The Protective Effect of Myristica fragrans Houtt. Extracts Against Obesity and Inflammation by Regulating Free Fatty Acids Metabolism in Nonalcoholic Fatty Liver Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Nutmeg Extract Preparation and Function Identification
2.2. Cell Lines and Viability Assays
2.3. Animal Treatment
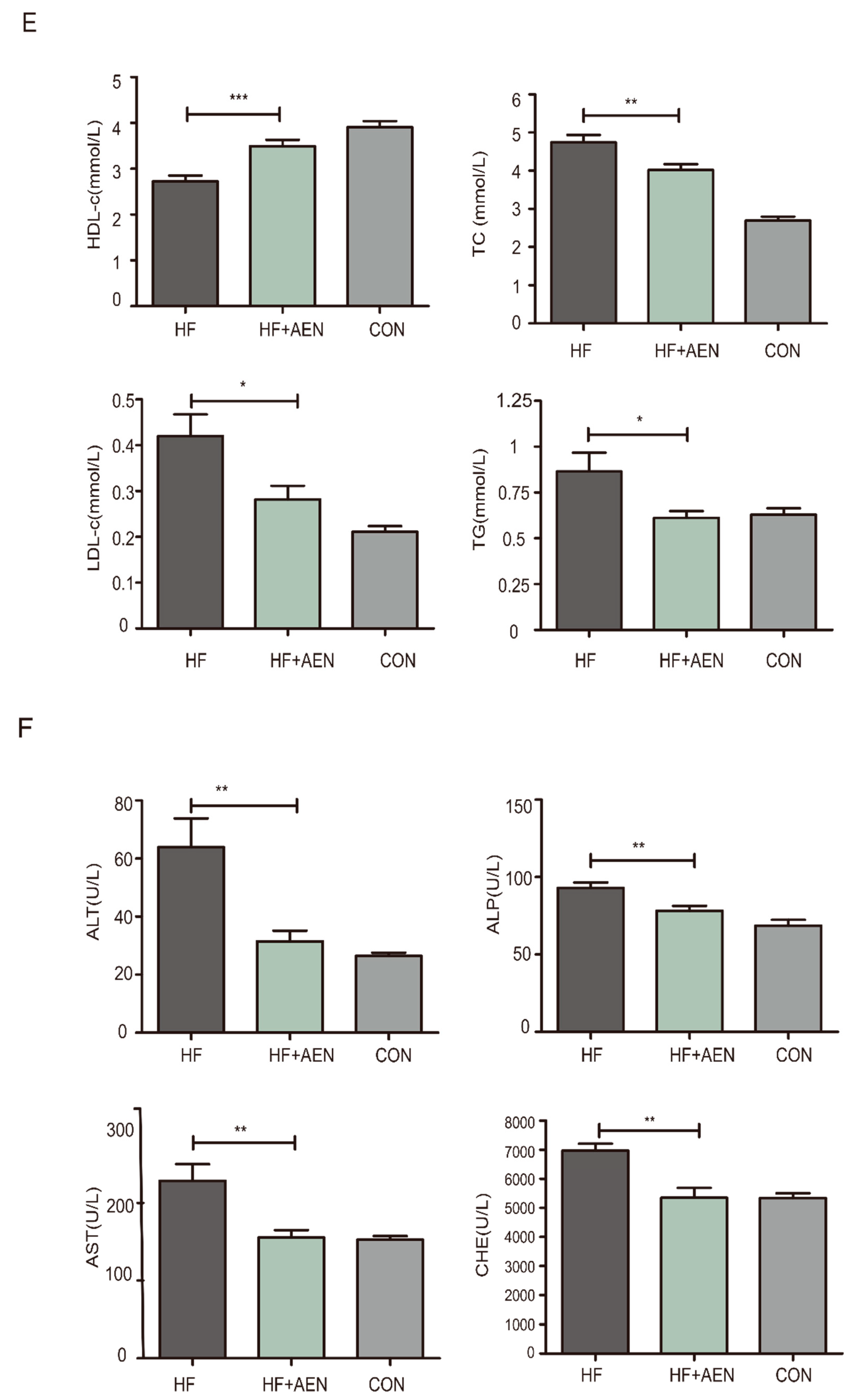
2.4. Serum Biochemical Index Determination
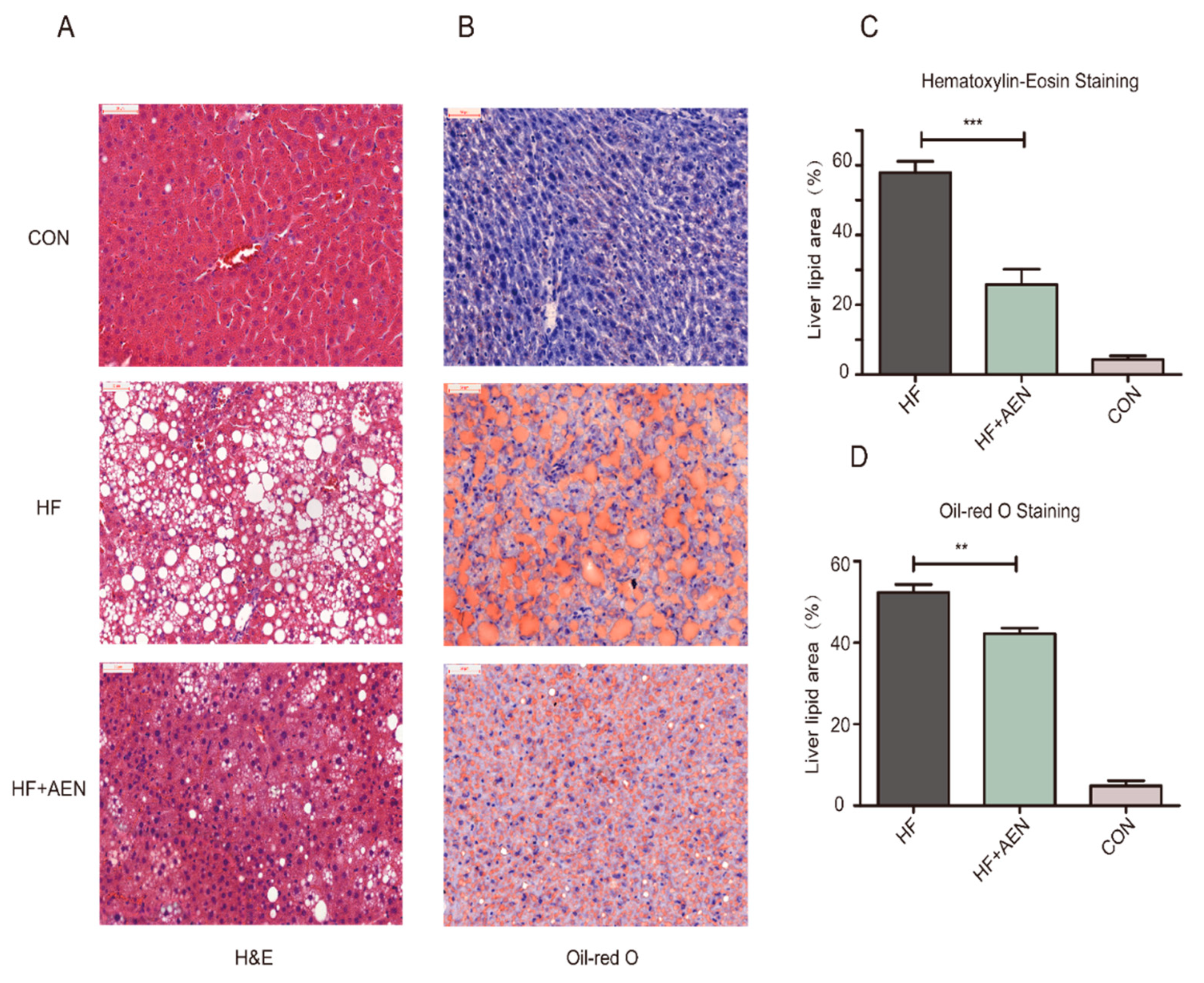
2.5. Hematoxylin and Eosin (H&E) and Oil Red O Staining
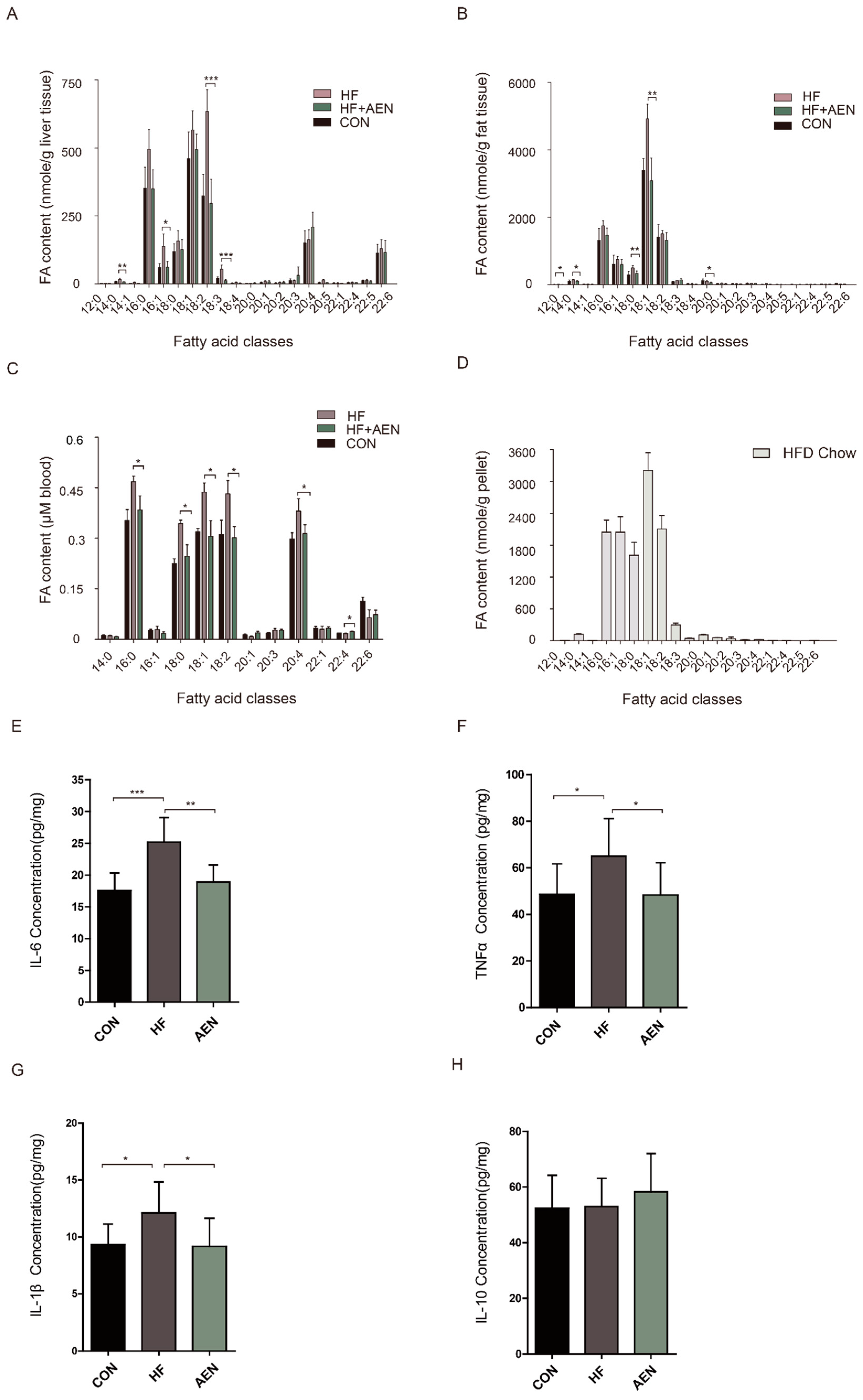
2.6. Fatty Acid Analysis of Tissue Samples
2.7. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR) Analysis
2.8. Cytokines Measurements
2.9. Western Blotting
2.10. Chemical Composition Analysis of AEN Using GC-MS
2.11. Statistical Analysis
3. Results
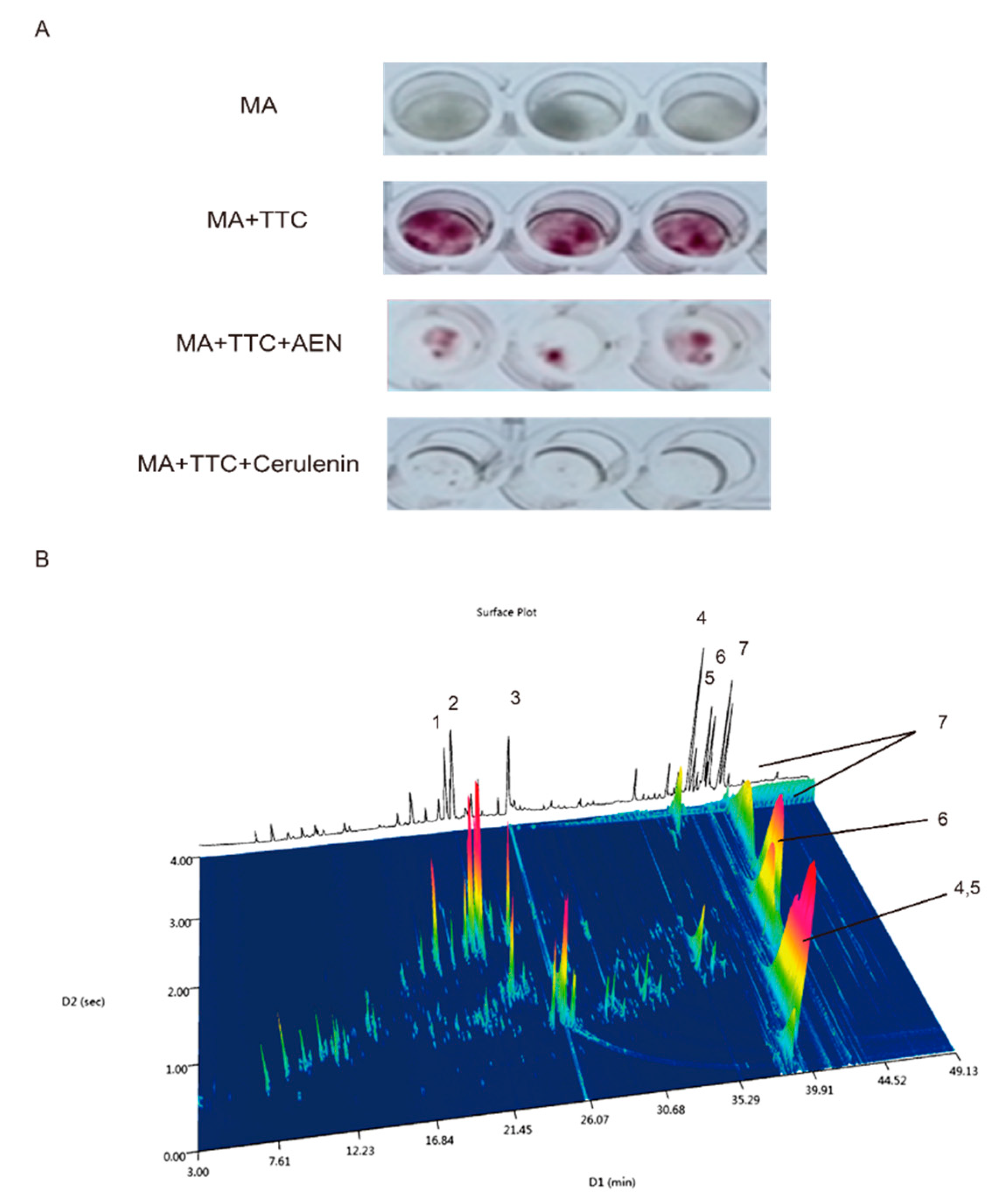
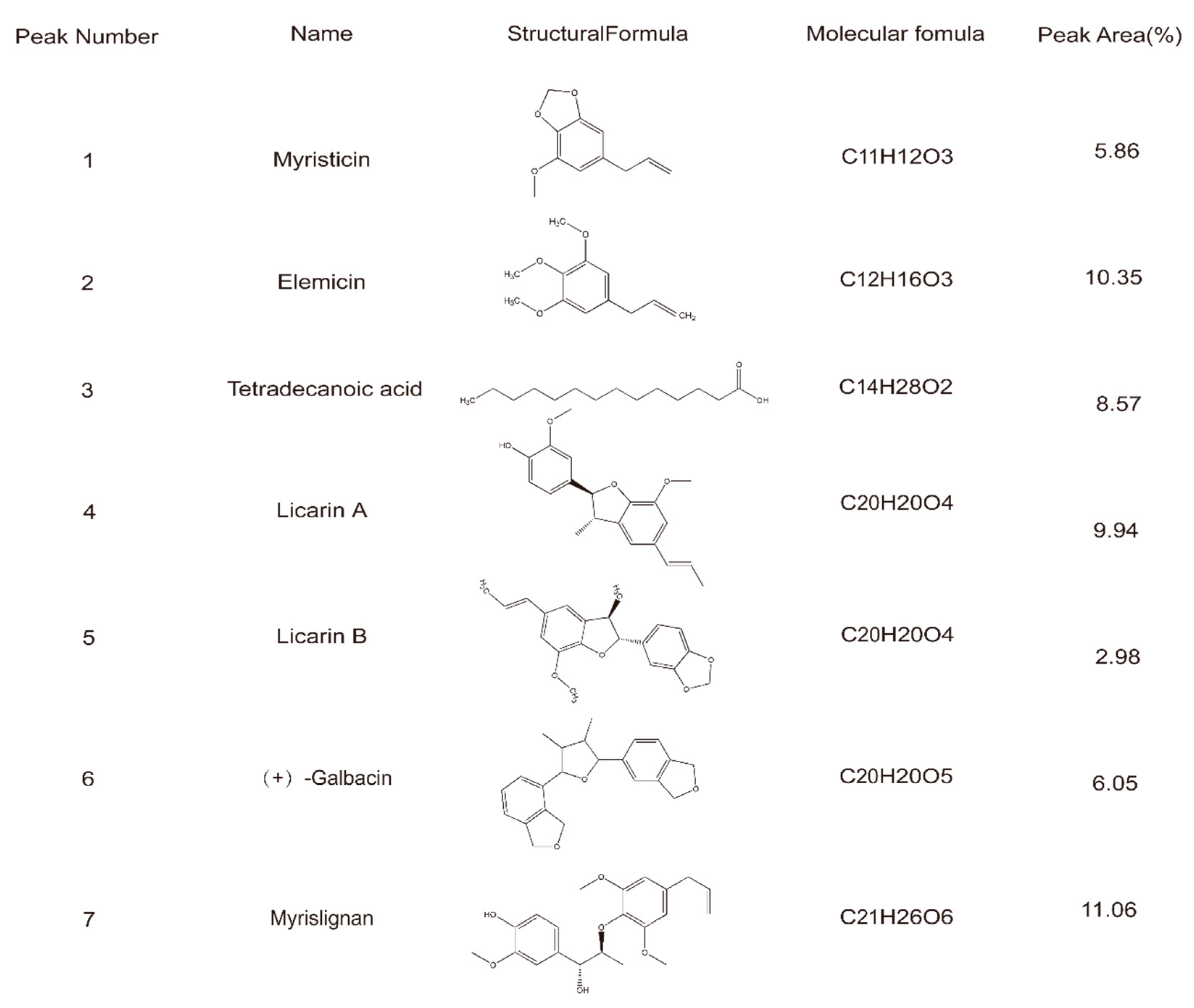
3.1. Preliminary Component Identification
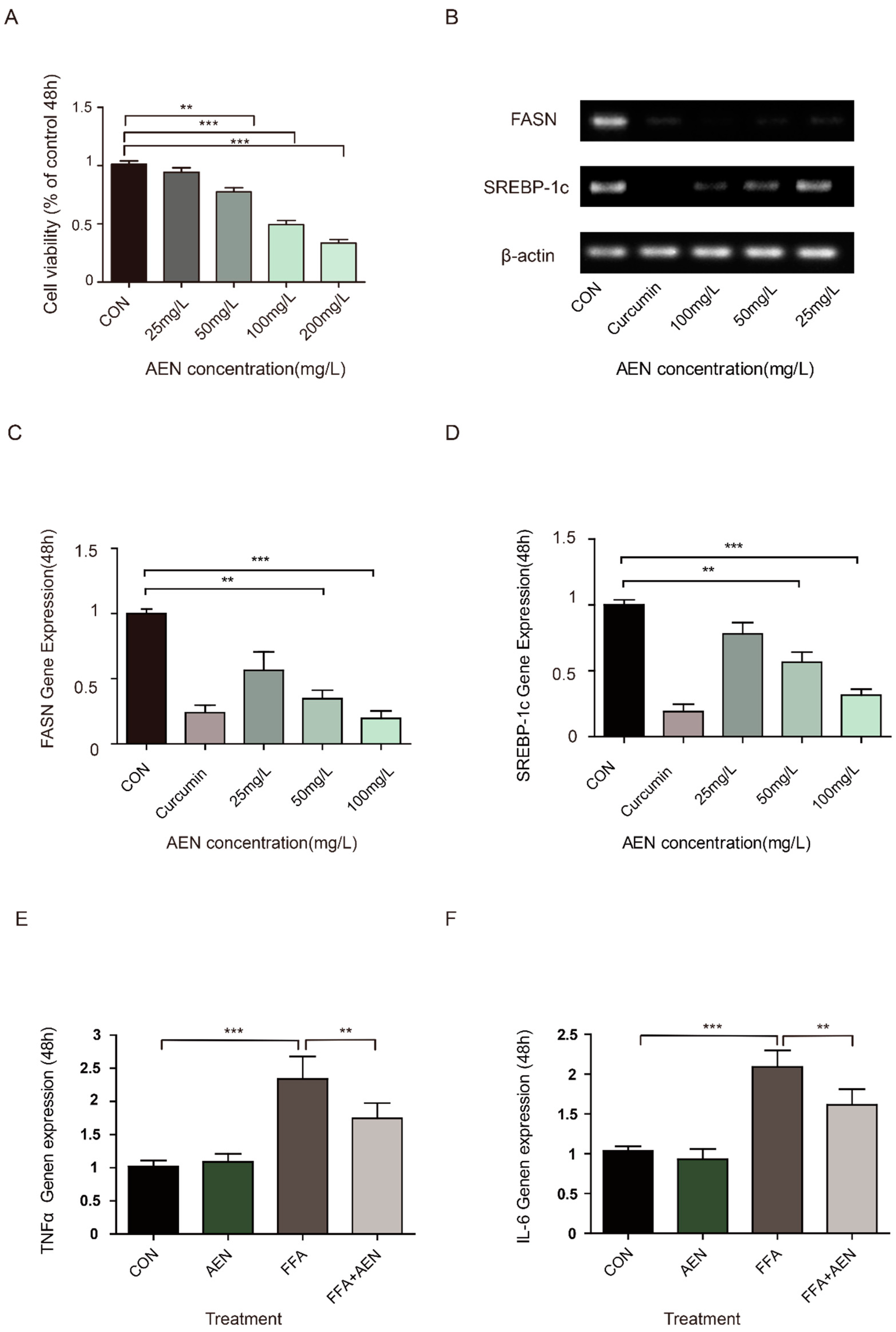
3.2. AEN Inhibited Gene Expression for Fatty Acid Synthesis and Inflammatory In Vitro
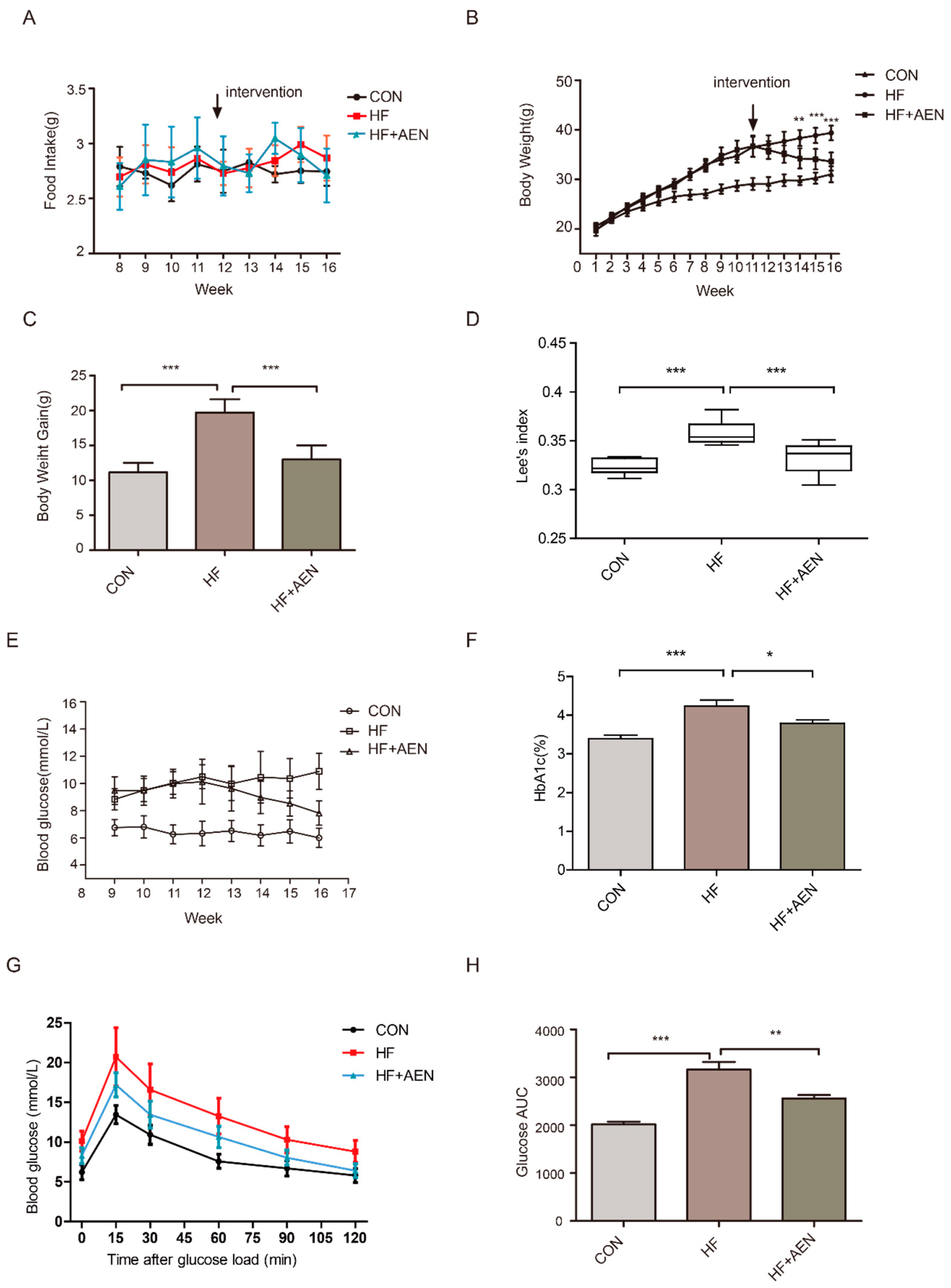
3.3. AEN Treatment Reduced Obesity and Blood Glucose in Mice Induced by a High-Fat Diet
3.4. AEN Improved Hepatocyte Steatosis and Liver Function in Mice
3.5. AEN Regulated Fatty Acid Metabolism and Inflammatory Reaction in Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Younossi, Z.M.; Anstee, Q.M.; Marietti, M.; Hardy, T.; Henry, L.; Eslam, M.; George, J.; Bugianesi, E. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 11. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.C.; Horton, J.D.; Hobbs, H.H. Human fatty liver disease: Old questions and new insights. Science 2011, 332, 1519–1523. [Google Scholar] [CrossRef] [PubMed]
- Brunt, E.M.; Wong, V.W.S.; Nobili, V.; Day, C.P.; Sookoian, S.; Maher, J.J.; Bugianesi, E.; Sirlin, C.B.; Neuschwandertetri, B.A.; Rinella, M.E. Nonalcoholic fatty liver disease. Nat. Rev. Dis. Primers 2015, 1, 15080. [Google Scholar] [CrossRef]
- Loomba, R.; Sanyal, A.J. The global NAFLD epidemic. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 686–690. [Google Scholar] [CrossRef] [PubMed]
- Willebrords, J.; Pereira, I.V.A.; Maes, M.; Yanguas, S.C.; Colle, I.; Bossche, B.V.D.; Silva, T.C.D.; Oliveira, C.P.; Andraus, W.; Alves, V.A.F. Strategies, models and biomarkers in experimental non-alcoholic fatty liver disease research. Prog. Lipid Res. 2015, 59, 106–125. [Google Scholar] [CrossRef] [PubMed]
- Woods, A.; Williams, J.R.; Muckett, P.J.; Mayer, F.V.; Liljevald, M.; Bohloolyy, M.; Carling, D. Liver-specific activation of AMPK prevents steatosis on a high-fructose diet. Cell Rep. 2017, 18, 3043. [Google Scholar] [CrossRef]
- Terangarcia, M.; Adamson, A.W.; Yu, G.; Rufo, C.; Suchankova, G.; Dreesen, T.D.; Tekle, M.; Clarke, S.D.; Gettys, T.W. Polyunsaturated fatty acid suppression of fatty acid synthase (FASN): Evidence for dietary modulation of NF-Y binding to the fasn promoter by SREBP-1c. Biochem. J. 2007, 402, 591–600. [Google Scholar] [CrossRef]
- Zhang, M.; Sun, W.; Zhou, M.; Tang, Y. MicroRNA-27a regulates hepatic lipid metabolism and alleviates NAFLD via repressing FAS and SCD1. Sci. Rep. 2017, 7, 14493. [Google Scholar] [CrossRef]
- Koutsari, C.; Mundi, M.S.; Ali, A.H.; Patterson, B.W.; Jensen, M.D. Systemic free fatty acid disposal into very low-density lipoprotein triglycerides. Diabetes 2013, 62, 2386–2395. [Google Scholar] [CrossRef]
- Choi, S.; Diehl, A.M. Role of inflammation in nonalcoholic steatohepatitis. Curr. Opin. Gastroenterol. 2005, 21, 702–707. [Google Scholar] [CrossRef]
- Wieckowska, A.; Papouchado, B.G.; Li, Z.Z.; Lopez, R.; Zein, N.N.; Feldstein, A.E. Increased hepatic and circulating interleukin-6 levels in human nonalcoholic steatohepatitis. Am. J. Gastroenterol. 2008, 103, 1372–1379. [Google Scholar] [CrossRef] [PubMed]
- Leggate, M. The IL-6 System and Its Interaction with Chronic Low-Grade Inflammation and High Intensity Intermittent Exercise. Ph.D. Thesis, Loughborough University, Leicestershire, UK, 2012. [Google Scholar]
- Jiao, P.; Ma, J.; Feng, B.; Zhang, H.; Xu, H. FFA-induced adipocyte inflammation and insulin resistance: Involvement of ER stress and IKKβ pathways. Obesity 2011, 19, 483–491. [Google Scholar] [CrossRef]
- Wymann, M.P.; Schneiter, R. Lipid signalling in disease. Nat. Rev. Mol. Cell Biol. 2008, 9, 162–176. [Google Scholar] [CrossRef] [PubMed]
- Viñas, G.; Oliveras, G.; Perez-Bueno, F.; Giro, A.; Blancafort, A.; Puig-Vives, M.; Marcos-Gragera, R.; Dorca, J.; Brunet, J.; Puig, T. Abstract P4-09-11: Fatty Acid Synthase (FASN) expression in Triple-Negative Breast Cancer. In Proceedings of the AACR, San Antonio, TX, USA, 4–8 December 2012. [Google Scholar]
- Fabbrini, E.; DeHaseth, D.; Deivanayagam, S.; Mohammed, B.S.; Vitola, B.E.; Klein, S. Alterations in fatty acid kinetics in obese adolescents with increased intrahepatic triglyceride content. Obesity 2009, 17, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Carling, D.; Thornton, C.; Woods, A.; Sanders, M.J. AMP-activated protein kinase: New regulation, new roles? Biochem. J. 2012, 445, 11–27. [Google Scholar] [CrossRef] [PubMed]
- Dandapani, M.; Hardie, D.G. AMPK: Opposing the metabolic changes in both tumour cells and inflammatory cells? Biochem. Soc. Trans. 2013, 41, 687–693. [Google Scholar] [CrossRef]
- Li, Y.; Xu, S.; Mihaylova, M.M.; Zheng, B.; Hou, X.; Jiang, B.; Park, O.; Luo, Z.; Lefai, E.; Shyy, J.Y.-J. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab. 2011, 13, 376–388. [Google Scholar] [CrossRef]
- Gwinn, D.M.; Shackelford, D.B.; Egan, D.F.; Mihaylova, M.M.; Mery, A.; Vasquez, D.S.; Turk, B.E.; Shaw, R.J. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol. Cell 2008, 30, 214–226. [Google Scholar] [CrossRef]
- Shao, W.; Yu, Z.; Chiang, Y.; Yang, Y.; Chai, T.; Foltz, W.; Lu, H.; Fantus, I.G.; Jin, T. Curcumin prevents high fat diet induced insulin resistance and obesity via attenuating lipogenesis in liver and inflammatory pathway in adipocytes. PLoS ONE 2012, 7, e28784. [Google Scholar] [CrossRef]
- Tang, Y. Curcumin targets multiple pathways to halt hepatic stellate cell activation: Updated mechanisms in vitro and in vivo. Dig. Dis. Sci. 2015, 60, 1554–1564. [Google Scholar] [CrossRef]
- Nabavi, S.F.; Daglia, M.; Moghaddam, A.H.; Habtemariam, S.; Nabavi, S.M. Curcumin and liver disease: From chemistry to medicine. Compr. Rev. Food Sci. Food Saf. 2014, 13, 62–77. [Google Scholar] [CrossRef]
- Ding, Y.; Gu, Z.; Wang, Y.; Wang, S.; Chen, H.; Zhang, H.; Chen, W.; Chen, Y.Q. Clove extract functions as a natural fatty acid synthesis inhibitor and prevents obesity in a mouse model. Food Funct. 2017. [Google Scholar] [CrossRef] [PubMed]
- Pathak, S.P.; Ojha, V.N. The component glycerides of nutmeg butter (Myristica fragrans). J. Sci. Food Agric. 1957, 8, 537–540. [Google Scholar] [CrossRef]
- Maya, K.M.; Zachariah, T.J.; Krishnamurthy, K.S.; Rema, J.; Krishnamoorthy, B. Fatty acids and leaf amino acids in Myristica fragrans Houtt. and related taxa. Indian J. Hortic. 2006, 63, 316–318. [Google Scholar]
- Abourashed, E.A.; El-Alfy, A.T. Chemical diversity and pharmacological significance of the secondary metabolites of nutmeg (Myristica fragrans Houtt.). Phytochem. Rev. Proc. Phytochem. Soc. Eur. 2016, 15, 1035. [Google Scholar] [CrossRef]
- Olaleye, M.T.; Akinmoladun, C.A.; Akindahunsi, A.A. Antioxidant properties of myristica fragrans (Houtt) and its effect on selected organs of albino rats. Afr. J. Biotechnol. 2006, 5, 1274–1278. [Google Scholar]
- Acuña, U.M.; Carcache, P.J.B.; Matthew, S.; Blanco, E.J.C.D. New acyclic bis phenylpropanoid and neolignans, from Myristica fragrans Houtt., exhibiting PARP-1 and NF-κB inhibitory effects. Food Chem. 2016, 202, 269–275. [Google Scholar]
- Le, T.V.T.; Nguyen, P.H.; Hong, S.C.; Yang, J.L.; Kang, K.W.; Ahn, S.G.; Oh, W.K. Diarylbutane-type lignans from Myristica fragrans (Nutmeg) show the cytotoxicity against breast cancer cells through activation of AMP-activated protein kinase. Nat. Prod. Sci. 2017, 23, 21. [Google Scholar] [CrossRef]
- Zhang, W.K.; Tao, S.S.; Li, T.T.; Li, Y.S.; Li, X.J.; Tang, H.B.; Cong, R.H.; Ma, F.L.; Wan, C.J. Nutmeg oil alleviates chronic inflammatory pain through inhibition of COX-2 expression and substance P release in vivo. Food Nutr. Res. 2016, 60, 30849. [Google Scholar] [CrossRef]
- Gupta, A.D.; Bansal, V.K.; Babu, V.; Maithil, N. Chemistry, antioxidant and antimicrobial potential of nutmeg (Myristica fragrans Houtt.). J. Genet. Eng. Biotechnol. 2013, 11, 25–31. [Google Scholar] [CrossRef]
- Sangalli, B.C.; Sangalli, B.; Chiang, W. Toxicology of nutmeg abuse. J. Toxicol. Clin. Toxicol. 2000, 38, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Forrester, M.B. Nutmeg intoxication in Texas, 1998–2004. Hum. Exp. Toxicol. 2005, 24, 563–566. [Google Scholar] [CrossRef] [PubMed]
- Stein, U.; Greyer, H.; Hentschel, H. Nutmeg (myristicin) poisoning—Report on a fatal case and a series of cases recorded by a poison information centre. Forensic Sci. Int. 2001, 118, 87–90. [Google Scholar] [CrossRef]
- Yuliana, N.D.; Iqbal, M.; Jahangir, M.; Wijaya, C.H.; Korthout, H.; Kottenhage, M.; Kim, H.K.; Verpoorte, R. Screening of selected Asian spices for anti obesity-related bioactivities. Food Chem. 2011, 126, 1724–1729. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.H.; Le, T.V.T.; Hu, W.K.; Chae, J.; Sang, K.K.; Kwon, K.I.; Seo, D.B.; Sang, J.L.; Oh, W.K. AMP-activated protein kinase (AMPK) activators from myristica fragrans (nutmeg) and their anti-obesity effect. Bioorganic Med. Chem. Lett. 2010, 20, 4128–4131. [Google Scholar] [CrossRef]
- Sailesh, K.S.; Padmanabha. A comparative study of the anti diabetic effect of oral administration of cinnamon, nutmeg and peppermint in wistar albino rats. Int. J. Health Sci. Res. 2014, 4, 61–67. [Google Scholar]
- Ji, Y.L.; Park, W. Anti-inflammatory effect of myristicin on RAW 264.7 macrophages stimulated with polyinosinic-polycytidylic acid. Molecules 2011, 16, 7132–7142. [Google Scholar]
- Yimam, M.; Jiao, P.; Hong, M.; Jia, Q. Hepatoprotective activity of an herbal composition, MAP, a standardized blend comprising myristica fragrans, astragalus membranaceus, and poria cocos. J. Med. Food 2016, 19, 953–960. [Google Scholar] [CrossRef]
- Yang, X.N.; Liu, X.M.; Fang, J.H.; Zhu, X.; Yang, X.W.; Xiao, X.R.; Huang, J.F.; Gonzalez, F.J.; Li, F. PPARα mediates the hepatoprotective effects of nutmeg. J. Proteome Res. 2018, 17, 1887–1897. [Google Scholar] [CrossRef]
- Leng, L.; Jiang, Z.Q.; Ji, G.Y. Effects of soybean isoflavone on liver lipid metabolism in nonalcoholic fatty liver rats. Zhonghua Yu Fang Yi Xue Za Zhi Chin. J. Prev. Med. 2011, 45, 335–339. [Google Scholar]
- Jiang, Y.; Gao, Y.Q.; Zhu, M.Q.; Man, L.I.; Zhang, X.; Hepatology, D.O. Establishment of the nonalcoholic fatty liver disease model in C57BL/6 mice. J. Bengbu Med. Coll. 2018, 43, P573–P576. [Google Scholar]
- Wojcikowski, K.; Gobe, G. Animal studies on medicinal herbs: Predictability, dose conversion and potential value. Phytother. Res. 2014, 28, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef] [PubMed]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Ken′Ichi, I.; Yumeto, F. Preparation of fatty acid methyl esters for gas-liquid chromatography. J. Lipid Res. 2010, 51, 635. [Google Scholar]
- Donnelly, K.L.; Smith, C.I.; Schwarzenberg, S.J.; Jessurun, J.; Boldt, M.O.; Parks, E.J.; Boldt, M.D. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J. Clin. Investig. 2005, 115, 1343–1351. [Google Scholar] [CrossRef]
- Hodson, L.; Gunn, P.J. The regulation of hepatic fatty acid synthesis and partitioning: The effect of nutritional state. Nat. Rev. Endocrinol. 2019, 15, 689–700. [Google Scholar] [CrossRef]
- Shoelson, S.E.; Herrero, L.; Naaz, A. Obesity, inflammation, and insulin resistance. Gastroenterology 2014, 132, 2169–2180. [Google Scholar] [CrossRef]
- Zivkovic, A.M.; German, J.B.; Sanyal, A.J. Comparative review of diets for the metabolic syndrome: Implications for nonalcoholic fatty liver disease. Am. J. Clin. Nutr. 2007, 86, 285–300. [Google Scholar] [CrossRef]
- Bodemar, G.; Mathiesen, U.L.; Thorelius, L.; Kechagias, S.; Ekstedt, M.; Holmqvist, M.; Franzen, J.L. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology 2010, 44, 865–873. [Google Scholar]
- Fuchs, C.D.; Claudel, T.; Trauner, M. Role of metabolic lipases and lipolytic metabolites in the pathogenesis of NAFLD. Trends Endocrinol. Metab. 2014, 25, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Yang, L.; Li, P.; Hofmann, O.; Dicker, L.; Hide, W.; Lin, X.; Watkins, S.M.; Ivanov, A.R.; Hotamisligil, G.S. Aberrant lipid metabolism disrupts calcium homeostasis causing liver endoplasmic reticulum stress in obesity. Nature 2011, 473, 528. [Google Scholar] [CrossRef] [PubMed]
- Adams, L.A.; Angulo, P.; Lindor, K.D. Nonalcoholic fatty liver disease. Ann. Epidemiol. 2007, 17, 863–869. [Google Scholar] [CrossRef] [PubMed]
- Fabbrini, E.; Sullivan, S.; Klein, S. Obesity and nonalcoholic fatty liver disease: Biochemical, metabolic, and clinical implications. Hepatology 2010, 51, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, M. Non-alcoholic fatty liver disease: The bile acid-activated farnesoid x receptor as an emerging treatment target. J. Lipids 2012, 2012, 1–8. [Google Scholar] [CrossRef]
- Berlanga, A.; Guiu-Jurado, E.; Porras, J.A.; Auguet, T. Molecular pathways in non-alcoholic fatty liver disease. Clin. Exp. Gastroenterol. 2014, 7, 221. [Google Scholar]
- Kohjima, M.; Enjoji, M.; Higuchi, N.; Kato, M.; Kotoh, K.; Yoshimoto, T.; Fujino, T.; Yada, M.; Yada, R.; Harada, N. Re-evaluation of fatty acid metabolism-related gene expression in nonalcoholic fatty liver disease. Int. J. Mol. Med. 2007, 20, 351–358. [Google Scholar] [CrossRef]
- Leamy, A.K.; Egnatchik, R.A.; Young, J.D. Molecular mechanisms and the role of saturated fatty acids in the progression of non-alcoholic fatty liver disease. Prog. Lipid Res. 2013, 52, 165–174. [Google Scholar] [CrossRef]
- Wang, D.; Wei, Y.; Pagliassotti, M.J. Saturated fatty acids promote endoplasmic reticulum stress and liver injury in rats with hepatic steatosis. Endocrinology 2006, 147, 943. [Google Scholar] [CrossRef]
- Loison, C.; Mendy, F.O.; Sérougne, C.; Lutton, C. Dietary myristic acid modifies the HDL-cholesterol concentration and liver scavenger receptor BI expression in the hamster. Br. J. Nutr. 2002, 87, 199–210. [Google Scholar] [CrossRef]
- Morita, T.; Jinno, K.; Kawagishi, H.; Arimoto, Y.; Sugiyama, K. Hepatoprotective effect of myristicin from nutmeg (Myristica fragrans) on lipopolysaccharide/d-galactosamine-induced liver injury. J. Agric. Food Chem. 2003, 51, 1560. [Google Scholar] [CrossRef] [PubMed]
- Shyni, G.; Sasidharan, K.; Francis, S.K.; Das, A.A.; Nair, M.S.; Raghu, K. Licarin B from myristica fragrans improves insulin sensitivity via PPARγ and activation of GLUT4 in the IRS-1/PI3K/AKT pathway in 3T3-L1 adipocytes. RSC Adv. 2016, 6, 79859–79870. [Google Scholar] [CrossRef]
- Lal, M.; Dutta, S.; Munda, S.; Pandey, S.K. Novel high value elemicin-rich germplasm of lemon grass (Cymbopogon khasianus (Hack) Stapf (ex Bor) from north east India. Ind. Crop. Prod. 2018, 115, 98–103. [Google Scholar] [CrossRef]
- Ghorbanian, D.; Ghasemi-Kasman, M.; Hashemian, M.; Gorji, E.; Gol, M.; Feizi, F.; Kazemi, S.; Ashrafpour, M.; Moghadamnia, A.A. Myristica fragrans Houtt extract attenuates neuronal loss and glial activation in pentylenetetrazol-induced kindling model. Iran. J. Pharm. Res. Ijpr 2019, 18, 812. [Google Scholar]
- Rioux, V.; Catheline, D.; Bouriel, M.; Legrand, P. Dietary myristic acid at physiologically relevant levels increases the tissue content of C20:5 n-3 and C20:3 n-6 in the rat. Reprod Nutr. Dev. 2005, 45, 599–612. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, W.; Song, F.; Hu, D.; Chen, H.; Zhai, Q.; Lu, W.; Zhao, J.; Zhang, H.; Chen, W.; Gu, Z.; et al. The Protective Effect of Myristica fragrans Houtt. Extracts Against Obesity and Inflammation by Regulating Free Fatty Acids Metabolism in Nonalcoholic Fatty Liver Disease. Nutrients 2020, 12, 2507. https://doi.org/10.3390/nu12092507
Zhao W, Song F, Hu D, Chen H, Zhai Q, Lu W, Zhao J, Zhang H, Chen W, Gu Z, et al. The Protective Effect of Myristica fragrans Houtt. Extracts Against Obesity and Inflammation by Regulating Free Fatty Acids Metabolism in Nonalcoholic Fatty Liver Disease. Nutrients. 2020; 12(9):2507. https://doi.org/10.3390/nu12092507
Chicago/Turabian StyleZhao, Wenyu, Fanfen Song, Diangeng Hu, Haiqin Chen, Qixiao Zhai, Wenwei Lu, Jianxin Zhao, Hao Zhang, Wei Chen, Zhennan Gu, and et al. 2020. "The Protective Effect of Myristica fragrans Houtt. Extracts Against Obesity and Inflammation by Regulating Free Fatty Acids Metabolism in Nonalcoholic Fatty Liver Disease" Nutrients 12, no. 9: 2507. https://doi.org/10.3390/nu12092507
APA StyleZhao, W., Song, F., Hu, D., Chen, H., Zhai, Q., Lu, W., Zhao, J., Zhang, H., Chen, W., Gu, Z., & Wang, G. (2020). The Protective Effect of Myristica fragrans Houtt. Extracts Against Obesity and Inflammation by Regulating Free Fatty Acids Metabolism in Nonalcoholic Fatty Liver Disease. Nutrients, 12(9), 2507. https://doi.org/10.3390/nu12092507




