Early Nutritional Interventions with Zinc, Selenium and Vitamin D for Raising Anti-Viral Resistance Against Progressive COVID-19
Abstract
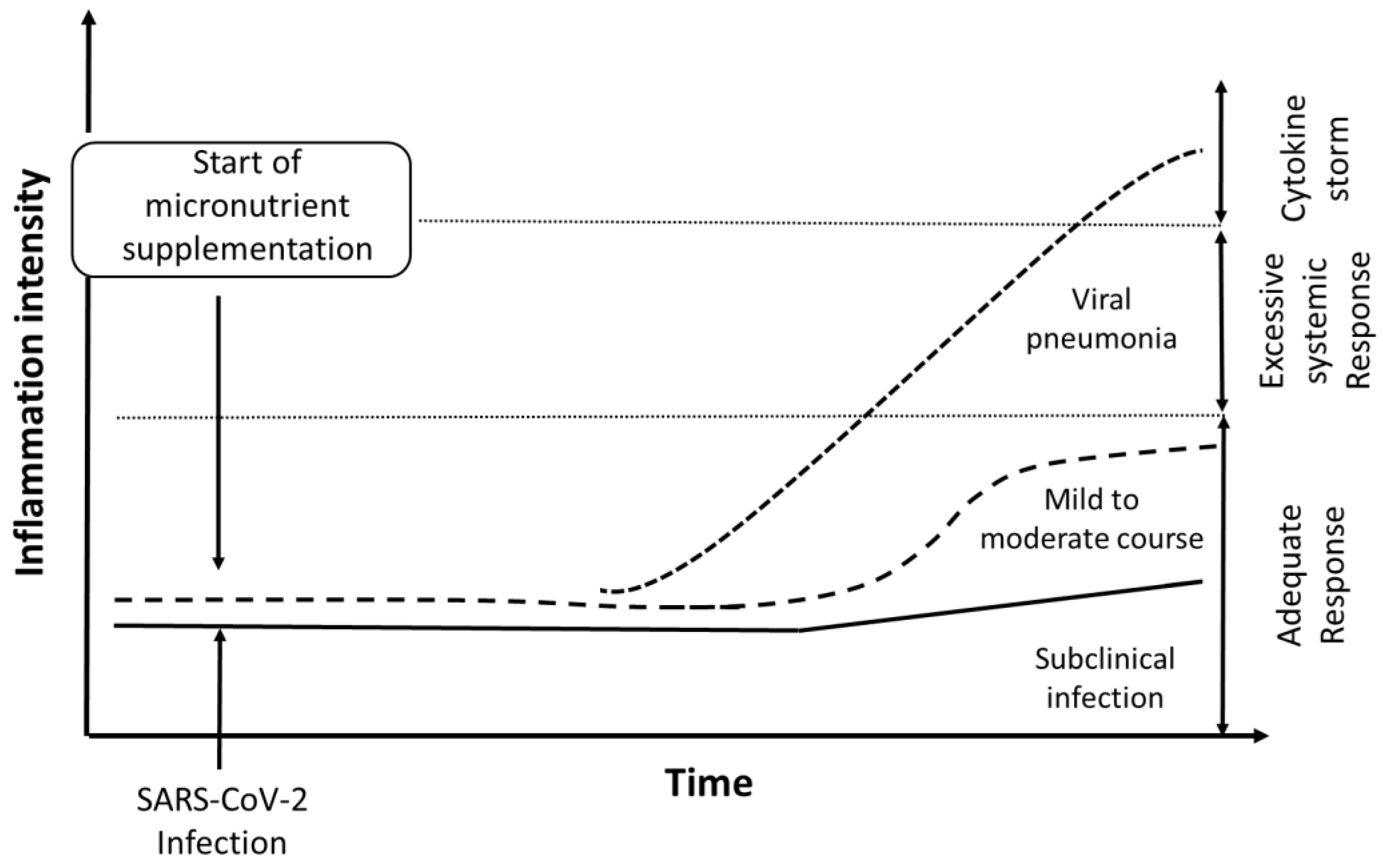
1. Introduction
2. Nutritional Interventions as a Preventive Approach
2.1. Zinc
2.2. Selenium
2.3. Selenium Plus Cofactors
2.4. Vitamin D
3. Discussion and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, Y.; Liu, Q.; Guo, D. Emerging coronaviruses: Genome structure, replication, and pathogenesis. J. Med. Virol. 2020, 92, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, M.; Cao, H.; Zhu, Y.; Zheng, J.; Zhou, H. Extraordinary GU-rich single-strand RNA identified from SARS coronavirus contributes an excessive innate immune response. Microbes Infect. 2013, 15, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Shimabukuro-Vornhagen, A.; Godel, P.; Subklewe, M.; Stemmler, H.J.; Schlosser, H.A.; Schlaak, M.; Kochanek, M.; Boll, B.; von Bergwelt-Baildon, M.S. Cytokine release syndrome. J. Immunother. Cancer 2018, 6, 56. [Google Scholar] [CrossRef]
- Conti, P.; Ronconi, G.; Caraffa, A.; Gallenga, C.E.; Ross, R.; Frydas, I.; Kritas, S.K. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): Anti-inflammatory strategies. J. Biol. Regul. Homeost. Agents 2020, 34. [Google Scholar] [CrossRef]
- Arabi, Y.M.; Fowler, R.; Hayden, F.G. Critical care management of adults with community-acquired severe respiratory viral infection. Intensive Care Med. 2020, 46, 315–328. [Google Scholar] [CrossRef]
- Russell, C.D.; Millar, J.E.; Baillie, J.K. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet 2020, 395, 473–475. [Google Scholar] [CrossRef]
- Lew, T.W.; Kwek, T.K.; Tai, D.; Earnest, A.; Loo, S.; Singh, K.; Kwan, K.M.; Chan, Y.; Yim, C.F.; Bek, S.L.; et al. Acute respiratory distress syndrome in critically ill patients with severe acute respiratory syndrome. JAMA 2003, 290, 374–380. [Google Scholar] [CrossRef]
- Weiss, P.; Murdoch, D.R. Clinical course and mortality risk of severe COVID-19. Lancet 2020, 395, 1014–1015. [Google Scholar] [CrossRef]
- Zheng, Y.Y.; Ma, Y.T.; Zhang, J.Y.; Xie, X. COVID-19 and the cardiovascular system. Nat. Rev. Cardiol. 2020, 17, 259–260. [Google Scholar] [CrossRef]
- Yang, Y.; Li, W.; Zhang, Q.; Zhang, L.; Cheung, T.; Xiang, Y.T. Mental health services for older adults in China during the COVID-19 outbreak. Lancet Psychiatry 2020, 7, e19. [Google Scholar] [CrossRef]
- Hoffman, R. Micronutrient deficiencies in the elderly—Could ready meals be part of the solution? J. Nutr. Sci. 2017, 6, e2. [Google Scholar] [CrossRef] [PubMed]
- Lipsitch, M.; Swerdlow, D.L.; Finelli, L. Defining the Epidemiology of Covid-19—Studies Needed. N. Engl. J. Med. 2020, 382, 1194–1196. [Google Scholar] [CrossRef] [PubMed]
- Bjorklund, G.; Dadar, M.; Pivina, L.; Dosa, M.D.; Semenova, Y.; Aaseth, J. The role of zinc and copper in insulin resistance and diabetes mellitus. Curr. Med. Chem. 2019. [Google Scholar] [CrossRef] [PubMed]
- Farrokhian, A.; Bahmani, F.; Taghizadeh, M.; Mirhashemi, S.M.; Aarabi, M.H.; Raygan, F.; Aghadavod, E.; Asemi, Z. Selenium Supplementation Affects Insulin Resistance and Serum hs-CRP in Patients with Type 2 Diabetes and Coronary Heart Disease. Horm. Metab. Res. 2016, 48, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Holmberg, S.; Rignell-Hydbom, A.; Lindh, C.H.; Jonsson, B.A.; Thelin, A.; Rylander, L. High levels of vitamin D associated with less ischemic heart disease—A nested case-control study among rural men in Sweden. Ann. Agric. Environ. Med. 2017, 24, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M. Metabolic syndrome: Connecting and reconciling cardiovascular and diabetes worlds. J. Am. Coll. Cardiol. 2006, 47, 1093–1100. [Google Scholar] [CrossRef]
- Sanada, F.; Taniyama, Y.; Muratsu, J.; Otsu, R.; Shimizu, H.; Rakugi, H.; Morishita, R. Source of Chronic Inflammation in Aging. Front. Cardiovasc Med. 2018, 5, 12. [Google Scholar] [CrossRef]
- Maggini, S.; Pierre, A.; Calder, P.C. Immune Function and Micronutrient Requirements Change over the Life Course. Nutrients 2018, 10, 1531. [Google Scholar] [CrossRef]
- Tainer, J.A.; Getzoff, E.D.; Richardson, J.S.; Richardson, D.C. Structure and mechanism of copper, zinc superoxide dismutase. Nature 1983, 306, 284–287. [Google Scholar] [CrossRef]
- Maares, M.; Haase, H. Zinc and immunity: An essential interrelation. Arch. Biochem. Biophys 2016, 611, 58–65. [Google Scholar] [CrossRef]
- Tuerk, M.J.; Fazel, N. Zinc deficiency. Curr. Opin. Gastroenterol. 2009, 25, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Barnett, J.B.; Hamer, D.H.; Meydani, S.N. Low zinc status: A new risk factor for pneumonia in the elderly? Nutr. Rev. 2010, 68, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Bonaventura, P.; Benedetti, G.; Albarede, F.; Miossec, P. Zinc and its role in immunity and inflammation. Autoimmun Rev. 2015, 14, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Kulik, L.; Maywald, M.; Kloubert, V.; Wessels, I.; Rink, L. Zinc deficiency drives Th17 polarization and promotes loss of Treg cell function. J. Nutr. Biochem. 2019, 63, 11–18. [Google Scholar] [CrossRef]
- Mariani, E.; Cattini, L.; Neri, S.; Malavolta, M.; Mocchegiani, E.; Ravaglia, G.; Facchini, A. Simultaneous evaluation of circulating chemokine and cytokine profiles in elderly subjects by multiplex technology: Relationship with zinc status. Biogerontology 2006, 7, 449–459. [Google Scholar] [CrossRef]
- Finzi, E. Treatment of SARS-CoV-2 with high dose oral zinc salts: A report on four patients. Int. J. Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Sattar, Y.; Connerney, M.; Rauf, H.; Saini, M.; Ullah, W.; Mamtani, S.; Syed, U.; Luddington, S.; Walfish, A. Three Cases of COVID-19 Disease With Colonic Manifestations. Am. J. Gastroenterol. 2020, 115, 948–950. [Google Scholar] [CrossRef]
- Lazzerini, M.; Wanzira, H. Oral zinc for treating diarrhoea in children. Cochrane Database Syst Rev. 2016, 12, CD005436. [Google Scholar] [CrossRef]
- Skalny, A.V.; Rink, L.; Ajsuvakova, O.P.; Aschner, M.; Gritsenko, V.A.; Alekseenko, S.I.; Svistunov, A.A.; Petrakis, D.; Spandidos, D.A.; Aaseth, J.; et al. Zinc and respiratory tract infections: Perspectives for COVID19 (Review). Int. J. Mol. Med. 2020, 46, 17–26. [Google Scholar] [CrossRef]
- Lassi, Z.S.; Moin, A.; Bhutta, Z.A. Zinc supplementation for the prevention of pneumonia in children aged 2 months to 59 months. Cochrane Database Syst Rev. 2016, 12, CD005978. [Google Scholar] [CrossRef]
- Haider, B.A.; Lassi, Z.S.; Ahmed, A.; Bhutta, Z.A. Zinc supplementation as an adjunct to antibiotics in the treatment of pneumonia in children 2 to 59 months of age. Cochrane Database Syst Rev. 2011. [Google Scholar] [CrossRef]
- Bhatnagar, S.; Wadhwa, N.; Aneja, S.; Lodha, R.; Kabra, S.K.; Natchu, U.C.; Sommerfelt, H.; Dutta, A.K.; Chandra, J.; Rath, B.; et al. Zinc as adjunct treatment in infants aged between 7 and 120 days with probable serious bacterial infection: A randomised, double-blind, placebo-controlled trial. Lancet 2012, 379, 2072–2078. [Google Scholar] [CrossRef]
- Science, M.; Johnstone, J.; Roth, D.E.; Guyatt, G.; Loeb, M. Zinc for the treatment of the common cold: A systematic review and meta-analysis of randomized controlled trials. CMAJ 2012, 184, E551–E561. [Google Scholar] [CrossRef]
- Hemila, H. Zinc lozenges may shorten the duration of colds: A systematic review. Open Respir. Med. J. 2011, 5, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Basnet, S.; Mathisen, M.; Strand, T.A. Oral zinc and common childhood infections—An update. J. Trace Elem. Med. Biol. 2015, 31, 163–166. [Google Scholar] [CrossRef] [PubMed]
- Ko, W.S.; Guo, C.H.; Hsu, G.S.; Chiou, Y.L.; Yeh, M.S.; Yaun, S.R. The effect of zinc supplementation on the treatment of chronic hepatitis C patients with interferon and ribavirin. Clin. Biochem. 2005, 38, 614–620. [Google Scholar] [CrossRef]
- te Velthuis, A.J.; van den Worm, S.H.; Sims, A.C.; Baric, R.S.; Snijder, E.J.; van Hemert, M.J. Zn(2+) inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathog. 2010, 6, e1001176. [Google Scholar] [CrossRef]
- Iyigundogdu, Z.U.; Demir, O.; Asutay, A.B.; Sahin, F. Developing Novel Antimicrobial and Antiviral Textile Products. Appl. Biochem. Biotechnol. 2017, 181, 1155–1166. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Y. Potential interventions for novel coronavirus in China: A systematic review. J. Med. Virol. 2020, 92, 479–490. [Google Scholar] [CrossRef]
- EU Scientific Committee on Food. Opinion of the Scientific Committee on Food on the Tolerable Upper Intake Level of Zinc; European Commission: Brussels, Belgium, 2003. [Google Scholar]
- Fairweather-Tait, S.J.; Bao, Y.; Broadley, M.R.; Collings, R.; Ford, D.; Hesketh, J.E.; Hurst, R. Selenium in human health and disease. Antioxid Redox Signal. 2011, 14, 1337–1383. [Google Scholar] [CrossRef]
- Beck, M.A.; Matthews, C.C. Micronutrients and host resistance to viral infection. Proc. Nutr. Soc. 2000, 59, 581–585. [Google Scholar] [CrossRef] [PubMed]
- Long, J.; Liu, Y.; Zhou, X.; He, L. Dietary Serine Supplementation Regulates Selenoprotein Transcription and Selenoenzyme Activity in Pigs. Biol. Trace Elem. Res. 2020. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Taylor, E.W.; Bennett, K.; Saad, R.; Rayman, M.P. Association between regional selenium status and reported outcome of COVID-19 cases in China. Am. J. Clin. Nutr. 2020, 111, 1297–1299. [Google Scholar] [CrossRef]
- Moghaddam, A.; Heller, R.A.; Sun, Q.; Seelig, J.; Cherkezov, A.; Seibert, L.; Hackler, J.; Seemann, P.; Diegmann, J.; Pilz, M.; et al. Selenium Deficiency Is Associated with Mortality Risk from COVID-19. Nutrients 2020, 12, 2098. [Google Scholar] [CrossRef]
- Gordon, D.E.; Jang, G.M.; Bouhaddou, M.; Xu, J.; Obernier, K.; White, K.M.; O’Meara, M.J.; Rezelj, V.V.; Guo, J.Z.; Swaney, D.L.; et al. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature 2020, 583, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Seale, L.A.; Torres, D.J.; Berry, M.J.; Pitts, M.W. A role for selenium-dependent GPX1 in SARS-CoV-2 virulence. Am. J. Clin. Nutr. 2020. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Parnham, M.J. Potential therapeutic use of ebselen for COVID-19 and other respiratory viral infections. Free Radic. Biol. Med. 2020, 156, 107–112. [Google Scholar] [CrossRef]
- Vavougios, G.D. Selenium—Associated gene signatures within the SARS-CoV-2—Host genomic interaction interface. Free Radic. Biol. Med. 2020. [Google Scholar] [CrossRef]
- Guillin, O.M.; Vindry, C.; Ohlmann, T.; Chavatte, L. Selenium, Selenoproteins and Viral Infection. Nutrients 2019, 11, 2101. [Google Scholar] [CrossRef]
- Pearson, H.; Clarke, T.; Abbott, A.; Knight, J.; Cyranoski, D. SARS: What have we learned? Nature 2003, 424, 121–126. [Google Scholar] [CrossRef]
- Harthill, M. Review: Micronutrient selenium deficiency influences evolution of some viral infectious diseases. Biol. Trace Elem. Res. 2011, 143, 1325–1336. [Google Scholar] [CrossRef] [PubMed]
- Alehagen, U.; Alexander, J.; Aaseth, J.; Larsson, A. Decrease in inflammatory biomarker concentration by intervention with selenium and coenzyme Q10: A subanalysis of osteopontin, osteoprotergerin, TNFr1, TNFr2 and TWEAK. J. Inflamm. 2019, 16, 5. [Google Scholar] [CrossRef] [PubMed]
- Avery, J.C.; Hoffmann, P.R. Selenium, Selenoproteins, and Immunity. Nutrients 2018, 10, 1203. [Google Scholar] [CrossRef] [PubMed]
- Beck, M.A.; Nelson, H.K.; Shi, Q.; Van Dael, P.; Schiffrin, E.J.; Blum, S.; Barclay, D.; Levander, O.A. Selenium deficiency increases the pathology of an influenza virus infection. FASEB J. 2001, 15, 1481–1483. [Google Scholar] [CrossRef] [PubMed]
- Beck, M.A. Selenium as an antiviral agent. In Selenium. Its Molecular Biology and Role in Human Health; Hatfield, D.L., Ed.; Kluwer Academic Publishers: Dordrect, The Netherlands, 2001; pp. 235–245. [Google Scholar]
- Yu, L.; Sun, L.; Nan, Y.; Zhu, L.Y. Protection from H1N1 influenza virus infections in mice by supplementation with selenium: A comparison with selenium-deficient mice. Biol. Trace Elem. Res. 2011, 141, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Shojadoost, B.; Kulkarni, R.R.; Yitbarek, A.; Laursen, A.; Taha-Abdelaziz, K.; Negash Alkie, T.; Barjesteh, N.; Quinteiro-Filho, W.M.; Smith, T.K.; Sharif, S. Dietary selenium supplementation enhances antiviral immunity in chickens challenged with low pathogenic avian influenza virus subtype H9N2. Vet. Immunol. Immunopathol. 2019, 207, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Ivory, K.; Prieto, E.; Spinks, C.; Armah, C.N.; Goldson, A.J.; Dainty, J.R.; Nicoletti, C. Selenium supplementation has beneficial and detrimental effects on immunity to influenza vaccine in older adults. Clin. Nutr. 2017, 36, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Steinbrenner, H.; Al-Quraishy, S.; Dkhil, M.A.; Wunderlich, F.; Sies, H. Dietary selenium in adjuvant therapy of viral and bacterial infections. Adv. Nutr. 2015, 6, 73–82. [Google Scholar] [CrossRef]
- Kafai, M.R.; Ganji, V. Sex, age, geographical location, smoking, and alcohol consumption influence serum selenium concentrations in the USA: Third National Health and Nutrition Examination Survey, 1988–1994. J. Trace Elem. Med. Biol. 2003, 17, 13–18. [Google Scholar] [CrossRef]
- Alehagen, U.; Johansson, P.; Bjornstedt, M.; Rosen, A.; Post, C.; Aaseth, J. Relatively high mortality risk in elderly Swedish subjects with low selenium status. Eur. J. Clin. Nutr. 2016, 70, 91–96. [Google Scholar] [CrossRef]
- Vogt, B.L.; Richie, J.P., Jr. Glutathione depletion and recovery after acute ethanol administration in the aging mouse. Biochem. Pharmacol. 2007, 73, 1613–1621. [Google Scholar] [CrossRef] [PubMed]
- Lang, C.A.; Naryshkin, S.; Schneider, D.L.; Mills, B.J.; Lindeman, R.D. Low blood glutathione levels in healthy aging adults. J. Lab. Clin. Med. 1992, 120, 720–725. [Google Scholar] [PubMed]
- Lang, C.A.; Mills, B.J.; Mastropaolo, W.; Liu, M.C. Blood glutathione decreases in chronic diseases. J. Lab. Clin. Med. 2000, 135, 402–405. [Google Scholar] [CrossRef] [PubMed]
- Chaves, F.J.; Mansego, M.L.; Blesa, S.; Gonzalez-Albert, V.; Jimenez, J.; Tormos, M.C.; Espinosa, O.; Giner, V.; Iradi, A.; Saez, G.; et al. Inadequate cytoplasmic antioxidant enzymes response contributes to the oxidative stress in human hypertension. Am. J. Hypertens. 2007, 20, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Khomich, O.A.; Kochetkov, S.N.; Bartosch, B.; Ivanov, A.V. Redox Biology of Respiratory Viral Infections. Viruses 2018, 10, 392. [Google Scholar] [CrossRef]
- Shetty, R.; Udupa, N.; Mutalik, S.; Kulkarni, V.; Rao, V. Mechanisms and Therapeutics of N-acetylcysteine: A Recent Update. Res. J. Pharm. Technol. 2019, 12, 2584–2588. [Google Scholar] [CrossRef]
- Lai, K.Y.; Ng, W.Y.; Osburga Chan, P.K.; Wong, K.F.; Cheng, F. High-dose N-acetylcysteine therapy for novel H1N1 influenza pneumonia. Ann. Intern. Med. 2010, 152, 687–688. [Google Scholar] [CrossRef]
- Horowitz, R.I.; Freeman, P.R.; Bruzzese, J. Efficacy of glutathione therapy in relieving dyspnea associated with COVID-19 pneumonia: A report of 2 cases. Respir Med. Case Rep. 2020. [Google Scholar] [CrossRef]
- Alehagen, U.; Lindahl, T.L.; Aaseth, J.; Svensson, E.; Johansson, P. Levels of sP-selectin and hs-CRP Decrease with Dietary Intervention with Selenium and Coenzyme Q10 Combined: A Secondary Analysis of a Randomized Clinical Trial. PLoS ONE 2015, 10, e0137680. [Google Scholar] [CrossRef]
- Alehagen, U.; Alexander, J.; Aaseth, J. Supplementation with Selenium and Coenzyme Q10 Reduces Cardiovascular Mortality in Elderly with Low Selenium Status. A Secondary Analysis of a Randomised Clinical Trial. PLoS ONE 2016, 11, e0157541. [Google Scholar] [CrossRef]
- Zhai, J.; Bo, Y.; Lu, Y.; Liu, C.; Zhang, L. Effects of Coenzyme Q10 on Markers of Inflammation: A Systematic Review and Meta-Analysis. PLoS ONE 2017, 12, e0170172. [Google Scholar] [CrossRef] [PubMed]
- Kalen, A.; Appelkvist, E.L.; Dallner, G. Age-related changes in the lipid compositions of rat and human tissues. Lipids 1989, 24, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Broman, L.M.; Bernardson, A.; Bursell, K.; Wernerman, J.; Flaring, U.; Tjader, I. Serum selenium in critically ill patients: Profile and supplementation in a depleted region. Acta Anaesthesiol. Scand. 2020, 64, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Manzanares, W.; Lemieux, M.; Elke, G.; Langlois, P.L.; Bloos, F.; Heyland, D.K. High-dose intravenous selenium does not improve clinical outcomes in the critically ill: A systematic review and meta-analysis. Crit. Care 2016, 20, 356. [Google Scholar] [CrossRef]
- EU Scientific Committee on Food. Opinion of the Scientific Committee on Food on the Tolerable Upper Intake Level of Selenium; European Commission: Brussels, Belgium, 2000. [Google Scholar]
- Liu, L.C.; Voors, A.A.; van Veldhuisen, D.J.; van der Veer, E.; Belonje, A.M.; Szymanski, M.K.; Sillje, H.H.; van Gilst, W.H.; Jaarsma, T.; de Boer, R.A. Vitamin D status and outcomes in heart failure patients. Eur. J. Heart Fail. 2011, 13, 619–625. [Google Scholar] [CrossRef]
- Tangpricha, V.; Pearce, E.N.; Chen, T.C.; Holick, M.F. Vitamin D insufficiency among free-living healthy young adults. Am. J. Med. 2002, 112, 659–662. [Google Scholar] [CrossRef]
- Holick, M.F. The vitamin D deficiency pandemic: Approaches for diagnosis, treatment and prevention. Rev. Endocr. Metab. Disord. 2017, 18, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Ilie, P.C.; Stefanescu, S.; Smith, L. The role of vitamin D in the prevention of coronavirus disease 2019 infection and mortality. Aging Clin. Exp. Res. 2020, 32, 1195–1198. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, J.M.; Subramanian, S.; Laird, E.; Kenny, R.A. Editorial: Low population mortality from COVID-19 in countries south of latitude 35 degrees North supports vitamin D as a factor determining severity. Aliment. Pharmacol. Ther. 2020, 51, 1434–1437. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.S.; Ding, Y.; Freund, M.K.; Johnson, R.; Schwarz, T.; Yabu, J.M.; Hazlett, C.; Chiang, J.N.; Wulf, A.; Geschwind, D.H.; et al. Prior diagnoses and medications as risk factors for COVID-19 in a Los Angeles Health System. medRxiv 2020. [Google Scholar] [CrossRef]
- Panagiotou, G.; Tee, S.A.; Ihsan, Y.; Athar, W.; Marchitelli, G.; Kelly, D.; Boot, C.S.; Stock, N.; Macfarlane, J.; Martineau, A.R.; et al. Low serum 25-hydroxyvitamin D (25[OH]D) levels in patients hospitalised with COVID-19 are associated with greater disease severity. Clin. Endocrinol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Mendy, A.; Apewokin, S.; Wells, A.A.; Morrow, A.L. Factors Associated with Hospitalization and Disease Severity in a Racially and Ethnically Diverse Population of COVID-19 Patients. medRxiv 2020. [Google Scholar] [CrossRef]
- Hughes, D.A.; Norton, R. Vitamin D and respiratory health. Clin. Exp. Immunol. 2009, 158, 20–25. [Google Scholar] [CrossRef] [PubMed]
- de Haan, K.; Groeneveld, A.B.; de Geus, H.R.; Egal, M.; Struijs, A. Vitamin D deficiency as a risk factor for infection, sepsis and mortality in the critically ill: Systematic review and meta-analysis. Crit. Care 2014, 18, 660. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Zhang, J.; Ma, C.; Yue, Y.; Zou, Z.; Yu, C.; Yin, F. Link between community-acquired pneumonia and vitamin D levels in older patients. Z. Gerontol. Geriatr. 2018, 51, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Remmelts, H.H.; van de Garde, E.M.; Meijvis, S.C.; Peelen, E.L.; Damoiseaux, J.G.; Grutters, J.C.; Biesma, D.H.; Bos, W.J.; Rijkers, G.T. Addition of vitamin D status to prognostic scores improves the prediction of outcome in community-acquired pneumonia. Clin. Infect. Dis. 2012, 55, 1488–1494. [Google Scholar] [CrossRef] [PubMed]
- Holter, J.C.; Ueland, T.; Norseth, J.; Brunborg, C.; Froland, S.S.; Husebye, E.; Aukrust, P.; Heggelund, L. Vitamin D Status and Long-Term Mortality in Community-Acquired Pneumonia: Secondary Data Analysis from a Prospective Cohort. PLoS ONE 2016, 11, e0158536. [Google Scholar] [CrossRef]
- Dancer, R.C.; Parekh, D.; Lax, S.; D’Souza, V.; Zheng, S.; Bassford, C.R.; Park, D.; Bartis, D.G.; Mahida, R.; Turner, A.M.; et al. Vitamin D deficiency contributes directly to the acute respiratory distress syndrome (ARDS). Thorax 2015, 70, 617–624. [Google Scholar] [CrossRef]
- Thickett, D.R.; Moromizato, T.; Litonjua, A.A.; Amrein, K.; Quraishi, S.A.; Lee-Sarwar, K.A.; Mogensen, K.M.; Purtle, S.W.; Gibbons, F.K.; Camargo, C.A., Jr.; et al. Association between prehospital vitamin D status and incident acute respiratory failure in critically ill patients: A retrospective cohort study. BMJ Open Respir Res. 2015, 2, e000074. [Google Scholar] [CrossRef]
- Telcian, A.G.; Zdrenghea, M.T.; Edwards, M.R.; Laza-Stanca, V.; Mallia, P.; Johnston, S.L.; Stanciu, L.A. Vitamin D increases the antiviral activity of bronchial epithelial cells in vitro. Antiviral Res. 2017, 137, 93–101. [Google Scholar] [CrossRef]
- Schogler, A.; Muster, R.J.; Kieninger, E.; Casaulta, C.; Tapparel, C.; Jung, A.; Moeller, A.; Geiser, T.; Regamey, N.; Alves, M.P. Vitamin D represses rhinovirus replication in cystic fibrosis cells by inducing LL-37. Eur. Respir. J. 2016, 47, 520–530. [Google Scholar] [CrossRef] [PubMed]
- Brockman-Schneider, R.A.; Pickles, R.J.; Gern, J.E. Effects of vitamin D on airway epithelial cell morphology and rhinovirus replication. PLoS ONE 2014, 9, e86755. [Google Scholar] [CrossRef] [PubMed]
- Hansdottir, S.; Monick, M.M.; Lovan, N.; Powers, L.; Gerke, A.; Hunninghake, G.W. Vitamin D decreases respiratory syncytial virus induction of NF-kappaB-linked chemokines and cytokines in airway epithelium while maintaining the antiviral state. J. Immunol. 2010, 184, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Bernheim, A.; Mei, X.; Huang, M.; Yang, Y.; Fayad, Z.A.; Zhang, N.; Diao, K.; Lin, B.; Zhu, X.; Li, K.; et al. Chest CT Findings in Coronavirus Disease-19 (COVID-19): Relationship to Duration of Infection. Radiology 2020. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, A.M.; Wongtrakool, C.; Welch, T.; Steinmeyer, A.; Zugel, U.; Roman, J. Vitamin D inhibition of pro-fibrotic effects of transforming growth factor beta1 in lung fibroblasts and epithelial cells. J. Steroid Biochem. Mol. Biol. 2010, 118, 142–150. [Google Scholar] [CrossRef]
- Remmelts, H.H.; Spoorenberg, S.M.; Oosterheert, J.J.; Bos, W.J.; de Groot, M.C.; van de Garde, E.M. The role of vitamin D supplementation in the risk of developing pneumonia: Three independent case-control studies. Thorax 2013, 68, 990–996. [Google Scholar] [CrossRef]
- Ingels, C.; Vanhorebeek, I.; Van Cromphaut, S.; Wouters, P.J.; Derese, I.; Dehouwer, A.; Moller, H.J.; Hansen, T.K.; Billen, J.; Mathieu, C.; et al. Effect of Intravenous 25OHD Supplementation on Bone Turnover and Inflammation in Prolonged Critically Ill Patients. Horm. Metab. Res. 2020, 52, 168–178. [Google Scholar] [CrossRef]
- Scientific Opinion on the Tolerable Upper Intake Level of vitamin D. EFSA J. 2012, 10, 2813. [CrossRef]
- Calder, P.C.; Carr, A.C.; Gombart, A.F.; Eggersdorfer, M. Optimal Nutritional Status for a Well-Functioning Immune System Is an Important Factor to Protect against Viral Infections. Nutrients 2020, 12, 1181. [Google Scholar] [CrossRef]
- Hemila, H.; Chalker, E. Vitamin C as a Possible Therapy for COVID-19. Infect. Chemother 2020, 52, 222–223. [Google Scholar] [CrossRef]
- Hemila, H. Vitamin C and Infections. Nutrients 2017, 9, 339. [Google Scholar] [CrossRef] [PubMed]
- Caccialanza, R.; Laviano, A.; Lobascio, F.; Montagna, E.; Bruno, R.; Ludovisi, S.; Corsico, A.G.; Di Sabatino, A.; Belliato, M.; Calvi, M.; et al. Early nutritional supplementation in non-critically ill patients hospitalized for the 2019 novel coronavirus disease (COVID-19): Rationale and feasibility of a shared pragmatic protocol. Nutrition 2020, 74, 110835. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alexander, J.; Tinkov, A.; Strand, T.A.; Alehagen, U.; Skalny, A.; Aaseth, J. Early Nutritional Interventions with Zinc, Selenium and Vitamin D for Raising Anti-Viral Resistance Against Progressive COVID-19. Nutrients 2020, 12, 2358. https://doi.org/10.3390/nu12082358
Alexander J, Tinkov A, Strand TA, Alehagen U, Skalny A, Aaseth J. Early Nutritional Interventions with Zinc, Selenium and Vitamin D for Raising Anti-Viral Resistance Against Progressive COVID-19. Nutrients. 2020; 12(8):2358. https://doi.org/10.3390/nu12082358
Chicago/Turabian StyleAlexander, Jan, Alexey Tinkov, Tor A. Strand, Urban Alehagen, Anatoly Skalny, and Jan Aaseth. 2020. "Early Nutritional Interventions with Zinc, Selenium and Vitamin D for Raising Anti-Viral Resistance Against Progressive COVID-19" Nutrients 12, no. 8: 2358. https://doi.org/10.3390/nu12082358
APA StyleAlexander, J., Tinkov, A., Strand, T. A., Alehagen, U., Skalny, A., & Aaseth, J. (2020). Early Nutritional Interventions with Zinc, Selenium and Vitamin D for Raising Anti-Viral Resistance Against Progressive COVID-19. Nutrients, 12(8), 2358. https://doi.org/10.3390/nu12082358






