Supplementation with Fermented Rice Bran Attenuates Muscle Atrophy in a Diabetic Rat Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals
2.3. Histological Analysis
2.4. Blood Glucose Level, Plasma Insulin Level, and Blood Profile
2.5. Western Blotting
2.6. RNA Extraction and Quantitative Reverse Transcriptase (RT)-PCR
2.7. Statistical Analysis
3. Results
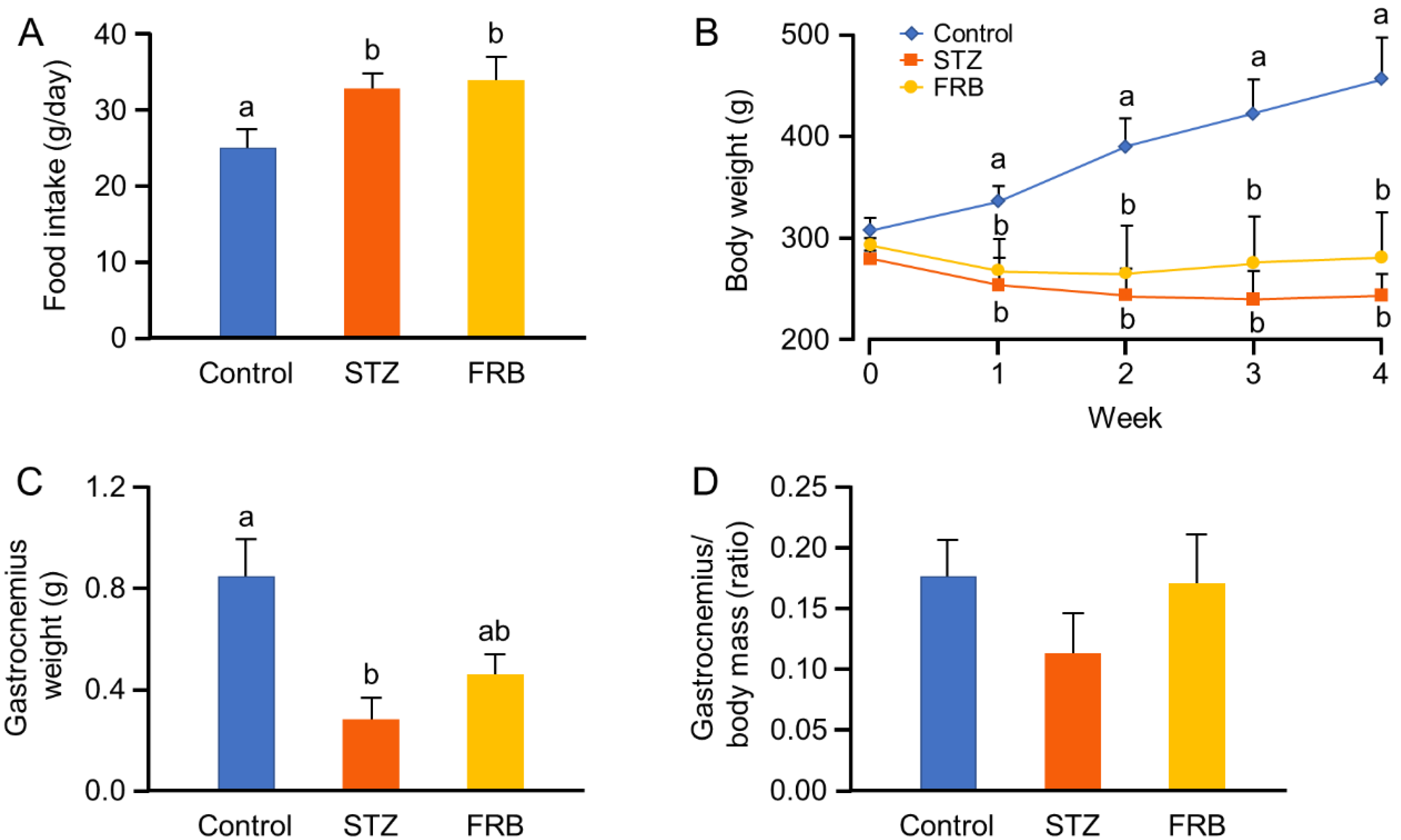
3.1. Effect of FRB on the Body Weight of Rats with STZ-Induced Diabetes
3.2. Effect of FRB on the Blood Profile of Rats with STZ-induced Diabetes
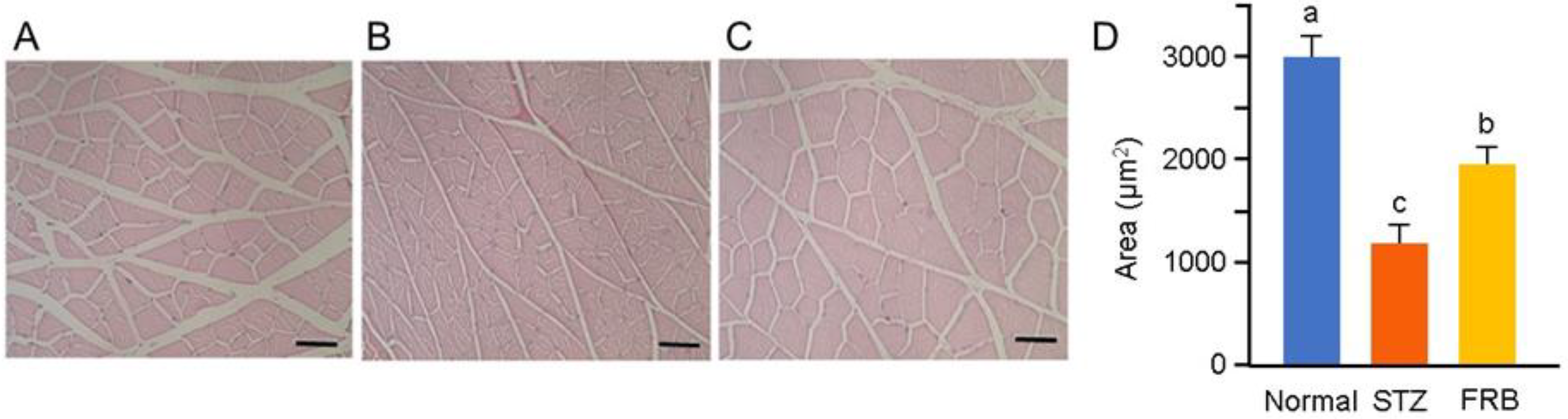
3.3. Effect of FRB on the Muscle Size of Rats with STZ-induced Diabetes
3.4. Effect of FRB on Diabetic Muscle Regulatory Genes in Rats with STZ-induced Diabetes
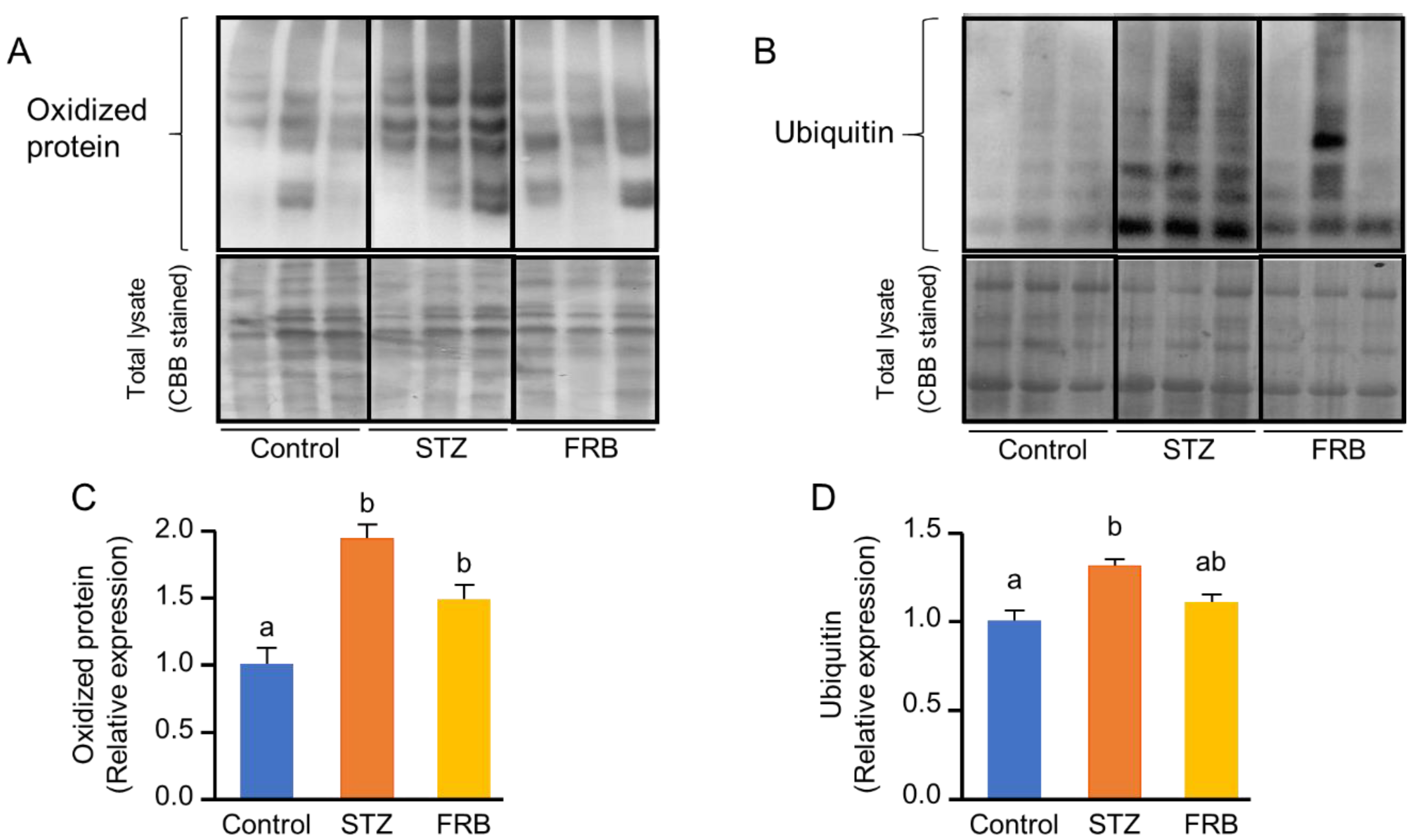
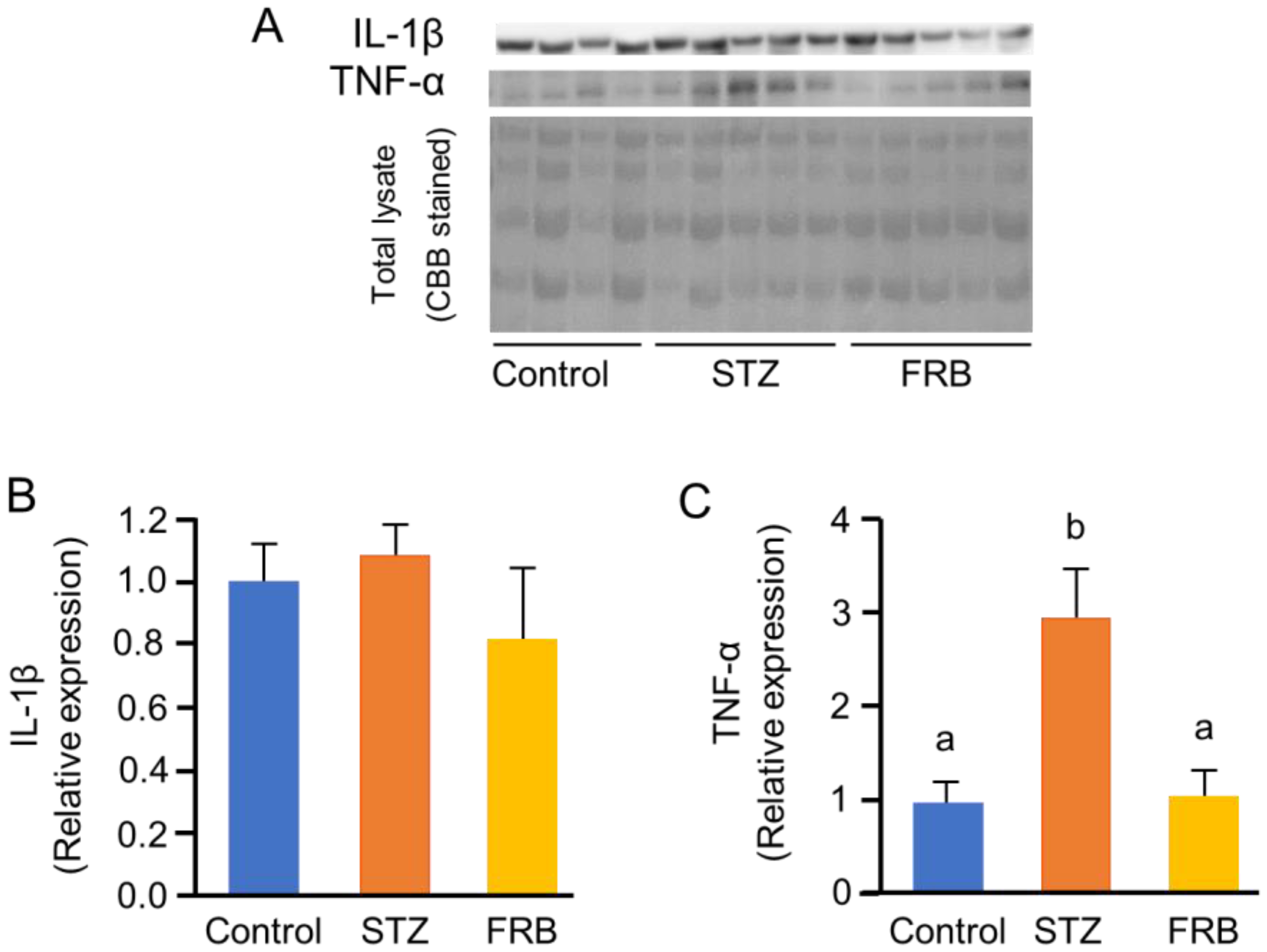
3.5. Effect of FRB on the Level of Oxidized and Ubiquitinated Proteins in the Muscles of Rats with STZ-Induced Diabetes
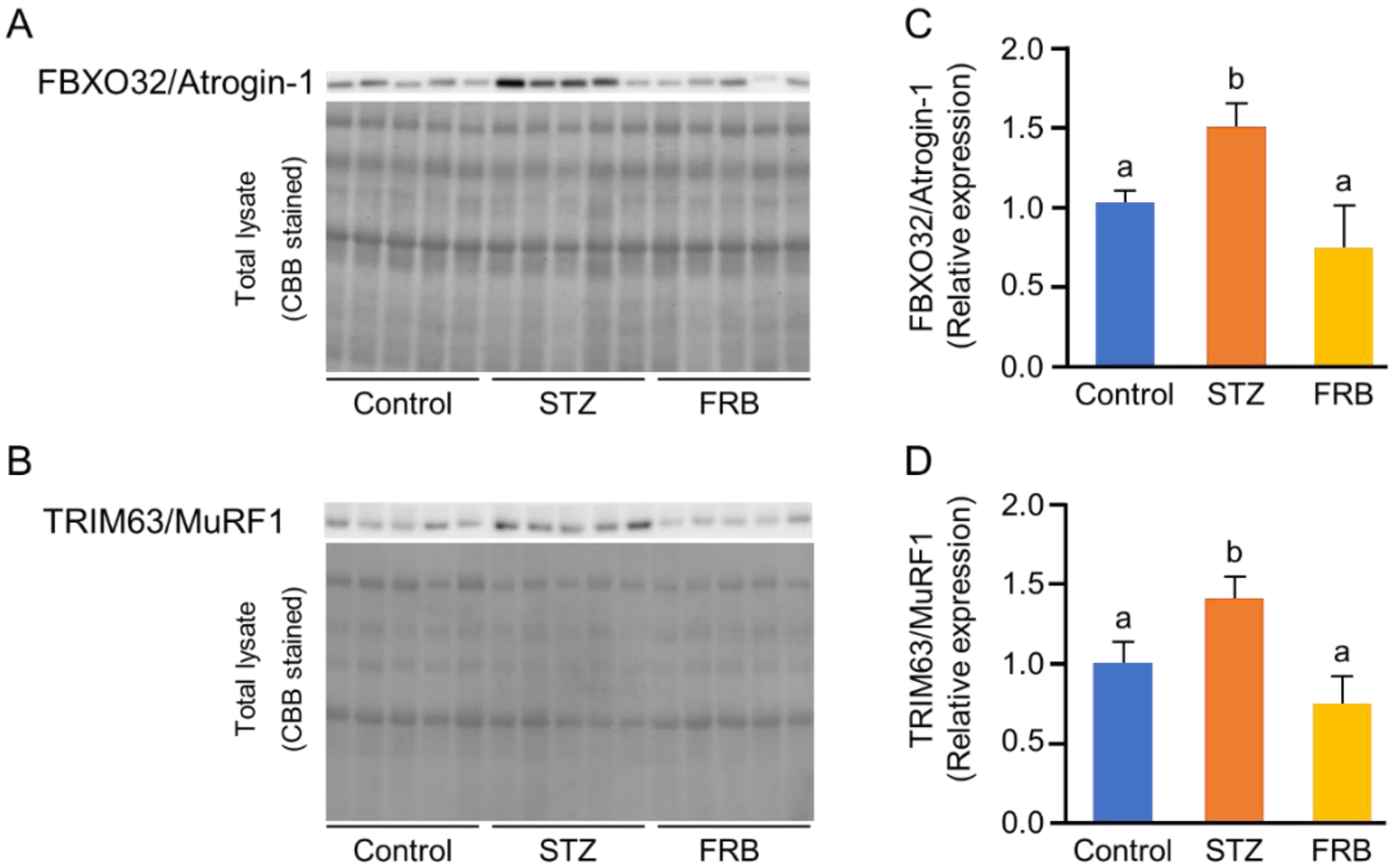
3.6. Effect of FRB on the Protein Degradation Markers in the Muscles of Rats with STZ-induced Diabetes
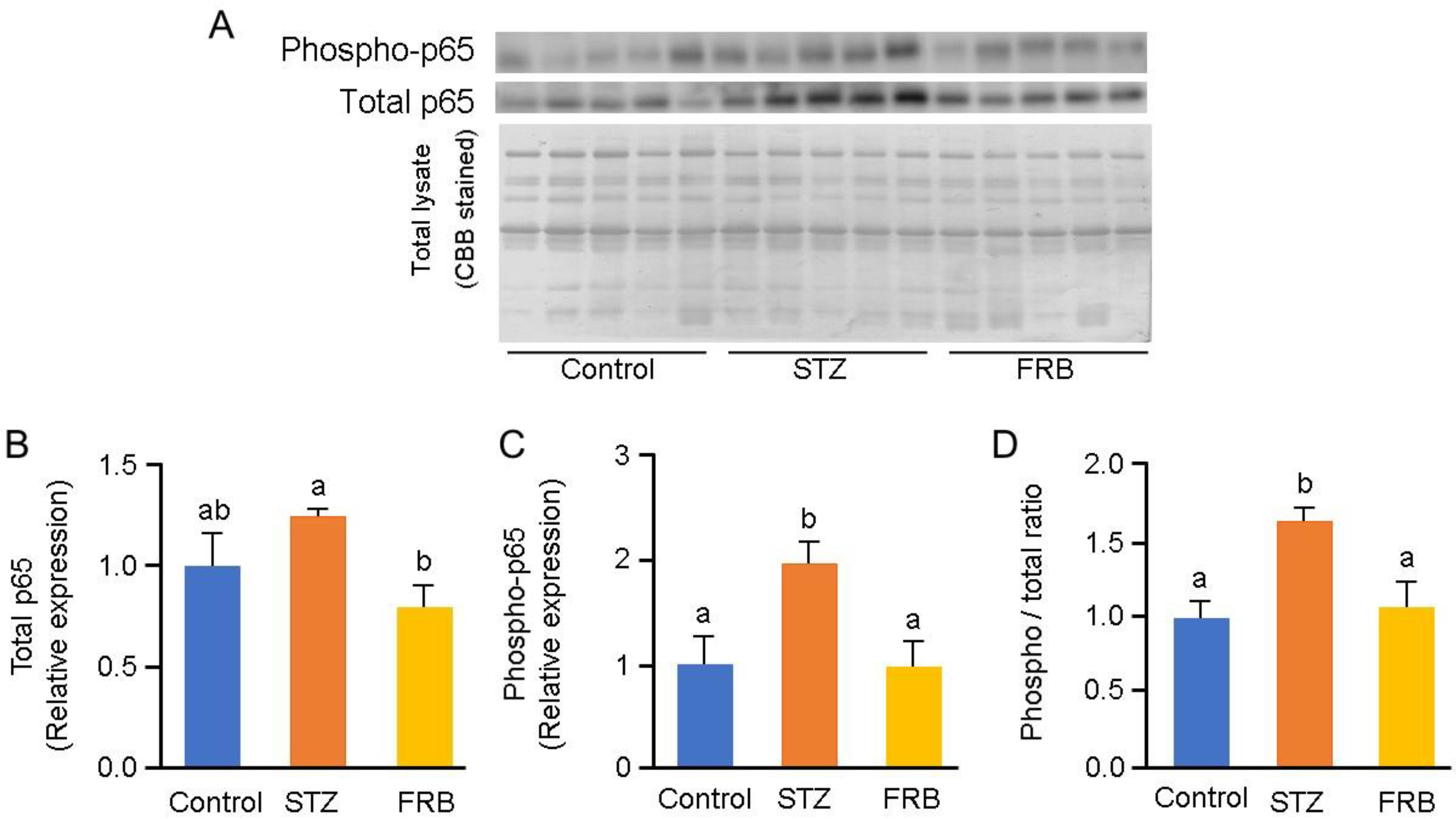
3.7. Effect of FRB on the Phosphorylation of NF-κB p65 in the Muscles of Rats with STZ-induced Diabetes
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Faulkner, J.A.; Larkin, L.M.; Claflin, D.R.; Brooks, S.V. Age-related changes in the structure and function of skeletal muscles. Clin. Exp. Pharm. Physiol. 2007, 34, 1091–1096. [Google Scholar] [CrossRef]
- Matsumoto, H.; Hagino, H.; Wada, T.; Kobayashi, E. Locomotive syndrome presents a risk for falls and fractures in the elderly Japanese population. Osteoporos. Sarcopenia 2016, 2, 156–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beaudart, C.; Rizzoli, R.; Bruyère, O.; Reginster, J.-Y.; Biver, E. Sarcopenia: Burden and challenges for public health. Arch. Public Health 2014, 72, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Neill, B.T.; Bhardwaj, G.; Penniman, C.M.; Krumpoch, M.T.; Beltran, P.A.S.; Klaus, K.; Poro, K.; Li, M.; Pan, H.; Dreyfuss, J.M.; et al. FoxO transcription factors are critical regulators of diabetes-related muscle atrophy. Diabetes 2019, 68, 556–570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oguntibeju, O.O. Type 2 diabetes mellitus, oxidative stress and inflammation: Examining the links. Int. J. Physiol. Pathophysiol. Pharm. 2019, 11, 45–63. [Google Scholar]
- Moylan, J.S.; Reid, M.B. Oxidative stress, chronic disease, and muscle wasting. Muscle Nerve 2007, 35, 411–429. [Google Scholar] [CrossRef]
- Meng, S.-J.; Yu, L.-J. Oxidative stress, molecular inflammation and sarcopenia. Int. J. Mol. Sci. 2010, 11, 1509–1526. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Yan, L.-J. Streptozotocin-induced type 1 diabetes in rodents as a model for studying mitochondrial mechanisms of diabetic β cell glucotoxicity. Diabetes Metab. Syndr. Obes. Targets 2015, 8, 181–188. [Google Scholar] [CrossRef] [Green Version]
- Ono, T.; Takada, S.; Kinugawa, S.; Tsutsui, H. Curcumin ameliorates skeletal muscle atrophy in type 1 diabetic mice by inhibiting protein ubiquitination. Exp. Physiol. 2015, 100, 1052–1063. [Google Scholar] [CrossRef] [Green Version]
- Hirata, Y.; Nomura, K.; Senga, Y.; Okada, Y.; Kobayashi, K.; Okamoto, S.; Minokoshi, Y.; Imamura, M.; Takeda, S.; Hosooka, T.; et al. Hyperglycemia induces skeletal muscle atrophy via a WWP1/KLF15 axis. JCI Insight 2019, 4. [Google Scholar] [CrossRef] [Green Version]
- Sandri, M. Protein breakdown in muscle wasting: Role of autophagy-lysosome and ubiquitin-proteasome. Int. J. Biochem. Cell Biol. 2013, 45, 2121–2129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonaldo, P.; Sandri, M. Cellular and molecular mechanisms of muscle atrophy. Dis. Model. Mech. 2013, 6, 25–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schreiber, A.; Peter, M. Substrate recognition in selective autophagy and the ubiquitin–proteasome system. Biochim. Biophys. Acta BBA Mol. Cell Res. 2014, 1843, 163–181. [Google Scholar] [CrossRef] [Green Version]
- Bodine, S.C.; Baehr, L.M. Skeletal muscle atrophy and the E3 ubiquitin ligases MuRF1 and MAFbx/atrogin-1. Am. J. Physiol. Endocrinol. Metab. 2014, 307, E469–E484. [Google Scholar] [CrossRef] [Green Version]
- Sandri, M. Signaling in muscle atrophy and hypertrophy. Physiology 2008, 23, 160–170. [Google Scholar] [CrossRef] [Green Version]
- Gomes, M.D.; Lecker, S.H.; Jagoe, R.T.; Navon, A.; Goldberg, A.L. Atrogin-1, a muscle-specific F-box protein highly expressed during muscle atrophy. Proc. Natl. Acad. Sci. USA 2001, 98, 14440–14445. [Google Scholar] [CrossRef] [Green Version]
- Eley, H.L.; Tisdale, M.J. Skeletal muscle atrophy, a link between depression of protein synthesis and increase in degradation. J. Biol. Chem. 2007, 282, 7087–7097. [Google Scholar] [CrossRef] [Green Version]
- Roy, B. Biomolecular basis of the role of diabetes mellitus in osteoporosis and bone fractures. World J. Diabetes 2013, 4, 101–113. [Google Scholar] [CrossRef]
- Whitehouse, A.S.; Tisdale, M.J. Increased expression of the ubiquitin–proteasome pathway in murine myotubes by proteolysis-inducing factor (PIF) is associated with activation of the transcription factor NF-κB. Br. J. Cancer 2003, 89, 1116–1122. [Google Scholar] [CrossRef]
- Wyke, S.M.; Russell, S.T.; Tisdale, M.J. Induction of proteasome expression in skeletal muscle is attenuated by inhibitors of NF-κB activation. Br. J. Cancer 2004, 91, 1742–1750. [Google Scholar] [CrossRef] [Green Version]
- Magne, H.; Auzeloux, I.S.; Rémond, D.; Dardevet, D. Nutritional strategies to counteract muscle atrophy caused by disuse and to improve recovery. Nutr. Res. Rev. 2013, 26, 149–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiménez, J.D.B.; Lluch, G.L.; Martínez, I.S.; Jiménez, A.M.; Bies, E.R.; Navas, P. Sarcopenia, implications of physical exercise in its pathophysiology. prevention and treatment. Rev. Med. Deporte 2011, 4, 158–166. [Google Scholar]
- Ternero, C.P.; Werner, C.M.; Nickel, A.G.; Herrera, M.D.; Motilva, M.-J.; Böhm, M.; Sotomayor, M.A.d.; Laufs, U. Ferulic acid, a bioactive component of rice bran, improves oxidative stress and mitochondrial biogenesis and dynamics in mice and in human mononuclear cells. J. Nutr. Biochem. 2017, 48, 51–61. [Google Scholar] [CrossRef]
- Islam, M.S.; Nagasaka, R.; Ohara, K.; Hosoya, T.; Ozaki, H.; Ushio, H.; Hori, M. Biological abilities of rice bran-derived antioxidant phytochemicals for medical therapy. Curr. Top. Med. Chem. 2011, 11, 1847–1853. [Google Scholar] [CrossRef]
- Ternero, C.P.; Pulgarin, B.B.; Sotomayor, M.A.d.; Herrera, M.D. Atherosclerosis-related inflammation and oxidative stress are improved by rice bran enzymatic extract. J. Funct. Foods 2016, 26, 610–621. [Google Scholar] [CrossRef]
- Alauddin, M.; Shirakawa, H.; Koseki, T.; Kijima, N.; Budijanto, S.; Islam, J.; Goto, T.; Komai, M. Fermented rice bran supplementation mitigates metabolic syndrome in stroke-prone spontaneously hypertensive rats. BMC Complement. Altern. Med. 2016, 16, 442. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Zhang, J.; Wang, J.; Sun, B. The anti-cancer activity and potential clinical application of rice bran extracts and fermentation products. RSC Adv. 2019, 9, 18060–18069. [Google Scholar] [CrossRef] [Green Version]
- Ardiansyah, N.; Shirakawa, H.; Sugita, Y.; Koseki, T.; Komai, M. Anti-metabolic syndrome effects of adenosine ingestion in stroke-prone spontaneously hypertensive rats fed a high-fat diet. Br. J. Nutr. 2010, 104, 48–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Islam, J.; Koseki, T.; Watanabe, K.; Ardiansyah, A.; Budijanto, S.; Oikawa, A.; Alauddin, M.; Goto, T.; Aso, H.; Komai, M.; et al. Dietary supplementation of fermented rice bran effectively alleviates dextran sodium sulfate-induced colitis in mice. Nutrients 2017, 9, 747. [Google Scholar] [CrossRef]
- Shibayama, J.; Kuda, T.; Shikano, A.; Fukunaga, M.; Takahashi, H.; Kimura, B.; Ishizaki, S. Effects of rice bran and fermented rice bran suspensions on caecal microbiota in dextran sodium sulphate-induced inflammatory bowel disease model mice. Food Biosci. 2018, 25, 8–14. [Google Scholar] [CrossRef]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C. AIN-93 purified diets for laboratory rodents: Final report of the american institute of nutrition Ad Hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [CrossRef] [PubMed]
- Fortes, M.A.S.; Nassr, G.N.M.; Vitzel, K.F.; Pinheiro, C.H.d.J.; Newsholme, P.; Curi, R. Housekeeping proteins: How useful are they in skeletal muscle diabetes studies and muscle hypertrophy models? Anal. Biochem. 2016, 504, 38–40. [Google Scholar] [CrossRef] [PubMed]
- Fischer, Y.W.; Garyantes, T. Improving the Reliability and Utility of Streptozotocin-Induced Rat Diabetic Model. Available online: https://www.hindawi.com/journals/jdr/2018/8054073/ (accessed on 26 March 2020).
- Rai, S.; Hajam, Y.A.; Basheer, M. Biochemical and histopathological inflections in hepato-renal tissues of streptozotocin (STZ) induced diabetic male rats: Impact of exogenous melatonin administration. J. Clin. Res. Bioeth. 2016, 7. [Google Scholar] [CrossRef]
- Wang, X.; Hu, Z.; Hu, J.; Du, J.; Mitch, W.E. Insulin resistance accelerates muscle protein degradation: Activation of the ubiquitin-proteasome pathway by defects in muscle cell signaling. Endocrinology 2006, 147, 4160–4168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalyani, R.R.; Corriere, M.; Ferrucci, L. Age-related and disease-related muscle loss: The effect of diabetes, obesity, and other diseases. Lancet Diabetes Endocrinol. 2014, 2, 819–829. [Google Scholar] [CrossRef] [Green Version]
- Junior, D.C.P.; Silva, K.S.; Michalani, M.L.; Yonamine, C.Y.; Esteves, J.V.; Fabre, N.T.; Thieme, K.; Catanozi, S.; Okamoto, M.M.; Seraphim, P.M.; et al. Advanced glycation end products-induced insulin resistance involves repression of skeletal muscle GLUT4 expression. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef]
- Coleman, S.K.; Rebalka, I.A.; D’Souza, D.M.; Deodhare, N.; Desjardins, E.M.; Hawke, T.J. Myostatin inhibition therapy for insulin-deficient type 1 diabetes. Sci. Rep. 2016, 6, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Federici, M.; Hribal, M.L.; Menghini, R.; Kanno, H.; Marchetti, V.; Porzio, O.; Sunnarborg, S.W.; Rizza, S.; Serino, M.; Cunsolo, V.; et al. Timp3 deficiency in insulin receptor–haploinsufficient mice promotes diabetes and vascular inflammation via increased TNF-α. J. Clin. Investig. 2005, 115, 3494–3505. [Google Scholar] [CrossRef] [Green Version]
- Sandri, M.; Sandri, C.; Gilbert, A.; Skurk, C.; Calabria, E.; Picard, A.; Walsh, K.; Schiaffino, S.; Lecker, S.H.; Goldberg, A.L. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell 2004, 117, 399–412. [Google Scholar] [CrossRef] [Green Version]
- Alzamil, H. Elevated serum TNF- α is related to obesity in type 2 diabetes mellitus and is associated with glycemic control and insulin resistance. J. Obes. 2020, 2020, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Thoma, A.; Lightfoot, A.P. NF-kB and inflammatory cytokine signalling: Role in skeletal muscle atrophy. In Muscle Atrophy; Xiao, J., Ed.; Springer: Singapore, 2018; pp. 267–279. ISBN 9789811314353. [Google Scholar]
- Li, H.; Malhotra, S.; Kumar, A. Nuclear factor-kappa B signaling in skeletal muscle atrophy. J. Mol. Med. 2008, 86, 1113–1126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, L.; Han, J.; Meng, Q.; Xi, Q.; Zhuang, Q.; Jiang, Y.; Han, Y.; Zhang, B.; Fang, J.; Wu, G. Muscle-specific E3 ubiquitin ligases are involved in muscle atrophy of cancer cachexia: An in vitro and in vivo study. Oncol. Rep. 2015, 33, 2261–2268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, D.; Frantz, J.D.; Tawa, N.E.; Melendez, P.A.; Oh, B.-C.; Lidov, H.G.W.; Hasselgren, P.-O.; Frontera, W.R.; Lee, J.; Glass, D.J.; et al. IKKβ/NF-κB activation causes severe muscle wasting in mice. Cell 2004, 119, 285–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Components (g/kg) | Control Group | Diabetes Groups | |
|---|---|---|---|
| STZ | FRB | ||
| Standard Diet | 10% FRB | ||
| Tert-Butylhydroquinone | 0.008 | 0.0072 | |
| l-Cystine | 1.8 | 1.62 | |
| Choline bitartrate | 2.5 | 2.25 | |
| Vitamin mixture (AIN-93G) | 10 | 9 | |
| Mineral mixture (AIN-93M) | 35 | 31.5 | |
| Soybean oil | 40 | 36 | |
| Cellulose | 50 | 45 | |
| Sucrose | 100 | 90 | |
| Casein | 140 | 126 | |
| Cornstarch | 620.7 | 558.623 | |
| Fermented rice bran | 0 | 100 | |
| Gene Name | Forward Primer (5′-3′) | Reverse Primer (5′- 3′) |
|---|---|---|
| Ager | GAAACCGGTGATGAAGGACAAG | TCGAGTCTGGGTTGTCGTTTT |
| Tnfa | GACGTGGAACTGGCAGAAGAG | TCTGGAAGCCCCCCATCT |
| Mstn | TACCATGCCTACCGAGTCTGA | CAGCTGGGCCTTTACCACTT |
| Il1b | CTGTGTCTTTCCCGTGGACC | CAGCTCATATGGGTCCGACA |
| Il6 | AGAGGAGACTTCACAGAGGATACCA | AATCAGAATTGCCATTGCACAAC |
| Foxo1 | CTAGGAGTTAGTGAGCAGGCAA | CTGCCAAGTCTGACGAAAGG |
| Foxo3 | GGGGAACTTCACTGGTGCTAA | AGTTTGAGGGTCTGCTTTGC |
| Foxo4 | AGGCTCCTACACTTCTGTTACTGG | CTTCAGTAGGAGATGCAAGCACAG |
| Trim63/Murf1 | CCTGTGAAGTTGCCCCCTTA | GCTGTTTTCCTTGGTCACTCG |
| Fbxo32/Atrogin1 | ACTAAGGAGCGCCATGGAT | CAGGGATGTGAGCTGTGACTT |
| Gabarapl1 | ACAACACGATCCACAGCCTT | TCTGCCCAAAGTGACGAGAG |
| Bnip3l | GCTGGCCTCAACAGTTCCTG | TGGATGGAAGACGAGGAAGGA |
| Eef1a1 | GATGGCCCCAAATTCTTGAAG | GGACCATGTCAACAATGGCAG |
| Parameter | Unit | Group | ||
|---|---|---|---|---|
| Control | STZ | FRB | ||
| Total protein | g/dL | 6.97 ± 0.51 a | 5.62 ± 0.38 b | 5.98 ± 0.72 b |
| Albumin | g/dL | 4.58 ± 0.35 a | 3.62 ± 0.22 b | 3.68 ± 0.45 b |
| Amylase | IU/L | 2308.33 ± 204 a | 1045.50 ± 326 b | 1329.00 ± 399 b |
| Triglyceride | mg/dL | 359.50 ± 139 a | 69.17 ± 30.2 b | 130.00 ± 101 b |
| HDL-cholesterol | mg/dL | 33.17 ± 3.25 a | 43.67 ± 8.66 b | 44.40 ± 10.30 b |
| Glucose | mg/dL | 153.83 ± 11.36 a | 680.00 ± 125.65 b | 614.00 ± 68.7 b |
| Insulin | ng/mL | 4.42 ± 0.99 a | 0.44 ± 0.13 b | 0.51 ± 0.29 b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rusbana, T.B.; Agista, A.Z.; Saputra, W.D.; Ohsaki, Y.; Watanabe, K.; Ardiansyah, A.; Budijanto, S.; Koseki, T.; Aso, H.; Komai, M.; et al. Supplementation with Fermented Rice Bran Attenuates Muscle Atrophy in a Diabetic Rat Model. Nutrients 2020, 12, 2409. https://doi.org/10.3390/nu12082409
Rusbana TB, Agista AZ, Saputra WD, Ohsaki Y, Watanabe K, Ardiansyah A, Budijanto S, Koseki T, Aso H, Komai M, et al. Supplementation with Fermented Rice Bran Attenuates Muscle Atrophy in a Diabetic Rat Model. Nutrients. 2020; 12(8):2409. https://doi.org/10.3390/nu12082409
Chicago/Turabian StyleRusbana, Tubagus Bahtiar, Afifah Zahra Agista, Wahyu Dwi Saputra, Yusuke Ohsaki, Kouichi Watanabe, Ardy Ardiansyah, Slamet Budijanto, Takuya Koseki, Hisashi Aso, Michio Komai, and et al. 2020. "Supplementation with Fermented Rice Bran Attenuates Muscle Atrophy in a Diabetic Rat Model" Nutrients 12, no. 8: 2409. https://doi.org/10.3390/nu12082409
APA StyleRusbana, T. B., Agista, A. Z., Saputra, W. D., Ohsaki, Y., Watanabe, K., Ardiansyah, A., Budijanto, S., Koseki, T., Aso, H., Komai, M., & Shirakawa, H. (2020). Supplementation with Fermented Rice Bran Attenuates Muscle Atrophy in a Diabetic Rat Model. Nutrients, 12(8), 2409. https://doi.org/10.3390/nu12082409








