Effects of Whey and Pea Protein Supplementation on Post-Eccentric Exercise Muscle Damage: A Randomized Trial
Abstract
1. Introduction
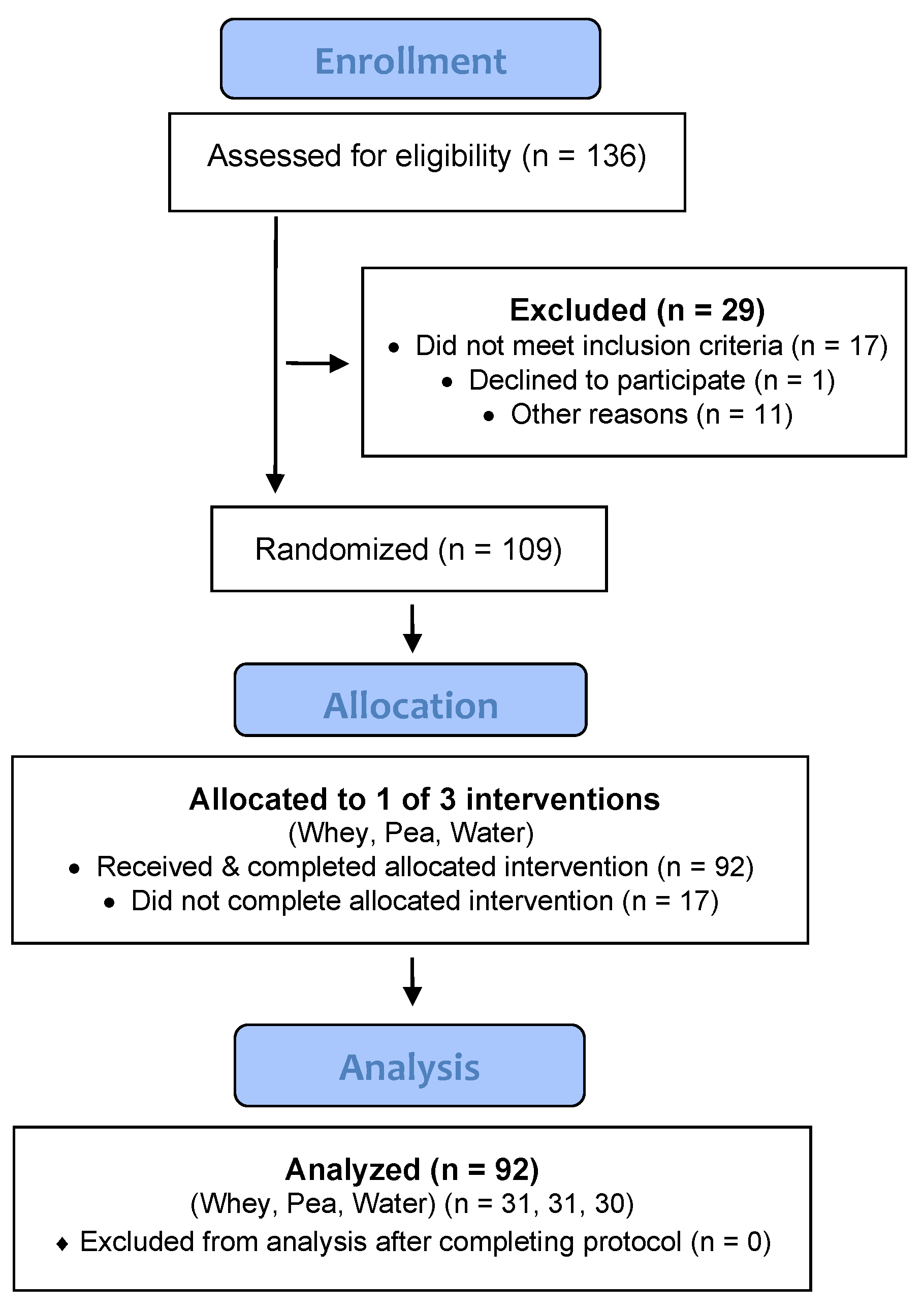
2. Materials and Methods
2.1. Study Participants
2.2. Study Design and Protein Supplementation Protocol
2.3. Blood Sample Analysis
2.4. Statistical Analysis
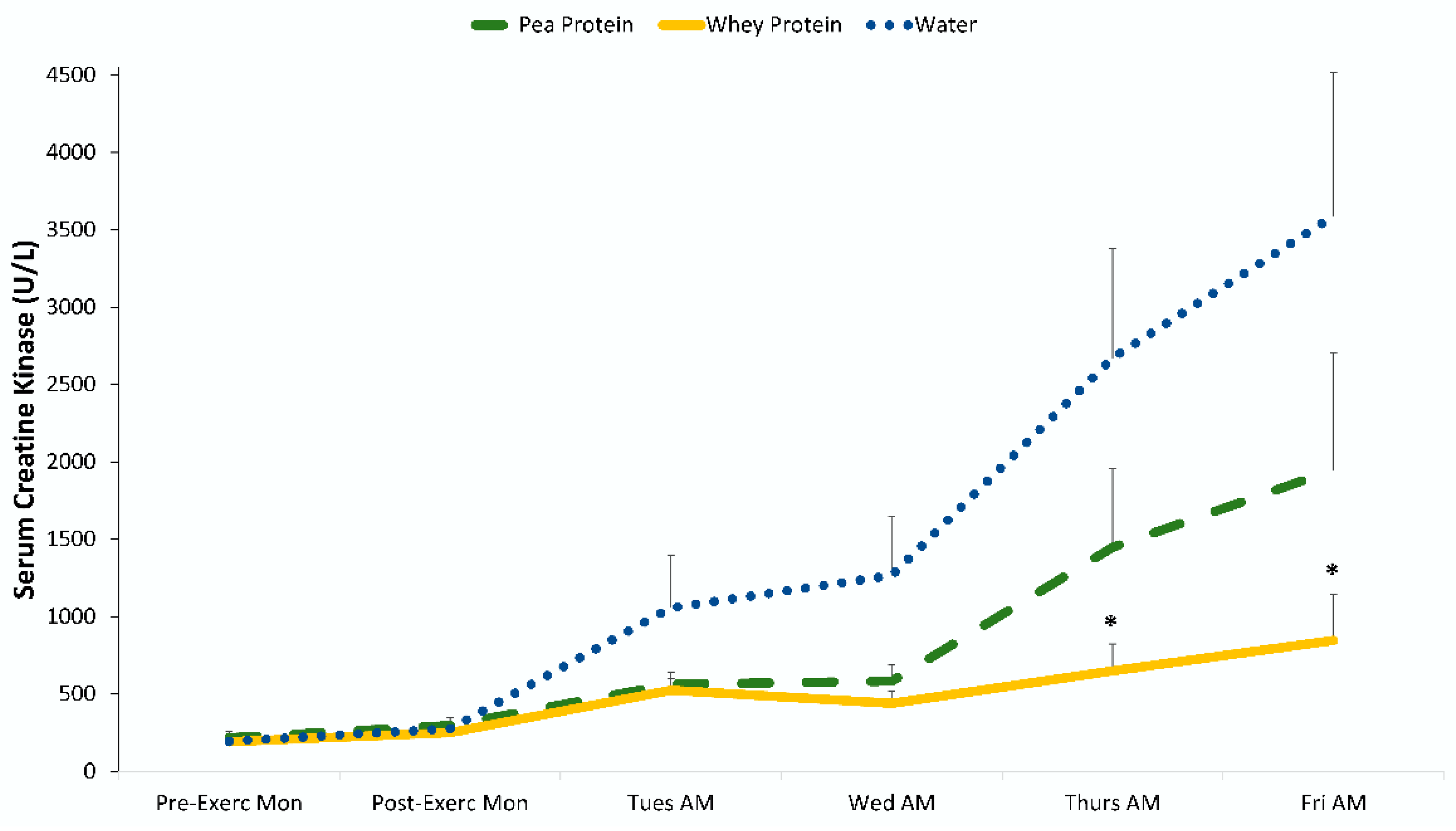
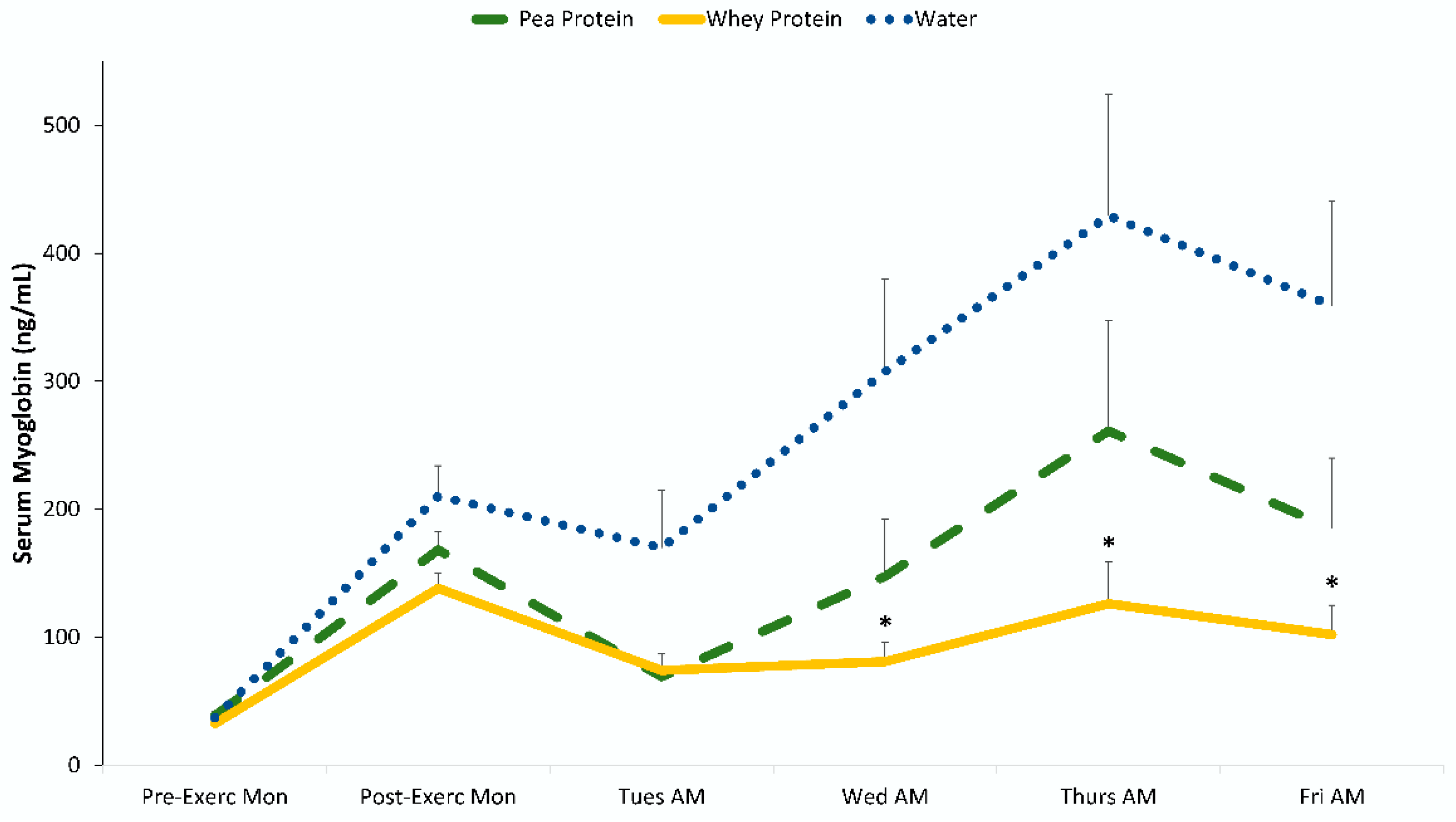
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wagenmakers, A.J. Muscle amino acid metabolism at rest and during exercise: Role in human physiology and metabolism. Exerc. Sport Sci. Rev. 1998, 26, 287–314. [Google Scholar] [CrossRef] [PubMed]
- Wagenmakers, A.J. Tracers to investigate protein and amino acid metabolism in human subjects. Proc. Nutr Soc. 1999, 58, 987–1000. [Google Scholar] [CrossRef] [PubMed]
- Mazzulla, M.; Sawan, S.A.; Williamson, E.; Hannaian, S.J.; Volterman, K.A.; West, D.W.D.; Moore, D.R. Protein intake to maximize whole-body anabolism during postexercise recovery in resistance-trained men with high habitual intakes is severalfold greater than the current Recommended Dietary Allowance. J. Nutr. 2020, 150, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Rahbek, S.K.; Farup, J.; Moller, A.B.; Vendelbo, M.H.; Holm, L.; Jessen, N.; Vissing, K. Effects of divergent resistance exercise contraction mode and dietary supplementation type on anabolic signalling, muscle protein synthesis and muscle hypertrophy. Amino Acids 2014, 46, 2377–2392. [Google Scholar] [CrossRef] [PubMed]
- Farnfield, M.M.; Breen, L.; Carey, K.A.; Garnham, A.; Cameron-Smith, D. Activation of mTOR signalling in young and old human skeletal muscle in response to combined resistance exercise and whey protein ingestion. Appl. Physiol. Nutr. Metab. 2012, 37, 21–30. [Google Scholar] [CrossRef]
- Trommelen, J.; Betz, M.W.; van Loon, L.J.C. The muscle protein synthetic response to meal ingestion following resistance-type exercise. Sports Med. 2019, 49, 185–197. [Google Scholar] [CrossRef]
- Camera, D.M.; West, D.W.; Burd, N.A.; Phillips, S.M.; Garnham, A.P.; Hawley, J.A.; Coffey, V.G. Low muscle glycogen concentration does not suppress the anabolic response to resistance exercise. J. Appl. Physiol. 2012, 113, 206–214. [Google Scholar] [CrossRef]
- Reitelseder, S.; Agergaard, J.; Doessing, S.; Helmark, I.C.; Lund, P.; Kristensen, N.B.; Frystyk, J.; Flyvbjerg, A.; van Hall, G. Whey and casein labeled with L-[1-13C]leucine and muscle protein synthesis: Effect of resistance exercise and protein ingestion. Am. J. Physiol. Endocrinol. Metab. 2011, 300, E231–E242. [Google Scholar] [CrossRef]
- Jackman, S.R.; Witard, O.C.; Philp, A.; Wallis, G.A.; Baar, K.; Tipton, K.D. Branched-chain amino acid ingestion stimulates muscle myofibrillar protein synthesis following resistance exercise in humans. Front. Physiol. 2017, 8, 390. [Google Scholar] [CrossRef]
- Biolo, G.; Tipton, K.D.; Klein, S.; Wolfe, R.R. An abundant supply of amino acids enhances the metabolic effect of exercise on muscle protein. Am. J. Physiol. Endocrinol. Metab. 1997, 273, E122–E129. [Google Scholar] [CrossRef]
- Cermak, N.M.; Res, P.T.; de Groot, L.C.; Saris, W.H.; van Loon, L.J. Protein supplementation augments the adaptive response of skeletal muscle to resistance-type exercise training: A meta-analysis. Am. J. Clin. Nutr. 2012, 96, 1454–1464. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.M. The impact of protein quality on the promotion of resistance exercise-induced changes in muscle mass. Nutr. Metab. (Lond.) 2016, 13, 64. [Google Scholar] [CrossRef]
- Jäger, R.; Kerksick, C.M.; Campbell, B.I.; Cribb, P.J.; Wells, S.D.; Skwiat, T.M.; Purpura, M.; Ziegenfuss, T.N.; Ferrando, A.A.; Arent, S.M.; et al. International Society of Sports Nutrition Position Stand: Protein and exercise. J. Int. Soc. Sports Nutr. 2017, 14, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Burd, N.A.; Yang, Y.; Moore, D.R.; Tang, J.E.; Tarnopolsky, M.A.; Phillips, S.M. Greater stimulation of myofibrillar protein synthesis with ingestion of whey protein isolate v. micellar casein at rest and after resistance exercise in elderly men. Br. J. Nutr. 2012, 108, 958–962. [Google Scholar] [CrossRef] [PubMed]
- Devries, M.C.; Phillips, S.M. Supplemental protein in support of muscle mass and health: Advantage whey. J. Food Sci. 2015, 80, A8–A15. [Google Scholar] [CrossRef] [PubMed]
- Churchward-Venne, T.A.; Breen, L.; Di Donato, D.M.; Hector, A.J.; Mitchell, C.J.; Moore, D.R.; Stellingwerff, T.; Breuille, D.; Offord, E.A.; Baker, S.K.; et al. Leucine supplementation of a low-protein mixed macronutrient beverage enhances myofibrillar protein synthesis in young men: A double blind, randomized trial. Am. J. Clin. Nutr. 2014, 99, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Breen, L.; Burd, N.A.; Hector, A.J.; Churchward-Venne, T.A.; Josse, A.R.; Tarnopolsky, M.A.; Phillips, S.M. Resistance exercise enhances myofibrillar protein synthesis with graded intakes of whey protein in older men. Br. J. Nutr. 2012, 108, 1780–1788. [Google Scholar] [CrossRef]
- van Vliet, S.; Beals, J.W.; Holwerda, A.M.; Emmons, R.S.; Goessens, J.P.; Paluska, S.A.; De Lisio, M.; van Loon, L.J.C.; Burd, N.A. Time-dependent regulation of postprandial muscle protein synthesis rates after milk protein ingestion in young men. J. Appl. Physiol. 2019, 127, 1792–1801. [Google Scholar] [CrossRef]
- Gorissen, S.H.M.; Trommelen, J.; Kouw, I.W.K.; Holwerda, A.M.; Pennings, B.; Groen, B.B.L.; Wall, B.T.; Churchward-Venne, T.A.; Horstman, A.M.H.; Koopman, R.; et al. Protein type, protein dose, and age modulate dietary protein digestion and phenylalanine absorption kinetics and plasma phenylalanine availability in humans. J. Nutr. 2020, 150, 2041–2050. [Google Scholar] [CrossRef]
- Reidy, P.T.; Walker, D.K.; Dickinson, J.M.; Gundermann, D.M.; Drummond, M.J.; Timmerman, K.L.; Fry, C.S.; Borack, M.S.; Cope, M.B.; Mukherjea, R.; et al. Protein blend ingestion following resistance exercise promotes human muscle protein synthesis. J. Nutr. 2013, 143, 410–416. [Google Scholar] [CrossRef]
- O’Bryan, K.R.; Doering, T.M.; Morton, R.W.; Coffey, V.G.; Phillips, S.M.; Cox, G.R. Do multi-ingredient protein supplements augment resistance training-induced gains in skeletal muscle mass and strength? A systematic review and meta-analysis of 35 trials. Br. J. Sports Med. 2020, 54, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.M.; Chevalier, S.; Leidy, H.J. Protein “requirements” beyond the RDA: Implications for optimizing health. Appl. Physiol. Nutr. Metab. 2016, 41, 565–572. [Google Scholar] [CrossRef]
- van Vliet, S.; Burd, N.A.; van Loon, L.J. The skeletal muscle anabolic response to plant- versus animal-based protein consumption. J. Nutr. 2015, 145, 1981–1991. [Google Scholar] [CrossRef] [PubMed]
- Morton, R.W.; Murphy, K.T.; McKellar, S.R.; Schoenfeld, B.J.; Henselmans, M.; Helms, E.; Aragon, A.A.; Devries, M.C.; Banfield, L.; Krieger, J.W.; et al. A systematic review, meta-analysis and meta-regression of the effect of protein supplementation on resistance training-induced gains in muscle mass and strength in healthy adults. Br. J. Sports Med. 2018, 52, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Areta, J.L.; Burke, L.M.; Ross, M.L.; Camera, D.M.; West, D.W.; Broad, E.M.; Jeacocke, N.A.; Moore, D.R.; Stellingwerff, T.; Phillips, S.M.; et al. Timing and distribution of protein ingestion during prolonged recovery from resistance exercise alters myofibrillar protein synthesis. J. Physiol. 2013, 591, 2319–2331. [Google Scholar] [CrossRef] [PubMed]
- Snijders, T.; Res, P.T.; Smeets, J.S.; van Vliet, S.; van Kranenburg, J.; Maase, K.; Kies, A.K.; Verdijk, L.B.; van Loon, L.J. Protein ingestion before sleep increases muscle mass and strength gains during prolonged resistance-type exercise training in healthy young men. J. Nutr. 2015, 145, 1178–1184. [Google Scholar] [CrossRef] [PubMed]
- Pasiakos, S.M.; Lieberman, H.R.; McLellan, T.M. Effects of protein supplements on muscle damage, soreness and recovery of muscle function and physical performance: A systematic review. Sports Med. 2014, 44, 655–670. [Google Scholar] [CrossRef]
- Poulios, A.; Georgakouli, K.; Draganidis, D.; Deli, C.K.; Tsimeas, P.D.; Chatzinikolaou, A.; Papanikolaou, K.; Batrakoulis, A.; Mohr, M.; Jamurtas, A.Z.; et al. Protein-based supplementation to enhance recovery in team sports: What is the evidence? J. Sports Sci. Med. 2019, 18, 523–536. [Google Scholar]
- Buckley, J.D.; Thomson, R.L.; Coates, A.M.; Howe, P.R.; DeNichilo, M.O.; Rowney, M.K. Supplementation with a whey protein hydrolysate enhances recovery of muscle force-generating capacity following eccentric exercise. J. Sci. Med. Sport. 2010, 13, 178–181. [Google Scholar] [CrossRef]
- Howatson, G.; Hoad, M.; Goodall, S.; Tallent, J.; Bell, P.G.; French, D.N. Exercise-induced muscle damage is reduced in resistance-trained males by branched chain amino acids: A randomized, double-blind, placebo controlled study. J. Int. Soc. Sports Nutr. 2012, 9, 20. [Google Scholar] [CrossRef]
- Shimomura, Y.; Inaguma, A.; Watanabe, S.; Yamamoto, Y.; Muramatsu, Y.; Bajotto, G.; Sato, J.; Shimomura, N.; Kobayashi, H.; Mawatari, K. Branched-chain amino acid supplementation before squat exercise and delayed-onset muscle soreness. Int. J. Sport Nutr. Exerc. Metab. 2010, 20, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Etheridge, T.; Philp, A.; Watt, P.W. A single protein meal increases recovery of muscle function following an acute eccentric exercise bout. Appl. Physiol. Nutr. Metab. 2008, 33, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Shan, Z.; Rehm, C.D.; Rogers, G.; Ruan, M.; Wang, D.D.; Hu, F.B.; Mozaffarian, D.; Zhang, F.F.; Bhupathiraju, S.N. Trends in dietary carbohydrate, protein, and fat intake and diet quality among US adults, 1999–2016. JAMA 2019, 322, 1178–1187. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Fung, T.T.; Hu, F.B.; Willett, W.C.; Longo, V.D.; Chan, A.T.; Giovannucci, E.L. Association of animal and plant protein intake with all-cause and cause-specific mortality. JAMA Intern. Med. 2016, 176, 1453–1463. [Google Scholar] [CrossRef] [PubMed]
- Babault, N.; Païzis, C.; Deley, G.; Guérin-Deremaux, L.; Saniez, M.H.; Lefranc-Millot, C.; Allaert, F.A. Pea proteins oral supplementation promotes muscle thickness gains during resistance training: A double-blind, randomized, placebo-controlled clinical trial vs. whey protein. J. Int. Soc. Sports Nutr. 2015, 12, 3. [Google Scholar] [CrossRef]
- Xia, Z.; Cholewa, J.M.; Dardevet, D.; Huang, T.; Zhao, Y.; Shang, H.; Yang, Y.; Ding, X.; Zhang, C.; Wang, H.; et al. Effects of oat protein supplementation on skeletal muscle damage, inflammation and performance recovery following downhill running in untrained collegiate men. Food Funct. 2018, 9, 4720–4729. [Google Scholar] [CrossRef]
- Smith, L.L.; Brunetz, M.H.; Chenier, T.C.; McCammon, M.R.; Houmard, J.A.; Franklin, M.E.; Israel, R.G. The effects of static and ballistic stretching on delayed onset muscle soreness and creatine kinase. Res. Q. Exerc. Sport 1993, 64, 103–107. [Google Scholar] [CrossRef]
- What We Eat in America, NHANES 2015-2016, Individuals 2 years and Over (Excluding Breast-Fed Children), Day 1. Available online: www.ars.usda.gov/nea/bhnrc/fsrg (accessed on 15 June 2020).
- Wu, G. Dietary protein intake and human health. Food Funct. 2016, 7, 1251–1265. [Google Scholar] [CrossRef]
- Nieman, D.C.; Gillitt, N.D.; Shanely, R.A.; Dew, D.; Meaney, M.P.; Luo, B. Vitamin D2 supplementation amplifies eccentric exercise-induced muscle damage in NASCAR pit crew athletes. Nutrients 2013, 6, 63–75. [Google Scholar] [CrossRef]
- Harty, P.S.; Cottet, M.L.; Malloy, J.K.; Kerksick, C.M. Nutritional and supplementation strategies to prevent and attenuate exercise-induced muscle damage: A brief review. Sports Med. Open. 2019, 5, 1. [Google Scholar] [CrossRef]
- Owens, D.J.; Twist, C.; Cobley, J.N.; Howatson, G.; Close, G.L. Exercise-induced muscle damage: What is it, what causes it and what are the nutritional solutions? Eur. J. Sport Sci. 2019, 19, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Foure, A.; Bendahan, D. Is branched-chain amino acids supplementation an efficient nutritional strategy to alleviate skeletal muscle damage? A systematic review. Nutrients 2017, 9, 1047. [Google Scholar] [CrossRef] [PubMed]
- VanDusseldorp, T.A.; Escobar, K.A.; Johnson, K.E.; Stratton, M.T.; Moriarty, T.; Cole, N.; McCormick, J.J.; Kerksick, C.M.; Vaughan, R.A.; Dokladny, K.; et al. Effect of branched-chain amino acid supplementation on recovery following acute eccentric exercise. Nutrients 2018, 10, 1389. [Google Scholar] [CrossRef] [PubMed]
- Davies, R.W.; Carson, B.P.; Jakeman, P.M. The effect of whey protein supplementation on the temporal recovery of muscle function following resistance training: A systematic review and meta-analysis. Nutrients 2018, 10, 221. [Google Scholar] [CrossRef] [PubMed]
- Pallottini, A.C.; Sales, C.H.; Vieira, D.A.D.S.; Marchioni, D.M.; Fisberg, R.M. Dietary BCAA intake is associated with demographic, socioeconomic and lifestyle factors in residents of São Paulo, Brazil. Nutrients 2017, 9, 449. [Google Scholar] [CrossRef] [PubMed]
| Grams/100 g Protein | Pea Protein Isolate | Whey Protein Isolate |
|---|---|---|
| Branched Chain Amino Acids | ||
| Isoleucine | 4.7 | 5.6 |
| Leucine | 8.2 | 12.7 |
| Valine | 5.0 | 5.4 |
| Other Amino Acids | ||
| Alanine | 4.3 | 4.9 |
| Arginine | 8.7 | 2.4 |
| Aspartic acid | 11.5 | 11.4 |
| Cystine | 0.1 | 2.8 |
| Glutamic acid | 16.7 | 16.1 |
| Glycine | 4.0 | 1.7 |
| Histidine | 2.5 | 2.0 |
| Lysine | 7.1 | 10.2 |
| Methionine | 1.1 | 2.3 |
| Phenylalanine | 5.5 | 3.5 |
| Proline | 4.3 | 4.7 |
| Serine | 5.1 | 3.3 |
| Threonine | 3.8 | 4.7 |
| Tryptophan | 0.1 | 2.9 |
| Tyrosine | 3.8 | 3.6 |
| Dumbbell Incline Bench Press | 3 sets, 5 eccentric reps, 23 kg (adjustment as needed). Rest intervals between sets, 30–45 s. |
| Bench Press | 3 sets, 20 s to fatigue, 43 kg, then 3 sets, 3 eccentric reps with concentric assist. Rest intervals, 1 min. |
| Supine Medicine Ball Catch and Throw | 3 sets, 20 s, explosive catch and throw of 6.4 kg medicine ball, with participant in a supine position on floor with knees bent. Rest intervals, 30 s. |
| Bent Arm Hangs | 4 sets, 90° bent arm hang to fatigue. Rest intervals, 30 s. |
| Eccentric Lat Pulls | 4 sets, 8 reps, lat pulls, weight adjusted for eccentric focus. Rest intervals, 1 min. |
| Downhill Treadmill Run | 3 sets, 2 min, 13.7 km/hour, 10% decline. Rest intervals, 1 min. |
| Drop Jumps with Rebound Vertical Jumps | 2 sets, 10 reps, drop jumps from a 41 cm box with explosive vertical jumps. Rest intervals, 30 s. |
| Explosive Tuck Jumps | 3 sets, 20 s, continuous explosive tuck jumps. Rest intervals, 30 s. |
| Eccentric Leg Extensions | 3 sets, 8 reps, each leg, maximal effort against applied resistance for leg extension in 5 s. Rest intervals, 30 s. |
| Eccentric Leg Curls | 2 sets, 8 reps, each leg, maximal effort against applied resistance for leg curl in 5 s, prone position. Rest intervals, 30 s. |
| Vertical Skips | 3 sets, 30 s, skip in place as high as possible. Rest intervals, 1 min. |
| Split Leg Squats | 3 sets, 15 reps, each leg. Rest intervals, 30 secs. |
| Shrug Walk | Walk 0.53 km on a treadmill with 4.5 kg dumbbells in each hand and a shoulder shrug every two steps. |
| Bottle Shakers | 3 sets, 15 s, isometric abdominal curl with medicine ball (6.4 kg) twisting side to side. Rest intervals, 30 s. |
| Abdominal Crunches | 3 sets, 20 s, supine position with legs bent, repeatedly reaching forward 10 cm. Rest intervals, 30 s. |
| Plank | 2 sets, 45 s, prone position on toes and elbows. Rest intervals, 1 min. |
| Variable | Pea Protein (n = 31) | Whey Protein (n = 31) | Water (n = 30) |
|---|---|---|---|
| Age (years) | 37.3 ± 1.6 | 40.3 ± 1.7 | 38.1 ± 1.9 |
| Weight (kg) | 81.5 ± 1.4 | 81.5 ± 1.4 | 80.3 ± 1.8 |
| Height (cm) | 179 ± 1.2 | 178 ± 1.0 | 179 ± 1.2 |
| BMI (kg/m2) | 25.4 ± 0.4 | 25.8 ± 0.4 | 25.0 ± 0.5 |
| Body Fat (%) | 21.1 ± 1.1 | 20.4 ± 1.0 | 21.2 ± 1.1 |
| Performance Measure | Group | Mon PreEx | Mon PostEx | Tuesday | Wednesday | Thursday | Friday | Time; Treatment; Interaction p Values |
|---|---|---|---|---|---|---|---|---|
| Vertical Jump (in) | Pea | 19.2 ± 0.7 | 19.6 ± 0.8 | 19.1 ± 0.8 | 19.1 ± 0.7 | 19.5 ± 0.8 | 19.8 ± 0.8 | <0.001; 0.390; 0.723 |
| Whey | 18.0 ± 0.6 | 18.5 ± 0.6 | 18.1 ± 0.7 | 18.3 ± 0.7 | 18.5 ± 0.7 | 18.9 ± 0.7 | ||
| Water | 18.2 ± 0.4 | 18.2 ± 0.5 | 18.4 ± 0.4 | 17.9 ± 0.5 | 18.4 ± 0.5 | 18.7 ± 0.4 | ||
| Leg/Back Strength (kg/kg body mass) | Pea | 1.64 ± 0.08 | 1.55 ± 0.09 | 1.64 ± 0.07 | 1.63 ± 0.07 | 1.68 ± 0.07 | 1.74 ± 0.07 | 0.002; 0.481; 0.137 |
| Whey | 1.64 ± 0.10 | 1.68 ± 0.10 | 1.58 ± 0.08 | 1.62 ± 0.08 | 1.68 ± 0.09 | 1.69 ± 0.08 | ||
| Water | 1.56 ± 0.07 | 1.52 ± 0.08 | 1.51 ± 0.06 | 1.50 ± 0.07 | 1.52 ± 0.07 | 1.62 ± 0.07 | ||
| Bench Press (repetitions) | Pea | 14.0 ± 1.3 | 10.9 ± 1.5 | 12.8 ± 1.6 | 13.5 ± 1.7 | 13.9 ± 1.7 | 14.4 ± 1.7 | <0.001; 0.141; 0.251 |
| Whey | 12.9 ± 1.1 | 9.48 ± 1.22 | 11.9 ± 1.3 | 12.8 ± 1.3 | 13.2 ± 1.3 | 14.3 ± 1.3 | ||
| Water | 11.6 ± 1.2 | 7.13 ± 1.16 | 8.80 ± 1.29 | 9.23 ± 1.21 | 10.1 ± 1.3 | 11.1 ± 1.4 | ||
| Wingate Peak Power (watts/kg) | Pea | 8.36 ± 0.30 | 7.54 ± 0.35 | 8.12 ± 0.26 | 8.05 ± 0.31 | 8.29 ± 0.30 | 8.41 ± 0.29 | <0.001; 0.464; 0.321 |
| Whey | 7.92 ± 0.25 | 7.27 ± 0.28 | 7.72 ± 0.21 | 7.82 ± 0.23 | 7.91 ± 0.22 | 7.99 ± 0.24 | ||
| Water | 8.54 ± 0.33 | 7.80 ± 0.38 | 8.45 ± 0.29 | 8.16 ± 0.33 | 8.26 ± 0.33 | 8.16 ± 0.32 | ||
| Wingate Mean Power (watts/kg) | Pea | 6.50 ± 0.16 | 5.84 ± 0.22 | 6.37 ± 0.15 | 6.32 ± 0.18 | 6.44 ± 0.16 | 6.51 ± 0.15 | <0.001; 0.926; 0.588 |
| Whey | 6.52 ± 0.17 | 5.96 ± 0.21 | 6.38 ± 0.18 | 6.45 ± 0.19 | 6.61 ± 0.18 | 6.56 ± 0.18 | ||
| Water | 6.55 ± 0.23 | 6.09 ± 0.32 | 6.55 ± 0.24 | 6.41 ± 0.25 | 6.51 ± 0.26 | 6.50 ± 0.25 | ||
| Delayed Onset Muscle Soreness | Pea | 1.45 ± 0.11 | 3.69 ± 0.40 | 6.23 ± 0.27 | 6.00 ± 0.25 | 4.06 ± 0.29 | 2.45 ± 0.25 | <0.001; 0.283; 0.210 |
| Whey | 1.42 ± 0.13 | 4.08 ± 0.34 | 6.19 ± 0.30 | 5.50 ± 0.31 | 3.66 ± 0.25 | 2.42 ± 0.20 | ||
| Water | 1.22 ± 0.07 | 4.25 ± 0.40 | 6.72 ± 0.33 | 6.37 ± 0.47 | 4.68 ± 0.45 | 3.03 ± 0.38 |
| Blood Measure | Group | Mon PreEx | Mon PostEx | Tuesday | Wednesday | Thursday | Friday | Time; Treatment; Interaction p Values |
|---|---|---|---|---|---|---|---|---|
| AST (IU/L) | Pea | 24.8 ± 1.7 | 27.8 ± 1.8 | 32.3 ± 1.9 | 30.7 ± 1.6 | 40.4 ± 6.8 | 49.5 ± 10.4 | <0.001; 0.018; 0.025 |
| Whey | 22.1 ± 1.1 | 24.6 ± 6.7 | 30.5 ± 2.1 | 29.6 ± 2.3 | 30.3 ± 2.8 | 33.4 ± 4.1 | ||
| Water | 22.7 ± 1.2 | 26.3 ± 1.3 | 43.8 ± 7.1 | 47.8 ± 9.1 | 63.7 ± 13.0 | 79.9 ± 17.3 | ||
| ALT (IU/L) | Pea | 22.1 ± 1.2 | 23.5 ± 1.3 | 22.3 ± 1.2 | 23.9 ± 1.2 | 27.0 ± 1.9 | 30.6 ± 3.4 | <0.001; 0.196; 0.029 |
| Whey | 22.2 ± 1.8 | 23.7 ± 1.9 | 23.2 ± 1.7 | 25.7 ± 2.1 | 25.8 ± 1.8 | 26.9 ± 1.9 | ||
| Water | 22.1 ± 1.5 | 24.2 ± 1.4 | 25.2 ± 1.7 | 27.9 ± 2.2 | 32.9 ± 3.3 | 40.1 ± 4.9 | ||
| BUN (mg/dL) | Pea | 13.6 ± 0.5 | 14.7 ± 0.5 * | 15.5 ± 0.6 * | 15.3 ± 0.6 * | 15.1 ± 0.7 * | 15.3 ± 0.5 | 0.015; <0.001 0.001 |
| Whey | 15.8 ± 0.5 | 17.6 ± 0.5 * | 17.9 ± 0.6 * | 17.9 ± 0.5 * | 17.3 ± 0.6 * | 18.2 ± 0.4 * | ||
| Water | 14.4 ± 0.6 | 14.7 ± 0.6 | 13.5 ± 0.5 | 12.9 ± 0.6 | 13.2 ± 0.6 | 13.8 ± 0.7 | ||
| CRP (mg/L) | Pea | 1.08 ± 0.25 | 1.11 ± 0.26 | 1.84 ± 0.42 | 1.71 ± 0.36 | 1.37 ± 0.34 | 1.34 ± 0.39 | <0.001; 0.133 0.549 |
| Whey | 0.64 ± 0.16 | 0.69 ± 0.16 | 1.10 ± 0.23 | 0.89 ± 0.18 | 0.74 ± 0.13 | 0.71 ± 0.11 | ||
| Water | 0.84 ± 0.14 | 0.92 ± 0.16 | 2.09 ± 0.44 | 1.69 ± 0.41 | 1.30 ± 0.31 | 1.30 ± 0.35 | ||
| Albumin (g/dL) | Pea | 4.55 ± 0.04 | 4.81 ± 0.04 | 4.52 ± 0.04 | 4.51 ± 0.05 | 4.51 ± 0.05 | 4.56 ± 0.05 | <0.001; 0.057 0.042 |
| Whey | 4.65 ± 0.04 | 4.85 ± 0.04 | 4.66 ± 0.04 | 4.59 ± 0.04 | 4.56 ± 0.04 | 4.65 ± 0.05 | ||
| Water | 4.68 ± 0.03 | 5.01 ± 0.05 | 4.58 ± 0.04 | 4.59 ± 0.04 | 4.60 ± 0.03 | 4.70 ± 0.04 | ||
| Glucose (mg/dL) | Pea | 92.4 ± 1.6 | 104 ± 5.2 | 93.8 ± 1.7 | 94.5 ± 1.5 | 93.2 ± 1.5 | 94.1 ± 1.5 | 0.002; 0.908 0.068 |
| Whey | 95.5 ± 1.3 | 95.3 ± 2.2 | 94.7 ± 1.9 | 95.4 ± 1.5 | 96.4 ± 1.4 | 95.5 ± 1.6 | ||
| Water | 94.6 ± 1.3 | 103 ± 3.8 | 94.3 ± 1.0 | 92.7 ± 0.9 | 92.6 ± 1.3 | 92.2 ± 1.5 | ||
| LDH (IU/L) | Pea | 162 ± 4.7 | 185 ± 4.5 | 172 ± 4.6 | 172 ± 4.6 | 193 ± 14.6 | 204 ± 20.4 | <0.001; 0.044; 0.054 |
| Whey | 164 ± 5.1 | 188 ± 5.6 | 174 ± 5.5 | 173 ± 5.8 | 174 ± 7.2 | 181 ± 9.5 | ||
| Water | 167 ± 3.8 | 198 ± 5.3 | 185 ± 7.2 | 194 ± 10.2 | 235 ± 22.5 | 251 ± 28.3 | ||
| Estimated GFR (mL/min/1.73) | Pea | 90.1 ± 2.4 | 72.6 ± 2.5 | 87.0 ± 3.2 | 90.7 ± 2.2 | 93.7 ± 2.5 | 91.9 ± 2.2 | <0.001; 0.930; 0.316 |
| Whey | 90.6 ± 2.5 | 75.8 ± 2.4 | 90.5 ± 2.4 | 94.6 ± 2.2 | 95.7 ± 2.3 | 93.7 ± 2.0 | ||
| Water | 99.0 ± 2.5 | 81.5 ± 2.3 | 93.8 ± 2.9 | 99.1 ± 2.8 | 101 ± 3.0 | 99.3 ± 3.4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nieman, D.C.; Zwetsloot, K.A.; Simonson, A.J.; Hoyle, A.T.; Wang, X.; Nelson, H.K.; Lefranc-Millot, C.; Guérin-Deremaux, L. Effects of Whey and Pea Protein Supplementation on Post-Eccentric Exercise Muscle Damage: A Randomized Trial. Nutrients 2020, 12, 2382. https://doi.org/10.3390/nu12082382
Nieman DC, Zwetsloot KA, Simonson AJ, Hoyle AT, Wang X, Nelson HK, Lefranc-Millot C, Guérin-Deremaux L. Effects of Whey and Pea Protein Supplementation on Post-Eccentric Exercise Muscle Damage: A Randomized Trial. Nutrients. 2020; 12(8):2382. https://doi.org/10.3390/nu12082382
Chicago/Turabian StyleNieman, David C., Kevin A. Zwetsloot, Andrew J. Simonson, Andrew T. Hoyle, Xintang Wang, Heather K. Nelson, Catherine Lefranc-Millot, and Laetitia Guérin-Deremaux. 2020. "Effects of Whey and Pea Protein Supplementation on Post-Eccentric Exercise Muscle Damage: A Randomized Trial" Nutrients 12, no. 8: 2382. https://doi.org/10.3390/nu12082382
APA StyleNieman, D. C., Zwetsloot, K. A., Simonson, A. J., Hoyle, A. T., Wang, X., Nelson, H. K., Lefranc-Millot, C., & Guérin-Deremaux, L. (2020). Effects of Whey and Pea Protein Supplementation on Post-Eccentric Exercise Muscle Damage: A Randomized Trial. Nutrients, 12(8), 2382. https://doi.org/10.3390/nu12082382







