A Review of Recent Findings on Meal Sequence: An Attractive Dietary Approach to Prevention and Management of Type 2 Diabetes
Abstract
1. Introduction
2. Secretion and Function of Glucagon-Like Peptide-1 (GLP-1)
3. Preloading Protein and/or Fats before Carbohydrates
4. Preloading Dietary Fiber before Carbohydrates
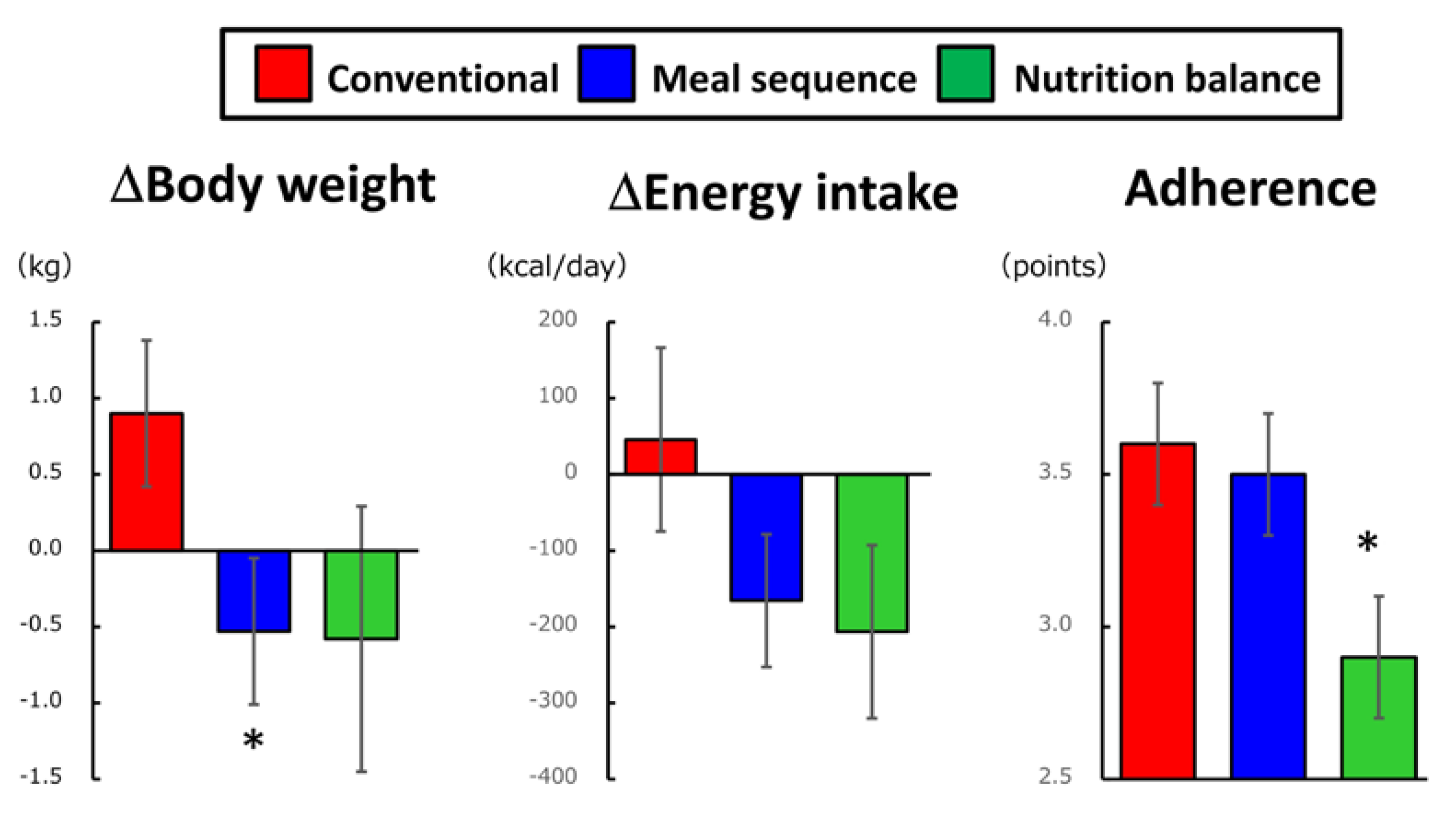
5. Long-Term Effects of Preload-Based Dietary Strategies
6. Conclusive Remarks and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Monnier, L.; Colette, C. Contributions of fasting and postprandial glucose to hemoglobin A1c. Endocr. Pract. 2006, 12 (Suppl. 1), 42–46. [Google Scholar] [CrossRef] [PubMed]
- Smith-Palmer, J.; Brandle, M.; Trevisan, R.; Orsini Federici, M.; Liabat, S.; Valentine, W. Assessment of the association between glycemic variability and diabetes-related complications in type 1 and type 2 diabetes. Diabetes Res. Clin. Pract. 2014, 105, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Bhupathiraju, S.N.; Tobias, D.K.; Malik, V.S.; Pan, A.; Hruby, A.; Manson, J.E.; Willett, W.C.; Hu, F.B. Glycemic index, glycemic load, and risk of type 2 diabetes: Results from 3 large US cohorts and an updated meta-analysis. Am. J. Clin. Nutr. 2014, 100, 218–232. [Google Scholar] [CrossRef] [PubMed]
- Borch-Johnsen, K.; Neil, A.; Balkau, B.; Larsen, S.; Nissinen, A.; Pekkanen, J.; Keinanen-Kiukaanniemi, S.; Hiltunen, L.; Kivela, S.L.; Jousilahti, P.; et al. Glucose tolerance and mortality: Comparison of WHO and American Diabetes Association diagnostic criteria. The DECODE study group. European Diabetes Epidemiology Group. Diabetes Epidemiology: Collaborative analysis Of Diagnostic criteria in Europe. Lancet 1999, 354, 617–621. [Google Scholar]
- Chiasson, J.L.; Josse, R.G.; Gomis, R.; Hanefeld, M.; Karasik, A.; Laakso, M. Acarbose treatment and the risk of cardiovascular disease and hypertension in patients with impaired glucose tolerance: The STOP-NIDDM trial. JAMA 2003, 290, 486–494. [Google Scholar] [CrossRef]
- Raz, I.; Ceriello, A.; Wilson, P.W.; Battioui, C.; Su, E.W.; Kerr, L.; Jones, C.A.; Milicevic, Z.; Jacober, S.J. Post hoc subgroup analysis of the HEART2D trial demonstrates lower cardiovascular risk in older patients targeting postprandial versus fasting/premeal glycemia. Diabetes Care 2011, 34, 1511–1513. [Google Scholar] [CrossRef]
- Seino, Y.; Kuwata, H.; Yabe, D. Incretin-based drugs for type 2 diabetes: Focus on East Asian perspectives. J. Diabetes Investig. 2016, 7 (Suppl. 1), 102–109. [Google Scholar] [CrossRef] [PubMed]
- Yabe, D.; Seino, Y.; Seino, Y. Incretin concept revised: The origin of the insulinotropic function of glucagon-like peptide-1—The gut, the islets or both? J. Diabetes Investig. 2018, 9, 21–24. [Google Scholar] [CrossRef]
- Seino, Y.; Yabe, D. Glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1: Incretin actions beyond the pancreas. J. Diabetes Investig. 2013, 4, 108–130. [Google Scholar] [CrossRef]
- Liu, Y.; Kubota, S.; Iizuka, K.; Yabe, D. Cardioprotective effects of GLP-1(28-36a): A degraded metabolite or GLP-1′s better half? J. Diabetes Investig. 2020. [Google Scholar] [CrossRef]
- Kobayashi, M.; Zochodne, D.W. Diabetic neuropathy and the sensory neuron: New aspects of pathogenesis and their treatment implications. J. Diabetes Investig. 2018, 9, 1239–1254. [Google Scholar] [CrossRef] [PubMed]
- Yabe, D.; Seino, Y. Cardiovascular safety trials of incretin-based drugs: What do they mean? J. Diabetes Investig. 2017, 8, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, S.L.; Rorth, R.; Jhund, P.S.; Docherty, K.F.; Sattar, N.; Preiss, D.; Køber, L.; Petrie, M.C.; McMurray, J.J.V. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: A systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2019, 7, 776–785. [Google Scholar] [CrossRef]
- Ma, J.; Stevens, J.E.; Cukier, K.; Maddox, A.F.; Wishart, J.M.; Jones, K.L.; Clifton, P.M.; Horowitz, M.; Rayner, C.K. Effects of a protein preload on gastric emptying, glycemia, and gut hormones after a carbohydrate meal in diet-controlled type 2 diabetes. Diabetes Care 2009, 32, 1600–1602. [Google Scholar] [CrossRef] [PubMed]
- Samocha-Bonet, D.; Wong, O.; Synnott, E.L.; Piyaratna, N.; Douglas, A.; Gribble, F.M.; Holst, J.J.; Chisholm, D.J.; Greenfield, J.R. Glutamine reduces postprandial glycemia and augments the glucagon-like peptide-1 response in type 2 diabetes patients. J. Nutr. 2011, 141, 1233–1238. [Google Scholar] [CrossRef]
- Bae, J.H.; Kim, L.K.; Min, S.H.; Ahn, C.H.; Cho, Y.M. Postprandial glucose-lowering effect of premeal consumption of protein-enriched, dietary fiber-fortified bar in individuals with type 2 diabetes mellitus or normal glucose tolerance. J. Diabetes Investig. 2018, 9, 1110–1118. [Google Scholar] [CrossRef]
- Akhavan, T.; Luhovyy, B.L.; Brown, P.H.; Cho, C.E.; Anderson, G.H. Effect of premeal consumption of whey protein and its hydrolysate on food intake and postmeal glycemia and insulin responses in young adults. Am. J. Clin. Nutr. 2010, 91, 966–975. [Google Scholar] [CrossRef]
- Gentilcore, D.; Chaikomin, R.; Jones, K.L.; Russo, A.; Feinle-Bisset, C.; Wishart, J.M.; Rayner, C.K.; Horowitz, M. Effects of fat on gastric emptying of and the glycemic, insulin, and incretin responses to a carbohydrate meal in type 2 diabetes. J. Clin. Endocrinol. Metab. 2006, 91, 2062–2067. [Google Scholar] [CrossRef]
- Kuwata, H.; Iwasaki, M.; Shimizu, S.; Minami, K.; Maeda, H.; Seino, S.; Nakada, K.; Nosaka, C.; Murotani, K.; Kurose, T. Meal sequence and glucose excursion, gastric emptying and incretin secretion in type 2 diabetes: A randomised, controlled crossover, exploratory trial. Diabetologia 2016, 59, 453–461. [Google Scholar] [CrossRef]
- Wu, T.; Little, T.J.; Bound, M.J.; Borg, M.; Zhang, X.; Deacon, C.F.; Horowitz, M.; Jones, K.L.; Rayner, C.K. A Protein Preload Enhances the Glucose-Lowering Efficacy of Vildagliptin in Type 2 Diabetes. Diabetes Care 2016, 39, 511–517. [Google Scholar] [CrossRef]
- Trico, D.; Baldi, S.; Tulipani, A.; Frascerra, S.; Macedo, M.P.; Mari, A.; Ferrannini, E.; Natali, A. Mechanisms through which a small protein and lipid preload improves glucose tolerance. Diabetologia 2015, 58, 2503–2512. [Google Scholar] [CrossRef] [PubMed]
- Trico, D.; Nesti, L.; Frascerra, S.; Baldi, S.; Mengozzi, A.; Natali, A. A Protein/Lipid Preload Attenuates Glucose-Induced Endothelial Dysfunction in Individuals with Abnormal Glucose Tolerance. Nutrients 2020, 12, 2053. [Google Scholar] [CrossRef] [PubMed]
- Itoh, K.; Moriguchi, R.; Yamada, Y.; Fujita, M.; Yamato, T.; Oumi, M.; Holst, J.J.; Seino, Y. High saturated fatty acid intake induces insulin secretion by elevating gastric inhibitory polypeptide levels in healthy individuals. Nutr. Res. 2014, 34, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Lardinois, C.K.; Starich, G.H.; Mazzaferri, E.L. The postprandial response of gastric inhibitory polypeptide to various dietary fats in man. J. Am. Coll. Nutr. 1988, 7, 241–247. [Google Scholar] [CrossRef]
- InterAct, C. Dietary fibre and incidence of type 2 diabetes in eight European countries: The EPIC-InterAct Study and a meta-analysis of prospective studies. Diabetologia 2015, 58, 1394–1408. [Google Scholar]
- Wong, J.M.W.; Jenkins, D.J.A. Carbohydrate Digestibility and Metabolic Effects. J. Nutr. 2007, 137, 2539S–2546S. [Google Scholar] [CrossRef]
- Sun, L.; Goh, H.J.; Govindharajulu, P.; Leow, M.K.; Henry, C.J. Postprandial glucose, insulin and incretin responses differ by test meal macronutrient ingestion sequence (PATTERN study). Clin. Nutr. 2020, 39, 950–957. [Google Scholar] [CrossRef]
- Trico, D.; Filice, E.; Trifiro, S.; Natali, A. Manipulating the sequence of food ingestion improves glycemic control in type 2 diabetic patients under free-living conditions. Nutr. Diabetes 2016, 6, e226. [Google Scholar] [CrossRef]
- Imai, S.; Matsuda, M.; Hasegawa, G.; Fukui, M.; Obayashi, H.; Ozasa, N.; Kajiyama, S. A simple meal plan of ‘eating vegetables before carbohydrate’ was more effective for achieving glycemic control than an exchange-based meal plan in Japanese patients with type 2 diabetes. Asia Pac. J. Clin. Nutr. 2011, 20, 161–168. [Google Scholar]
- Yabe, D.; Kuwata, H.; Fujiwara, Y.; Sakaguchi, M.; Moyama, S.; Makabe, N.; Murotani, K.; Asano, H.; Ito, S.; Mishima, H.; et al. Dietary instructions focusing on meal-sequence and nutritional balance for prediabetes subjects: An exploratory, cluster-randomized, prospective, open-label, clinical trial. J. Diabetes Complicat. 2019, 33, 107450. [Google Scholar] [CrossRef]
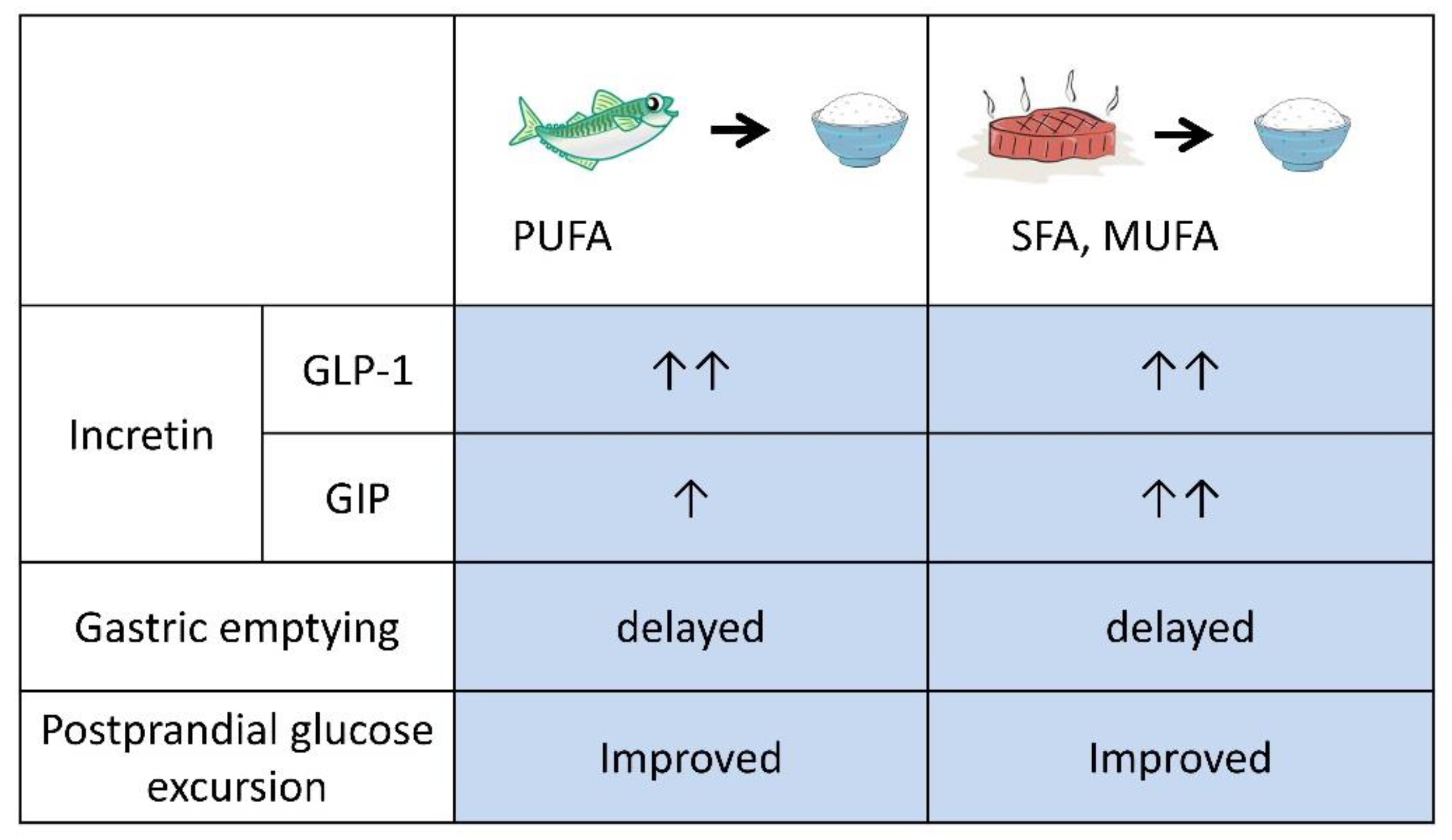
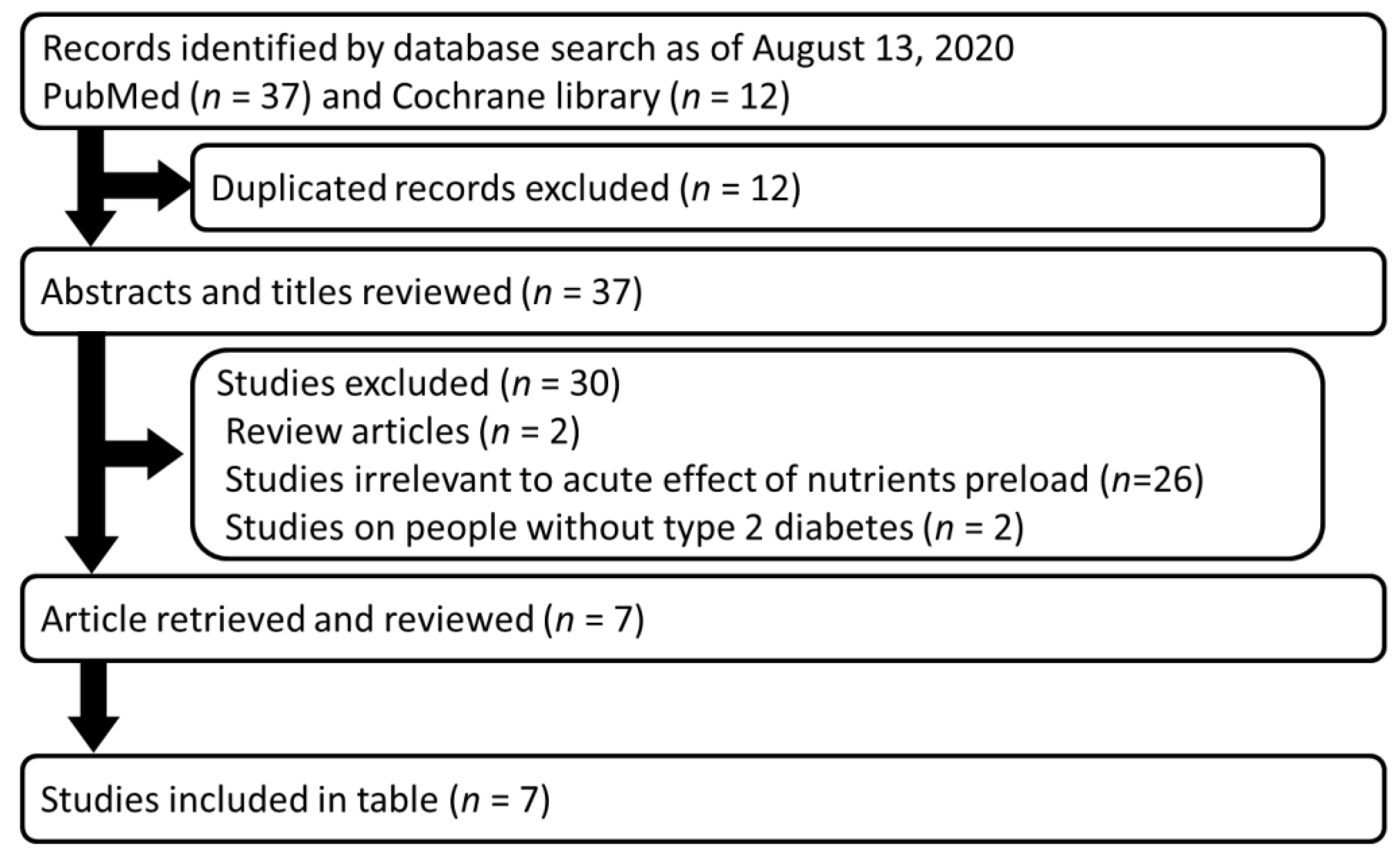
| Preload | Details of Main Meal | n | Outcomes | Ref | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type | Details | Timing | Glucose | Insulin | Glucagon | GLP-1 | GIP | CCK | GE | |||
| F | 30 ml olive oil | 30 min before C | 65 g mashed potato/20 g glucose (C 61 g) | 6 | Peak delayed | Peak delayed | ND | Enhanced | NC | ND | Delayed | [18] |
| P | 55 g whey protein | 30 min before C | 65 g mashed potato/20 g glucose (C 59.1 g; P 5.2 g; F 4.3 g) | 8 | Suppressed | Enhanced | ND | Enhanced | Enhanced | Enhanced | Delayed | [14] |
| P | 25 g whey protein | 30 min before C | 65 g mashed potato/20 g glucose/1 egg yolk | 22 | Suppressed | Enhanced | Enhanced | Enhanced | Enhanced | ND | Delayed | [20] |
| Mixed | 50 g cheese/one small-size boiled egg (C 2 g; P 23 g; F 17 g) | 30 min before C | 75 g glucose (C 75 g) | 10 | Suppressed | NC | Enhanced | Enhanced | Enhanced | ND | ND | [21] |
| Mixed | 100 g steamed mackerel (C 0g; P 15.1 g; F 17.7 g) | 15 min before C | 150 g rice (C 53.4 g; P 3.5 g; F 0.6g) | 12 | Suppressed | Enhanced | Enhanced | Enhanced | Enhanced | ND | Delayed | [19] |
| Mixed | 79 g grilled beef (C 0.2 g; P 16.4 g; F 17.1 g) | 15 min before C | 150 g rice (C 53.4 g; P 3.5 g; F 0.6g) | 12 | Suppressed | Enhanced | Enhanced | Enhanced | Enhanced* | ND | Delayed | [19] |
| Mixed | Protein enriched, dietary fiber fortified bar (C 0.4 g; P 10.7 g; F 0.3 g; Fiber 12.7 g) | 30 min before C | 286 g Bagle/70 g Cream cheese/95g Orange juice (C 79.5 g; P 15.5 g; F 50.5g) | 15 | Suppressed | Suppressed | ND | Enhanced | NC | ND | ND | [16] |
| Mixed | 50 g cheese/one small-size boiled egg (C 2 g; P 23 g; F 17 g) | 30 min before C | 75 g glucose (C 75 g) | 9 | Suppressed | NC | Enhanced | Enhanced | Enhanced | ND | ND | [22] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kubota, S.; Liu, Y.; Iizuka, K.; Kuwata, H.; Seino, Y.; Yabe, D. A Review of Recent Findings on Meal Sequence: An Attractive Dietary Approach to Prevention and Management of Type 2 Diabetes. Nutrients 2020, 12, 2502. https://doi.org/10.3390/nu12092502
Kubota S, Liu Y, Iizuka K, Kuwata H, Seino Y, Yabe D. A Review of Recent Findings on Meal Sequence: An Attractive Dietary Approach to Prevention and Management of Type 2 Diabetes. Nutrients. 2020; 12(9):2502. https://doi.org/10.3390/nu12092502
Chicago/Turabian StyleKubota, Sodai, Yanyan Liu, Katsumi Iizuka, Hitoshi Kuwata, Yutaka Seino, and Daisuke Yabe. 2020. "A Review of Recent Findings on Meal Sequence: An Attractive Dietary Approach to Prevention and Management of Type 2 Diabetes" Nutrients 12, no. 9: 2502. https://doi.org/10.3390/nu12092502
APA StyleKubota, S., Liu, Y., Iizuka, K., Kuwata, H., Seino, Y., & Yabe, D. (2020). A Review of Recent Findings on Meal Sequence: An Attractive Dietary Approach to Prevention and Management of Type 2 Diabetes. Nutrients, 12(9), 2502. https://doi.org/10.3390/nu12092502







