An Investigation of the Influence of Age and Saliva Flow on the Oral Retention of Whey Protein and Its Potential Effect on the Perception and Acceptance of Whey Protein Beverages
Abstract
1. Introduction
- (1)
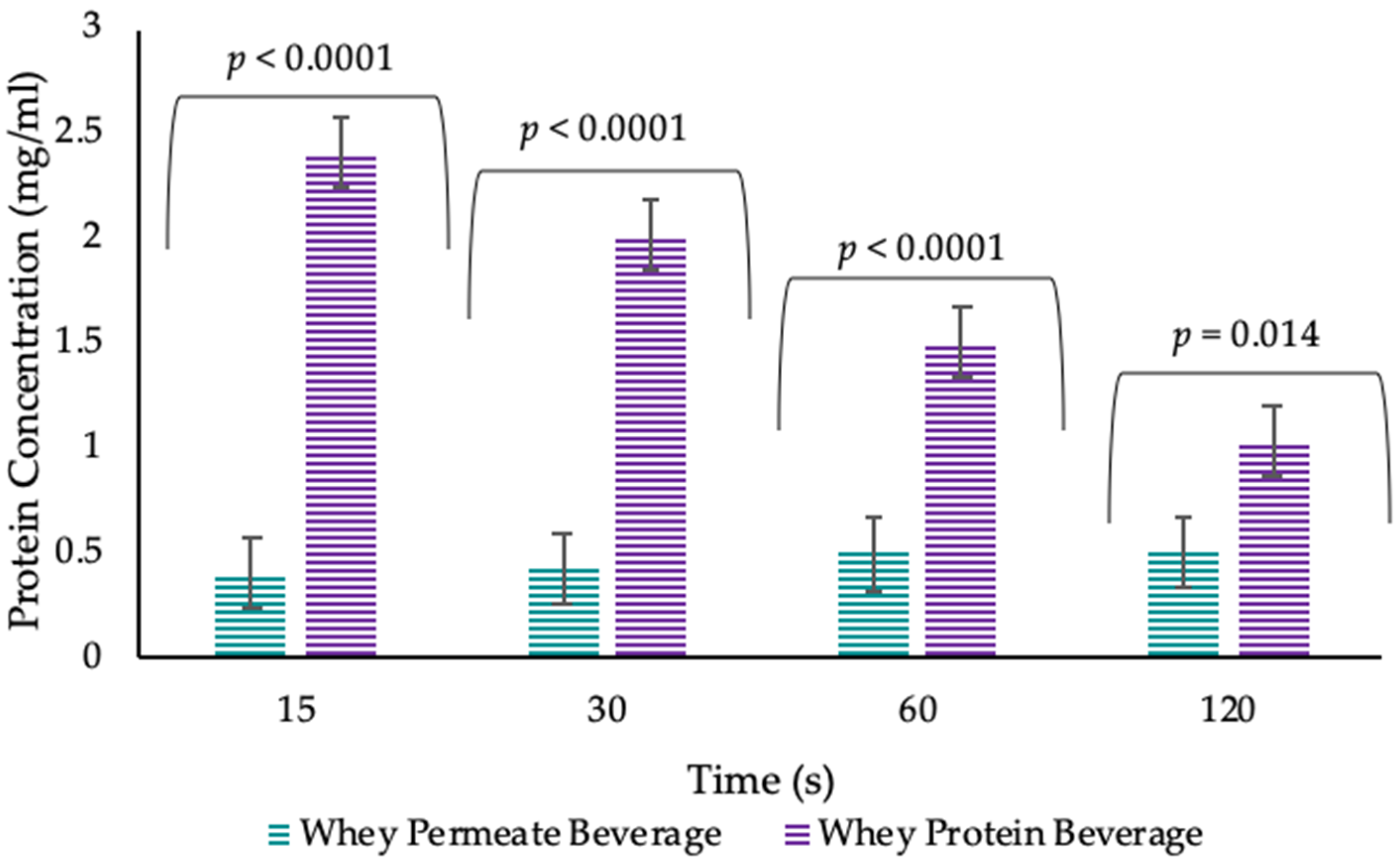
- A pilot study was carried out with the objective of establishing whether the protein measured in the oral cavity post WPB consumption resulted from mucoadhesion of the WPB (rather than resulting from consumption-induced release of salivary protein). The pilot study was conducted in younger adults and measured protein concentration of saliva samples post beverage consumption (WPB and whey permeate beverage (WPeB)) at 4 different timepoints (15 s, 30 s, 60 s and 120 s), in order to validate the oral retention method.
- (2)
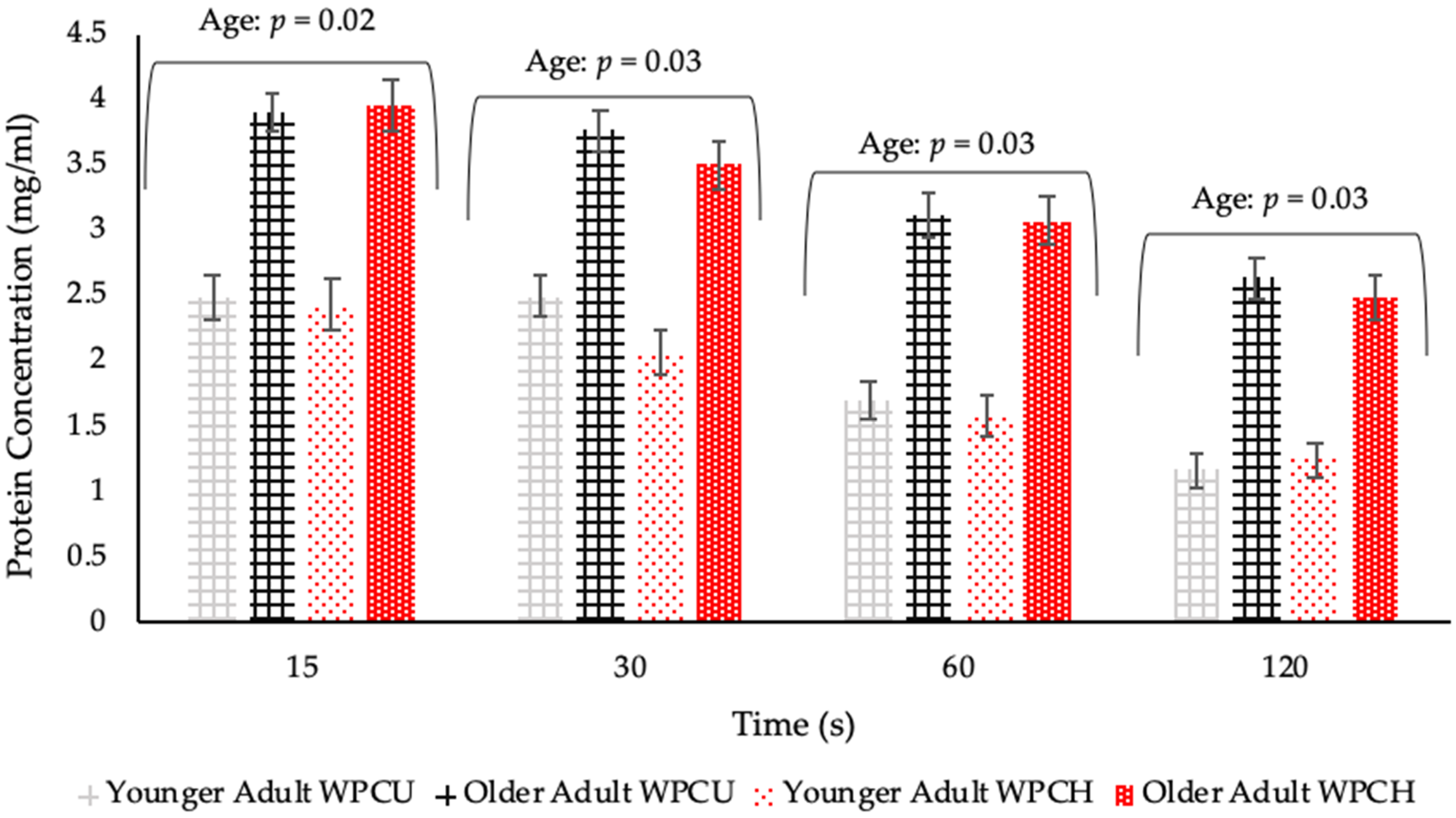
- The main study had the following objectives: (a) to measure salivary flow rates from unstimulated and stimulated saliva, (b) to determine if protein adheres to the oral cavity of older adults to a greater extent than younger adults, (c) to determine if salivary flow rates influence mucoadhesion and perception of WPBs, and (d) to evaluate whether heat treatment of protein in WPB causes mouthdrying and reduced acceptance within each volunteer group. This study recruited younger and older adults to test these objectives.
2. Materials and Methods
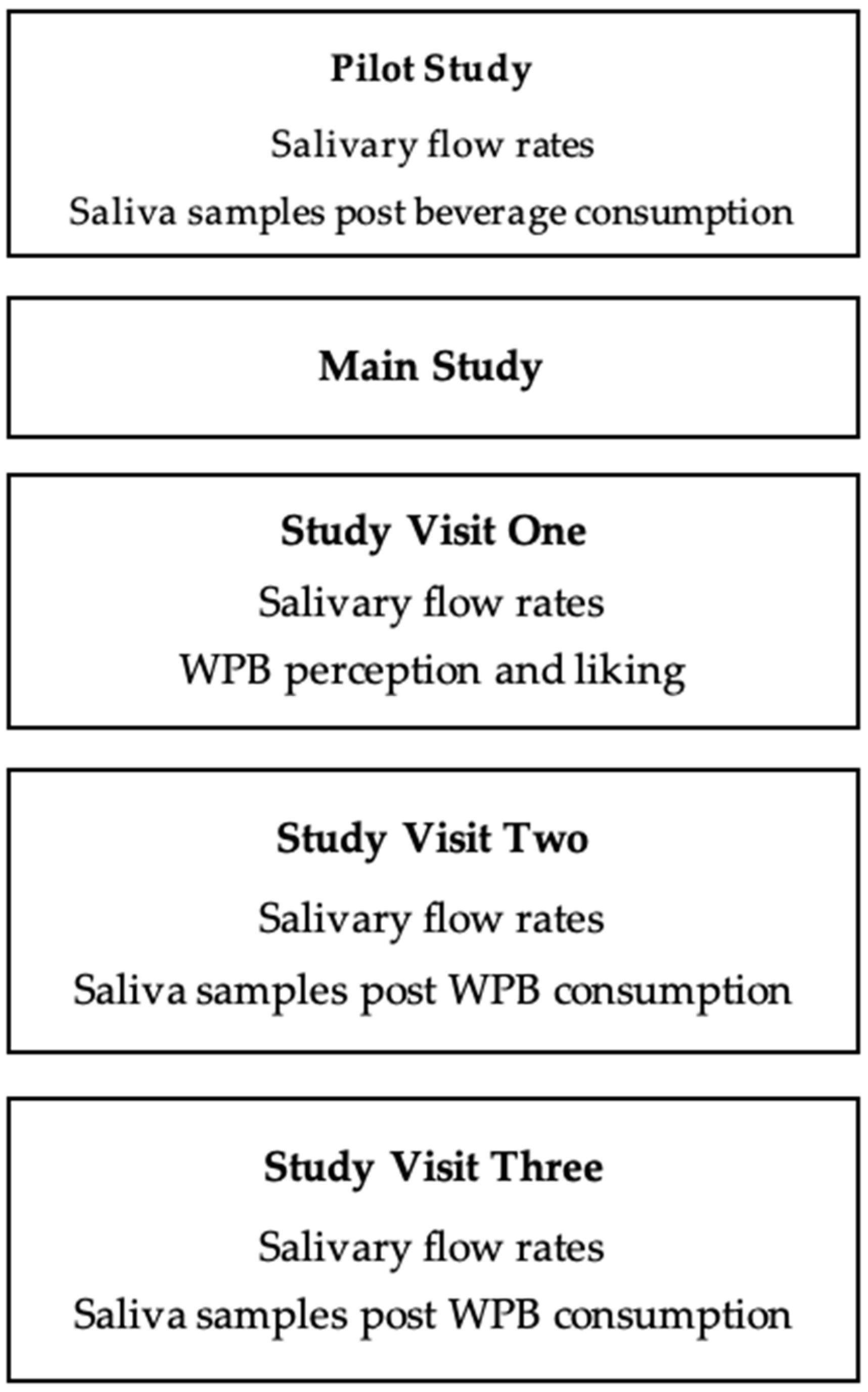
2.1. Overview of Pilot and Main Study
2.2. Materials
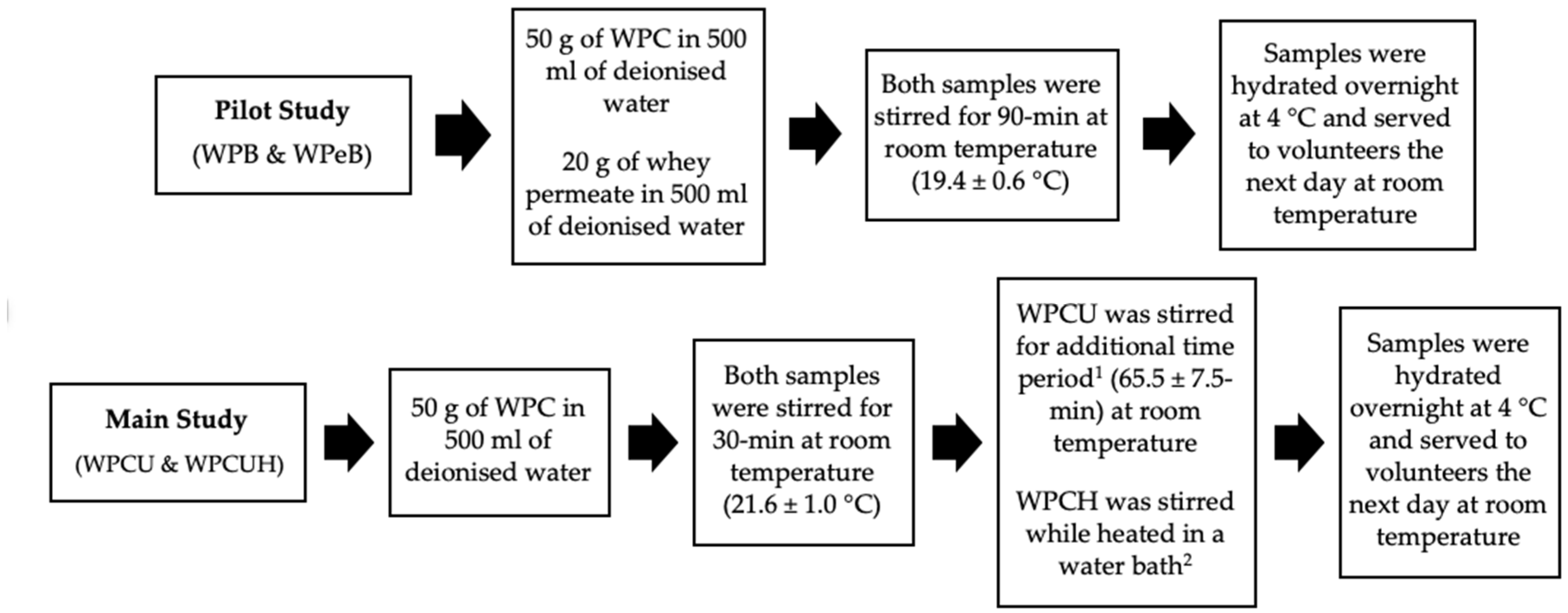
2.3. Liquid Model Preparation
2.3.1. Salivary Flow Rates
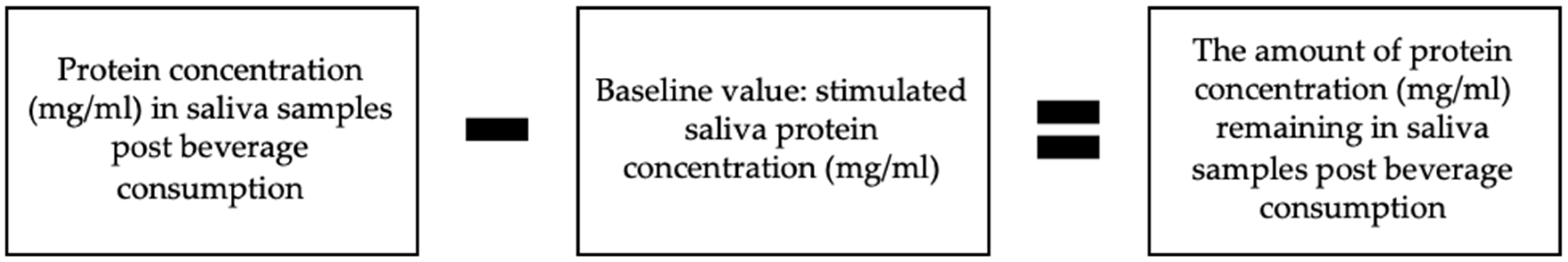
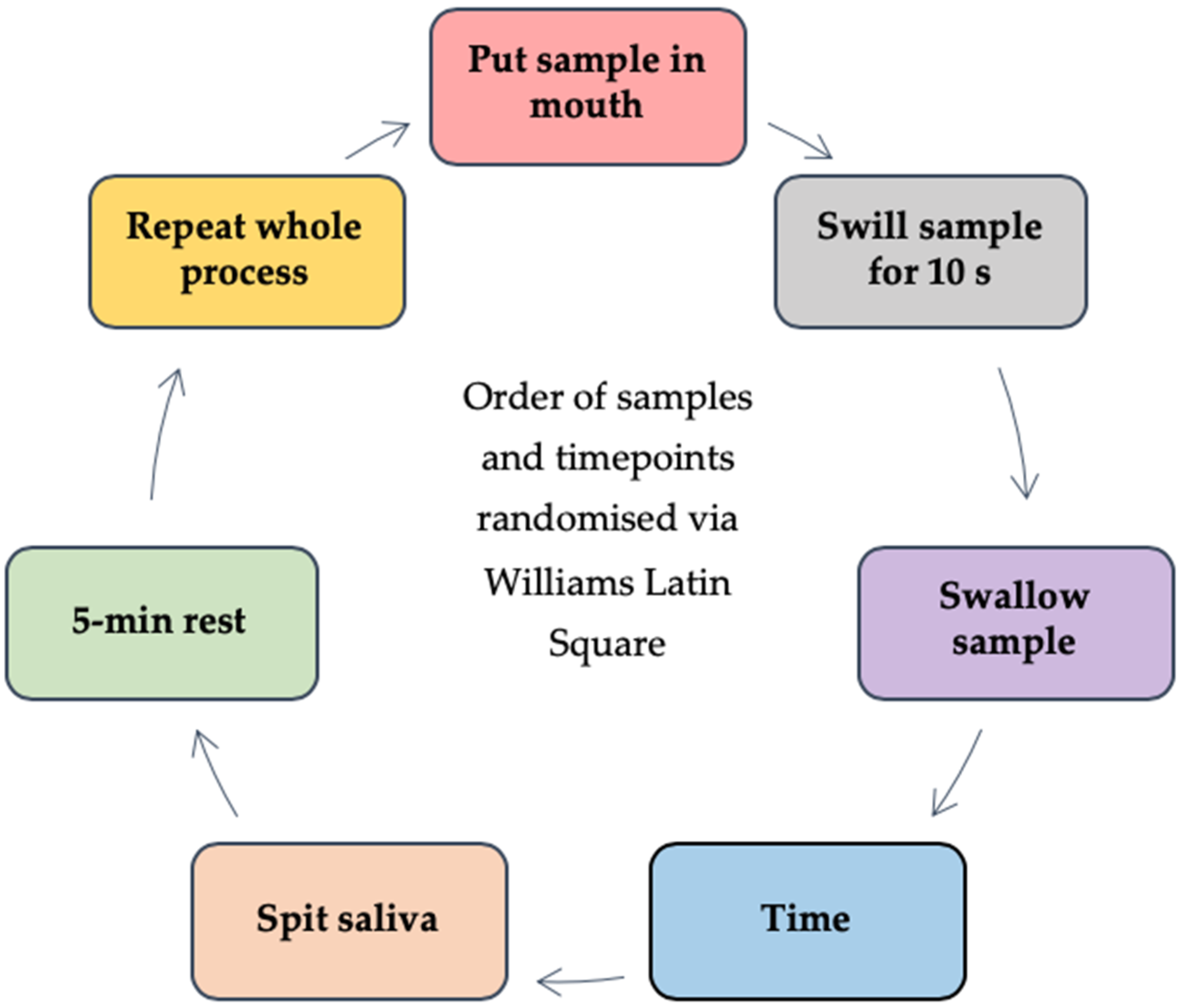
2.3.2. Saliva Samples Post Beverage Consumption
2.3.3. Protein Analysis of Saliva Samples
2.3.4. WPB Individual Perception and Liking
2.4. Statistical Analysis
2.4.1. Pilot Study
2.4.2. Main Study
3. Results
3.1. Pilot Study (Salivary Flow Rates and Saliva Samples Post Beverage Consumption)
3.2. Main Study
3.2.1. Salivary Flow Rates
3.2.2. Saliva Samples Post WPB Consumption
3.2.3. WPB Individual Perception and Liking
3.2.4. Preference, Penalty Analysis and Qualitative Feedback
4. Discussion
4.1. Mucoadhesion and WPB
4.2. Salivary Flow Rates
4.3. Saliva Samples Post WPB Consumption
4.4. WPB Individual Perception and Liking
4.5. Saliva and WPB Individual Perception and Liking
4.6. Age Related Changes in WPB Individual Perception and Liking
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
Oral Retention Method Development


| Sample | Protein Concentration | Confidence Interval | |
|---|---|---|---|
| Lower | Upper | ||
| WPB 15 s | 2.40 ± 0.18 | 1.86 | 2.93 |
| WPB 30 s | 2.01 ± 0.18 | 1.46 | 2.56 |
| WPB 60 s | 1.50 ± 0.18 | 1.06 | 1.94 |
| WPB 120 s | 1.03 ± 0.18 | 0.50 | 1.56 |
| Timepoint | Whey Permeate Beverage | Confidence Interval | Stimulated Saliva | Confidence Interval | Significance of Sample (p Value) | ||
|---|---|---|---|---|---|---|---|
| Lower | Upper | Lower | Upper | ||||
| 15 s | 0.40 ± 0.18 | 0.33 | 0.46 | 0.78 ± 0.20 | 0.67 | 0.89 | <0.0001 |
| 30 s | 0.42 ± 0.18 | 0.35 | 0.49 | 0.78 ± 0.20 | 0.67 | 0.89 | <0.0001 |
| 60 s | 0.50 ± 0.18 | 0.42 | 0.57 | 0.78 ± 0.20 | 0.67 | 0.89 | <0.0001 |
| 120 s | 0.50 ± 0.18 | 0.39 | 0.60 | 0.78 ± 0.20 | 0.67 | 0.89 | <0.0001 |
References
- BAPEN (2018). Available online: https://www.bapen.org.uk/malnutrition-undernutrition/introduction-to-malnutrition?start=4 (accessed on 10 January 2019).
- Bauer, J.; Biolo, G.; Cederholm, T.; Cesari, M.; Cruz-Jentoft, A.J.; Morley, J.E.; Philips, S.; Sieber, C.; Stehle, P.; Teta, D.; et al. Evidence based recommendation for optimal dietary protein intake in older people: A position paper from the PROT-AGE study group. JAMA 2013, 14, 542–559. [Google Scholar] [CrossRef] [PubMed]
- Schiffman, S.; Graham, B. Taste and smell perception affect appetite and immunity in the elderly. Eur. J. Clin. Nutr. 2000, 54, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, G.H. The secretion, components, and properties of saliva. Annu. Rev. Food Sci. Technol. 2013, 4, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Vandenberghe-Descamps, M.; Laboure, H.; Prot, A.; Septier, C.; Tournier, C.; Feron, G.; Sulmont-Rosse, C. Salivary flow decreases in healthy elderly people independently of dental status and drug intake. J. Texture Stud. 2016, 47, 353–360. [Google Scholar] [CrossRef]
- Lee, S.K.; Lee, S.W.; Chung, S.C.; Kim, Y.K.; Kho, H.S. Analysis of residual saliva and minor salivary gland secretions in patients with dry mouth. Arch. Oral Biol. 2002, 47, 637–641. [Google Scholar] [CrossRef]
- Turner, M.D.; Ship, J.A. Dry mouth and its effects on the oral health of elderly people. J. Am. Dent. Assoc. 2007, 138, 15–20. [Google Scholar] [CrossRef]
- Chaudhury, N.M.A.; Shirlaw, P.; Pramanik, R.; Carpenter, G.H.; Proctor, G.B. Changes in saliva rheological properties and mucin glycosylation in dry mouth. J. Dent. Res. 2015, 94, 1660–1667. [Google Scholar] [CrossRef]
- Nagler, R.M.; Hershkovich, O. Relationships between age, drugs, oral sensorial complaints and salivary profile. Arch. Oral Biol. 2005, 50, 7–16. [Google Scholar] [CrossRef]
- Mioche, L.; Bourdiol, P.; Peyron, M. Influence of age on mastication: Effects on eating behaviour. Nutr. Res. Rev. 2004, 17, 43–54. [Google Scholar] [CrossRef]
- Munoz-Gonzalez, C.; Feron, G.; Canon, F. Main effects of human saliva on flavour perception and the potential contribution to food consumption. Proc. Nutr. Soc. 2018, 77, 423–431. [Google Scholar] [CrossRef]
- Pennings, B.; Boirie, Y.; Senden, J.M.; Gijsen, A.P.; Kuipers, H.; van Loon, L.J. Whey protein stimulates postprandial muscle protein accretion more effectively than do casein and casein hydrolysate in older men. Am. J. Clin. Nutr. 2011, 93, 997–1005. [Google Scholar] [CrossRef] [PubMed]
- Childs, J.L.; Drake, M.A. Consumer perception of astringency in clear acidic whey protein beverages. J. Food Sci. 2010, 75, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, O.; Law, C.; Methven, L.; Mottram, D.; Gosney, M. Investigating age related changes in taste and affects on sensory perceptions of oral nutritional supplements. Age Ageing 2010, 39, 733–738. [Google Scholar] [CrossRef] [PubMed]
- Gosney, M. Are we wasting our money on food supplements in elder care wards? J. Adv. Nurs. 2003, 43, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Methven, L.; Rahelu, K.; Economou, N.; Kinneavy, L.; Ladbrooke-Davis, L.; Kennedy, O.B.; Mottram, D.S.; Gosney, M.A. The effect of consumption volume of profile and liking of oral nutritional supplements of varied sweetness: Sequential profiling and boredom tests. Food Qual. Prefer. 2010, 21, 948–955. [Google Scholar] [CrossRef]
- Withers, C.; Gosney, M.A.; Methven, L. Perception of thickness, mouth coating and mouth drying of dairy beverages by younger and older volunteers. J. Sens. Stud. 2013, 28, 230–237. [Google Scholar] [CrossRef]
- Bull, S.P.; Hong, Y.; Khutoryanskiy, V.V.; Parker, J.K.; Faka, M.; Methven, L. Whey protein mouth drying influenced by thermal denaturation. Food Qual. Prefer. 2017, 56, 233–240. [Google Scholar] [CrossRef]
- Lemieux, L.; Simard, R.E. Astringency, a textural defect in dairy products. Lait 1994, 74, 217–240. [Google Scholar] [CrossRef]
- Gibbins, H.L.; Carpenter, G.H. Alternative mechanisms of astringency—what is the role of saliva? J. Texture Stud. 2013, 44, 364–375. [Google Scholar] [CrossRef]
- Draijier, R.; van Dorsten, F.A.; Zebregs, Y.E.; Hollebrands, B.; Peters, S.; Duchateau, G.S.; Grun, C.H. Impact of proteins on the uptake, distribution and excretion of phenolics in the human body. Nutrients 2016, 8, 814. [Google Scholar] [CrossRef]
- Vardhanabhuti, B.; Kelly, M.A.; Luck, P.J.; Drake, M.A.; Foegeding, E.A. Roles of charge interactions on astringency of whey proteins at low pH. J. Dairy Sci. 2010, 93, 1890–1899. [Google Scholar] [CrossRef] [PubMed]
- Ye, A.; Streicher, C.; Singh, H. Interactions between whey proteins and salivary proteins as related to astringency of whey protein beverages at low pH. J. Dairy Sci. 2011, 94, 5842–5850. [Google Scholar] [CrossRef]
- Sano, H.; Egashira, T.; Kinekawa, Y.; Kitabatake, N. Astringency of bovine milk whey protein. J. Dairy Sci. 2005, 88, 2312–2317. [Google Scholar] [CrossRef]
- Beecher, J.W.; Drake, M.A.; Luck, P.J.; Foegeding, E.A. Factors regulating astringency of whey protein beverages. J. Dairy Sci. 2008, 91, 2553–2560. [Google Scholar] [CrossRef] [PubMed]
- Withers, C.A.; Lewis, M.J.; Gosney, M.A.; Methven, L. Potential sources of mouth drying in beverages fortified with dairy proteins: A comparison of casein- and whey-rich ingredients. J. Dairy Sci. 2014, 97, 1233–1247. [Google Scholar] [CrossRef] [PubMed]
- Andrewes, P.; Kelly, M.; Vardhanabhuti, B.; Foegeding, E.A. Dynamic modelling of whey protein-saliva interactions in the mouth and relation to astringency in acidic beverages. Int. Dairy J. 2011, 21, 523–530. [Google Scholar] [CrossRef]
- Kelly, M.; Vardhanabhuti, B.; Luck, P.; Drake, M.A.; Osborne, J.; Foegeding, E.A. Role of protein concentration and protein–saliva interactions in the astringency of whey proteins at low pH. J. Dairy Sci. 2010, 93, 1900–1909. [Google Scholar] [CrossRef]
- Vardhanabhuti, B.; Cox, P.W.; Norton, I.T.; Foegeding, E.A. Lubricating properties of human whole saliva as affected by beta-lactoglobulin. Food Hydrocoll. 2011, 25, 1499–1506. [Google Scholar] [CrossRef]
- Ye, A.; Zheng, T.; Ye, J.Z.; Singh, H. Potential role of the binding of whey proteins to human buccal cells on the perception of astringency in whey proteins beverages. Physiol. Behav. 2012, 106, 645–650. [Google Scholar] [CrossRef]
- Withers, C.A.; Cook, M.T.; Methven, L.; Gosney, M.A.; Khutoryanskiy, V.V. Investigation of milk proteins binding to the oral mucosa. Food Funct. 2013, 4, 1668–1674. [Google Scholar] [CrossRef]
- Hsein, H.; Garrait, G.; Beyssac, E.; Hoffart, V. Whey protein mucoadhesive properties for oral drug delivery: Mucin whey protein interaction and mucoadhesive bond strength. Coll. Surf. B 2015, 136, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Bull, S.P.; Khutoryanskiy, V.V.; Parker, J.K.; Faka, M.; Methven, L. Oral retention of whey protein: Measurement and mechanisms. Food Chem. 2020. submitted for publication. [Google Scholar]
- Josephson, R.V.; Thomas, E.L.; Morr, C.V.; Coulter, S.T. Relation of heat-induced changes in protein-salt constituents to astringency in milk systems. J. Dairy Sci. 1967, 50, 1376–1383. [Google Scholar] [CrossRef]
- Smart, J.D. The basics and underlying mechanisms of mucoadhesion. Adv. Drug Deliv. Rev. 2005, 57, 1556–1568. [Google Scholar] [CrossRef] [PubMed]
- Andrews, G.P.; Laverty, T.P.; Jones, D.S. Mucoadhesive polymeric platforms for controlled drug delivery. Eur. J. Pharm. Biopharm. 2009, 71, 505–518. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, F.C.; Bruschi, M.L.; Evangelista, R.C.; Gremiao, M.P.D. Mucoadhesive drug delivery systems. Braz. J. Pharm. Sci. 2010, 46, 1–17. [Google Scholar] [CrossRef]
- Khutoryanskiy, V.V. Advances in mucoadhesion and mucoadhesive polymers. Macromol. Biosci. 2011, 11, 748–764. [Google Scholar] [CrossRef]
- Cook, S.L.; Bull, S.P.; Methven, L.; Parker, J.K.; Khutoryanskiy, V.V. Mucoadhesion: A food perspective. Food Hydrocoll. 2017, 72, 281–296. [Google Scholar] [CrossRef]
- Cook, S.L.; Woods, S.; Methven, L.; Parker, J.K.; Khutoryanskiy, V.V. Mucoadhesive polysaccharides modulate sodium retention, release and taste perception. Food Chem. 2018, 240, 482–489. [Google Scholar] [CrossRef]
- Luiking, Y.C.; Deutz, N.E.P.; Memelink, R.G.; Verlaan, S.; Wolfe, R.R. Postprandial muscle protein synthesis is higher after a high whey protein, leucine enriched supplement than after a dairy like product in healthy older people: A randomised controlled trial. Nutr. J. 2014, 13, 9. [Google Scholar] [CrossRef]
- Buzalaf, M.A.R.; Hannas, A.R.; Kato, M.T. Saliva and dental erosion. J. Appl. Oral Sci. 2012, 20, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Belitz, H.D.; Grosch, W.; Schieberle, P. Food Chemistry, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 2004; pp. 245–339. [Google Scholar]
- Boye, J.I.; Alli, I. Thermal denaturation of mixtures of a lactalbumin and b lactoglobulin: A differential scanning calorimetric study. Food Res. Int. 2000, 33, 673–682. [Google Scholar] [CrossRef]
- De Wit, J.N.; Swinkels, G.A.M. A differential scanning calorimetric study of the thermal denaturation of bovine beta lactoglobulin thermal behaviour at temperature up to 100 degrees. Biochim. Biophs. Acta 1980, 624, 40–50. [Google Scholar] [CrossRef]
- Pushpass, R.A.G.; Pellicciotta, N.; Kelly, C.; Proctor, G.; Carpenter, G.H. Reduced salivary mucin binding and glycosylation in older adults influences taste in an vitro cell model. Nutrients 2019, 11, 2280. [Google Scholar] [CrossRef] [PubMed]
- Feron, G. Unstimulated saliva: Background noise in taste molecules. J. Texture Stud. 2019, 50, 6–18. [Google Scholar] [CrossRef]
- Affoo, R.H.; Foley, N.; Garrick, R.; Siqueira, W.L.; Martin, R.E. Meta-analysis of salivary flow rates in young and older adults. J. Am. Geriatr. Soc. 2015, 63, 2142–2151. [Google Scholar] [CrossRef]
- Feron, G.; Ayed, C.; Qannari, E.M.; Courcoux, P.; Laboure, H.; Guichard, E. Understanding aroma release from model cheeses by a statistical multiblock approach on oral processing. PLoS ONE 2014, 9, e93113. [Google Scholar] [CrossRef]
- Munoz-Gonzalez, C.; Brule, M.; Feron, G.; Canon, F. Does interindividual variability of saliva affect the release and metabolization of aroma compounds Ex Vivo? The particular case of elderly suffering or not from hyposalivation. J. Texture Stud. 2019, 50, 36–44. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Zor, T.; Selinger, Z. Linerization of the Bradford protein assay increases its sensitivity: Theoretical and experimental studies. Anal. Biochem. 1996, 236, 302–308. [Google Scholar] [CrossRef]
- Bartoshuk, L.M.; Duffy, V.B.; Green, B.G.; Hoffman, H.J.; Ko, C.-W.; Lucchina, L.A.; Marks, L.E.; Snyder, D.J.; Weiffenbach, J.M. Valid across-group comparisons with labeled scales: The gLMS versus magnitude matching. Physiol. Behav. 2004, 82, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Torrico, D.D.; Jirangrat, W.; Wang, J.; Chompreeda, P.; Sriwattana, S.; Prinyawiwatkul, W. Novel modelling approaches to characterise and quantify carryover effects on sensory acceptability. Foods 2018, 7, 186. [Google Scholar] [CrossRef] [PubMed]
- Ennis, J.M.; Jesionka, V. The power of sensory discrimination methods revisited. J. Sens. Stud. 2011, 26, 371–382. [Google Scholar] [CrossRef]
- Bhattarai, K.R.; Kim, H.R.; Chae, H.J. Compliance with saliva collection protocol in healthy volunteers: Strategies for managing risk and errors. Int. J. Med. Sci. 2018, 15, 823–831. [Google Scholar] [CrossRef]
- Meurman, J.H.; Rantonen, P.; Pajukoski, H.; Sulkava, R. Salivary albumin and other constituents and their relation to oral and general health in the elderly. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2002, 94, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Ladgotra, A.; Verma, P.; Raj, S.S. Estimation of salivary and serum biomarkers in diabetic and non diabetic patients—A comparative study. J. Clin. Diagn. Res. 2016, 10, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Rudney, J.D. Does variability in salivary protein concentrations influence oral microbial ecology and oral health? Crit. Rev. Oral Biol. Med. 1995, 6, 343–367. [Google Scholar] [CrossRef] [PubMed]
- Fielding, R.A.; Vellas, B.; Evans, W.J.; Bhasin, S.; Morley, J.E.; Newman, A.B.; Van Kan, G.A.; Andrieu, S.; Bauer, J.; Breuillé, D.; et al. Sarcopenia: An undiagnosed condition in older adults. Current consensus definition: Prevalence, etiology and consequences. JAMA 2011, 12, 249–256. [Google Scholar]
- Hickson, M. Malnutrition and Ageing. Postgrad. Med. J. 2006, 82, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Santagiuliana, M.; Sampedro Marigomez, I.; Broers, L.; Hayes, J.E.; Piqueras-Fiszman, B.; Scholten, E.; Stieger, M. Exploring variability in detection thresholds of microparticles through participant characteristics. Food Funct. 2019, 10, 5386–5397. [Google Scholar] [CrossRef]
- Methven, L.; Allen, V.; Withers, C.; Gosney, M.A. Ageing and Taste. Proc. Nutr. Soc. 2012, 71, 556–565. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.H.; Logemann, J.; Burghardt, W.R.; Zecker, S.G.; Rademaker, A.W. Oral and oropharyngeal perceptions of fluid viscosity across the age span. Dysphagia 2006, 21, 209–217. [Google Scholar] [CrossRef] [PubMed]


| Proposed Cause | Description |
|---|---|
| The pH of WPB | Low pH can cause precipitation of the protein, however, there is evidence of mouthdrying from WPB at both low and neutral pH [18,22,23,24,25,26] |
| Saliva and protein interactions | Perception of mouthdrying has links to saliva and protein interactions [22,23,25,27,28] |
| Reduced lubrication of saliva | Increased friction within the oral cavity from reduced lubrication [29] |
| Adhesion and binding properties | Whey proteins binding to oral epithelial cells, proteins remaining on surfaces, mucoadhesive properties and increased oral retention [30,31,32,33] |
| Heating time | Mouthdrying is considered to increase with product heating time, potentially due to protein denaturation [18,34] |
| Whey Protein Beverage | Whey Permeate Beverage | ||||
|---|---|---|---|---|---|
| Per 5 mL 1 | Per 10 mL 2 | Per 100 mL | Per 10 mL 2 | Per 100 mL | |
| Nutritional Composition | |||||
| Energy (kcal) | 2.0 | 4.0 | 39.7 | 1.5 | 14.7 |
| Fat (g) | 0.04 | 0.07 | 0.7 | 0.0008 | 0.008 |
| of which saturates (g) | 0.01 | 0.03 | 0.3 | - | - |
| Carbohydrate (g) | 0.02 | 0.04 | 0.4 | 0.4 | 3.6 |
| of which sugars (g) | 0.02 | 0.04 | 0.4 | - | - |
| Protein (g) | 0.4 | 0.8 | 8.2 | 0.01 | 0.1 |
| Typical Mineral Profile | |||||
| Calcium (mg) | - | 5.5 | 55 | 2.2 | 21.6 |
| Magnesium (mg) | - | 0.5 | 5 | 0.4 | 4.4 |
| Phosphorus (mg) | - | 3.5 | 35 | 2.4 | 24.4 |
| Potassium (mg) | - | 5 | 50 | 5.7 | 57.2 |
| Sodium (mg) | - | 1.5 | 15 | 1.8 | 18.4 |
| Chloride (mg) | - | 0.5 | 5 | 1.8 | 18.4 |
| Chemical Properties | |||||
| Protein % | - | 0.8 | 8.2 | 0.01 | 0.1 |
| Moisture % | - | 0.05 | 0.5 | 0.004 | 0.04 |
| Ash % | - | 0.04 | 0.4 | 0.02 | 0.2 |
| Lactose % | - | 0.04 | 0.4 | 0.4 | 3.6 |
| Fat % | - | 0.07 | 0.7 | 0.008 | 0.008 |
| pH | - | 6.5 | 6.5 | 5.6 | 5.6 |
| Unstimulated Saliva Flow | Stimulated Saliva Flow | |||||
|---|---|---|---|---|---|---|
| Low (0.04–0.53) | Medium (0.53–0.77) | High (0.77–2.18) | Low (0.23–1.63) | Medium (1.63–2.76) | High (2.77–6.13) | |
| Total (n = 84) | 28 | 27 | 29 | 25 | 30 | 29 |
| Younger Adults (n = 42) | 9 | 14 | 19 | 9 | 18 | 15 |
| Older Adults (n = 42) | 19 | 13 | 10 | 16 | 12 | 14 |
| Overall (n = 84) | Age | Unstimulated Saliva Flow | |||||
|---|---|---|---|---|---|---|---|
| Significance of Sample (p Value) | Younger Adults (n = 42) | Older Adults (n = 42) | Low Saliva Flow (n = 27) | Medium Saliva Flow (n = 28) | High Saliva Flow (n = 29) | ||
| Overall Liking | |||||||
| WPCU | 3.7 ± 0.3 | 0.10 | 3.6 ± 0.4 A | 3.7 ± 0.3 | 3.5 ± 0.4 | 3.8 ± 0.3 A | 3.6 ± 0.4 |
| WPCH | 3.3 ± 0.3 | 2.8 ± 0.4 aB | 3.9 ± 0.3 b | 2.9 ± 0.4 | 3.3 ± 0.3 B | 3.7 ± 0.4 | |
| Easiness to Drink | |||||||
| WPCU | 3.9 ± 0.1 | 0.11 | 4.0 ± 0.2 | 3.9 ± 0.2 | 4.2 ± 0.2 A | 3.8 ± 0.2 | 3.8 ± 0.2 |
| WPCH | 3.7 ± 0.1 | 3.6 ± 0.3 | 3.8 ± 0.2 | 3.7 ± 0.2 B | 3.8 ± 0.2 | 3.6 ± 0.2 | |
| Easiness to Swallow | |||||||
| WPCU | 4.2 ± 0.1 | 0.0004 | 4.4 ± 0.2 A | 4.1 ± 0.2 | 4.4 ± 0.2 A | 4.2 ± 0.1 A | 3.9 ± 0.2 |
| WPCH | 3.9 ± 0.1 | 3.7 ± 0.2 B | 4.0 ± 0.2 | 3.9 ± 0.2 B | 3.9 ± 0.1 B | 3.9 ± 0.2 | |
| Mouthdrying | |||||||
| WPCU | 16.9 ± 3.5 | <0.0001 | 18.1 ± 5.2 A | 15.7 ± 3.8 A | 15.5 ± 4.8 A | 15.8 ± 4.6 A | 19.3 ± 5.0 A |
| WPCH | 28.0 ± 3.5 | 34.4 ± 5.2 B | 21.8 ± 3.8 B | 23.9 ± 4.8 B | 30.3 ± 4.6 B | 30.0 ± 5.0 B | |
| Sweetness | |||||||
| WPCU | 7.6 ± 1.1 | 0.04 | 7.1 ± 1.7 | 7.9 ± 1.2 | 8.7 ± 1.6 | 9.1 ± 1.5 A | 4.8 ± 1.6 |
| WPCH | 6.0 ± 1.1 | 6.4 ± 1.7 | 5.6 ± 1.2 | 8.2 ± 1.6 | 5.5 ± 1.5 B | 4.3 ± 1.6 | |
| Thickness | |||||||
| WPCU | 9.7 ± 2.0 | <0.0001 | 11.5 ± 2.9 A | 7.9 ± 2.1 A | 9.5 ± 2.7 A | 9.9 ± 2.6 A | 9.7 ± 2.9 A |
| WPCH | 17.3 ± 2.0 | 19.5 ± 2.9 B | 15.2 ± 2.1 B | 13.3 ± 2.7 B | 18.2 ± 2.6 B | 20.6 ± 2.9 B | |
| Overall (n = 84) | Age | Unstimulated Saliva Flow | Penalty Analysis (Mean Liking Drop Where Attribute Deviates From Just-About-Right) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Significance of Sample (p Value) | Younger Adults (n = 42) | Older Adults (n = 42) | Low Saliva Flow (n = 27) | Medium Saliva Flow (n = 28) | High Saliva Flow (n = 29) | Too Little (YA) | Too Much (YA) | Too Little (OA) | Too Much (OA) | ||
| JAR Flavour | |||||||||||
| WPCU | 2.3 ± 0.1 | 0.29 | 2.4 ± 0.3 | 2.2 ± 0.2 A | 2.7 ± 0.2 | 2.0 ± 0.2 | 2.2 ± 0.2 | 1.04 * | 2.18 | 1.21 * | 1.07 |
| WPCH | 2.5 ± 0.1 | 2.5 ± 0.3 | 2.5 ± 0.1 B | 2.6 ± 0.2 | 2.1 ± 0.2 | 2.7 ± 0.2 | 0.59 * | 0.71 | 0.80 * | 0.63 | |
| JAR Thickness | |||||||||||
| WPCU | 2.2 ± 0.1 | <0.0001 | 2.4 ± 0.2 A | 2.1 ± 0.1 A | 2.4 ± 0.2 | 2.2 ± 0.1 A | 2.0 ± 0.2 A | 1.06 * | 0.80 | 0.56 * | −0.58 |
| WPCH | 2.7 ± 0.1 | 2.9 ± 0.2 B | 2.6 ± 0.1 B | 2.6 ± 0.2 | 2.6 ± 0.1 B | 2.9 ± 0.2 B | 0.73 * | 0.29 | 1.17 a | 1.68 b | |
| Sample | Comments and Volunteers Details |
|---|---|
| WPCU | Tasteless and mouthdrying (v3, female, younger adult, aged 22). Bland flavour, unappealing colour, unsatisfying dry finish and aftertaste (v49, male, older adult, aged 65). |
| WPCH | It felt strange. It was thick and made my mouth feel dry afterwards. Almost as if all the moisture in my mouth had been sucked from it (v9, male, younger adult, aged 19). My mouth and teeth feel yucky. Like when you eat rhubarb, I would like to go and clean my teeth (v79, female, older adult, aged 75). |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Norton, V.; Lignou, S.; Bull, S.P.; Gosney, M.A.; Methven, L. An Investigation of the Influence of Age and Saliva Flow on the Oral Retention of Whey Protein and Its Potential Effect on the Perception and Acceptance of Whey Protein Beverages. Nutrients 2020, 12, 2506. https://doi.org/10.3390/nu12092506
Norton V, Lignou S, Bull SP, Gosney MA, Methven L. An Investigation of the Influence of Age and Saliva Flow on the Oral Retention of Whey Protein and Its Potential Effect on the Perception and Acceptance of Whey Protein Beverages. Nutrients. 2020; 12(9):2506. https://doi.org/10.3390/nu12092506
Chicago/Turabian StyleNorton, Victoria, Stella Lignou, Stephanie P. Bull, Margot A. Gosney, and Lisa Methven. 2020. "An Investigation of the Influence of Age and Saliva Flow on the Oral Retention of Whey Protein and Its Potential Effect on the Perception and Acceptance of Whey Protein Beverages" Nutrients 12, no. 9: 2506. https://doi.org/10.3390/nu12092506
APA StyleNorton, V., Lignou, S., Bull, S. P., Gosney, M. A., & Methven, L. (2020). An Investigation of the Influence of Age and Saliva Flow on the Oral Retention of Whey Protein and Its Potential Effect on the Perception and Acceptance of Whey Protein Beverages. Nutrients, 12(9), 2506. https://doi.org/10.3390/nu12092506





