Effect of Co-Administration of Panax ginseng and Brassica oleracea on Postmenopausal Osteoporosis in Ovariectomized Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. P. ginseng and B. oleracea Extract Powders and Reagents
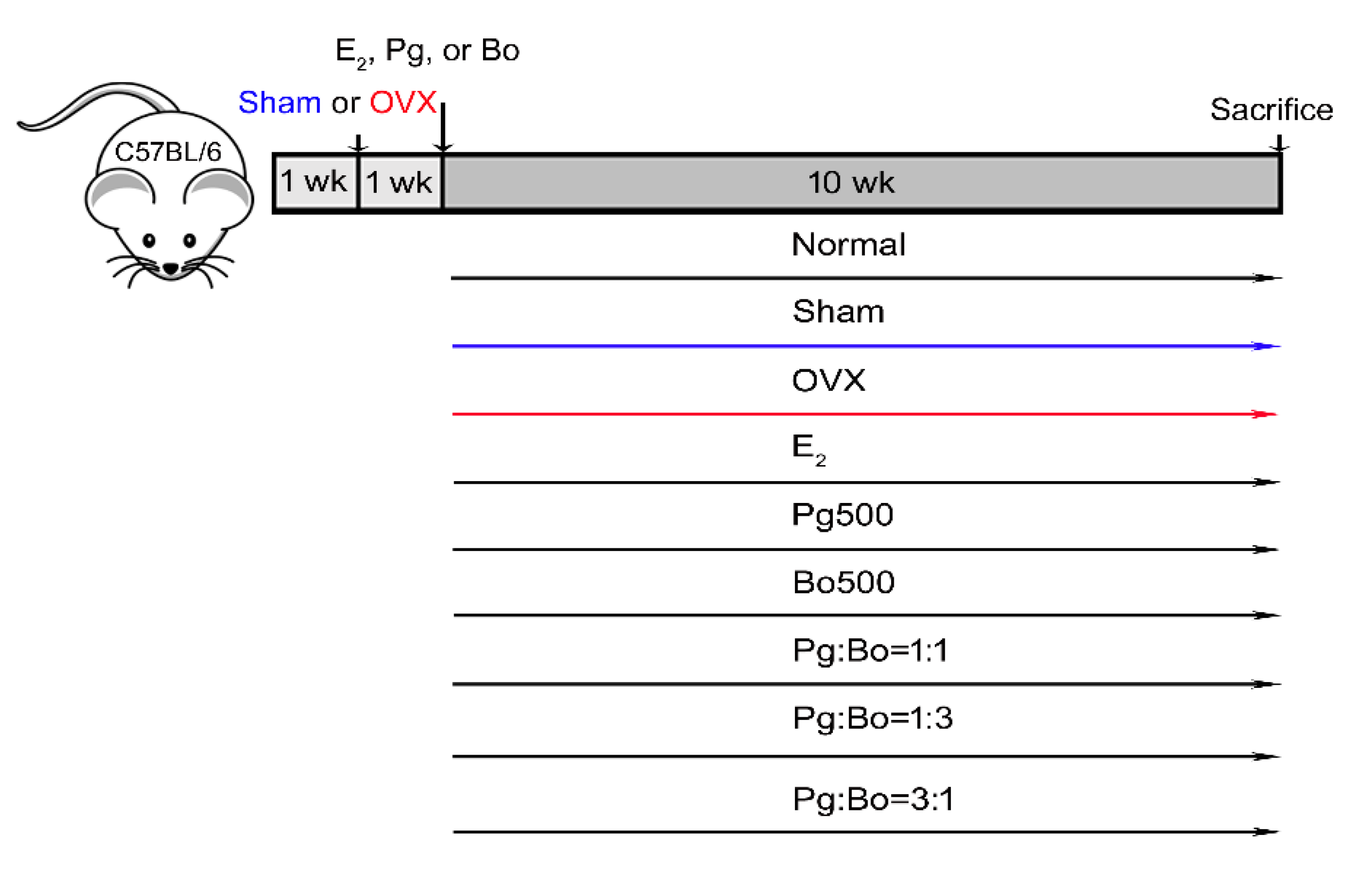
2.2. OVX Operation in Mice
2.3. Pg and Bo Treatment
2.4. Measurement of Bone Loss in OVX Mice
2.5. Histological Preparation and TRAP Staining
2.6. Blood Sampling and Analysis
2.7. OC Differentiation In Vitro
2.8. Statistical Analysis
3. Results
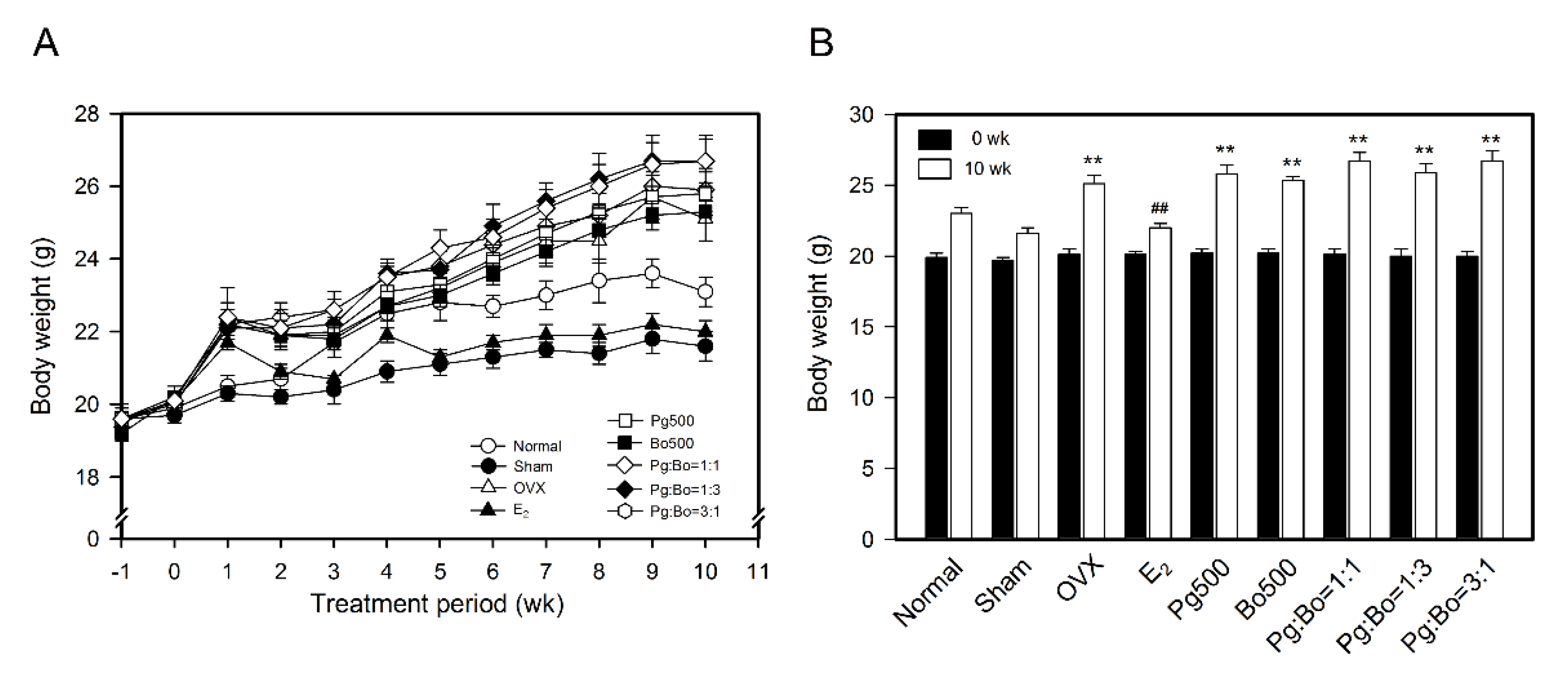
3.1. Effect of P. ginseng and B. oleracea on Body Weight
3.2. Effect of P. ginseng and B. oleracea on Bone Weight and Bone Mineral Density
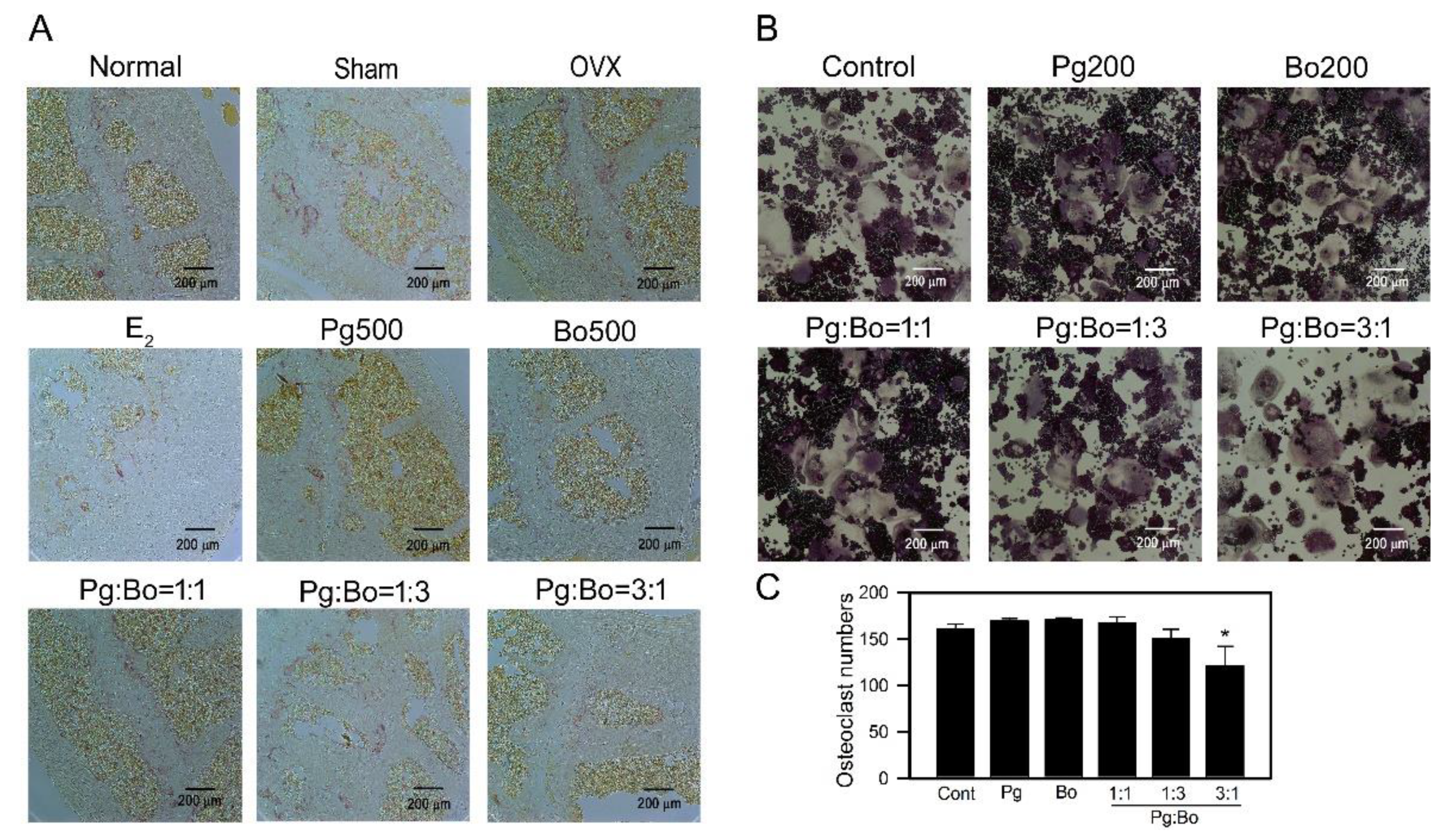
3.3. Effect of P. ginseng and B. oleracea on OC Formation
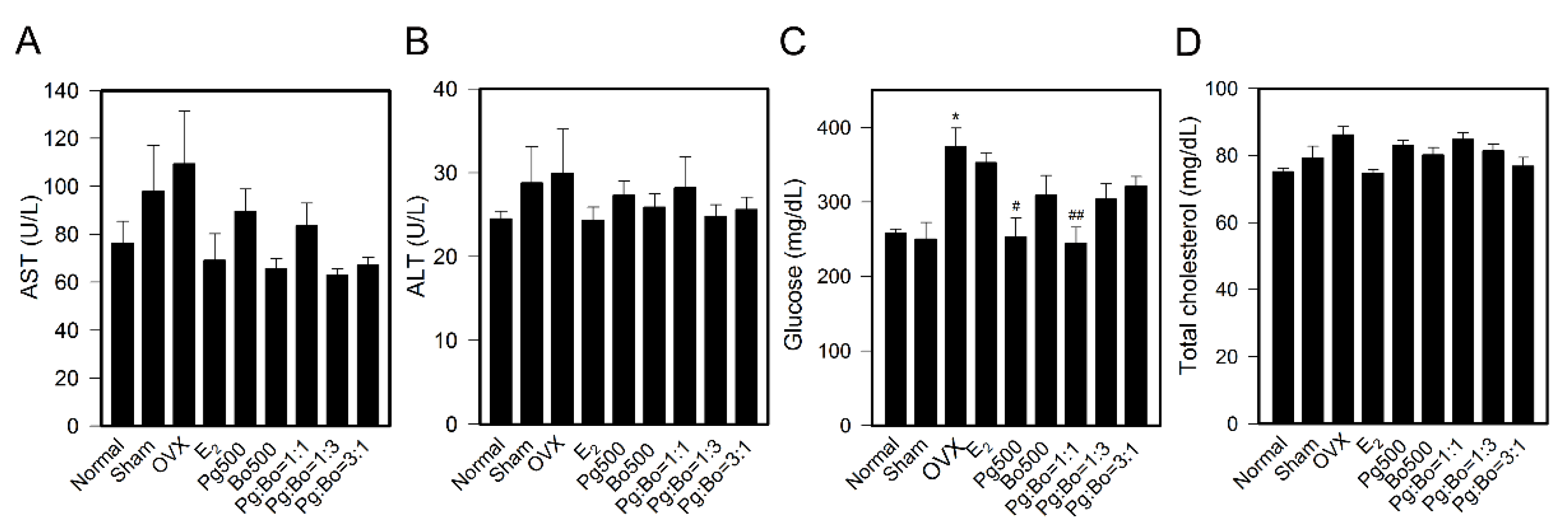
3.4. Effect of P. ginseng and B. oleracea on Blood Biochemical Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ALT | alanine aminotransferase |
| AST | aspartate aminotransferase |
| BMD | bone mineral density |
| Bo | Brassica oleracea extract |
| H&E | hematoxylin and eosin |
| OC | osteoclast |
| OP | osteoporosis |
| OVX | ovariectomized |
| Pg | P. ginseng extract |
| RANKL | receptor activator of nuclear factor kappa B (NF-κB) ligand |
| TRAP | tartrate-resistant acid phosphatase |
References
- International Osteoporosis Foundation. Available online: https://www.iofbonehealth.org/facts-statistics (accessed on 10 June 2019).
- Ji, M.X.; Yu, Q. Primary osteoporosis in postmenopausal women. Chronic Dis. Transl. Med. 2015, 1, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Li, H.W.R.; Brereton, R.E.; Anderson, R.A.; Wallace, A.M.; Ho, C.K. Vitamin D deficiency is common and associated with metabolic risk factors in patients with polycystic ovary syndrome. Metabolism 2011, 60, 1475–1481. [Google Scholar] [CrossRef] [PubMed]
- Bertone-Johnson, E.R.; Hankinson, S.E.; Bendich, A.; Johnson, S.R.; Willett, W.C.; Manson, J.E. Calcium and vitamin D intake and risk of incident premenstrual syndrome. Arch. Intern. Med. 2005, 165, 1246–1252. [Google Scholar] [CrossRef]
- Vitale, S.G.; Caruso, S.; Rapisarda, A.M.C.; Cianci, S.; Cianci, A. Isoflavones, calcium, vitamin D and inulin improve quality of life, sexual function, body composition and metabolic parameters in menopausal women: Result from a prospective, randomized, placebo-controlled, parallel-group study. Menopause Rev. 2018, 17, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Purdue-Smithe, A.C.; Whitcomb, B.W.; Szegda, K.L.; Boutot, M.E.; Manson, J.E.; Hankinson, S.E.; Rosner, B.A.; Troy, L.M.; Michels, K.B.; Bertone-Johnson, E.R. Vitamin D and calcium intake and risk of early menopause. Am. J. Clin. Nutr. 2017, 105, 1493–1501. [Google Scholar] [CrossRef]
- Choi, K.T. Botanical characteristics, pharmacological effects and medicinal components of Korean Panax ginseng C A Meyer. Acta Pharmacol. Sin. 2008, 29, 1109–1118. [Google Scholar] [CrossRef]
- Kiefer, D.; Pantuso, T. Panax ginseng. Am. Fam. Physician 2003, 68, 1539–1542. [Google Scholar]
- Park, H.-J.; Kim, D.-H.; Park, S.-J.; Kim, J.-M.; Ryu, J.H. Ginseng in traditional herbal prescriptions. J. Ginseng Res. 2012, 36, 225–241. [Google Scholar] [CrossRef]
- Attele, A.S.; Wu, J.A.; Yuan, C.S. Ginseng pharmacology: Multiple constituents and multiple actions. Biochem. Pharmacol. 1999, 58, 1685–1693. [Google Scholar] [CrossRef]
- Bhattacharya, S.K.; Mitra, S.K. Anxiolytic activity of Panax ginseng roots: An experimental study. J. Ethnopharmacol. 1991, 34, 87–92. [Google Scholar] [CrossRef]
- Siddiqi, M.H.; Siddiqi, M.Z.; Ahn, S.; Kang, S.; Kim, Y.-J.; Sathishkumar, N.; Yang, D.-U.; Yang, D.-C. Ginseng saponins and the treatment of osteoporosis: Mini literature review. J. Ginseng Res. 2013, 37, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, H.; Kang, K.S.; Chun, K.-H.; Hwang, G.S. Protective effect of Korean Red Ginseng against glucocorticoid-induced osteoporosis in vitro and in vivo. J. Ginseng Res. 2015, 39, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Avsar, U.; Karakus, E.; Halici, Z.; Bayir, Y.; Bilen, H.; Aydin, A.; Avsar, U.Z.; Ayan, A.; Aydin, S.; Karadeniz, A. Prevention of bone loss by Panax ginseng in a rat model of inflammation-induced bone loss. Cell. Mol. Biol. 2013, 59 (Suppl. OL), 1835–1841. [Google Scholar]
- Shen, Y.; Li, Y.-Q.; Li, S.-P.; Ma, L.; Ding, L.-J.; Ji, H. Alleviation of ovariectomy-induced osteoporosis in rats by Panax notoginseng saponins. J. Nat. Med. 2010, 64, 336–345. [Google Scholar] [CrossRef]
- Fan, J.-Z.; Wang, Y.; Meng, Y.; Li, G.-W.; Chang, S.-X.; Nian, H.; Liang, Y.-J. Panax notoginseng saponins mitigate ovariectomy-induced bone loss and inhibit marrow adiposity in rats. Menopause 2015, 22, 1343–1350. [Google Scholar] [CrossRef]
- Gong, Y.-S.; Chen, J.; Zhang, Q.-Z.; Zhang, J.-T. Effect of 17β-oestradiol and ginsenoside on osteoporosis in ovariectomised rats. J. Asian Nat. Prod. Res. 2006, 8, 649–656. [Google Scholar] [CrossRef]
- Kim, H.-R.; Cui, Y.; Hong, S.-J.; Shin, S.-J.; Kim, D.-S.; Kim, N.-M.; So, S.-H.; Lee, S.-K.; Kim, E.-C.; Chae, S.-W.; et al. Effect of ginseng mixture on osteoporosis in ovariectomized rats. Immunopharmacol. Immunotoxicol. 2008, 30, 333–345. [Google Scholar] [CrossRef]
- Huang, Q.; Gao, B.; Jie, Q.; Wei, B.-Y.; Fan, J.; Zhang, H.-Y.; Zhang, J.-K.; Li, X.-J.; Shi, J.; Luo, Z.; et al. Ginsenoside-Rb2 displays anti-osteoporosis effects through reducing oxidative damage and bone-resorbing cytokines during osteogenesis. Bone 2014, 66, 306–314. [Google Scholar] [CrossRef]
- Bei, J.; Zhang, X.; Wu, J.; Hu, Z.; Xu, B.; Lin, S.; Cui, L.; Wu, T.; Zou, L. Ginsenoside Rb1 does not halt osteoporotic bone loss in ovariectomized rats. PLoS ONE 2018, 13, e0202885. [Google Scholar] [CrossRef]
- Avato, P.; Argentieri, M.P. Brassicaceae: A rich source of health improving phytochemicals. Phytochem. Rev. 2015, 14, 1019–1033. [Google Scholar] [CrossRef]
- Park, S.; Arasu, M.V.; Lee, M.-K.; Chun, J.-H.; Seo, J.M.; Lee, S.-W.; Al-Dhabi, N.A.; Kim, J.-B. Quantification of glucosinolates, anthocyanins, free amino acids, and vitamin C in inbred lines of cabbage (Brassica oleracea L.). Food Chem. 2014, 145, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Šamec, D.; Pavlović, I.; Sondi, B.S. White cabbage (Brassica oleracea var. capitata f. alba): Botanical, phytochemical and pharmacological overview. Phytochem. Rev. 2016, 16, 117–135. [Google Scholar] [CrossRef]
- Clarke, D.B. Glucosinolates, structures and analysis in food. Anal. Methods 2010, 2, 310–325. [Google Scholar] [CrossRef]
- Fenwick, G.R.; Griffiths, N.M.; Heaney, R.K. Bitterness in Brussels sprouts (Brassica oleracea L. var. gemmifera): The role of 126 glucosinolates and their breakdown products. J. Sci. Food Agric. 1983, 34, 73–80. [Google Scholar] [CrossRef]
- Van Poppel, G.; Verhoeven, D.T.H.; Verhagen, H.; Goldbohm, R.A. Brassica vegetables and cancer prevention. Pharm. Biotechnol. 1999, 472, 159–168. [Google Scholar] [CrossRef]
- Hwang, E.-S.; Hong, E.-Y.; Kim, G. Determination of bioactive compounds and anti-cancer effect from extracts of Korean cabbage and cabbage. Korean J. Food Nutr. 2012, 25, 259–265. [Google Scholar] [CrossRef]
- Sousa, C.; Taveira, M.; Valentão, P.; Fernandes, F.; Pereira, J.A.; Estevinho, L.M.; Bento, A.A.; Ferreres, F.; Seabra, R.M.; Andrade, P.B. Inflorescences of Brassicacea species as source of bioactive compounds: A comparative study. Food Chem. 2008, 110, 953–961. [Google Scholar] [CrossRef]
- Ben Hadda, T.; ElSawy, N.A.; Header, E.A.M.; Mabkhot, Y.N.; Mubarak, M.S. Effect of garlic and cabbage on healing of gastric ulcer in experimental rats. Med. Chem. Res. 2014, 23, 5110–5119. [Google Scholar] [CrossRef]
- Ayshwarya, M.; Sudha Rameshwari, K. Antimicrobial activity of the plant extracts of Brassica oleracea var. capitate rubra. J. Int. Acad. Res. Multidisciplin. 2015, 3, 149–156. [Google Scholar]
- Waghulde, S.; Khan, N.A.; Gorde, N.; Kale, M.; Naik, P.; Yewale, R.P. Comparative antimicrobial activity study of Brassica oleceracea. Proceedings 2019, 9, 64. [Google Scholar] [CrossRef]
- Pereira, J.V.; Santos, H.B.; Agra, M.F.; Guedes, D.N.; Modesto-Filho, J. Use of cabbage leaves (Brassica oleracea var. acephala) in the stabilization of bone mass after menopause. Rev. Bras. Farm. 2006, 16, 345–349. [Google Scholar] [CrossRef]
- Tella, S.H.; Gallagher, J.C. Prevention and treatment of postmenopausal osteoporosis. J. Steroid Biochem. Mol. Biol. 2014, 142, 155–170. [Google Scholar] [CrossRef]
- Awasthi, H.; Mani, D.; Singh, D.; Gupta, A. The underlying pathophysiology and therapeutic approaches for osteoporosis. Med. Res. Rev. 2018, 38, 2024–2057. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, R.; Krege, J.H.; Marín, F.; Jin, L.; Stepan, J. Teriparatide for osteoporosis: Importance of the full course. Osteoporos. Int. 2016, 27, 2395–2410. [Google Scholar] [CrossRef] [PubMed]
- Gibaldi, M. Prevention and treatment of osteoporosis: Does the future belong to hormone replacement therapy? J. Clin. Pharmacol. 1997, 37, 1087–1099. [Google Scholar] [CrossRef]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. PLoS Biol. 2010, 8, e1000412. [Google Scholar] [CrossRef]
- De Franciscis, P.; Colacurci, N.; Riemma, G.; Conte, A.; Pittana, E.; Guida, M.; Schiattarella, A.; Franciscis, D. A nutraceutical approach to menopausal complaints. Medicina 2019, 55, 544. [Google Scholar] [CrossRef]
- Comhaire, F.H.; Depypere, H.T. Hormones, herbal preparations and nutriceuticals for a better life after the menopause: Part I. Climacteric 2015, 18, 358–363. [Google Scholar] [CrossRef]
- Xu, Y.; Ding, J.; Ma, X.-P.; Ma, Y.-H.; Liu, Z.; Lin, N. Treatment with Panax ginseng antagonizes the estrogen decline in ovariectomized mice. Int. J. Mol. Sci. 2014, 15, 7827–7840. [Google Scholar] [CrossRef]
- Lee, H.; Choi, J.; Shin, S.S.; Yoon, M. Effects of Korean red ginseng (Panax ginseng) on obesity and adipose inflammation in ovariectomized mice. J. Ethnopharmacol. 2016, 178, 229–237. [Google Scholar] [CrossRef]
- Wenxi, D.; Shufang, D.; Xiaoling, Y.; Liming, Y. Panax notoginseng saponins suppress radiation-induced osteoporosis by regulating bone formation and resorption. Phytomedicine 2015, 22, 813–819. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Lee, J.; Jang, J.H.; Lee, S.-H.; Nan, M.H.; Oh, B.-C.; Lee, S.G.; Kim, H.H.; Soung, N.-K.; Ahn, J.S.; et al. Ginsenoside Rh2 inhibits osteoclastogenesis through down-regulation of NF-κB, NFATc1 and c-Fos. Bone 2012, 50, 1207–1213. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Shiono, J.; Shimizu, K.; Yu, H.; Zhang, C.; Jin, F.; Kondo, R. 20(R)-ginsenoside Rh2, not 20(S), is a selective osteoclastgenesis inhibitor without any cytotoxicity. Bioorganic Med. Chem. Lett. 2009, 19, 3320–3323. [Google Scholar] [CrossRef] [PubMed]
- Prasannarong, M.; Vichaiwong, K.; Saengsirisuwan, V. Calorie restriction prevents the development of insulin resistance and impaired insulin signaling in skeletal muscle of ovariectomized rats. Biochim. Biophys. Acta 2012, 1822, 1051–1061. [Google Scholar] [CrossRef] [PubMed]
- Lu, N.; Sun, H.; Yu, J.; Wang, X.; Liu, N.; Zhao, L.; Sun, L.; Zhao, H.; Tao, B.; Liu, J.-M. Glucagon-like peptide-1 receptor agonist Liraglutide has anabolic bone effects in ovariectomized rats without diabetes. PLoS ONE 2015, 10, e0132744. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Bala, M.; Gupta, S.; Dua, A.; Dabur, R.; Injeti, E.; Mittal, A. Efficacy and risk profile of anti-diabetic therapies: Conventional vs traditional drugs—A mechanistic revisit to understand their mode of action. Pharmacol. Res. 2016, 113, 636–674. [Google Scholar] [CrossRef]
- Yuan, H.-D.; Kim, J.-T.; Kim, S.-H.; Chung, S.H. Ginseng and diabetes: The evidences from in vitro, animal and human studies. J. Ginseng Res. 2012, 36, 27–39. [Google Scholar] [CrossRef]
- Choi, J.; Kim, T.-H.; Choi, T.-Y.; Lee, M.S. Ginseng for health care: A systematic review of randomized controlled trials in Korean literature. PLoS ONE 2013, 8, e59978. [Google Scholar] [CrossRef]
- Park, S.J.; Nam, J.S.; Ahn, C.; Kim, Y.S. Anti-diabetic properties of different fractions of Korean red ginseng. J. Ethnopharmacol. 2019, 236, 220–230. [Google Scholar] [CrossRef]
- Louet, J.-F.; Lemay, C.; Mauvais-Jarvis, F. Antidiabetic actions of estrogen: Insight from human and genetic mouse models. Curr. Atheroscler. Rep. 2004, 6, 180–185. [Google Scholar] [CrossRef]
- Wang, J.; Hou, X.; Adeosun, S.; Hill, R.; Henry, S.; Paul, I.; Irwin, R.W.; Ou, X.-M.; Bigler, S.; Stockmeier, C.; et al. A dominant negative ERβ splice variant determines the effectiveness of early or late estrogen therapy after ovariectomy in rats. PLoS ONE 2012, 7, e33493. [Google Scholar] [CrossRef] [PubMed]
- Matsui, S.; Yasui, T.; Kasai, K.; Keyama, K.; Kato, T.; Uemura, H.; Kuwahara, A.; Matsuzaki, T.; Irahara, M. Changes of liver enzymes and triglyceride during the menopausal transition in Japanese women. J. Obstet. Gynaecol. 2016, 36, 806–811. [Google Scholar] [CrossRef] [PubMed]
- Hanley, A.J.; Wagenknecht, L.E.; Festa, A.; D’Agostino, R.B.; Haffner, S.M. Alanine aminotransferase and directly measured insulin sensitivity in a multiethnic cohort: The insulin resistance atherosclerosis study. Diabetes Care 2007, 30, 1819–1827. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, I.S.; Agidigbi, T.S.; Kwon, Y.M.; Kim, D.-G.; Kim, R.I.; In, G.; Lee, M.-H.; Kim, C. Effect of Co-Administration of Panax ginseng and Brassica oleracea on Postmenopausal Osteoporosis in Ovariectomized Mice. Nutrients 2020, 12, 2415. https://doi.org/10.3390/nu12082415
Kang IS, Agidigbi TS, Kwon YM, Kim D-G, Kim RI, In G, Lee M-H, Kim C. Effect of Co-Administration of Panax ginseng and Brassica oleracea on Postmenopausal Osteoporosis in Ovariectomized Mice. Nutrients. 2020; 12(8):2415. https://doi.org/10.3390/nu12082415
Chicago/Turabian StyleKang, In Soon, Taiwo Samuel Agidigbi, Young Min Kwon, Dong-Gyu Kim, Rang Ie Kim, Gyo In, Mi-Hyang Lee, and Chaekyun Kim. 2020. "Effect of Co-Administration of Panax ginseng and Brassica oleracea on Postmenopausal Osteoporosis in Ovariectomized Mice" Nutrients 12, no. 8: 2415. https://doi.org/10.3390/nu12082415
APA StyleKang, I. S., Agidigbi, T. S., Kwon, Y. M., Kim, D.-G., Kim, R. I., In, G., Lee, M.-H., & Kim, C. (2020). Effect of Co-Administration of Panax ginseng and Brassica oleracea on Postmenopausal Osteoporosis in Ovariectomized Mice. Nutrients, 12(8), 2415. https://doi.org/10.3390/nu12082415




