.P.; Mihalko, S.L.; Legault, C.; Miller, G.D.; Nicklas, B.J.; DeVita, P.; Beavers, D.P.; Hunter, D.J.; Lyles, M.F.; Eckstein, F.; et al. Effects of intensive diet and exercise on knee joint loads, inflammation, and clinical outcomes among overweight and obese adults with knee osteoarthritis: The IDEA randomized clinical trial. JAMA 2013, 310, 1263–1273. [Google Scholar] [CrossRef]
- Michalsen, A.; Li, C.; Kaiser, K.; Lüdtke, R.; Meier, L.; Stange, R.; Kessler, C. In-Patient Treatment of Fibromyalgia: A Controlled Nonrandomized Comparison of Conventional Medicine versus Integrative Medicine including Fasting Therapy. Evid. Based Complement. Altern. Med. 2013, 2013, 908610. [Google Scholar] [CrossRef] [PubMed]
- Riecke, B.F.; Christensen, R.; Christensen, P.; Leeds, A.R.; Boesen, M.; Lohmander, L.S.; Astrup, A.; Bliddal, H. Comparing two low-energy diets for the treatment of knee osteoarthritis symptoms in obese patients: A pragmatic randomized clinical trial. Osteoarthr. Cartil. 2010, 18, 746–754. [Google Scholar] [CrossRef] [PubMed]
- Marum, A.P.; Moreira, C.; Saraiva, F.; Tomas-Carus, P.; Sousa-Guerreiro, C. A low fermentable oligo-di-mono saccharides and polyols (FODMAP) diet reduced pain and improved daily life in fibromyalgia patients. Scand. J. Pain 2016, 13, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Ramsden, C.E.; Faurot, K.R.; Zamora, D.; Suchindran, C.M.; Macintosh, B.A.; Gaylord, S.; Ringel, A.; Hibbeln, J.R.; Feldstein, A.E.; Mori, T.A.; et al. Targeted alteration of dietary n-3 and n-6 fatty acids for the treatment of chronic headaches: A randomized trial. Pain 2013, 154, 2441–2451. [Google Scholar] [CrossRef]
- Soares, A.A.; Loucana, P.M.C.; Nasi, E.P.; Sousa, K.M.H.; Sa, O.M.S.; Silva-Neto, R.P. A double- blind, randomized, and placebo-controlled clinical trial with omega-3 polyunsaturated fatty acids (OPFA -3) for the prevention of migraine in chronic migraine patients using amitriptyline. Nutr. Neurosci. 2018, 21, 219–223. [Google Scholar] [CrossRef]
- Bunner, A.E.; Wells, C.L.; Gonzales, J.; Agarwal, U.; Bayat, E.; Barnard, N.D. A dietary intervention for chronic diabetic neuropathy pain: A randomized controlled pilot study. Nutr. Diabetes 2015, 5, e158. [Google Scholar] [CrossRef]
- Ferrara, L.A.; Pacioni, D.; Di Fronzo, V.; Russo, B.F.; Speranza, E.; Carlino, V.; Gargiulo, F.; Ferrara, F. Low-lipid diet reduces frequency and severity of acute migraine attacks. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 370–375. [Google Scholar] [CrossRef]
- Martínez-Rodríguez, A.; Leyva-Vela, B.; Martínez-García, A.; Nadal-Nicolás, Y. Effects of lacto-vegetarian diet and stabilization core exercises on body composition and pain in women with fibromyalgia: Randomized controlled trial. Nutr. Hosp. 2018, 35, 392–399. [Google Scholar] [PubMed]
- Maruki, J.; Sai, J.K.; Watanabe, S. Efficacy of low-fat diet against dyspepsia associated with nonalcoholic mild pancreatic disease diagnosed using the Rosemont criteria. Pancreas 2013, 42, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Towery, P.; Guffey, J.S.; Doerflein, C.; Stroup, K.; Saucedo, S.; Taylor, J. Chronic musculoskeletal pain and function improve with a plant-based diet. Complement. Med. 2018, 40, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Shell, W.E.; Pavlik, S.; Roth, B.; Silver, M.; Breitstein, M.L.; May, L.; Silver, D. Reduction in Pain and Inflammation Associated With Chronic Low Back Pain With the Use of the Medical Food Theramine. Am. J. 2016, 23, e1353–e1362. [Google Scholar] [CrossRef] [PubMed]
- Holton, K.F.; Taren, D.L.; Thomson, C.A.; Bennett, R.M.; Jones, K.D. The effect of dietary glutamate on fibromyalgia and irritable bowel symptoms. Clin. Exp. Rheumatol 2012, 30 (Suppl. 74), 10–17. [Google Scholar] [PubMed]
- Lucarelli, S.; Lastrucci, G.; Di Nardo, G.; D’Alfonso, Y.; Aloi, M.; Oliva, S.; Frediani, S.; Rossetti, D.; Frediani, T. Intestinal lymphoid nodular hyperplasia in children: The relationship to food allergy. Pediatr. Allergy Immunol. 2015, 26, 18–24. [Google Scholar] [CrossRef]
- Magiera, R.; Schürer-Maly, C.C.; Mortsiefer, A.; Abholz, H.H.; Maly, F.E.; Pentzek, M. Are there differences between patients with and without the homozygous--13910CC genetic variant in the MCM-6 gene upstream from the lactase gene?--A non-randomised, two armed intervention study without control group. Clin. Lab. 2014, 60, 1617–1625. [Google Scholar] [CrossRef]
- Rodrigo, L.; Blanco, I.; Bobes, J.; de Serres, F.J. Remarkable prevalence of coeliac disease in patients with irritable bowel syndrome plus fibromyalgia in comparison with those with isolated irritable bowel syndrome: A case-finding study. Arthritis Res. 2013, 15, R201. [Google Scholar] [CrossRef]
- Savaiano, D.A.; Ritter, A.J.; Klaenhammer, T.R.; James, G.M.; Longcore, A.T.; Chandler, J.R.; Walker, W.A.; Foyt, H.L. Improving lactose digestion and symptoms of lactose intolerance with a novel galacto-oligosaccharide (RP-G28): A randomized, double-blind clinical trial. Nutr. J. 2013, 12, 160. [Google Scholar] [CrossRef]
- Slim, M.; Calandre, E.P.; Garcia-Leiva, J.M.; Rico-Villademoros, F.; Molina-Barea, R.; Rodriguez-Lopez, C.M.; Morillas-Arques, P. The Effects of a Gluten-free Diet Versus a Hypocaloric Diet Among Patients With Fibromyalgia Experiencing Gluten Sensitivity-like Symptoms: A Pilot, Open-Label Randomized Clinical Trial. J. Clin. Gastroenterol. 2017, 51, 500–507. [Google Scholar] [CrossRef]
- Vellisca, M.Y.; Latorre, J.I. Monosodium glutamate and aspartame in perceived pain in fibromyalgia. Rheumatol. Int. 2014, 34, 1011–1013. [Google Scholar] [CrossRef] [PubMed]
- Anoushirvani, A.A.; Poorsaadat, L.; Aghabozorgi, R.; Kasravi, M. Comparison of the Effects of Omega 3 and Vitamin E on Palcitaxel-Induced Peripheral Neuropathy. Open Access Maced. J. Med. Sci. 2018, 6, 1857–1861. [Google Scholar] [CrossRef] [PubMed]
- Brady, S.R.E.; Naderpoor, N.; de Courten, M.P.J.; Scragg, R.; Cicuttini, F.; Mousa, A.; de Courten, B. Vitamin D supplementation may improve back pain disability in vitamin D deficient and overweight or obese adults. J. Steroid. Biochem. Mol. Biol. 2019, 185, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, R.; Singh, N.; Sachdev, V.; Upadhyay, A.D.; Saraya, A. Effect of antioxidant supplementation on surrogate markers of fibrosis in chronic pancreatitis: A randomized, placebo-controlled trial. Pancreas 2013, 42, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Gaul, C.; Diener, H.C.; Danesch, U.; Migravent Study, G. Improvement of migraine symptoms with a proprietary supplement containing riboflavin, magnesium and Q10: A randomized, placebo-controlled, double-blind, multicenter trial. J. Headache Pain 2015, 16, 516. [Google Scholar] [CrossRef]
- Gazerani, P.; Fuglsang, R.; Pedersen, J.G.; Sørensen, J.; Kjeldsen, J.L.; Yassin, H.; Nedergaard, B.S. A randomized, double-blinded, placebo-controlled, parallel trial of vitamin D(3) supplementation in adult patients with migraine. Curr. Med. Res. Opin. 2019, 35, 715–723. [Google Scholar] [CrossRef]
- Ghai, B.; Bansal, D.; Kanukula, R.; Gudala, K.; Sachdeva, N.; Dhatt, S.S.; Kumar, V. Vitamin D Supplementation in Patients with Chronic Low Back Pain: An Open Label, Single Arm Clinical Trial. Pain Physician 2017, 20, E99–E105. [Google Scholar] [CrossRef]
- Khan, Q.J.; Kimler, B.F.; Reddy, P.S.; Sharma, P.; Klemp, J.R.; Nydegger, J.L.; Yeh, H.W.; Fabian, C.J. Randomized trial of vitamin D3 to prevent worsening of musculoskeletal symptoms in women with breast cancer receiving adjuvant letrozole. The VITAL trial. Breast Cancer Res. Treat. 2017, 166, 491–500. [Google Scholar] [CrossRef]
- Rastelli, A.L.; Taylor, M.E.; Gao, F.; Armamento-Villareal, R.; Jamalabadi-Majidi, S.; Napoli, N.; Ellis, M.J. Vitamin D and aromatase inhibitor-induced musculoskeletal symptoms (AIMSS): A phase II, double-blind, placebo-controlled, randomized trial. Breast Cancer Res. Treat. 2011, 129, 107–116. [Google Scholar] [CrossRef]
- Rajanandh, M.G.; Kosey, S.; Prathiksha, G. Assessment of antioxidant supplementation on the neuropathic pain score and quality of life in diabetic neuropathy patients - a randomized controlled study. Pharm. Rep. 2014, 66, 44–48. [Google Scholar] [CrossRef]
- Brain, K.; Burrows, T.L.; Rollo, M.E.; Hayes, C.; Hodson, F.J.; Collins, C.E. The Effect of a Pilot Dietary Intervention on Pain Outcomes in Patients Attending a Tertiary Pain Service. Nutrients 2019, 16, 181. [Google Scholar] [CrossRef] [PubMed]
- Cassettari, V.M.G.; Machado, N.C.; Lourenção, P.; Carvalho, M.A.; Ortolan, E.V.P. Combinations of laxatives and green banana biomass on the treatment of functional constipation in children and adolescents: A randomized study. J. Pediatr (Rio J.) 2019, 95, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Smith, A.; Avalos, M.; South, S.; Crabtree, K.; Wang, W.; Kwon, Y.H.; Vijayagopal, P.; Juma, S. Blueberries Improve Pain, Gait Performance, and Inflammation in Individuals with Symptomatic Knee Osteoarthritis. Nutrients 2019, 11, 290. [Google Scholar] [CrossRef] [PubMed]
- Romano, C.; Comito, D.; Famiani, A.; Calamara, S.; Loddo, I. Partially hydrolyzed guar gum in pediatric functional abdominal pain. World J. Gastroenterol. 2013, 19, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Cassani, E.; Privitera, G.; Pezzoli, G.; Pusani, C.; Madio, C.; Iorio, L.; Barichella, M. Use of probiotics for the treatment of constipation in Parkinson’s disease patients. Minerva Gastroenterol. Dietol. 2011, 57, 117–121. [Google Scholar]
- Guerra, P.V.; Lima, L.N.; Souza, T.C.; Mazochi, V.; Penna, F.J.; Silva, A.M.; Nicoli, J.R.; Guimaraes, E.V. Pediatric functional constipation treatment with Bifidobacterium-containing yogurt: A crossover, double-blind, controlled trial. World J. Gastroenterol. 2011, 1, 3916–3921. [Google Scholar] [CrossRef]
- Roman, P.; Estevez, A.F.; Miras, A.; Sanchez-Labraca, N.; Canadas, F.; Vivas, A.B.; Cardona, D. A Pilot Randomized Controlled Trial to Explore Cognitive and Emotional Effects of Probiotics in Fibromyalgia. Sci. Rep. 2018, 8, 10965. [Google Scholar] [CrossRef]
- Sunagawa, Y.; Okamura, N.; Miyazaki, Y.; Shimizu, K.; Genpei, M.; Funamoto, M.; Shimizu, S.; Katanasaka, Y.; Morimoto, E.; Yamakage, H.; et al. Effects of Products Containing Bacillus subtilis var. natto on Healthy Subjects with Neck and Shoulder Stiffness, a Double-Blind, Placebo-Controlled, Randomized Crossover Study. Biol. Pharm. Bull. 2018, 41, 504–509. [Google Scholar]
- Waitzberg, D.L.; Logullo, L.C.; Bittencourt, A.F.; Torrinhas, R.S.; Shiroma, G.M.; Paulino, N.P.; Teixeira-da-Silva, M.L. Effect of synbiotic in constipated adult women - a randomized, double-blind, placebo-controlled study of clinical response. Clin. Nutr. 2013, 32, 27–33. [Google Scholar] [CrossRef]
- Patterson, R.E.; Laughlin, G.A.; LaCroix, A.Z.; Hartman, S.J.; Natarajan, L.; Senger, C.M.; Martinez, M.E.; Villasenor, A.; Sears, D.D.; Marinac, C.R.; et al. Intermittent Fasting and Human Metabolic Health. J. Acad. Nutr. Diet. 2015, 115, 1203–1212. [Google Scholar] [CrossRef]
- Michalsen, A.; Li, C. Fasting therapy for treating and preventing disease - current state of evidence. Komplementmed 2013, 20, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Aly, S.M. Role of intermittent fasting on improving health and reducing diseases. Int. J. Health Sci. (Qassim) 2014, 8, V–VI. [Google Scholar] [CrossRef] [PubMed]
- Dong, T.A.; Sandesara, P.B.; Dhindsa, D.S.; Mehta, A.; Arneson, L.C.; Dollar, A.L.; Taub, P.R.; Sperling, L.S. Intermittent Fasting: A Heart Healthy Dietary Pattern? Am. J. Med. 2020. [Google Scholar] [CrossRef] [PubMed]
- Longo, V.D.; Mattson, M.P. Fasting: Molecular mechanisms and clinical applications. Cell Metab. 2014, 19, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Moriarty, O.; Ruane, N.; O’Gorman, D.; Maharaj, C.H.; Mitchell, C.; Sarma, K.M.; Finn, D.P.; McGuire, B.E. Cognitive Impairment in Patients with Chronic Neuropathic or Radicular Pain: An Interaction of Pain and Age. Front. Behav. Neurosci. 2017, 11, 100. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P.; Moehl, K.; Ghena, N.; Schmaedick, M.; Cheng, A. Intermittent metabolic switching, neuroplasticity and brain health. Nat. Rev. Neurosci. 2018, 19, 63–80. [Google Scholar] [CrossRef]
- Sibille, K.T.; Bartsch, F.; Reddy, D.; Fillingim, R.B.; Keil, A. Increasing Neuroplasticity to Bolster Chronic Pain Treatment: A Role for Intermittent Fasting and Glucose Administration? J. Pain 2016, 17, 275–281. [Google Scholar] [CrossRef]
- McDonald, T.J.W.; Cervenka, M.C. Ketogenic Diets for Adult Neurological Disorders. Neurotherapeutics 2018, 15, 1018–1031. [Google Scholar] [CrossRef]
- McDonald, T.J.W.; Cervenka, M.C. The Expanding Role of Ketogenic Diets in Adult Neurological Disorders. Brain Sci. 2018, 8, 148. [Google Scholar] [CrossRef]
- Tan, B.-T.; Jiang, H.; Moulson, A.; Wu, X.-L.; Wang, W.-C.; Liu, J.; Plunet, W.; Tetzlaff, W. Neuroprotective effects of a ketogenic diet in combination with exogenous ketone salts following acute spinal cord injury. Neural Regen. Res. 2020, 15, 1912–1919. [Google Scholar] [CrossRef]
- Bell, R.F.; Borzan, J.; Kalso, E.; Simonnet, G. Food, pain, and drugs: Does it matter what pain patients eat? Pain 2012, 153, 1993–1996. [Google Scholar] [CrossRef] [PubMed]
- Deer, R.R.; Volpi, E. Protein intake and muscle function in older adults. Curr. Opin. Clin. Nutr. Metab. Care 2015, 1, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Perna, S.; Alalwan, T.A.; Al-Thawadi, S.; Negro, M.; Parimbelli, M.; Cerullo, G.; Gasparri, C.; Guerriero, F.; Infantino, V.; Diana, M.; et al. Evidence-Based Role of Nutrients and Antioxidants for Chronic Pain Management in Musculoskeletal Frailty and Sarcopenia in Aging. Geriatry 2020, 5, 16. [Google Scholar] [CrossRef] [PubMed]
- Batista, E.D.; Andretta, A.; de Miranda, R.C.; Nehring, J.; Dos Santos Paiva, E.; Schieferdecker, M.E. Food intake assessment and quality of life in women with fibromyalgia. Rev. Bras. Reum. Engl Ed. 2016, 56, 105–110. [Google Scholar] [CrossRef]
- Albuhairi, S.; Rachid, R. Novel Therapies for Treatment of Food Allergy. Immunol. Allergy Clin. North. Am. 2020, 40, 175–186. [Google Scholar] [CrossRef]
- Biesiekierski, J.R. What is gluten? J. Gastroenterol. Hepatol. 2017, 32 (Suppl. 1), 78–81. [Google Scholar] [CrossRef]
- Ludvigsson, J.F.; Leffler, D.A.; Bai, J.C.; Biagi, F.; Fasano, A.; Green, P.H.; Hadjivassiliou, M.; Kaukinen, K.; Kelly, C.P.; Leonard, J.N.; et al. The Oslo definitions for coeliac disease and related terms. Gut 2013, 62, 43–52. [Google Scholar] [CrossRef]
- Niland, B.; Cash, B.D. Health Benefits and Adverse Effects of a Gluten-Free Diet in Non-Celiac Disease Patients. Gastroenterol Hepatol (N Y) 2018, 14, 82–91. [Google Scholar]
- Leonard, M.M.; Vasagar, B. US perspective on gluten-related diseases. Clin. Exp. Gastroenterol. 2014, 7, 25–37. [Google Scholar]
- Wu, J.H.; Neal, B.; Trevena, H.; Crino, M.; Stuart-Smith, W.; Faulkner-Hogg, K.; Yu Louie, J.C.; Dunford, E. Are gluten-free foods healthier than non-gluten-free foods? An evaluation of supermarket products in Australia. Br. J. Nutr. 2015, 114, 448–454. [Google Scholar] [CrossRef]
- Sharma, N.; Bhatia, S.; Chunduri, V.; Kaur, S.; Sharma, S.; Kapoor, P.; Kumari, A.; Garg, M. Pathogenesis of Celiac Disease and Other Gluten Related Disorders in Wheat and Strategies for Mitigating Them. Front. Nutr. 2020, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- El Khoury, D.; Balfour-Ducharme, S.; Joye, I.J. A Review on the Gluten-Free Diet: Technological and Nutritional Challenges. Nutrients 2018, 10, 1410. [Google Scholar] [CrossRef] [PubMed]
- Micic, D.; Rao, V.L.; Rubin, D.T. Clinical Approach to Lactose Intolerance. JAMA 2019. [Google Scholar] [CrossRef] [PubMed]
- Flynn, A.; Kehoe, L.; Hennessy, A.; Walton, J. Estimating safe maximum levels of vitamins and minerals in fortified foods and food supplements. Eur. J. Nutr. 2017, 56, 2529–2539. [Google Scholar] [CrossRef] [PubMed]
- Majewski, M.; Kozlowska, A.; Thoene, M.; Lepiarczyk, E.; Grzegorzewski, W.J. Overview of the role of vitamins and minerals on the kynurenine pathway in health and disease. J. Physiol. Pharm. 2016, 67, 3–19. [Google Scholar]
- Liu, Y.; Dickerson, T.; Waddingham, P.; Clarkson, P.J. Improving people’s access to community-based back pain treatment through an inclusive design approach. Appl. Erg. 2019, 81, 102876. [Google Scholar] [CrossRef]
- Zahari, Z.; Ishak, A.; Justine, M. The effectiveness of patient education in improving pain, disability and quality of life among older people with low back pain: A systematic review. J. Back Musculoskelet. Rehabil. 2020, 33, 245–254. [Google Scholar] [CrossRef]
- Pandey, K.R.; Naik, S.R.; Vakil, B.V. Probiotics, prebiotics and synbiotics- a review. J. Food Sci. Technol. 2015, 52, 7577–7587. [Google Scholar] [CrossRef]
- Boonstra, A.M.; Schiphorst Preuper, H.R.; Reneman, M.F.; Posthumus, J.B.; Stewart, R.E. Reliability and validity of the visual analogue scale for disability in patients with chronic musculoskeletal pain. Int. J. Rehabil. Res. 2008, 31, 165–169. [Google Scholar] [CrossRef]
- Couper, M.P.; Tourangeau, R.; Conrad, F.G.; Singer, E. Evaluating the Effectiveness of Visual Analog Scales: A Web Experiment. Soc. Sci. Comput. Rev. 2006, 24, 227–245. [Google Scholar] [CrossRef]
- Delgado, D.A.; Lambert, B.S.; Boutris, N.; McCulloch, P.C.; Robbins, A.B.; Moreno, M.R.; Harris, J.D. Validation of Digital Visual Analog Scale Pain Scoring With a Traditional Paper-based Visual Analog Scale in Adults. J. Am. Acad. Orthop. Surg. Glob. Res. Rev. 2018, 2, e088. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.R.; Bernardo, A.; Costa, J.; Cardoso, A.; Santos, P.; de Mesquita, M.F.; Vaz Patto, J.; Moreira, P.; Silva, M.L.; Padrao, P. Dietary interventions in fibromyalgia: A systematic review. Ann. Med. 2019, 51 (Suppl. 1), 2–14. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.; Di Lollo, A.C.; Guzzo, M.P.; Giacomelli, C.; Atzeni, F.; Bazzichi, L.; Di Franco, M. Fibromyalgia and nutrition: What news? Clin. Exp. Rheumatol. 2015, 33 (Suppl. 88), S117–S125. [Google Scholar] [PubMed]
- Aman, M.M.; Jason Yong, R.; Kaye, A.D.; Urman, R.D. Evidence-Based Non-Pharmacological Therapies for Fibromyalgia. Curr. Pain Headache Rep. 2018, 22, 33. [Google Scholar] [CrossRef] [PubMed]
- Bjorklund, G.; Dadar, M.; Chirumbolo, S.; Aaseth, J. Fibromyalgia and nutrition: Therapeutic possibilities? Biomed. Pharm. 2018, 103, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Karras, S.; Rapti, E.; Matsoukas, S.; Kotsa, K. Vitamin D in Fibromyalgia: A Causative or Confounding Biological Interplay? Nutrients 2016, 8, 343. [Google Scholar] [CrossRef]
- Messina, O.D.; Vidal Wilman, M.; Vidal Neira, L.F. Nutrition, osteoarthritis and cartilage metabolism. Aging Clin. Exp. Res. 2019, 31, 807–813. [Google Scholar] [CrossRef]
- Rios, C.; Irarrazaval, S. Weight loss exclusively through diet for knee osteoarthritis. Medwave 2019, 19, e7623. [Google Scholar]
- Ebell, M.H.; Grad, R. Top 20 Research Studies of 2016 for Primary Care Physicians. Am. Fam. Physician 2017, 95, 572–579. [Google Scholar]
- Amer, M.; Woodward, M.; Appel, L.J. Effects of dietary sodium and the DASH diet on the occurrence of headaches: Results from randomised multicentre DASH-Sodium clinical trial. Bmj Open 2014, 4, e006671. [Google Scholar] [CrossRef]
- Martin, K.; Jackson, C.F.; Levy, R.G.; Cooper, P.N. Ketogenic diet and other dietary treatments for epilepsy. Cochrane Database Syst. Rev. 2016, 2, CD001903. [Google Scholar] [CrossRef] [PubMed]
- Barbanti, P.; Fofi, L.; Aurilia, C.; Egeo, G.; Caprio, M. Ketogenic diet in migraine: Rationale, findings and perspectives. Neurol. Sci. 2017, 38 (Suppl. 1), 111–115. [Google Scholar] [CrossRef] [PubMed]
- Razeghi Jahromi, S.; Ghorbani, Z.; Martelletti, P.; Lampl, C.; Togha, M.; On behalf of the School of Advanced Studies of the European Headache Federation (EHF-SAS). Association of diet and headache. J. Headache Pain 2019, 20, 106. [Google Scholar] [CrossRef] [PubMed]
- Gazerani, P. Migraine and Diet. Nutrients 2020, 12, 1658. [Google Scholar] [CrossRef]
- Byrd, D.A.; Judd, S.E.; Flanders, W.D.; Hartman, T.J.; Fedirko, V.; Bostick, R.M. Development and Validation of Novel Dietary and Lifestyle Inflammation Scores. J. Nutr 2019, 149, 2206–2218. [Google Scholar] [CrossRef]
- Maher, T.; Clegg, M.E. Dietary lipids with potential to affect satiety: Mechanisms and evidence. Crit. Rev. Food Sci. Nutr. 2019, 59, 1619–1644. [Google Scholar] [CrossRef]
- Brown, Z.; Metcalf, R.; Bednarz, J.; Spargo, L.; Lee, A.; Hill, C.; Wechalekar, M.; Stavrou, C.; James, M.; Cleland, L.; et al. Modifiable Lifestyle Factors Associated With Response to Treatment in Early Rheumatoid Arthritis. Acr. Open Rheumatol. 2020, 2, 371–377. [Google Scholar] [CrossRef]
- Thomas, T.R.; Liu, Y.; Linden, M.A.; Rector, R.S. Interaction of exercise training and n-3 fatty acid supplementation on postprandial lipemia. Appl. Physiol. Nutr. Metab. 2007, 32, 473–480. [Google Scholar] [CrossRef]
- Soveyd, N.; Abdolahi, M.; Bitarafan, S.; Tafakhori, A.; Sarraf, P.; Togha, M.; Okhovat, A.A.; Hatami, M.; Sedighiyan, M.; Djalali, M.; et al. Molecular mechanisms of omega-3 fatty acids in the migraine headache. Iran. J. Neurol. 2017, 16, 210–217. [Google Scholar]
- Fischer, R.; Konkel, A.; Mehling, H.; Blossey, K.; Gapelyuk, A.; Wessel, N.; von Schacky, C.; Dechend, R.; Muller, D.N.; Rothe, M.; et al. Dietary omega-3 fatty acids modulate the eicosanoid profile in man primarily via the CYP-epoxygenase pathway. J. Lipid Res. 2014, 55, 1150–1164. [Google Scholar] [CrossRef]
- Maghsoumi-Norouzabad, L.; Mansoori, A.; Abed, R.; Shishehbor, F. Effects of omega-3 fatty acids on the frequency, severity, and duration of migraine attacks: A systematic review and meta-analysis of randomized controlled trials. Nutr. Neurosci. 2018, 21, 614–623. [Google Scholar] [CrossRef] [PubMed]
- Ramsden, C.E.; Domenichiello, A.F.; Yuan, Z.X.; Sapio, M.R.; Keyes, G.S.; Mishra, S.K.; Gross, J.R.; Majchrzak-Hong, S.; Zamora, D.; Horowitz, M.S.; et al. A systems approach for discovering linoleic acid derivatives that potentially mediate pain and itch. Sci Signal. 2017, 10. [Google Scholar] [CrossRef] [PubMed]
- Pace, A.; Giannarelli, D.; Galiè, E.; Savarese, A.; Carpano, S.; Della Giulia, M.; Pozzi, A.; Silvani, A.; Gaviani, P.; Scaioli, V.; et al. Vitamin E neuroprotection for cisplatin neuropathy. Random. Placebo-Control. Trial 2010, 74, 762–766. [Google Scholar]
- Kottschade, L.A.; Sloan, J.A.; Mazurczak, M.A.; Johnson, D.B.; Murphy, B.P.; Rowland, K.M.; Smith, D.A.; Berg, A.R.; Stella, P.J.; Loprinzi, C.L. The use of vitamin E for the prevention of chemotherapy-induced peripheral neuropathy: Results of a randomized phase III clinical trial. Support. Care Cancer 2011, 19, 1769–1777. [Google Scholar] [CrossRef]
- Afonseca, S.O.; Cruz, F.M.; Cubero Dde, I.; Lera, A.T.; Schindler, F.; Okawara, M.; Souza, L.F.; Rodrigues, N.P.; Giglio, A. Vitamin E for prevention of oxaliplatin-induced peripheral neuropathy: A pilot randomized clinical trial. Sao Paulo Med. J. 2013, 131, 35–38. [Google Scholar] [CrossRef]
- Eum, S.; Choi, H.D.; Chang, M.J.; Choi, H.C.; Ko, Y.J.; Ahn, J.S.; Shin, W.G.; Lee, J.Y. Protective effects of vitamin E on chemotherapy-induced peripheral neuropathy: A meta-analysis of randomized controlled trials. Int J. Vitam Nutr. Res. 2013, 83, 101–111. [Google Scholar] [CrossRef]
- Schloss, J.; Colosimo, M.; Vitetta, L. Herbal medicines and chemotherapy induced peripheral neuropathy (CIPN): A critical literature review. Crit. Rev. Food Sci. Nutr. 2017, 57, 1107–1118. [Google Scholar] [CrossRef]
- Schloss, J.; Colosimo, M.; Vitetta, L. New Insights into Potential Prevention and Management Options for Chemotherapy-Induced Peripheral Neuropathy. Asia Pac. J. Oncol. Nurs. 2016, 3, 73–85. [Google Scholar] [CrossRef]
- Brami, C.; Bao, T.; Deng, G. Natural products and complementary therapies for chemotherapy-induced peripheral neuropathy: A systematic review. Crit. Rev. Oncol. Hematol. 2016, 98, 325–334. [Google Scholar] [CrossRef]
- Baute, V.; Zelnik, D.; Curtis, J.; Sadeghifar, F. Complementary and Alternative Medicine for Painful Peripheral Neuropathy. Curr. Treat. Options Neurol. 2019, 21, 44. [Google Scholar] [CrossRef]
- Asadi, S.; Gholami, M.S.; Siassi, F.; Qorbani, M.; Sotoudeh, G. Beneficial effects of nano-curcumin supplement on depression and anxiety in diabetic patients with peripheral neuropathy: A randomized, double-blind, placebo-controlled clinical trial. Phytother Res. 2020, 34, 896–903. [Google Scholar] [CrossRef] [PubMed]
- Mazzawi, T.; Hausken, T.; Gundersen, D.; El-Salhy, M. Effect of dietary management on the gastric endocrine cells in patients with irritable bowel syndrome. Eur. J. Clin. Nutr. 2015, 69, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Daulatzai, M.A. Non-celiac gluten sensitivity triggers gut dysbiosis, neuroinflammation, gut-brain axis dysfunction, and vulnerability for dementia. Cns Neurol. Disord Drug Targets 2015, 14, 110–131. [Google Scholar] [CrossRef]
- Galligan, J.J.; Sternini, C. Insights into the Role of Opioid Receptors in the GI Tract: Experimental Evidence and Therapeutic Relevance. Handb Exp. Pharm. 2017, 239, 363–378. [Google Scholar]
- Ulbricht, K.; Layer, P.; Andresen, V. Chronic, non-infectious diarrhea: Diagnostics and therapy. Dtsch Med. Wochenschr 2016, 141, 1395–1402. [Google Scholar] [PubMed]
- Rodino-Janeiro, B.K.; Vicario, M.; Alonso-Cotoner, C.; Pascua-Garcia, R.; Santos, J. A Review of Microbiota and Irritable Bowel Syndrome: Future in Therapies. Advanced 2018, 35, 289–310. [Google Scholar] [CrossRef]
- Seo, M.; Heo, J.; Yoon, J.; Kim, S.Y.; Kang, Y.M.; Yu, J.; Cho, S.; Kim, H. Methanobrevibacter attenuation via probiotic intervention reduces flatulence in adult human: A non-randomised paired-design clinical trial of efficacy. PLoS ONE 2017, 12, e0184547. [Google Scholar] [CrossRef]
- Staudacher, H.M.; Lomer, M.C.E.; Farquharson, F.M.; Louis, P.; Fava, F.; Franciosi, E.; Scholz, M.; Tuohy, K.M.; Lindsay, J.O.; Irving, P.M.; et al. A Diet Low in FODMAPs Reduces Symptoms in Patients With Irritable Bowel Syndrome and A Probiotic Restores Bifidobacterium Species: A Randomized Controlled Trial. Gastroenterology 2017, 153, 936–947. [Google Scholar]
- Rondanelli, M.; Faliva, M.A.; Miccono, A.; Naso, M.; Nichetti, M.; Riva, A.; Guerriero, F.; De Gregori, M.; Peroni, G.; Perna, S. Food pyramid for subjects with chronic pain: Foods and dietary constituents as anti-inflammatory and antioxidant agents. Nutr. Res. Rev. 2018, 31, 131–151. [Google Scholar] [CrossRef]
- De Gregori, M.; Belfer, I.; De Giorgio, R.; Marchesini, M.; Muscoli, C.; Rondanelli, M.; Martini, D.; Mena, P.; Arranz, L.I.; Lorente-Cebrián, S.; et al. Second edition of SIMPAR’s “Feed Your Destiny” workshop: The role of lifestyle in improving pain management. J. Pain Res. 2018, 11, 1627–1636. [Google Scholar] [CrossRef]
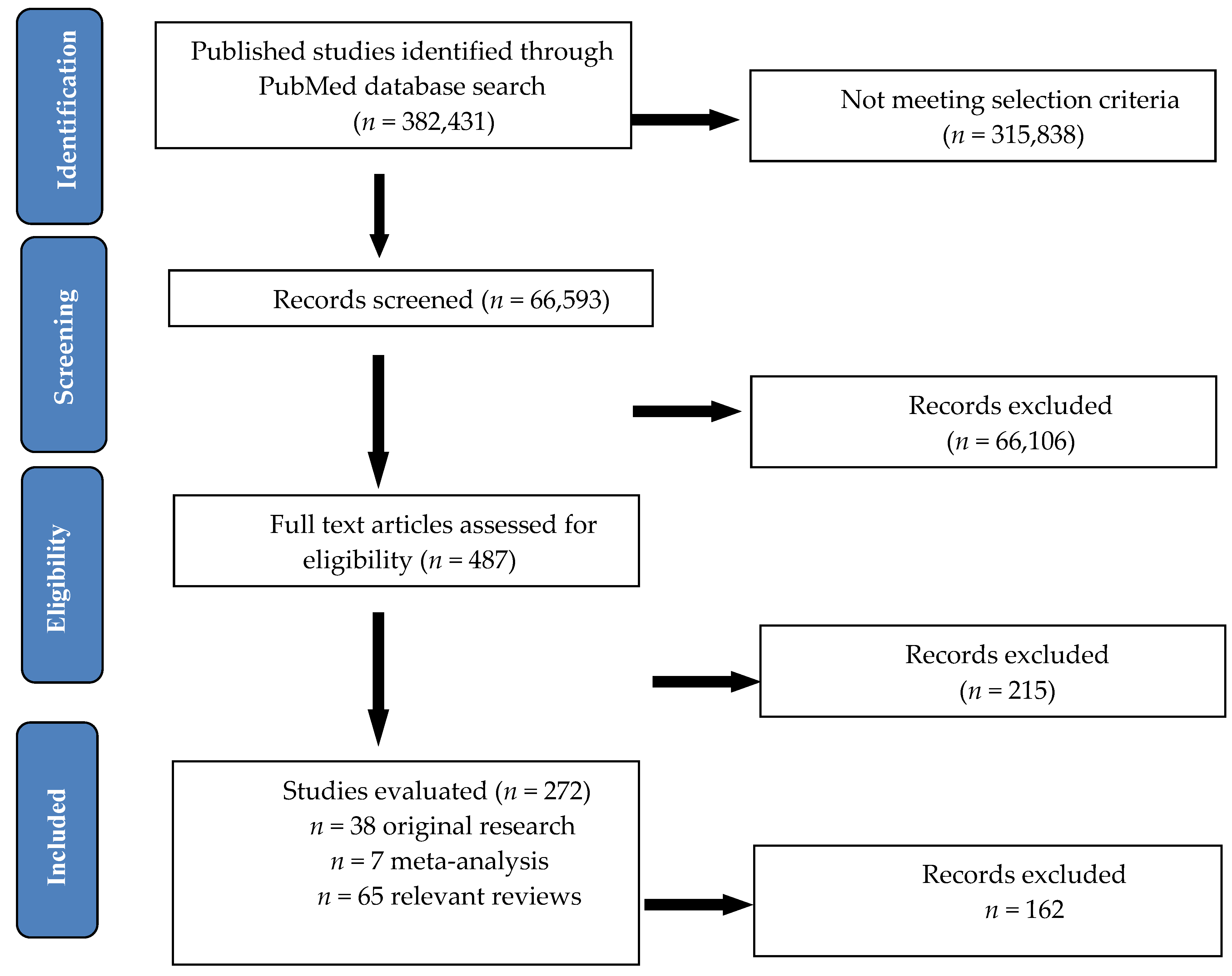
| Diet Type | Number | Authors and Year | Type of Study | Duration of Study | Number of Patients | Chronic Pain Condition | Diet Intervention | Effect on Pain |
|---|---|---|---|---|---|---|---|---|
| Primary/Secondary Outcomes | ||||||||
| Caloric restriction and fasting | 1. | Di Lorenzo et al. Eur. J. Neurol. 2015 [43] | two parallel groups, proof-of-concept study | 12 months | 96 subjects (96 female) | Headache due to starvation or a ketogenic diet | 1-month very-low-calorie ketogenic diet prescription followed by a 5-month standard low-calorie diet vs. 6-month standard low-calorie diet | Migraine improvement during short lasting ketogenesis from 1 month |
| Primary outcomes | ||||||||
| 2. | Messier et al., JAMA, 2013 [44] | single-blind randomized | 18 months | 399 subjects (325 female and 74 male) | Knee osteoarthritis in overweight and obesity | Calorie-restricted 800–1000 calories/day, 2 meal replacements supplements and exercise regimen vs. intensive diet-induced weight loss vs exercise | reductions in knee compressive force, weight loss and less pain in diet group and exercise regimen | |
| Primary outcomes | ||||||||
| 3. | Michalsen A et al., Evid Based Complement Alternat Med., 2013 [45] | controlled, nonrandomized pilot study | 12 weeks | 48 subjects (46 female and 2 male) | General body pain due to fibromyalgia | Conventional Medicine vs. Integrative Medicine Including Fasting Therapy | Improvements of pain scores after 2 weeks | |
| Primary outcomes | ||||||||
| 4. | Riecke et al., Osteoarthritis Cartilage, 2010 [46] | prospective, pragmatic randomized clinical trial, with blinded outcome assessors | 16 weeks | 192 subjects (155 female and 37 male) | knee osteoarthritis in obese patients | 8 weeks of low-energy diet (LED; 810 kcal/day) or a very-low-energy diet (VLED; 415 kcal/day) using formula foods | weight loss and highly significant improvements in pain symptoms | |
| Primary outcomes | ||||||||
| 5. | Marum et al., Scandinavian Journal of Pain, 2016 [47] | pilot, open label, randomized clinical trial | 4 weeks | 38 subjects 38 female) | General body pain in female patients diagnosed with fibromyalgia | low fermentable oligo-di-monosaccharides and polyols (FODMAP) diet | significant reduction in gastrointestinal disorders and fibromyalgia symptoms, including pain scores | |
| Primary outcomes | ||||||||
| Enriched polyunsaturated fatty acid diets | 1. | Ramsden CE et al. Pain. 2013 [48] | randomized, single-blind, parallel-group clinical trial | 12 weeks | 67 subjects (58 female and 9 male) | chronic daily headaches | dietary high n-3 and low n-6 fatty acids supplementation | reduced headache pain, improved quality-of-life |
| Primary outcomes | ||||||||
| 2. | Soares AA et al., Nutr Neurosci. 2018 [49] | prospective, experimental, controlled, double-blind | 60 days | 51 subjects (36 female and 15 male) | Chronic migraine | Omega-3 dietary supplements vs placebo | reduction of days of headache and prevention of migraine attacks | |
| Primary outcomes | ||||||||
| Low-fat plant-based diet | 1. | Bunner AE et al., Nutr Diabetes. 2015 [50] | randomized parallel aassignment | 20 weeks | 33 subjects (19 female and 14 male) | painful diabetic neuropathy in type 2 diabetes patients | a low-fat, plant-based diet in combination with a vitamin B12 supplement vs control group (only with vitamin B12 supplement) | improvement of clinical and pain symptoms, pain scales and quality of life |
| Primary outcomes | ||||||||
| 2. | Ferrara LA et al., Nutr Metab Cardiovasc Dis. 2015 [51] | randomized, crossover intervention trial | 6 months | 83 subjects (63 female and 20 male) | migraine crises | two dietary regimens a low-lipid vs. normal-lipid diet | Reduced numbers of crises and severity of pain, with a significant difference in favor of the low-lipid diet. | |
| Primary outcomes | ||||||||
| 3. | Martínez-Rodríguez et al., Nutr Hosp. 2018 [52] | randomized, placebo-controlled study | 4 weeks | 21 subjects (21 female) | lower back pain in women with fibromyalgia | lacto-vegetarian diet and stabilization core exercises vs placebo + lacto-vegetarian diet vs control | pain reduction and improved body composition in first group | |
| Primary outcomes | ||||||||
| 4. | Maruki J et al., Pancreas. 2013 [53] | randomized, controlled trial | 4 weeks | 45 subjects | upper abdominal pain in nonalcoholic mild pancreatic disease | low-fat diet (<20 g of fat/day) | improvement of visual analog scale score | |
| Secondary outcomes | ||||||||
| 5. | Towery et al., Complement Ther Med, 2018 [54] | randomized, controlled trial | 8 weeks | 14 subjects | Chronic musculoskeletal pain | plant-based diet consisted of grains, fruits, vegetables and legumes | decreased pain and improvement and quality of life | |
| Primary outcomes | ||||||||
| High-protein diet | 1. | Shell, Am J Ther., 2016 [55] | double-blind controlled study | 28 days | 122 subjects | chronic lower back pain | ibuprofen alone (400 mg daily) vs amino acid blend alone (two 355 mg capsules twice daily) vs the combined use of ibuprofen (400 mg daily) and amino acid blend (two 355 mg capsules twice daily) | substantial improvement in chronic back pain |
| Primary outcomes | ||||||||
| Elimination diets | 1. | Holton et al., Clinical experimental rheumatology, 2012 [56] | double-blind, placebo-controlled, cross-over clinical trial | 4 weeks | 37 subjects (34 women and 3 male) | General body pain in patients with fibromyalgia with irritable bowel syndrome | diet excluding monosodium glutamate vs. placebo | improvement of pain symptoms and tender point number |
| Secondary outcomes | ||||||||
| 2. | Lucarelli S et al., Pediatr Allergy Immunol. 2015 [57] | prospective, parallel multiarm, randomized clinical trial | 8 weeks | 72 children | Abdominal pain due to intestinal lymphoid nodular hyperplasia in children | Elimination diet vs. mesalamine vs. symptomatic treatment | diet had no effect on pain symptoms compared to symptomatic therapy | |
| Secondary outcomes | ||||||||
| 3. | Magiera R et al., Clin Lab. 2014 [58] | Nonrandomized, Two Armed Intervention Study without Control Group | 2 months | 210 subjects | Chronic abdominal pain in lactose intolerance | Lactose restricted diet | improvement of abdominal pain symptoms | |
| Primary outcomes | ||||||||
| 4. | Rodrigo L et al., Arthritis Res Ther. 2014 [59] | case-control study | 1 year | 229 subjects (197 female and 32 male) | General body pain in irritable bowel syndrome plus fibromyalgia with/without lymphocytic enteritis | gluten-free diet | significant improvement in all symptoms and pain scales in group with lymphocytic enteritis | |
| Primary outcomes | ||||||||
| 5. | Savaiano DA AT et al., Nutr J. 2013 [60] | randomized, double-blind, parallel group, placebo-controlled study | 66 days | 61 subjects | Abdominal pain in lactose intolerance | RP-G28 novel galacto-oligosaccharide (GOS) vs placebo (corn syrup) | reduction in abdominal pain and improve of all symptoms of the lactose intolerance | |
| Primary outcomes | ||||||||
| 6. | Slim M et al., J Clin Gastroenterol 2017 [61] | pilot, open-labe, randomized clinical trial | 24 weeks | 75 subjects (73 female and 2 male) | General body pain in patients with fibromyalgia experiencing gluten sensitivity symptoms | gluten-free diet vs. hypocaloric diet | similar beneficial outcomes in alleviating pain | |
| Secondary outcomes | ||||||||
| 7. | Vellisca MY et al., Rheumatol Int. 2014 [62] | case-control study | 3 months | 72 subjects (72 female) | General body pain due to fibromyalgia | discontinuation of dietary monosodium glutamate and aspartame vs waiting list | no improvement of pain symptoms | |
| Primary outcomes | ||||||||
| Antioxidant vitamins and minerals | 1. | Anoushirvani AA. et al., Open Access Maced J Med Sci. 2018, [63] | randomized, placebo-controlled study | 3 months | 63 Subjects (46 female and 17 male) | Paclitaxel-induced peripheral neuropathy | 640 mg omega-3 three times a day Vs 640 mg omega-3 three times a day Vs placebo | vitamin E and omega-3 may greatly enhance quality of life |
| Secondary outcomes | ||||||||
| 2. | Brady SRE et al., Steroid Biochem. Mol. Biol. 2019 [64] | randomized, placebo-controlled study | 16 weeks | 49 subjects (18 female and 31 male) | low back pain in overweight or obese adults with vitamin D deficit | bolus oral dose of 100,000 IU followed by 4000 IU cholecalciferol/day vs. placebo | improvement of back pain disability in subjects with vitamin D deficit | |
| Primary outcomes | ||||||||
| 3. | Dhingra R et al., Pancreas. 2013 [65] | randomized, placebo -controlled trial | 3 months | 61 subjects (18 female and 43 male) | Abdominal pain in chronic pancreatitis | Antioxidants vs. placebo (Antioxidants supplements daily doses of 600 ug organic selenium,0.54 g vit C, 9000 IU b-carotene, 270 IU vit E and 2 g methionine) | pain relief | |
| Primary outcomes | ||||||||
| 4. | Gaul et al., J Headache Pain. 2015 [66] | randomized, placebo-controlled, parallel-arm, double-blind, prospective multicenter study | 12 weeks | 112 subjects (97 female and 15 male) | migraine crises in adults under 65 years | Multivitamins: 400 mg B2, 600 mg Mg,150 mg Q10, 750 ug vitamin A, 200 mg vitamin C, 134 mg vitamin E, 5 mg B1, 20 mg B 3,5 mg B 6,6 ug B12, 400 lg B 9,5 ug vitamin D, 10 mg B5, 165 ug B 7, 0.8 mg Fe, 5 mg Zn, 2 mg Mn, 0.5 mg Cu, 30 lg Cr, 60 ug Mo, 50 ug Se,5 mg bioflavonoids vs. placebo | Improvement of migraine pain and no reduction of migraine days | |
| Primary outcomes | ||||||||
| 5. | Gazerani et al., Curr Med Res Opin. 2019 [67] | randomized, double-blinded, placebo-controlled, parallel trial | 28 weeks | 48 subjects (36 female and 12 male) | Migraine in adults | 100 μg/day D3-Vitamin vs placebo | Improvement only of migraine frequency | |
| Primary outcomes | ||||||||
| 6. | Ghai B et al., Pain Physician. 2017 [68] | open label, single arm clinical trial | 6 months | 68 subjects (31 female and 37 male) | chronic low back pain in adults with insufficient or vitamin D deficit | 60,000 IU oral vitamin-D3 supplementation every week for 8 weeks | improvement of pain intensity and functional disability | |
| Primary outcomes | ||||||||
| 7. | Khan et al., Breast cancer research treatment, 2017 [69] | randomized, placebo-controlled trial | 4 months | 160 subjects (160 female) | Musculoskeletal pain due to breast cancer | 30,000 IU oral VitD3/week + daily supplement of 1200 mg calcium and 600 IU vitamin D3 vs placebo + daily supplement of 1200 mg calcium and 600 IU vitamin D3 | no change of musculoskeletal symptoms | |
| Primary outcomes | ||||||||
| 8. | Rastelli et al., Breast cancer research treatment, 2011 [70] | double-blind placebo-controlled randomized phase II trial | 4 months | 60 subjects (60 female) | Musculoskeletal symptoms in breast cancer induced by aromatase inhibitor | 50,000 IU Vitamin D2 vs. placebo | improvement of musculoskeletal symptoms, pain scores and severity improved | |
| Primary outcomes | ||||||||
| 9. | Rajanandh et al., Pharmacol. Rep. 2014 [71] | controlled randomized trial | 12 weeks | 92 subjects | diabetic neuropathy | vitamin-E 300 bid | reduction in total pain score in all questionnaires applied | |
| Primary outcomes | ||||||||
| Fruits and fibers | 1. | Brain K et al., Nutrients. 2019 [72] | controlled randomized trial | 6 weeks | 60 subjects (41 female and 19 male) | Generalized chronic musculoskeletal pain | personalized dietary consultations and active fruit juice vs personalized dietary consultations and placebo fruit juice vs waitlist control group and active fruit juice vs waitlist control group and placebo fruit juice | significant improvement in 3 of 5 pain scores and quality of life in dietary intervention groups |
| Primary outcomes | ||||||||
| 2. | Cassettari VMG et al., J Pediatr (Rio J.). 2019 [73] | prospective, interventional, randomized clinical study | 8 weeks | 80 subjects (43 female and 37 male) | Abdominal pain due to functional constipation in children and adolescents | green banana biomass alone vs green banana biomass plus PEG 3350 with electrolytes vs green banana biomass plus sodium picosulfate vs PEG 3350 with electrolytes alone vs sodium picosulfate alone | alleviation of abdominal pain and pain defecation by adding green banana biomass | |
| Secondary outcomes | ||||||||
| 3. | Du et al., Nutrient, 2019 [74] | randomized, double-blind trial | 4 months | 49 subjects (35 female and 14 male) | knee osteoarthritis | Blueberry powder vs. placebo powder | pain and quality of life improvement | |
| Primary outcomes | ||||||||
| 4. | Romano C et al. World J. Gastroenterol. 2013 [75] | randomized double-blind pilot study | 8 weeks | 60 subjects (37 female and 23 male) | chronic abdominal pain due to irritable bowel syndrome in pediatric patients | Partially hydrolyzed guar gum vs. placebo | tendency toward normalization of bowel habit and pain control | |
| Primary outcomes | ||||||||
| Prebiotics and probiotics | 1. | Cassani E et al., Minerva Gastroenterol. Dietol. 2011 [76] | pilot study | 6 weeks | 40 subjects | Abdominal pain due to constipation in Parkinson disease | Probiotic supplementation with Lactobacillus casei Shirota | improvement of abdominal pain, decreased bloating, normalization of stools |
| Primary outcomes | ||||||||
| 2. | Guerra PV et al., World J. Gastroenterol. 2011 [77] | crossover, double-blind formula controlled trial | 5 weeks | 59 subjects (47 female and 12 male) | Chronic abdominal pain due to functional constipation and defecation in students | Probiotic goat yogurt with Bifidobacterium vs. yogurt alone | significant improvement in abdominal and defecation pain | |
| Primary outcomes | ||||||||
| 3. | Roman et al., Scientific Reports, 2018 [78] | double-blind, placebo-controlled, parallel assignment | 8 weeks | 60 (28 female and 3 male) | General body pain due to fibromyalgia | Lactobacillus acidophilus vs. Lactobacillus Rhamnosus GG vs. placebo | no improvement of pain symptoms | |
| Secondary outcomes | ||||||||
| 4. | Sunagawa Y et al., Biol. Pharm. Bull. 2018 [79] | double-blind placebo-controlled randomized crossover study | 4 weeks | 29 subjects (8 female and 21 male) | neck and shoulder stiffness, headaches | 250 mg of NKCP®, a natto-derived dietary food supplement with bacillopeptidase F vs placebo | alleviation of headaches and chronic neck and shoulder stiffness and pain | |
| Primary outcomes | ||||||||
| 5. | Waitzberg DL et al., Clinical Nutrition, 2013 [80] | randomized, double-blind, placebo-controlled study | 30 days | 100 subjects (100 female) | abdominal pain due to chronic constipation | synbiotic, combining fructooligosaccharides with Lactobacillus and Bifidobacterium strains (LACTOFOS®) vs. maltodextrin (placebo group) | no improvement of pain symptoms | |
| Primary outcomes |
| Chronic Pain Category. | Chronic Pain Type | Dietary Pattern/Intervention | References |
|---|---|---|---|
| Chronic musculo-skeletal pain | Fibromyalgia | Low FODMAPs diet | [47] |
| Elimination diet (MSD and aspartame) | [56,62] | ||
| Gluten-free | [59,61] | ||
| Fasting | [45] | ||
| Probiotics | [77] | ||
| Low back pain | Lacto-vegetarian diet | [52] | |
| High-protein diet (amino acids supplementation) | [55] | ||
| Vitamin D3 supplementation | [64,68] | ||
| Knee osteoarthritis in obese patients | Calorie-restricted diet | [44,46] | |
| Blueberry polyphenols supplementation | [74] | ||
| Neck pain and stiffness | Probiotics | [79] | |
| Musculoskeletal pain due to breast cancer treatment | Vitamin D2/D3 supplementation | [69,70] | |
| Generalized chronic musculoskeletal pain | Plant-based low-fat diet | [54] | |
| Fruit juice (apple/cherry) | [72] | ||
| Chronic headache | Chronic headache or migraine | Very-low-calorie ketogenic diet | [43] |
| Fatty acids supplementation (high n-3 and low n-6) | [48] | ||
| Omega-3 supplementation | [49] | ||
| Low-fat diet | [51] | ||
| Multivitamins and vitamin D3 supplementation | [66,67] | ||
| Neuropathic pain | Diabetic neuropathy | Low-fat, plant-based diet with vitamin B12 supplementation | [50] |
| Vitamin E supplementation | [71] | ||
| Taxol-induced neuropathic pain | Vitamin E or omega-3 supplementation | [63] | |
| Chronic abdominal pain | Upper abdominal pain in pancreatic disease | Low-fat diet | [53] |
| Antioxidants supplementation | [65] | ||
| Intestinal metaplasia in children | Lactose elimination diet | [57] | |
| Lactose intolerance | Lactose elimination diet | [58] | |
| Novel food RP-G28 galacto-oligosaccharide | [60] | ||
| Functional constipation | Increased fibers | [73] | |
| Probiotics/synbiotics | [76,77,80] | ||
| Irritable bowel syndrome | Increased fibers | [75] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dragan, S.; Șerban, M.-C.; Damian, G.; Buleu, F.; Valcovici, M.; Christodorescu, R. Dietary Patterns and Interventions to Alleviate Chronic Pain. Nutrients 2020, 12, 2510. https://doi.org/10.3390/nu12092510
Dragan S, Șerban M-C, Damian G, Buleu F, Valcovici M, Christodorescu R. Dietary Patterns and Interventions to Alleviate Chronic Pain. Nutrients. 2020; 12(9):2510. https://doi.org/10.3390/nu12092510
Chicago/Turabian StyleDragan, Simona, Maria-Corina Șerban, Georgiana Damian, Florina Buleu, Mihaela Valcovici, and Ruxandra Christodorescu. 2020. "Dietary Patterns and Interventions to Alleviate Chronic Pain" Nutrients 12, no. 9: 2510. https://doi.org/10.3390/nu12092510
APA StyleDragan, S., Șerban, M.-C., Damian, G., Buleu, F., Valcovici, M., & Christodorescu, R. (2020). Dietary Patterns and Interventions to Alleviate Chronic Pain. Nutrients, 12(9), 2510. https://doi.org/10.3390/nu12092510






