A High Docosahexaenoic Acid Diet Alters the Lung Inflammatory Response to Acute Dust Exposure
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Preparation of Organic Dust Extract
2.3. Animal Care
2.4. In Vivo Model of Dust Exposure
2.5. Neutrophil Extracellular Trap (NET) Scoring
2.6. Analysis of Cytokines and Chemokines
2.7. Fatty Acid Blood Levels
2.8. NanoString Gene Expression
2.9. Statistical Analyses
3. Results
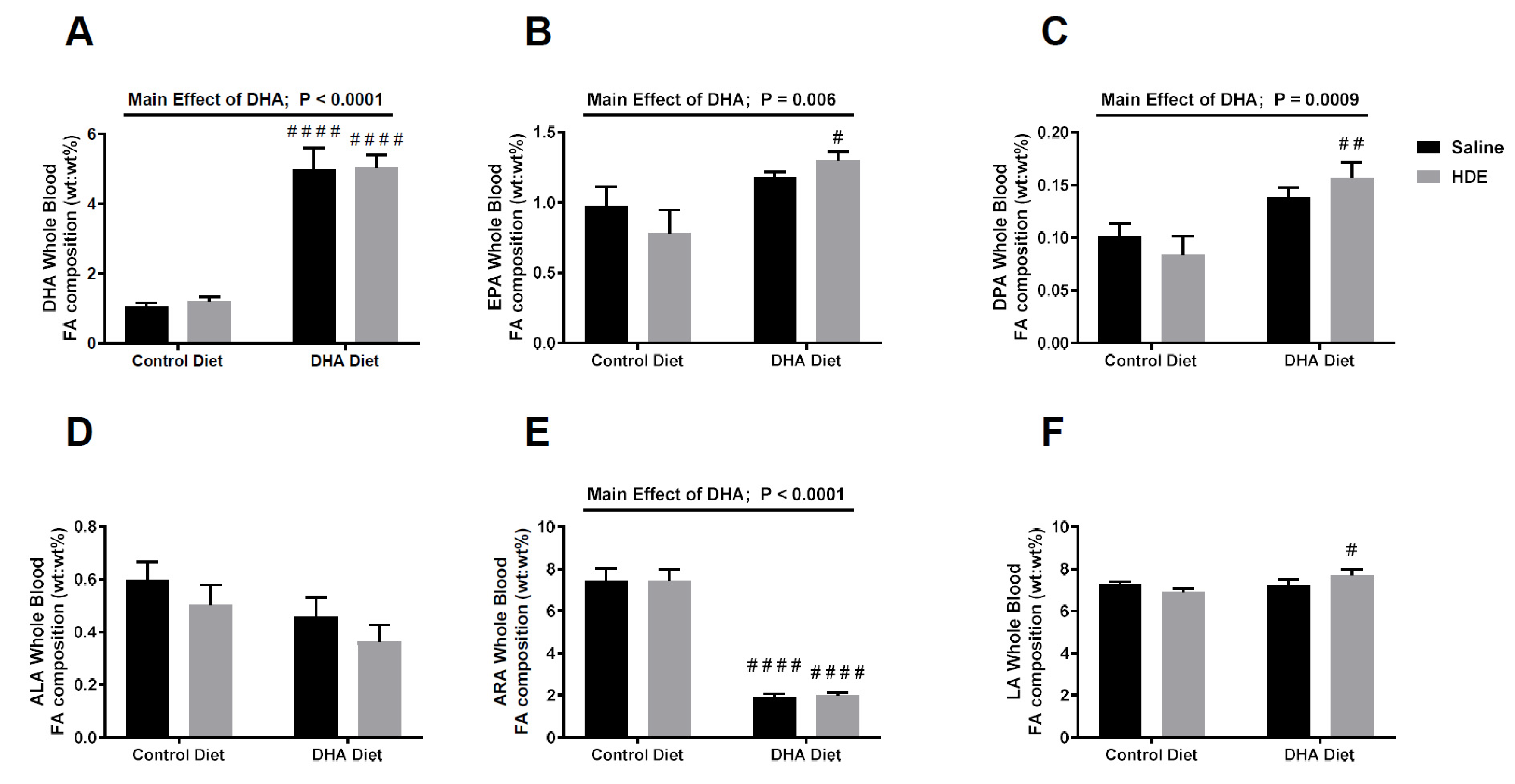
3.1. A High-DHA Diet Alters Blood Omega-3 and Omega-6 PUFA Levels
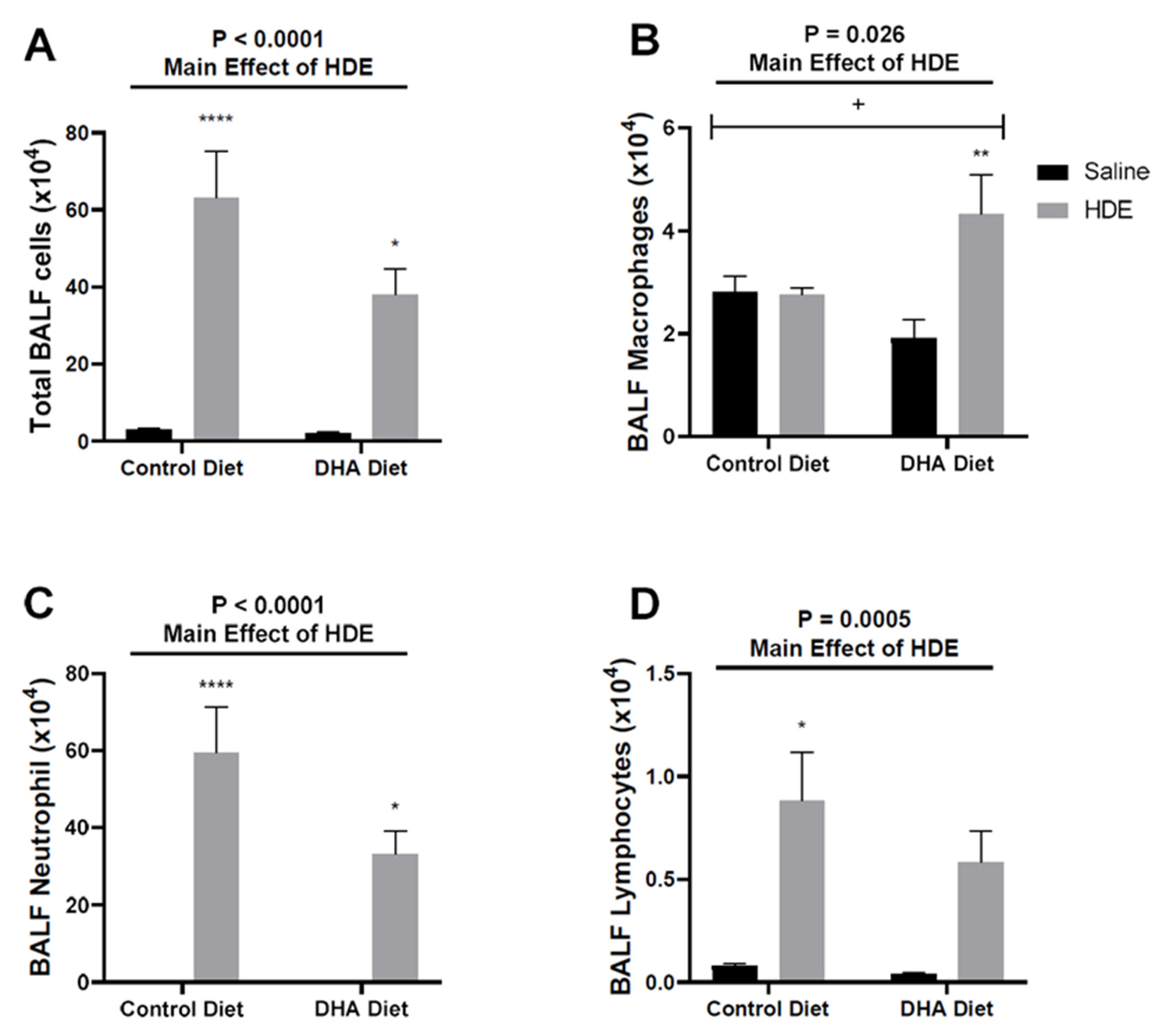
3.2. A High-DHA Diet Impacts Overall Lung Cellular Influx in Mice Following Acute HDE Exposure
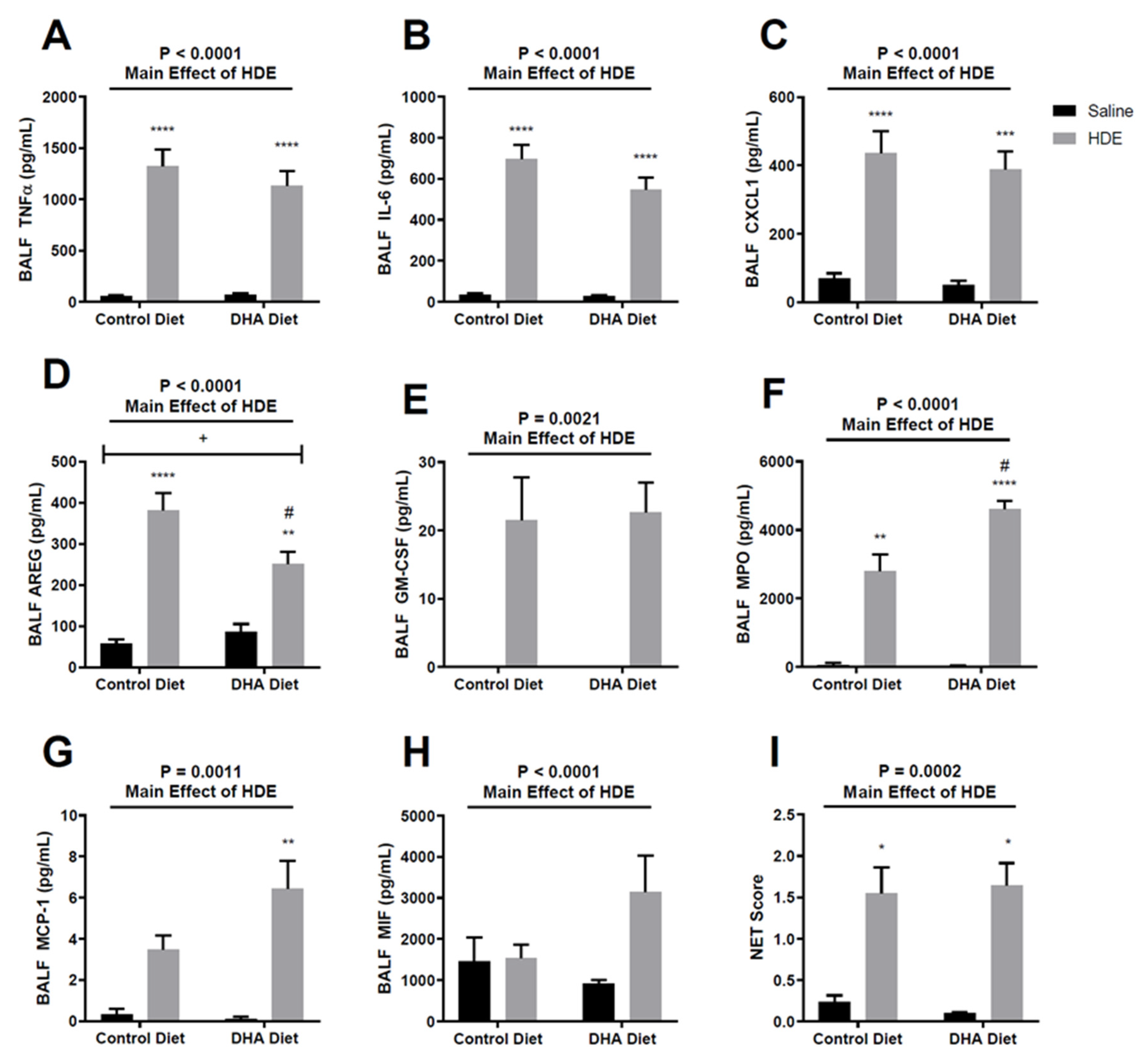
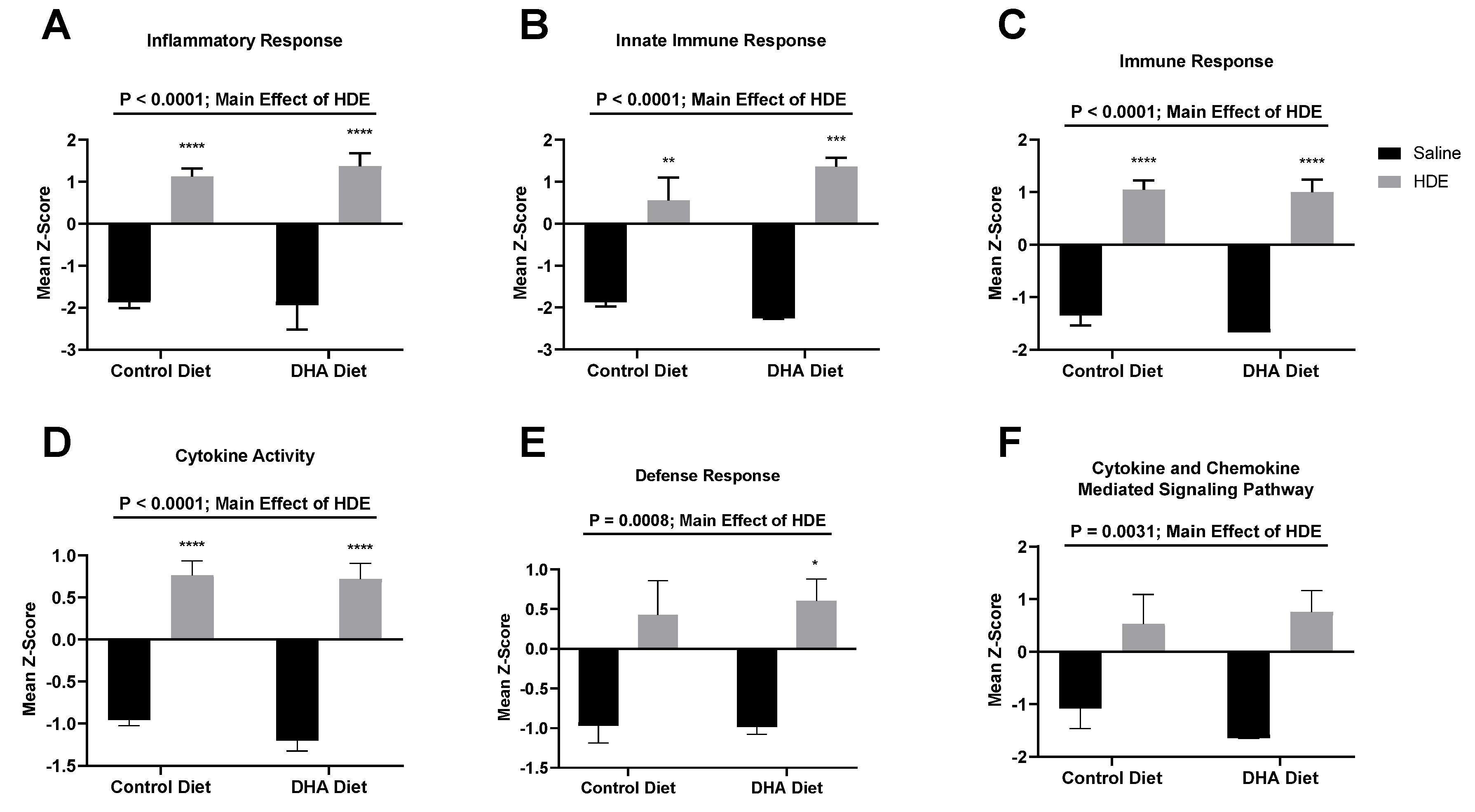
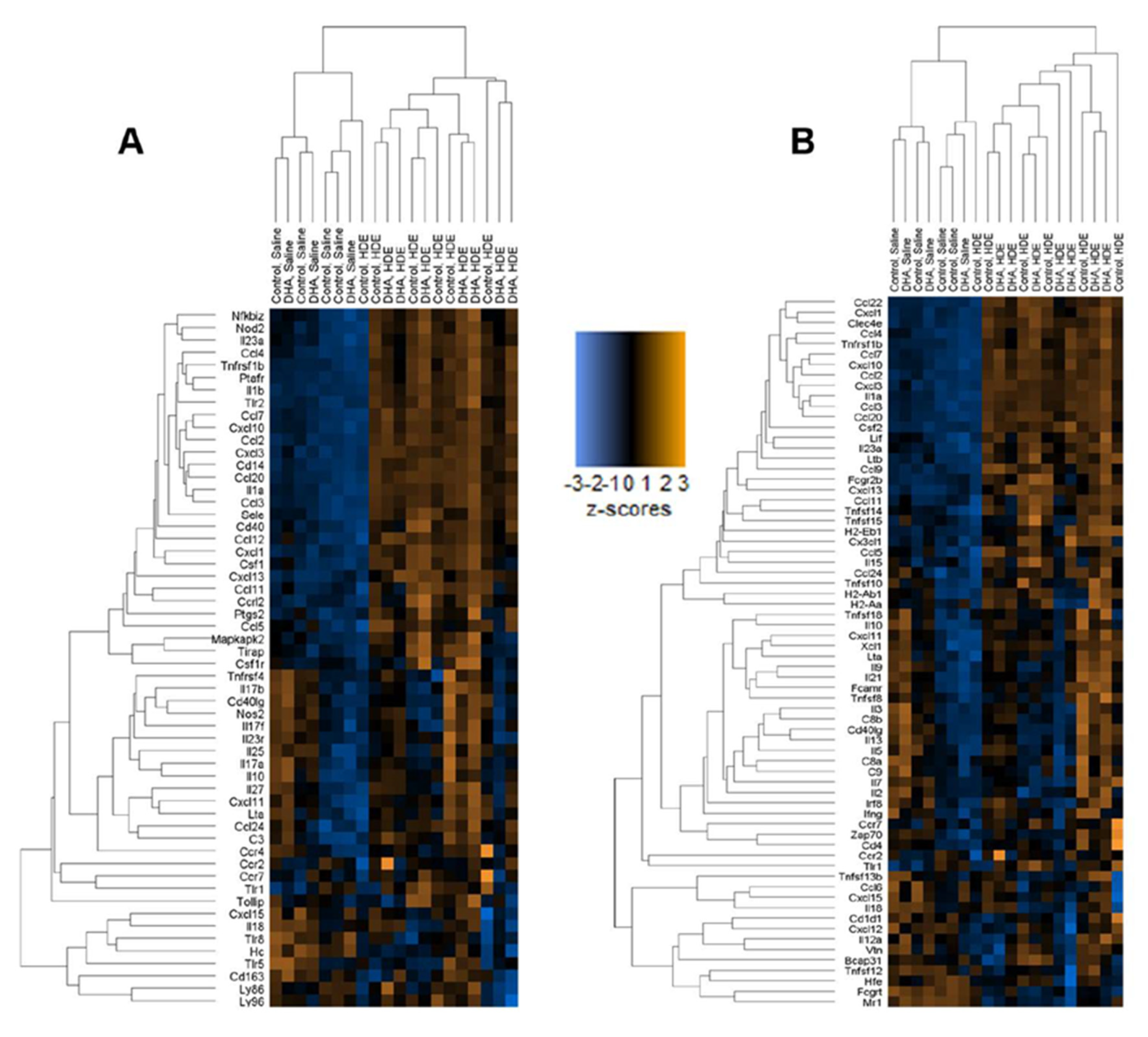
3.3. A DHA-Rich Diet Alters HDE-Induced Changes in Pro-Inflammatory Cytokine/Chemokine Release
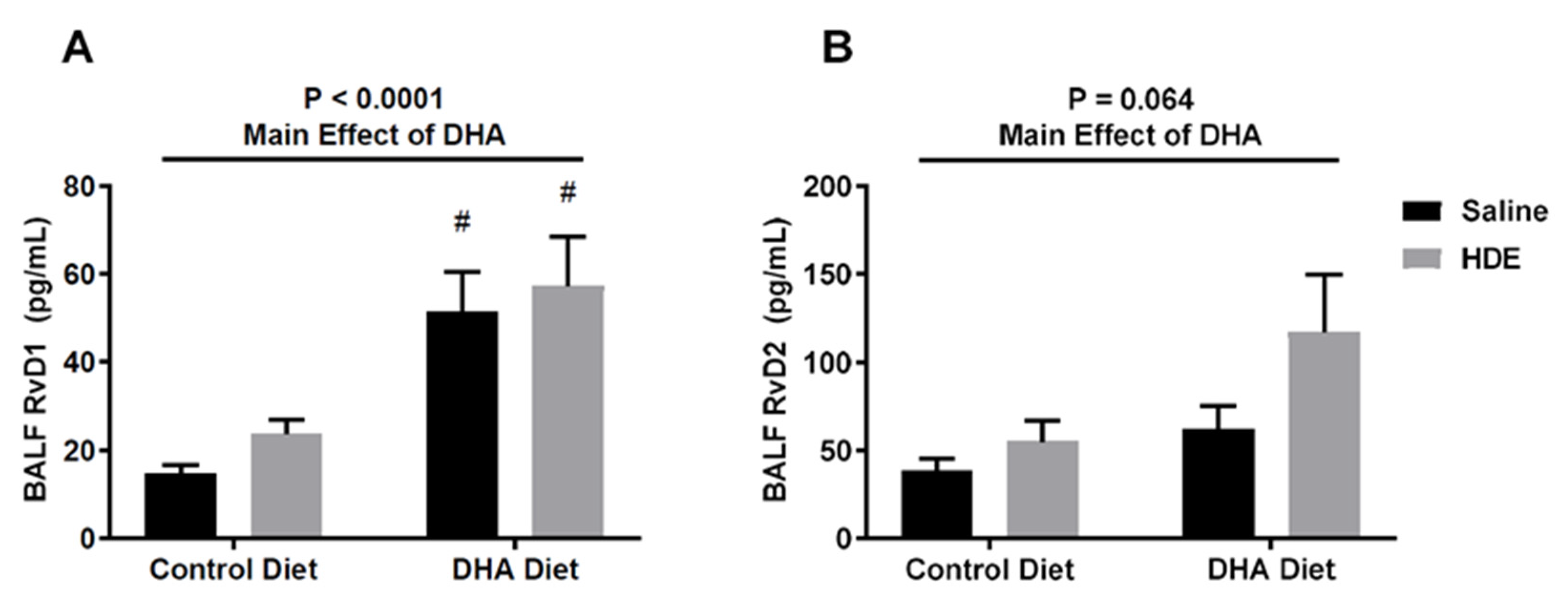
3.4. A High-DHA Diet Is Associated with Increased Production of Resolvin D1
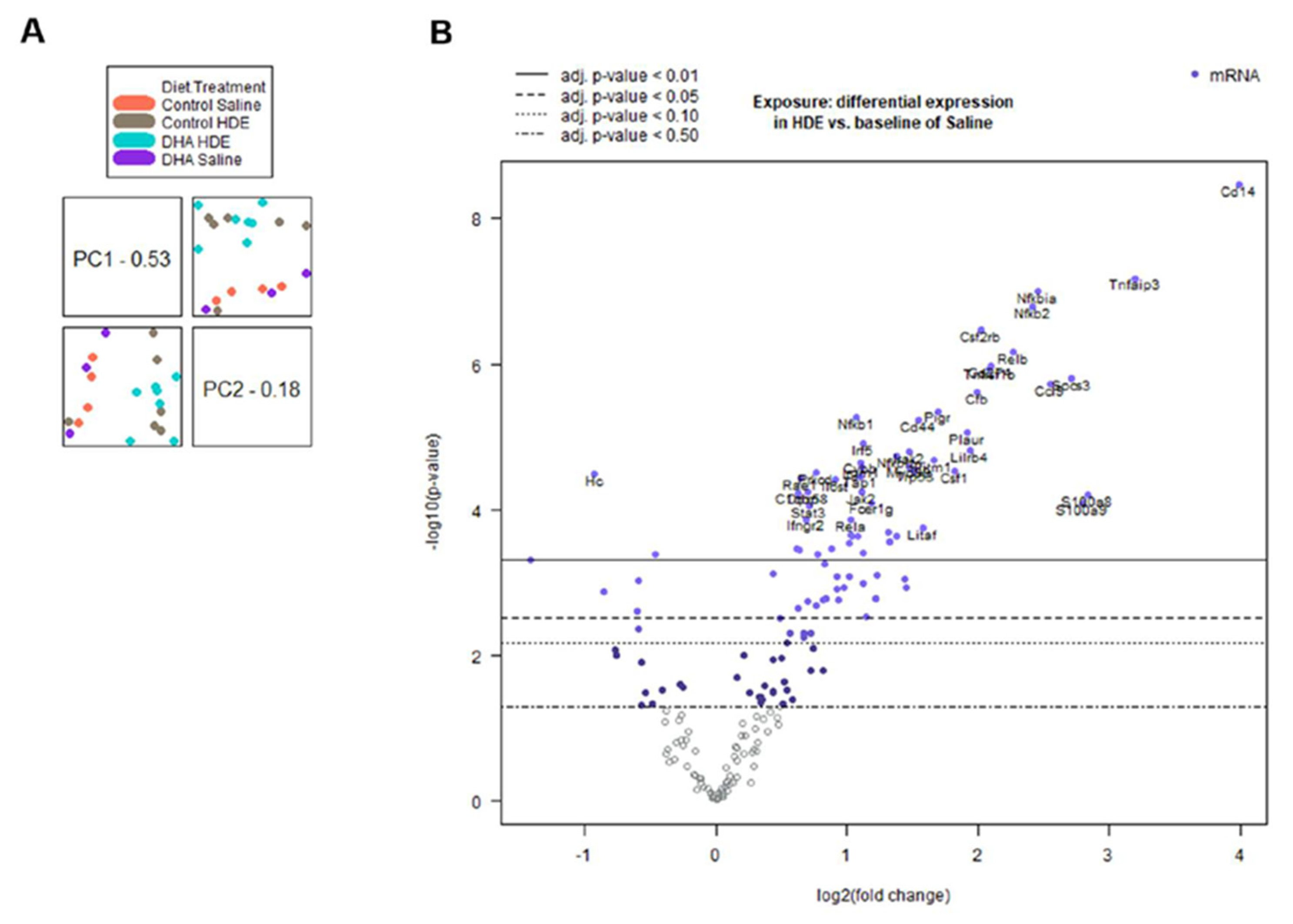
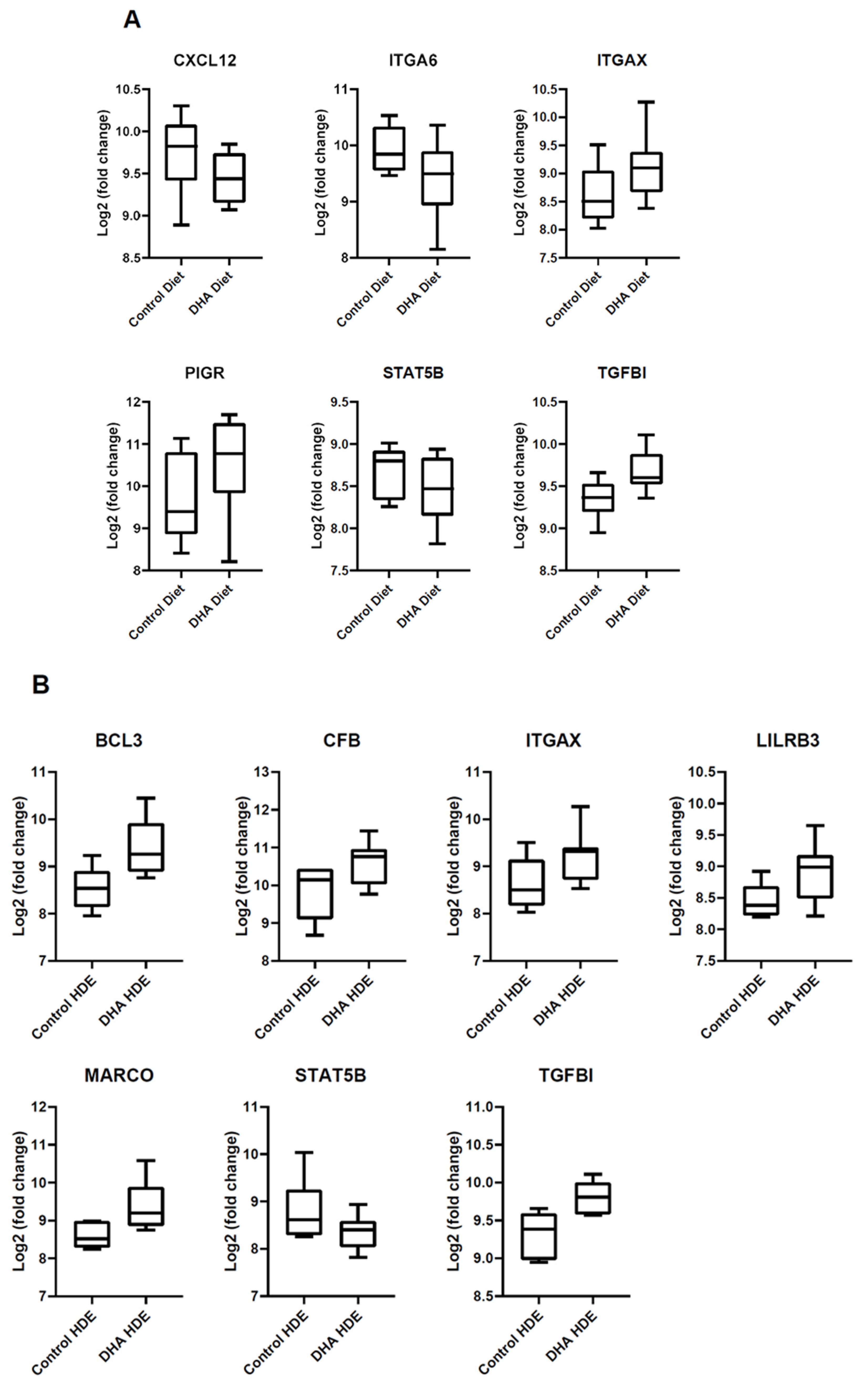
3.5. In Vivo Gene Expression Changes in DHA-Diet-Fed Mice Following Exposure to HDE
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kirkhorn, S.R.; Garry, V.F. Agricultural lung diseases. Environ. Health Perspect. 2000, 108 (Suppl. 4), 705–712. [Google Scholar] [CrossRef] [PubMed]
- Langley, R.L. Consequences of respiratory exposures in the farm environment. N. C. Med. J. 2011, 72, 477–480. [Google Scholar] [PubMed]
- Respiratory Health Hazards in Agriculture. Am. J. Respir. Crit. Care Med. 1998, 158, S1–S76. [CrossRef] [PubMed]
- Von Essen, S.; Donham, K. Illness and injury in animal confinement workers. Occup. Med. 1999, 14, 337–350. [Google Scholar] [PubMed]
- Bongers, P.; Houthuijs, D.; Remijn, B.; Brouwer, R.; Biersteker, K. Lung function and respiratory symptoms in pig farmers. Br. J. Ind. Med. 1987, 44, 819–823. [Google Scholar] [CrossRef]
- Eduard, W.; Pearce, N.; Douwes, J. Chronic bronchitis, COPD, and lung function in farmers: The role of biological agents. Chest 2009, 136, 716–725. [Google Scholar] [CrossRef]
- Von Essen, S.; Romberger, D. The respiratory inflammatory response to the swine confinement building environment: The adaptation to respiratory exposures in the chronically exposed worker. J. Agric. Saf. Health 2003, 9, 185–196. [Google Scholar] [CrossRef]
- Szczyrek, M.; Krawczyk, P.; Milanowski, J.; Jastrzebska, I.; Zwolak, A.; Daniluk, J. Chronic obstructive pulmonary disease in farmers and agricultural workers—An overview. Ann. Agric. Environ. Med. 2011, 18, 310–313. [Google Scholar]
- Boissy, R.J.; Romberger, D.J.; Roughead, W.A.; Weissenburger-Moser, L.; Poole, J.A.; LeVan, T.D. Shotgun pyrosequencing metagenomic analyses of dusts from swine confinement and grain facilities. PLoS ONE 2014, 9, e95578. [Google Scholar] [CrossRef]
- Romberger, D.J.; Heires, A.J.; Nordgren, T.M.; Souder, C.P.; West, W.; Liu, X.-D.; Poole, J.A.; Toews, M.L.; Wyatt, T.A. Proteases in agricultural dust induce lung inflammation through PAR-1 and PAR-2 activation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2015, 309, L388–L399. [Google Scholar] [CrossRef]
- Wang, Z.; Larsson, K.; Palmberg, L.; Malmberg, P.; Larsson, P.; Larsson, L. Inhalation of swine dust induces cytokine release in the upper and lower airways. Eur. Respir. J. 1997, 10, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Romberger, D.J.; Bodlak, V.; Essen, S.G.V.; Mathisen, T.; Wyatt, T.A. Hog barn dust extract stimulates IL-8 and IL-6 release in human bronchial epithelial cells via PKC activation. J. Appl. Physiol. 2002, 93, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, D.C.; Schenker, M.B. Protection against breathing dust: Behavior over time in Californian farmers. J. Agric. Saf. Health 2008, 14, 189–203. [Google Scholar] [CrossRef]
- Schenker, M.B.; Orenstein, M.R.; Samuels, S.J. Use of protective equipment among California farmers. Am. J. Ind. Med. 2002, 42, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Moldoveanu, B.; Otmishi, P.; Jani, P.; Walker, J.; Sarmiento, X.; Guardiola, J.; Saad, M.; Yu, J. Inflammatory mechanisms in the lung. J. Inflamm. Res. 2009, 2, 1–11. [Google Scholar]
- Sansbury, B.E.; Spite, M. Resolution of Acute Inflammation and the Role of Resolvins in Immunity, Thrombosis, and Vascular Biology. Circ. Res. 2016, 119, 113–130. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharm. 2002, 56, 365–379. [Google Scholar] [CrossRef]
- Giudetti, A.M.; Cagnazzo, R. Beneficial effects of n-3 PUFA on chronic airway inflammatory diseases. Prostaglandins Other Lipid Mediat. 2012, 99, 57–67. [Google Scholar] [CrossRef]
- Levy, B.D.; Clish, C.B.; Schmidt, B.; Gronert, K.; Serhan, C.N. Lipid mediator class switching during acute inflammation: Signals in resolution. Nat. Immunol. 2001, 2, 612–619. [Google Scholar] [CrossRef]
- Patterson, E.; Wall, R.; Fitzgerald, G.; Ross, R.; Stanton, C. Health implications of high dietary omega-6 polyunsaturated fatty acids. J. Nutr. Metab. 2012, 2012, 539426. [Google Scholar] [CrossRef]
- Serhan, C.N.; Chiang, N.; Van Dyke, T.E. Resolving inflammation: Dual anti-inflammatory and pro-resolution lipid mediators. Nat. Rev. Immunol. 2008, 8, 349–361. [Google Scholar] [CrossRef]
- Serhan, C.N.; Savill, J. Resolution of inflammation: The beginning programs the end. Nat. Immunol. 2005, 6, 1191–1197. [Google Scholar] [CrossRef]
- Bannenberg, G.L. Therapeutic applicability of anti-inflammatory and proresolving polyunsaturated fatty acid-derived lipid mediators. Sci. World J. 2010, 10, 676–712. [Google Scholar] [CrossRef]
- Serhan, C.N.; Dalli, J.; Karamnov, S.; Choi, A.; Park, C.-K.; Xu, Z.-Z.; Ji, R.-R.; Zhu, M.; Petasis, N.A. Macrophage proresolving mediator maresin 1 stimulates tissue regeneration and controls pain. FASEB J. 2012, 26, 1755–1765. [Google Scholar] [CrossRef]
- Wang, W.; Zhu, J.; Lyu, F.; Panigrahy, D.; Ferrara, K.W.; Hammock, B.; Zhang, G. ω-3 Polyunsaturated fatty acids-derived lipid metabolites on angiogenesis, inflammation and cancer. Prostaglandins Other Lipid Mediat. 2014, 113–115, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Duvall, M.G.; Levy, B.D. DHA- and EPA-derived resolvins, protectins, and maresins in airway inflammation. Eur. J. Pharmacol. 2016, 785, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, H.-M.; Sapinoro, R.E.; Thatcher, T.H.; Croasdell, A.; Levy, E.P.; Fulton, R.A.; Olsen, K.C.; Pollock, S.J.; Serhan, C.N.; Phipps, R.P.; et al. A novel anti-inflammatory and pro-resolving role for resolvin D1 in acute cigarette smoke-induced lung inflammation. PLoS ONE 2013, 8, e58258. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N. Novel Lipid Mediators and Resolution Mechanisms in Acute Inflammation: To Resolve or Not? Am. J. Pathol. 2010, 177, 1576–1591. [Google Scholar] [CrossRef]
- Nordgren, T.M.; Friemel, T.D.; Heires, A.J.; Poole, J.A.; Wyatt, T.A.; Romberger, D.J. The Omega-3 Fatty Acid Docosahexaenoic Acid Attenuates Organic Dust-Induced Airway Inflammation. Nutrients 2014, 6, 5434–5452. [Google Scholar] [CrossRef]
- Nordgren, T.M.; Heires, A.J.; Bailey, K.L.; Katafiasz, D.M.; Toews, M.L.; Wichman, C.S.; Romberger, D.J. Docosahexaenoic acid enhances amphiregulin-mediated bronchial epithelial cell repair processes following organic dust exposure. Am. J. Physiol. Lung Cell. Mol. Physiol. 2018, 314, L421–L431. [Google Scholar] [CrossRef]
- Poole, J.A.; Wyatt, T.A.; Oldenburg, P.J.; Elliott, M.K.; West, W.W.; Sisson, J.H.; Von Essen, S.G.; Romberger, D.J. Intranasal organic dust exposure-induced airway adaptation response marked by persistent lung inflammation and pathology in mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 2009, 296, L1085–L1095. [Google Scholar] [CrossRef] [PubMed]
- Poole, J.A.; Romberger, D.J. Immunological and inflammatory responses to organic dust in agriculture. Curr. Opin. Allergy Clin. Immunol. 2012, 12, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Perez, S.E.; Berg, B.M.; Moore, K.A.; He, B.; Counts, S.E.; Fritz, J.J.; Hu, Y.S.; Lazarov, O.; Lah, J.J.; Mufson, E.J. DHA diet reduces AD pathology in young APPswe/PS1 Delta E9 transgenic mice: Possible gender effects. J. Neurosci. Res. 2010, 88, 1026–1040. [Google Scholar] [CrossRef] [PubMed]
- Vargas, A.; Boivin, R.; Cano, P.; Murcia, Y.; Bazin, I.; Lavoie, J.-P. Neutrophil extracellular traps are downregulated by glucocorticosteroids in lungs in an equine model of asthma. Respir. Res. 2017, 18, 207. [Google Scholar] [CrossRef]
- Nordgren, T.M.; Bauer, C.D.; Heires, A.J.; Poole, J.A.; Wyatt, T.A.; West, W.W.; Romberger, D.J. Maresin-1 reduces airway inflammation associated with acute and repetitive exposures to organic dust. Transl. Res. 2015, 166, 57–69. [Google Scholar] [CrossRef]
- Linaker, C.; Smedley, J. Respiratory illness in agricultural workers. Occup. Med. 2002, 52, 451–459. [Google Scholar] [CrossRef]
- Kromhout, D.; Bosschieter, E.B.; Coulander, C.D.L. The Inverse Relation between Fish Consumption and 20-Year Mortality from Coronary Heart Disease. N. Engl. J. Med. 1985, 312, 1205–1209. [Google Scholar] [CrossRef]
- Kromhout, D.; Feskens, E.J.M.; Bowles, C.H. The Protective Effect of a Small Amount of Fish on Coronary Heart Disease Mortality in an Elderly Population. Int. J. Epidemiol. 1995, 24, 340–345. [Google Scholar] [CrossRef]
- Marik, P.E.; Varon, J. Omega-3 dietary supplements and the risk of cardiovascular events: A systematic review. Clin. Cardiol. 2009, 32, 365–372. [Google Scholar] [CrossRef]
- Hanson, C.; Lyden, E.; Weissenburger-Moser, L.; Furtado, J.; Hinds, J.; LeVan, T. Serum Level of Nutritional Antioxidants are Decreased in Veteran Smokers with COPD. Mil. Veterans Health 2016, in press. [Google Scholar]
- Chazaud, B. Macrophages: Supportive cells for tissue repair and regeneration. Immunobiology 2014, 219, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Oishi, Y.; Manabe, I. Macrophages in inflammation, repair and regeneration. Int. Immunol. 2018, 30, 511–528. [Google Scholar] [CrossRef] [PubMed]
- Levy, B. Resolvin D1 and Resolvin E1 Promote the Resolution of Allergic Airway Inflammation via Shared and Distinct Molecular Counter-Regulatory Pathways. Front. Immunol. 2012, 3. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Hong, S.; Gronert, K.; Colgan, S.P.; Devchand, P.R.; Mirick, G.; Moussignac, R.-L. Resolvins: A Family of Bioactive Products of Omega-3 Fatty Acid Transformation Circuits Initiated by Aspirin Treatment that Counter Proinflammation Signals. J. Exp. Med. 2002, 196, 1025–1037. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Dong, J.; Wu, W.; Yang, T.; Wang, T.; Guo, L.; Chen, L.; Xu, D.; Wen, F. Resolvin D1 attenuates inflammation in lipopolysaccharide-induced acute lung injury through a process involving the PPARγ/NF-κB pathway. Respir. Res. 2012, 13, 110. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.; Levy, B.D. Resolvins: Natural agonists for resolution of pulmonary inflammation. Prog. Lipid Res. 2011, 50, 75–88. [Google Scholar] [CrossRef]
- Nordgren, T.M.; Heires, A.J.; Zempleni, J.; Swanson, B.J.; Wichman, C.; Romberger, D.J. Bovine milk-derived extracellular vesicles enhance inflammation and promote M1 polarization following agricultural dust exposure in mice. J. Nutr. Biochem. 2019, 64, 110–120. [Google Scholar] [CrossRef]
- Nummela, P.; Lammi, J.; Soikkeli, J.; Saksela, O.; Laakkonen, P.; Hölttä, E. Transforming Growth Factor Beta-Induced (TGFBI) Is an Anti-Adhesive Protein Regulating the Invasive Growth of Melanoma Cells. Am. J. Pathol. 2012, 180, 1663–1674. [Google Scholar] [CrossRef]
- Thapa, N.; Lee, B.-H.; Kim, I.-S. TGFBIp/βig-h3 protein: A versatile matrix molecule induced by TGF-β. Int. J. Biochem. Cell Biol. 2007, 39, 2183–2194. [Google Scholar] [CrossRef]
- Cao, W.; Tan, P.; Lee, C.H.; Zhang, H.; Lu, J. A transforming growth factor–β–induced protein stimulates endocytosis and is up-regulated in immature dendritic cells. Blood 2006, 107, 2777–2785. [Google Scholar] [CrossRef]
- Rawe, I.M.; Zhan, Q.; Burrows, R.; Bennett, K.; Cintron, C. Beta-ig. Molecular cloning and in situ hybridization in corneal tissues. Investig. Ophthalmol. Vis. Sci. 1997, 38, 893–900. [Google Scholar]
- Chapman, H.A.; Li, X.; Alexander, J.P.; Brumwell, A.; Lorizio, W.; Tan, K.; Sonnenberg, A.; Wei, Y.; Vu, T.H. Integrin α6β4 identifies an adult distal lung epithelial population with regenerative potential in mice. J. Clin. Investig. 2011, 121, 2855–2862. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, D. Functions of Pulmonary Epithelial Integrins: From Development to Disease. Physiol. Rev. 2003, 83, 673–686. [Google Scholar] [CrossRef] [PubMed]
- Guth, A.M.; Janssen, W.J.; Bosio, C.M.; Crouch, E.C.; Henson, P.M.; Dow, S.W. Lung environment determines unique phenotype of alveolar macrophages. Am. J. Physiol. Lung Cell. Mol. Physiol. 2009, 296, L936–L946. [Google Scholar] [CrossRef] [PubMed]
- Poole, J.A.; Gleason, A.M.; Bauer, C.; West, W.W.; Alexis, N.; van Rooijen, N.; Reynolds, S.J.; Romberger, D.J.; Kielian, T.L. CD11c(+)/CD11b(+) cells are critical for organic dust-elicited murine lung inflammation. Am. J. Respir. Cell Mol. Biol. 2012, 47, 652–659. [Google Scholar] [CrossRef]
- Murthy, S.; Larson-Casey, J.L.; Ryan, A.J.; He, C.; Kobzik, L.; Carter, A.B. Alternative activation of macrophages and pulmonary fibrosis are modulated by scavenger receptor, macrophage receptor with collagenous structure. FASEB J. 2015, 29, 3527–3536. [Google Scholar] [CrossRef]
- Zhang, L.; Nie, L.; Cai, S.-Y.; Chen, J.; Chen, J. Role of a macrophage receptor with collagenous structure (MARCO) in regulating monocyte/macrophage functions in ayu, Plecoglossus altivelis. Fish Shellfish Immunol. 2018, 74, 141–151. [Google Scholar] [CrossRef]
- An, H.; Chandra, V.; Piraino, B.; Borges, L.; Geczy, C.; McNeil, H.P.; Bryant, K.; Tedla, N. Soluble LILRA3, a potential natural antiinflammatory protein, is increased in patients with rheumatoid arthritis and is tightly regulated by interleukin 10, tumor necrosis factor-alpha, and interferon-gamma. J. Rheumatol. 2010, 37, 1596–1606. [Google Scholar] [CrossRef]
- Hirayasu, K.; Arase, H. Functional and genetic diversity of leukocyte immunoglobulin-like receptor and implication for disease associations. J. Hum. Genet. 2015, 60, 703–708. [Google Scholar] [CrossRef]
- Kang, X.; Kim, J.; Deng, M.; John, S.; Chen, H.; Wu, G.; Phan, H.; Zhang, C.C. Inhibitory leukocyte immunoglobulin-like receptors: Immune checkpoint proteins and tumor sustaining factors. Cell Cycle 2016, 15, 25–40. [Google Scholar] [CrossRef]
- Kaetzel, C.S. The polymeric immunoglobulin receptor: Bridging innate and adaptive immune responses at mucosal surfaces. Immunol. Rev. 2005, 206, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Kaetzel, C.S. Cooperativity among secretory IgA, the polymeric immunoglobulin receptor, and the gut microbiota promotes host–microbial mutualism. Immunol. Lett. 2014, 162, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Kreisel, D.; Sugimoto, S.; Tietjens, J.; Zhu, J.; Yamamoto, S.; Krupnick, A.S.; Carmody, R.J.; Gelman, A.E. Bcl3 prevents acute inflammatory lung injury in mice by restraining emergency granulopoiesis. J. Clin. Investig. 2011, 121, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Taube, C.; Thurman, J.M.; Takeda, K.; Joetham, A.; Miyahara, N.; Carroll, M.C.; Dakhama, A.; Giclas, P.C.; Holers, V.M.; Gelfand, E.W. Factor B of the alternative complement pathway regulates development of airway hyperresponsiveness and inflammation. Proc. Natl. Acad. Sci. USA 2006, 103, 8084. [Google Scholar] [CrossRef]
- Negrete-García, M.C.; Velazquez, J.R.; Popoca-Coyotl, A.; Montes-Vizuet, A.R.; Juárez-Carvajal, E.; Teran, L.M. Chemokine (C-X-C Motif) Ligand 12/Stromal Cell-Derived Factor-1 Is Associated With Leukocyte Recruitment in Asthma. Chest 2010, 138, 100–106. [Google Scholar] [CrossRef]
- Caldenhoven, E.; van Dijk, T.B.; Tijmensen, A.; Raaijmakers, J.A.M.; Lammers, J.-W.J.; Koenderman, L.; de Groot, R.P. Differential Activation of Functionally Distinct STAT5 Proteins by IL-5 and GM-CSF During Eosinophil and Neutrophil Differentiation from Human CD34+ Hematopoietic Stem Cells. Stem Cells 1998, 16, 397–403. [Google Scholar] [CrossRef]
- Kagami, S.-I.; Nakajima, H.; Kumano, K.; Suzuki, K.; Suto, A.; Imada, K.; Davey, H.W.; Saito, Y.; Takatsu, K.; Leonard, W.J.; et al. Both Stat5a and Stat5b are required for antigen-induced eosinophil and T-cell recruitment into the tissue. Blood 2000, 95, 1370–1377. [Google Scholar] [CrossRef]
- Stout, B.A.; Bates, M.E.; Liu, L.Y.; Farrington, N.N.; Bertics, P.J. IL-5 and Granulocyte-Macrophage Colony-Stimulating Factor Activate STAT3 and STAT5 and Promote Pim-1 and Cyclin D3 Protein Expression in Human Eosinophils. J. Immunol. 2004, 173, 6409. [Google Scholar] [CrossRef]
| Experimental Group | Omega-6 PUFA/Omega-3 PUFA Ratio (95% CI) | p-Value * |
|---|---|---|
| Control Diet, Saline | 6.20: 1 (3.58:1–8.82:1) | -- |
| Control Diet, HDE | 7.38:1 (4.91:1–9.85:1) | 0.79 |
| DHA Diet, Saline | 2.34:1 (1.15:1–3.52:1) | 0.039 |
| DHA Diet, HDE | 2.36:1 (1.40:1–3.31:1) | 0.021 |
| Experimental Group | Number of Samples per Group | Differentially Regulated Genes | Total Number of Differentially Regulated Genes | |
|---|---|---|---|---|
| Up | Down | |||
| DHA vs. Control Diet | 20 | 3 | 3 | 6 |
| HDE vs. Saline | 20 | 94 | 16 | 110 |
| DHA vs. Control Diet Exposed to HDE | 13 | 6 | 1 | 7 |
| Gene Symbol | Fold Change In Each Data Set | |
|---|---|---|
| DHA vs. Control Diet | DHA vs. Control Diet, Exposed to HDE | |
| BCL3 | b Below Threshold | ↑ 2.24 |
| CFB | a ↑ 1.48 | ↑ 1.57 |
| CXCL12 | ↓ 1.41 | a ↓ 1.37 |
| ITGA6 | ↓ 1.52 | a ↓ 1.40 |
| ITGAX | ↑ 1.31 | ↑ 1.47 |
| LILRB3 | b Below Threshold | ↑ 1.59 |
| MARCO | b Below Threshold | ↑ 2.07 |
| PIGR | ↑ 1.44 | a ↑ 1.57 |
| STAT5B | ↓ 1.45 | ↓ 1.61 |
| TGFBI | ↑ 1.2 | ↑ 1.31 |
| Gene Symbol | Fold Change/Regulation | ||
|---|---|---|---|
| Control Diet + HDE | DHA Diet + Saline | DHA Diet + HDE | |
| CFB | ↑ 3.66 | a ↑ 1.32 | ↑ 5.77 |
| CXCL12 | a ↓ 1.35 | a ↓ 1.47 | ↓ 1.83 |
| ITGA6 | a ↓ 1.37 | a ↓ 1.74 | ↓ 1.93 |
| ITGAX | a ↑ 1.01 | a ↑ 1.07 | ↑ 1.47 |
| PIGR | ↑ 2.89 | a ↑ 1.21 | ↑ 4.54 |
| STAT5B | a ↑ 1.03 | a ↓ 1.20 | ↓ 1.56 |
| TGFBI | a ↓ 1.08 | a ↑ 1.02 | a ↑ 1.06 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dominguez, E.C.; Heires, A.J.; Pavlik, J.; Larsen, T.D.; Guardado, S.; Sisson, J.H.; Baack, M.L.; Romberger, D.J.; Nordgren, T.M. A High Docosahexaenoic Acid Diet Alters the Lung Inflammatory Response to Acute Dust Exposure. Nutrients 2020, 12, 2334. https://doi.org/10.3390/nu12082334
Dominguez EC, Heires AJ, Pavlik J, Larsen TD, Guardado S, Sisson JH, Baack ML, Romberger DJ, Nordgren TM. A High Docosahexaenoic Acid Diet Alters the Lung Inflammatory Response to Acute Dust Exposure. Nutrients. 2020; 12(8):2334. https://doi.org/10.3390/nu12082334
Chicago/Turabian StyleDominguez, Edward C., Art J. Heires, Jacqueline Pavlik, Tricia D. Larsen, Stephanie Guardado, Joseph H. Sisson, Michelle L. Baack, Debra J. Romberger, and Tara M. Nordgren. 2020. "A High Docosahexaenoic Acid Diet Alters the Lung Inflammatory Response to Acute Dust Exposure" Nutrients 12, no. 8: 2334. https://doi.org/10.3390/nu12082334
APA StyleDominguez, E. C., Heires, A. J., Pavlik, J., Larsen, T. D., Guardado, S., Sisson, J. H., Baack, M. L., Romberger, D. J., & Nordgren, T. M. (2020). A High Docosahexaenoic Acid Diet Alters the Lung Inflammatory Response to Acute Dust Exposure. Nutrients, 12(8), 2334. https://doi.org/10.3390/nu12082334





