The Effect of Voluntary Exercise on Gut Microbiota in Partially Hydrolyzed Guar Gum Intake Mice under High-Fat Diet Feeding
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal and Experimental Design
2.2. Diet and PHGG
2.3. Voluntary Exercise
2.4. Measurement of Maximum Oxygen Consumption ( O2max)
2.5. Glucose Tolerance Test (GTT)
2.6. Measurement Body Mass, Food Intake, Tissue Mass, and Secum Contents
2.7. Fecal pH Measurement and Analysis of Gut Microbiota
2.8. Analysis of Short-Chain Fatty Acids (SCFAs)
2.9. Statistical Analysis
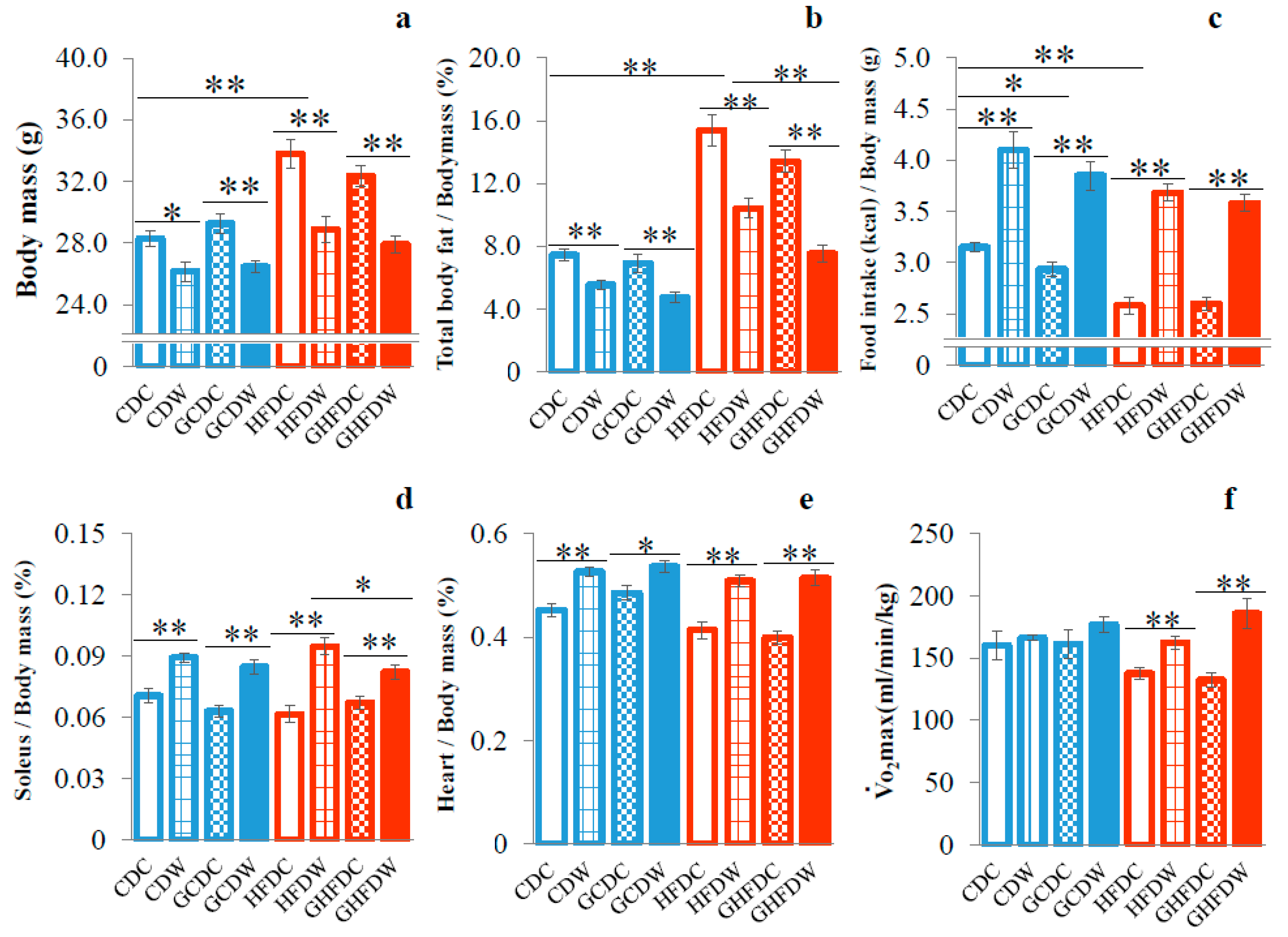
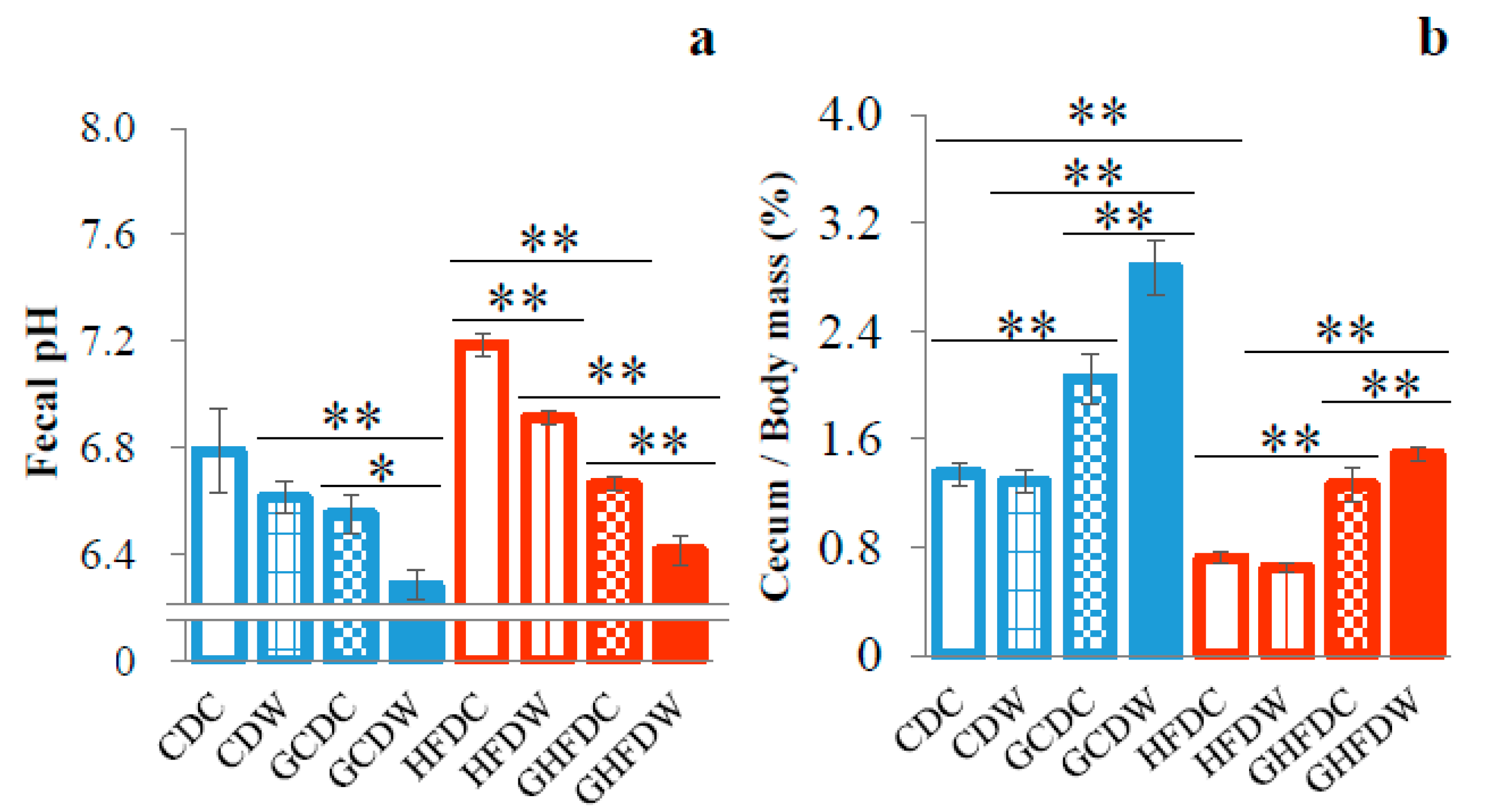
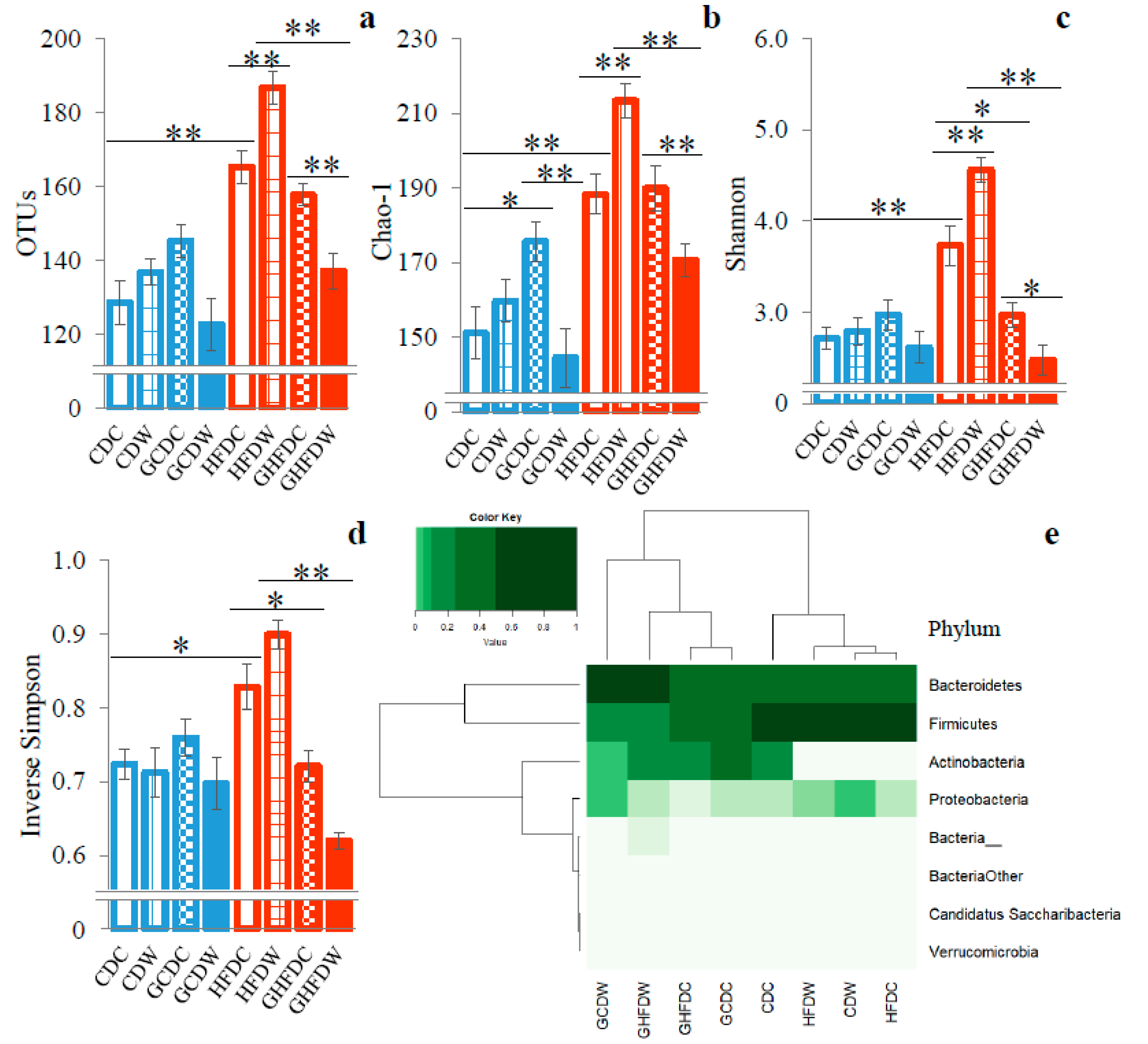
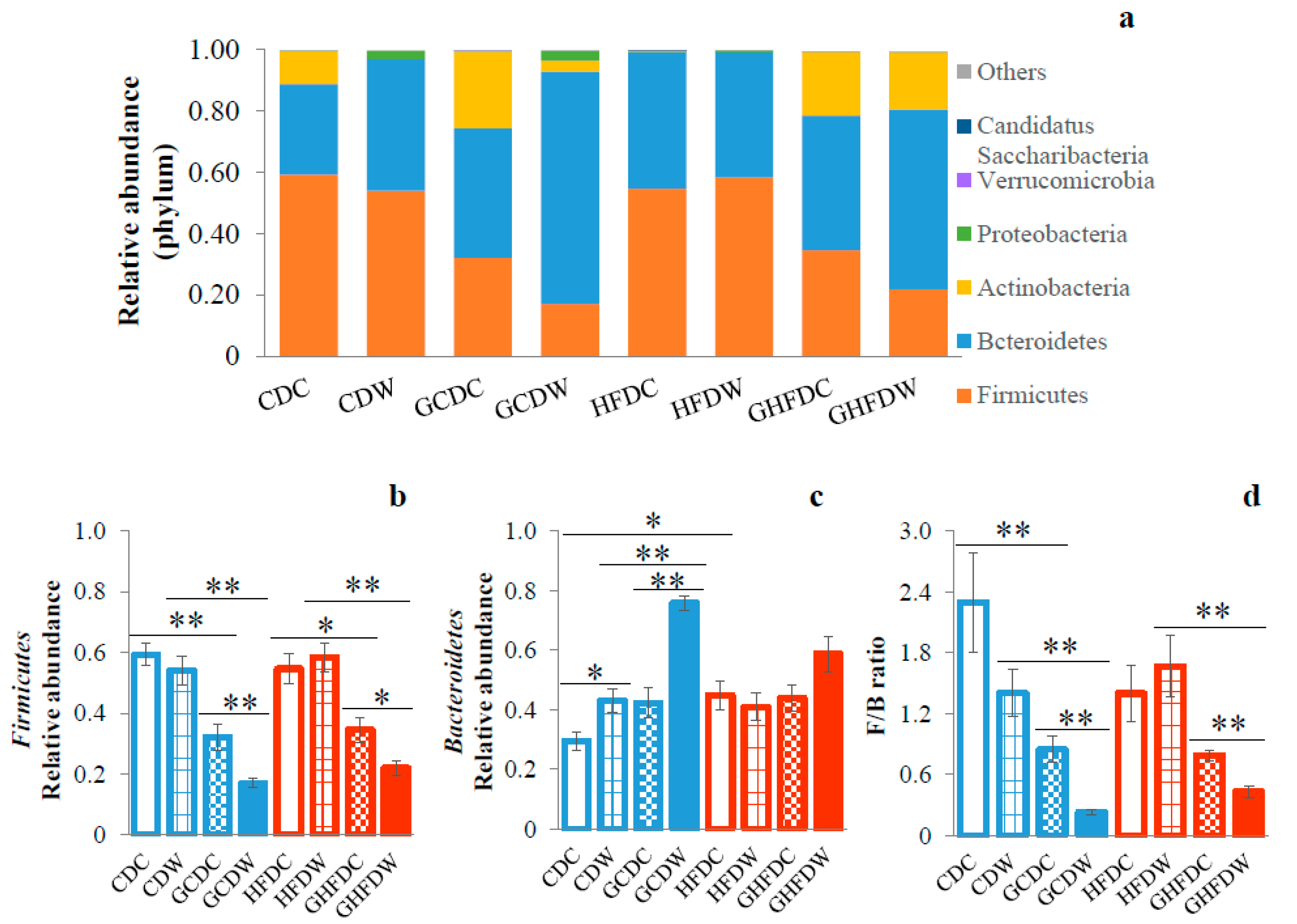
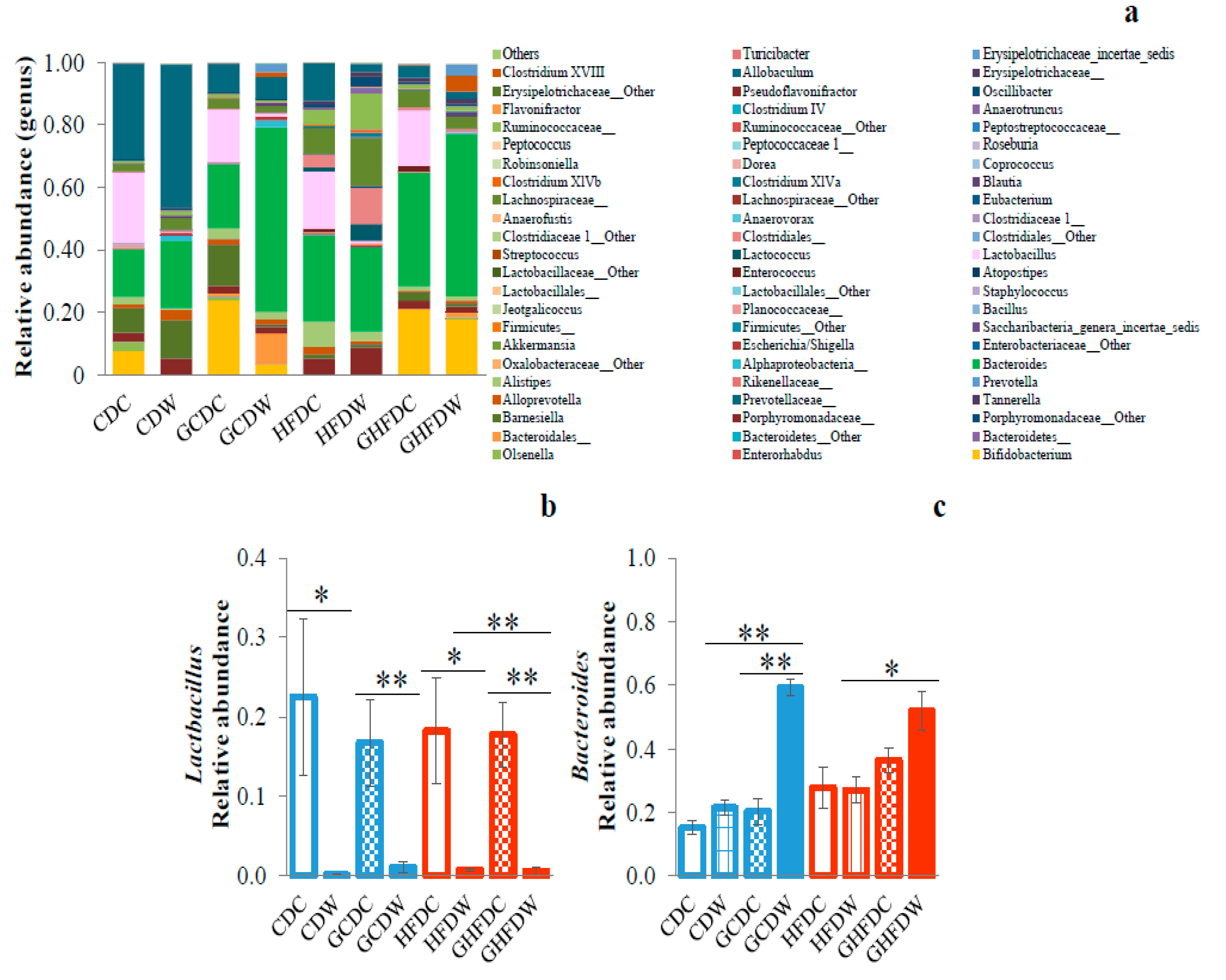
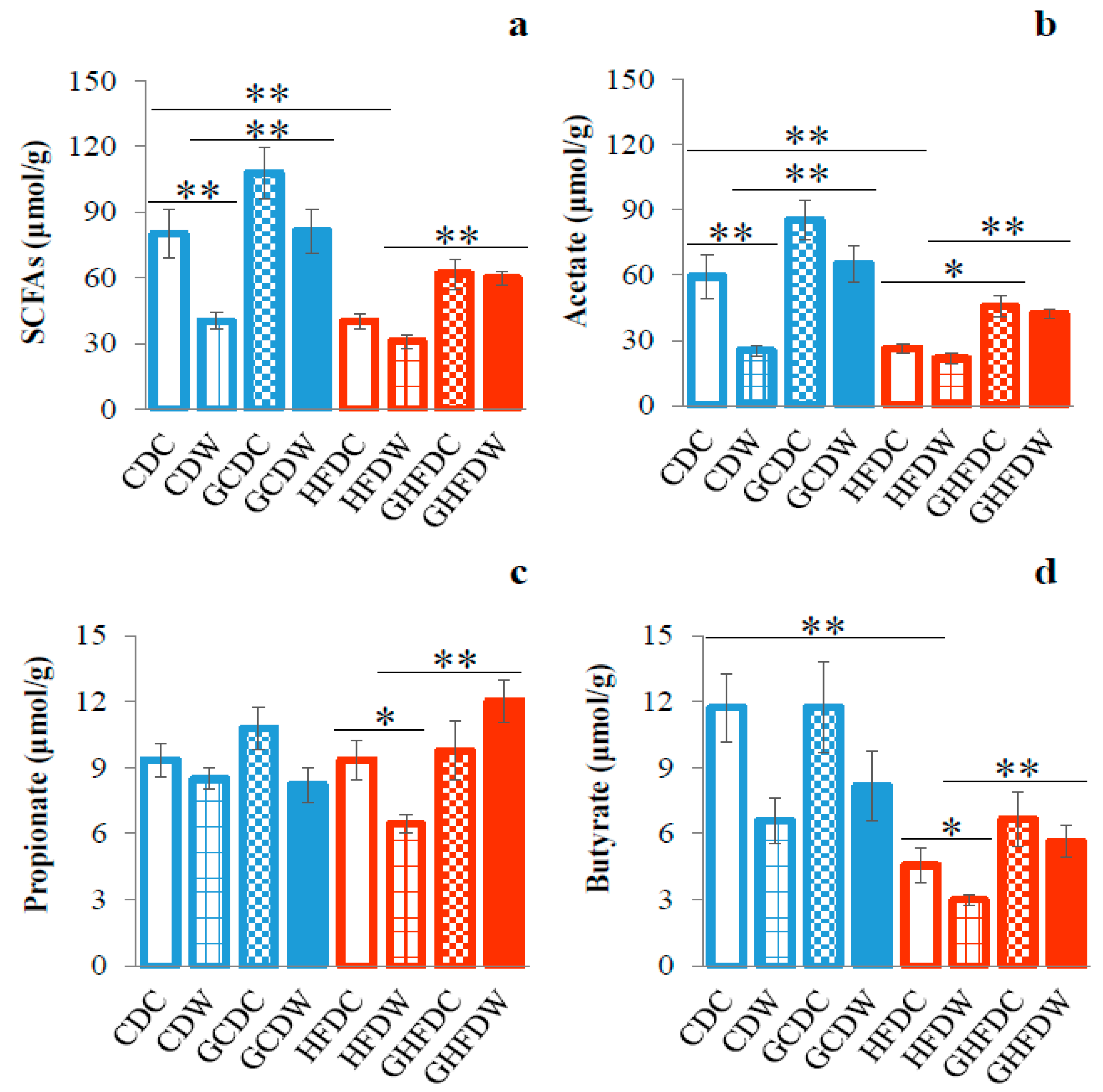
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Slavin, J. Fiber and Prebiotics: Mechanisms and Health Benefits. Nutrients 2013, 5, 1417–1435. [Google Scholar] [CrossRef]
- Markowiak, P.; Śliżewska, K. Effects of Probiotics, Prebiotics, and Synbiotics on Human Health. Nutrients 2017, 9, 1021. [Google Scholar] [CrossRef]
- McRorie, J.W., Jr.; McKeown, N.M. Understanding the Physics of Functional Fibers in the Gastrointestinal Tract: An Evidence-Based Approach to Resolving Enduring Misconceptions about Insoluble and Soluble Fiber. J. Acad. Nutr. Diet. 2017, 117, 251–264. [Google Scholar] [CrossRef]
- Yasukawa, Z.; Naito, Y.; Takagi, T.; Mizushima, K.; Tokunaga, M.; Ishihara, N.; Juneja, L.R.; Yoshikawa, T. Partially Hydrolyzed Guar Gum Affects the Expression of Genes Involved in Host Defense Functions and Cholesterol Absorption in Colonic Mucosa of db/db Male Mice. J. Clin. Biochem. Nutr. 2012, 51, 33–38. [Google Scholar] [CrossRef]
- Dall’Alba, V.; Silva, F.M.; Antonio, J.P.; Steemburgo, T.; Royer, C.P.; Almeida, J.C.; Gross, J.L.; Azevedo, M.J. Improvement of the Metabolic Syndrome Profile by Soluble Fibre-Guar Gum-in Patients with Type 2 Diabetes: A Randomised Clinical Trial. Br. J. Nutr. 2013, 110, 1601–1610. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Li, R.; Zhang, T.; Li, M.; Mou, H. Study on the Ability of Partially Hydrolyzed Guar Gum to Modulate the Gut Microbiota and Relieve Constipation. J. Food Biochem. 2019, 43, e12715. [Google Scholar] [CrossRef]
- Giannini, E.G.; Mansi, C.; Dulbecco, P.; Savarino, V. Role of Partially Hydrolyzed Guar Gum in the Treatment of Irritable Bowel Syndrome. Nutrition 2006, 22, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Alam, N.H.; Ashraf, H.; Sarker, S.A.; Olesen, M.; Troup, J.; Salam, M.A.; Gyr, N.; Meier, R. Efficacy of Partially Hydrolyzed Guar Gum-Added Oral Rehydration Solution in the Treatment of Severe Cholera in Adults. Digestion 2008, 78, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Furnari, M.; Parodi, A.; Gemignani, L.; Giannini, E.G.; Marenco, S.; Savarino, E.; Assandri, L.; Fazio, V.; Bonfanti, D.; Inferrera, S.; et al. Clinical Trial: The Combination of Rifaximin with Partially Hydrolysed Guar Gum Is More Effective than Rifaximin Alone in Eradicating Small Intestinal Bacterial Overgrowth. Aliment. Pharmacol. Ther. 2010, 32, 1000–1006. [Google Scholar] [CrossRef] [PubMed]
- Romano, C.; Comito, D.; Famiani, A.; Calamara, S.; Loddo, I. Partially Hydrolyzed Guar Gum in Pediatric Functional Abdominal Pain. World J. Gastroenterol. 2013, 19, 235–240. [Google Scholar] [CrossRef]
- Naito, Y.; Takagi, T.; Katada, K.; Uchiyama, K.; Kuroda, M.; Kokura, S.; Ichikawa, H.; Watabe, J.; Yoshida, N.; Okanoue, T.; et al. Partially Hydrolyzed Guar Gum Down-Regulates Colonic Inflammatory Response in Dextran Sulfate Sodium-Induced Colitis in Mice. J. Nutr. Biochem. 2006, 17, 402–409. [Google Scholar] [CrossRef]
- Weitkunat, K.; Stuhlmann, C.; Postel, A.; Rumberger, S.; Fankhänel, M.; Woting, A.; Petzke, K.J.; Gohlke, S.; Schulz, T.J.; Blaut, M.; et al. Short-chain fatty acids and inulin, but not guar gum, prevent diet-induced obesity and insulin resistance through differential mechanisms in mice. Sci. Rep. 2017, 7, 6109. [Google Scholar] [CrossRef]
- Suzuki, T.; Hara, H. Ingestion of Guar Gum Hydrolysate, a Soluble and Fermentable Nondigestible Saccharide, Improves Glucose Intolerance and Prevents Hypertriglyceridemia in Rats Fed Fructose. J. Nutr. 2004, 134, 1942–1947. [Google Scholar] [CrossRef] [PubMed]
- Kirwan, J.P.; Sacks, J.; Nieuwoudt, S. The Essential Role of Exercise in the Management of Type 2 Diabetes. Clevel. Clin. J. Med. 2017, 84, S15–S21. [Google Scholar] [CrossRef] [PubMed]
- Ruegsegger, G.N.; Booth, F.W. Health Benefits of Exercise. Cold Spring Harb. Perspect. Med. 2018, 8, a029694. [Google Scholar] [CrossRef]
- Karl, J.P.; Hatch, A.M.; Arcidiacono, S.M.; Pearce, S.C.; Pantoja-Feliciano, I.G.; Doherty, L.A.; Soares, J.W. Effects of Psychological, Environmental and Physical Stressors on the Gut Microbiota. Front. Microbiol. 2018, 9, 2013. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, C.M.; Davy, B.M.; Hulver, M.W.; Neilson, A.P.; Bennett, B.J.; Davy, K.P. Does Exercise Alter Gut Microbial Composition? A Systematic Review. Med. Sci. Sports Exerc. 2019, 51, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Ticinesi, A.; Lauretani, F.; Milani, C.; Nouvenne, A.; Tana, C.; Del Rio, D.; Maggio, M.; Ventura, M.; Meschi, T. Aging Gut Microbiota at the Cross-Road between Nutrition, Physical Frailty, and Sarcopenia: Is There a Gut–Muscle Axis? Nutrients 2017, 9, 1303. [Google Scholar] [CrossRef]
- Weiss, G.A.; Hennet, T. Mechanisms and consequences of intestinal dysbiosis. Cell Mol. Life Sci. 2017, 74, 2959–2977. [Google Scholar] [CrossRef]
- Du, H.; Zhao, A.; Wang, Q.; Yang, X.; Ren, D. Supplementation of Inulin with Various Degree of Polymerization Ameliorates Liver Injury and Gut Microbiota Dysbiosis in High Fat-Fed Obese Mice. J. Agric. Food Chem. 2020, 68, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.S.; Teng, C.Y.; Kalyanam, N.; Ho, C.T.; Pan, M.H. Garcinol Reduces Obesity in High-Fat-Diet-Fed Mice by Modulating Gut Microbiota Composition. Mol. Nutr. Food Res. 2019, 63, e1800390. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Smolin, J.; Nayak, J.; Ayala, J.E.; Scott, D.A.; Peterson, S.N.; Freeze, H.H. Mannose Alters Gut Microbiome, Prevents Diet-Induced Obesity, and Improves Host Metabolism. Cell Rep. 2018, 24, 3087–3098. [Google Scholar] [CrossRef]
- Ishihara, K.; Taniguchi, H. Fat Max as an Index of Aerobic Exercise Performance in Mice during Uphill Running. PLoS ONE 2018, 13, e0193470. [Google Scholar] [CrossRef] [PubMed]
- Bradley, R.L.; Jeon, J.Y.; Liu, F.F.; Maratos-Flier, E. Voluntary Exercise Improves Insulin Sensitivity and Adipose Tissue Inflammation in Diet-Induced Obese Mice. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E586–E594. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.L.; Zhu, Y.Y.; Li, L.; Shen, R.L.; Li, H. Effect of Oat Soluble and Insoluble β-glucan on Lipid Metabolism and Intestinal Lactobacillus in High-fat Diet-induced Obese Mice. J. Food Nutr. Res. 2014, 2, 510–516. [Google Scholar]
- Cole, J.R.; Wang, Q.; Fish, J.A.; Chai, B.; McGarrell, D.M.; Sun, Y.; Brown, C.T.; Porras-Alfaro, A.; Kuske, C.R.; Tiedje, J.M. Ribosomal Database Project: Data and Tools for High Throughput rRNA Analysis. Nucleic Acids Res. 2014, 42, D633–D642. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME Allows Analysis of Highthroughput Community Sequencing Data. Nat. Methods. 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Aoe, S.; Nakamura, F.; Fujiwara, S. Effect of Wheat Bran on Fecal Butyrate-Producing Bacteria and Wheat Bran Combined with Barley on Bacteroides Abundance in Japanese Healthy Adults. Nutrients 2018, 10, 1980. [Google Scholar] [CrossRef]
- den Besten, G.; Gerding, A.; van Dijk, T.H.; Ciapaite, J.; Bleeker, A.; van Eunen, K.; Havinga, R.; Groen, A.K.; Reijngoud, D.J.; Bakker, B.M. Protection against the Metabolic Syndrome by Guar Gum-Derived Short-Chain Fatty Acids Depends on Peroxisome Proliferator-Activated Receptor γ and Glucagon-Like Peptide-1. PLoS ONE 2015, 10, e0136364. [Google Scholar] [CrossRef]
- Ley, R.E.; Bäckhed, F.; Turnbaugh, P.; Lozupone, C.A.; Knight, R.D.; Gordon, J.I. Obesity Alters Gut Microbial Ecology. Proc. Natl. Acad. Sci. USA 2005, 102, 11070–11075. [Google Scholar] [CrossRef]
- Liu, D.; Huang, J.; Luo, Y.; Wen, B.; Wu, W.; Zeng, H.; Zhonghua, L. Fuzhuan Brick Tea Attenuates High-Fat Diet-Induced Obesity and Associated Metabolic Disorders by Shaping Gut Microbiota. J. Agric. Food Chem. 2019, 67, 13589–13604. [Google Scholar] [CrossRef] [PubMed]
- Ohue-Kitano, R.; Taira, S.; Watanabe, K.; Masujima, Y.; Kuboshima, T.; Miyamoto, J.; Nishitani, Y.; Kawakami, H.; Kuwahara, H.; Kimura, I. 3-(4-Hydroxy-3-Methoxyphenyl) Propionic Acid Produced from 4-Hydroxy-3-Methoxycinnamic Acid by Gut Microbiota Improves Host Metabolic Condition in Diet-Induced Obese Mice. Nutrients 2019, 11, 1036. [Google Scholar] [CrossRef] [PubMed]
- Durk, R.P.; Castillo, E.; Márquez-Magaña, L.; Grosicki, G.J.; Bolter, N.D.; Lee, C.M.; Bagley, J.R. Gut Microbiota Composition is Related to Cardiorespiratory Fitness in Healthy Young Adults. Int. J. Sport Nutr. Exerc. Metab. 2019, 29, 249–253. [Google Scholar] [CrossRef]
- Mika, A.; Van Treuren, W.; González, A.; Herrera, J.J.; Knight, R.; Fleshner, M. Exercise is More Effective at Altering Gut Microbial Composition and Producing Stable Changes in Lean Mass in Juvenile Versus Adult Male F344 Rats. PLoS ONE 2015, 10, e0125889. [Google Scholar] [CrossRef] [PubMed]
- Clarke, S.F.; Murphy, E.F.; O’Sullivan, O.; Lucey, A.J.; Humphreys, M.; Hogan, A.; Hayes, P.; O’Reilly, M.; Jeffery, I.B.; Wood-Martin, R.; et al. Exercise and Associated Dietary Extremes Impact on Gut Microbial Diversity. Gut 2014, 63, 1913–1920. [Google Scholar] [CrossRef] [PubMed]
- Oyanagi, E.; Uchida, M.; Kremenik, M.J.; Yano, H. Altered Gut Microbiota by Voluntary Exercise Induces High Physical Activity in High-Fat Diet Mice. J. Phys. Fit. Sports Med. 2018, 7, 81–85. [Google Scholar] [CrossRef][Green Version]
- Evans, C.C.; LePard, K.J.; Kwak, J.W.; Stancukas, M.C.; Laskowski, S.; Dougherty, J.; Moulton, L.; Glawe, A.; Wang, Y.; Leone, V.; et al. Exercise Prevents Weight Gain and Alters the Gut Microbiota in a Mouse Model of High Fat Diet-Induced Obesity. PLoS ONE 2014, 9, e92193. [Google Scholar] [CrossRef]
- Lambert, J.E.; Myslicki, J.P.; Bomhof, M.R.; Belke, D.D.; Shearer, J.; Reimer, R.A. Exercise Training Modifies Gut Microbiota in Normal and Diabetic Mice. Appl. Physiol. Nutr. Metab. 2015, 40, 749–752. [Google Scholar] [CrossRef]
- Armougom, F.; Henry, M.; Vialettes, B.; Raccah, D.; Raoult, D. Monitoring Bacterial Community of Human Gut Microbiota Reveals an Increase in Lactobacillus in Obese Patients and Methanogens in Anorexic Patients. PLoS ONE 2009, 4, e7125. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Hamady, M.; Yatsunenko, T.; Cantarel, B.L.; Duncan, A.; Ley, R.E.; Sogin, M.L.; Jones, W.J.; Roe, B.A.; Affourtit, J.P.; et al. A Core Gut Microbiome in Obese and Lean Twins. Nature 2009, 457, 480–484. [Google Scholar] [CrossRef]
- Million, M.; Angelakis, E.; Paul, M.; Armougom, F.; Leibovici, L.; Raoult, D. Comparative Meta-Analysis of the Effect of Lactobacillus Species on Weight Gain in Humans and Animals. Microb. Pathog. 2012, 53, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Miller, T.L. The Pathway of Formation of Acetate and Succinate from Pyruvate by Bacteroides succinogenes. Arch. Microbiol. 1978, 117, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Wrzosek, L.; Miquel, S.; Noordine, M.L.; Bouet, S.; Joncquel Chevalier-Curt, M.; Robert, V.; Philippe, C.; Bridonneau, C.; Cherbuy, C.; Robbe-Masselot, C.; et al. Bacteroides thetaiotaomicron and Faecalibacterium prausnitzii Influence the Production of Mucus Glycans and the Development of Goblet Cells in the Colonic Epithelium of a Gnotobiotic Model Rodent. BMC Biol. 2013, 11, 61. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Tang, H.; Zhang, C.; Zhao, Y.; Derrien, M.; Rocher, E.; van-Hylckama Vlieg, J.E.; Strissel, K.; Zhao, L.; Obin, M.; et al. Modulation of Gut Microbiota during Probiotic-Mediated Attenuation of Metabolic Syndrome in High Fat Diet-Fed Mice. ISME J. 2015, 9, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, B.M.; Guadagnini, D.; Tsukumo, D.M.L.; Schenka, A.A.; Latuf-Filho, P.; Vassallo, J.; Dias, J.C.; Kubota, L.T.; Carvalheira, J.B.C.; Saad, M.J.A. Modulation of Gut Microbiota by Antibiotics Improves Insulin Signalling in High-Fat Fed Mice. Diabetologia 2012, 55, 2823–2834. [Google Scholar] [CrossRef] [PubMed]
- Kondo, T.; Kishi, M.; Fushimi, T.; Kaga, T. Acetic Acid Upregulates the Expression of Genes for Fatty Acid Oxidation Enzymes in Liver to Suppress Body Fat Accumulation. J. Agric. Food Chem. 2009, 57, 5982–5986. [Google Scholar] [CrossRef]
- Yamashita, H.; Fujisawa, K.; Ito, E.; Idei, S.; Kawaguchi, N.; Kimoto, M.; Hiemori, M.; Tsuji, H. Improvement of Obesity and Glucose Tolerance by Acetate in Type 2 Diabetic Otsuka Long-Evans Tokushima Fatty (OLETF) Rats. Biosci. Biotechnol. Biochem. 2007, 71, 1236–1243. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aoki, T.; Oyanagi, E.; Watanabe, C.; Kobiki, N.; Miura, S.; Yokogawa, Y.; Kitamura, H.; Teramoto, F.; Kremenik, M.J.; Yano, H. The Effect of Voluntary Exercise on Gut Microbiota in Partially Hydrolyzed Guar Gum Intake Mice under High-Fat Diet Feeding. Nutrients 2020, 12, 2508. https://doi.org/10.3390/nu12092508
Aoki T, Oyanagi E, Watanabe C, Kobiki N, Miura S, Yokogawa Y, Kitamura H, Teramoto F, Kremenik MJ, Yano H. The Effect of Voluntary Exercise on Gut Microbiota in Partially Hydrolyzed Guar Gum Intake Mice under High-Fat Diet Feeding. Nutrients. 2020; 12(9):2508. https://doi.org/10.3390/nu12092508
Chicago/Turabian StyleAoki, Takafumi, Eri Oyanagi, Chihiro Watanabe, Nanako Kobiki, Suzuka Miura, Yuka Yokogawa, Hiromi Kitamura, Fusako Teramoto, Michael J. Kremenik, and Hiromi Yano. 2020. "The Effect of Voluntary Exercise on Gut Microbiota in Partially Hydrolyzed Guar Gum Intake Mice under High-Fat Diet Feeding" Nutrients 12, no. 9: 2508. https://doi.org/10.3390/nu12092508
APA StyleAoki, T., Oyanagi, E., Watanabe, C., Kobiki, N., Miura, S., Yokogawa, Y., Kitamura, H., Teramoto, F., Kremenik, M. J., & Yano, H. (2020). The Effect of Voluntary Exercise on Gut Microbiota in Partially Hydrolyzed Guar Gum Intake Mice under High-Fat Diet Feeding. Nutrients, 12(9), 2508. https://doi.org/10.3390/nu12092508




