Association between Biomarkers of Cardiovascular Diseases and the Blood Concentration of Carotenoids among the General Population without Apparent Illness
Abstract
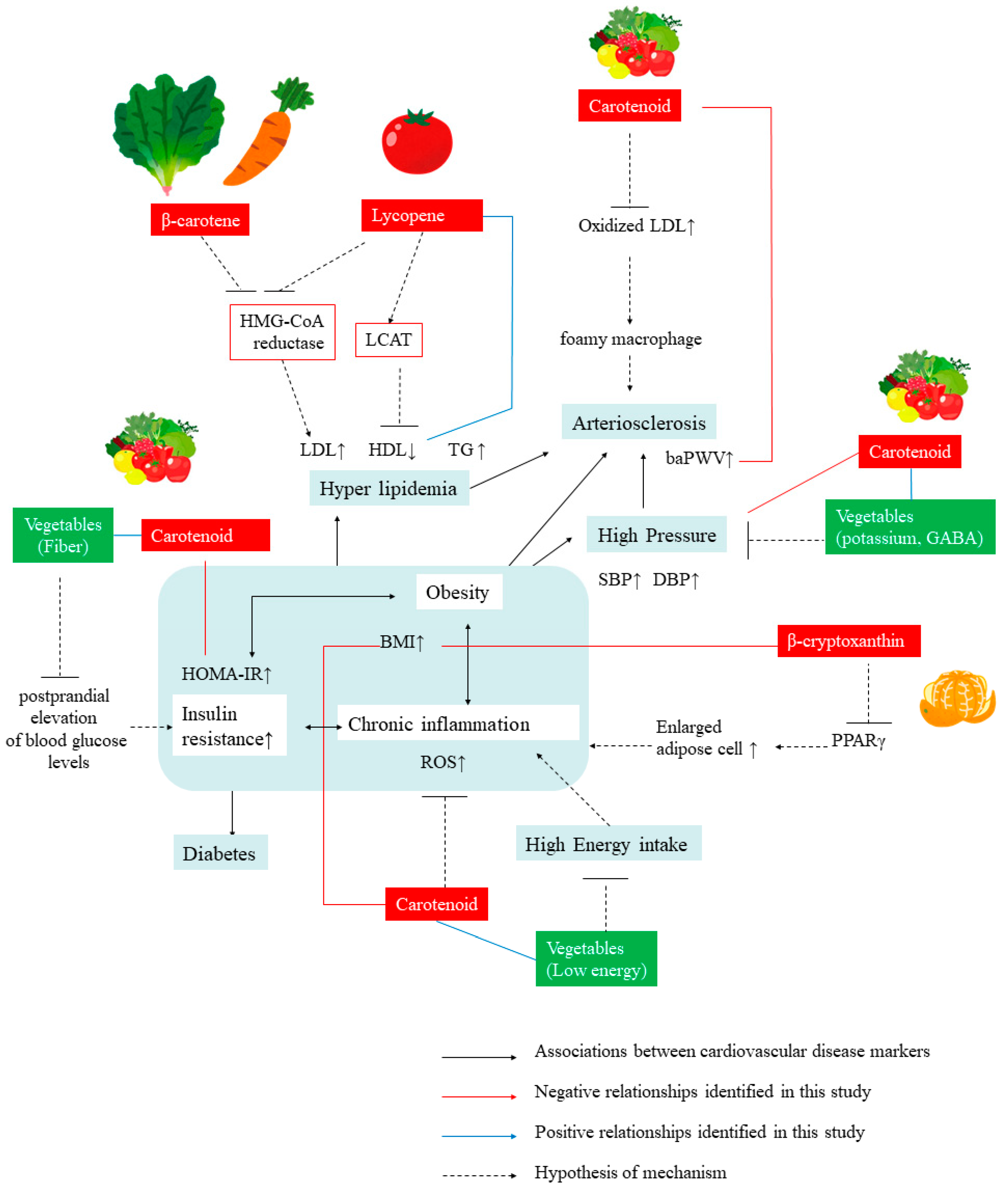
1. Introduction
2. Materials and Methods
2.1. Study Design and Subjects
2.2. Self-Administered Questionnaire
2.3. Body Measurements
2.4. Blood Sampling and Testing
2.5. Statistical Analyses
3. Results
3.1. Characteristics of the Study Subjects
3.2. Relationship between Serum Total Carotenoid Concentration and Vegetable Intake
3.3. Relationship between Serum Concentrations of Carotenoids and Markers of CVDs
3.4. Relationship between Individual Carotenoid Levels and Markers of CVDs
4. Discussion
4.1. Characteristics of the Study Subjects
4.2. Relationship between Serum Total Carotenoid Levels and Markers of CVDs
4.3. Relationship between Individual Carotenoid Levels and Markers of CVDs
4.4. Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shimazu, T.; Wakai, K.; Tamakoshi, A.; Tsuji, I.; Tanaka, K.; Matsuo, K.; Nagata, C.; Mizoue, T.; Inoue, M.; Tsugane, S.; et al. Association of vegetable and fruit intake with gastric cancer risk among Japanese: A pooled analysis of four cohort studies. Ann. Oncol. 2014, 25, 1228–1233. [Google Scholar] [CrossRef]
- Casari, I.; Falasca, M. Diet and Pancreatic Cancer Prevention. Cancers 2015, 7, 2309–2317. [Google Scholar] [CrossRef]
- Bhupathiraju, S.N.; Wedick, N.M.; Pan, A.; Manson, J.E.; Rexrode, K.M.; Willett, W.C.; Rimm, E.B.; Hu, F.B. Quantity and variety in fruit and vegetable intake and risk of coronary heart disease. Am. J. Clin. Nutr. 2013, 98, 1514–1523. [Google Scholar] [CrossRef] [PubMed]
- Takachi, R.; Inoue, M.; Ishihara, J.; Kurahashi, N.; Iwasaki, M.; Sasazuki, S.; Iso, H.; Tsubono, Y.; Tsugane, S. JPHC Study Group. Fruit and vegetable intake and risk of total cancer and cardiovascular disease: Japan Public Health Center-Based Prospective Study. Am. J. Epidemiol. 2008, 167, 59–70. [Google Scholar] [CrossRef]
- Oude Griep, L.M.; Verschuren, W.M.; Kromhout, D.; Ocké, M.C.; Geleijnse, J.M. Colors of fruit and vegetables and 10-year incidence of stroke. Stroke 2011, 42, 3190–3195. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.B.; Fan, J.H.; Dawsey, S.M.; Sinha, R.; Freedman, N.D.; Taylor, P.R.; Qiao, Y.L.; Abnet, C.C. Dietary components and risk of total, cancer and cardiovascular disease mortality in the Linxian Nutrition Intervention Trials cohort in China. Sci. Rep. 2016, 6, 22619. [Google Scholar] [CrossRef] [PubMed]
- Aune, D.; Giovannucci, E.; Boffetta, P.; Fadnes, L.T.; Keum, N.; Norat, T.; Greenwood, D.C.; Riboli, E.; Vatten, L.J.; Tonstad, S. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality-a systematic review and dose-response meta-analysis of prospective studies. Int. J. Epidemiol. 2017, 46, 1029–1056. [Google Scholar] [CrossRef] [PubMed]
- Britton, S.; Liaaen-Jensen, S.; Pfander, H. Carotenoids: Handbook; Birkhäuser: Boston, MA, USA, 2004. [Google Scholar]
- Stahl, W.; Schwarz, W.; Sundquist, A.R.; Sies, H. Cis-trans isomers of lycopene and beta-carotene in human serum and tissues. Arch. Biochem. Biophys. 1992, 294, 173–177. [Google Scholar] [CrossRef]
- Yeum, K.J.; Shang, F.M.; Schalch, W.M.; Russell, R.M.; Taylor, A. Fat-soluble Nutrient Concentrations in Different Layers of Human Cataractous Lens. Curr. Eye Res. 1999, 19, 502–505. [Google Scholar] [CrossRef]
- SanGiovanni, J.P.; Chew, E.Y.; Clemons, T.E.; Ferris, F.L., 3rd; Gensler, G.; Lindblad, A.S.; Milton, R.C.; Seddon, J.M.; Sperduto, R.D. The relationship of dietary carotenoid and vitamin A, E, and C intake with age-related macular degeneration in a case-control study: AREDS Report No. 22. Arch. Ophthalmol. 2007, 125, 1225–1232. [Google Scholar]
- Etminan, M.; Takkouche, B.; Caamaño-Isorna, F. The role of tomato products and lycopene in the prevention of prostate cancer: A meta-analysis of observational studies. Cancer Epidemiol. Biomark. Prev. 2004, 13, 340–345. [Google Scholar]
- Wang, Y.; Cui, R.; Xiao, Y.; Fang, J.; Xu, Q. Effect of Carotene and Lycopene on the Risk of Prostate Cancer: A Systematic Review and Dose-Response Meta-Analysis of Observational Studies. PLoS ONE 2015, 10, e0137427. [Google Scholar] [CrossRef]
- WHO/FAO. Diet., Nutrition and the Prevention of Chronic Diseases: Report of a Joint WHO/FAO Expert Consultation; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- Gammone, M.A.; Riccioni, G.; D’Orazio, N. Carotenoids: Potential allies of cardiovascular health? Food Nutr. Res. 2015, 59, 26762. [Google Scholar] [CrossRef] [PubMed]
- Campbell, D.R.; Gross, M.D.; Martini, M.C.; Grandits, G.A.; Slavin, J.L.; Potter, J.D. Plasma carotenoids as biomarkers of vegetable and fruit intake. Cancer Epidemiol. Prev. Biomark. 1994, 3, 493–500. [Google Scholar]
- Di Mascio, P.; Kaiser, S.; Sies, H. Lycopene as the Most Efficient Biological Carotenoid Singlet Oxygen Quencher. Arch. Biochem. Biophys. 1989, 274, 532–538. [Google Scholar] [CrossRef]
- Rimm, E.; Colditz, G. Smoking, Alcohol, and Plasma Levels of Carotenes and Vitamin E. Ann. Acad. Sci. 1993, 686, 323–333. [Google Scholar] [CrossRef]
- Kobayashi, S.; Honda, S.; Murakami, K.; Sasaki, S.; Okubo, H.; Hirota, N.; Notsu, A.; Fukui, M.; Date, C. Both comprehensive and brief self-administered diet history questionnaires satisfactorily rank nutrient intakes in Japanese adults. J. Epidemiol. 2012, 22, 151–159. [Google Scholar] [CrossRef]
- Ozato, N.; Saito, S.; Yamaguchi, T.; Katashima, M.; Tokuda, I.; Sawada, K.; Katsuragi, Y.; Imoto, S.; Ihara, K.; Nakaji, S. Association between Nutrients and Visceral Fat in Healthy Japanese Adults: A 2-Year Longitudinal Study Brief Title: Micronutrients Associated with Visceral Fat Accumulation. Nutrients 2019, 11, 2698. [Google Scholar] [CrossRef]
- Oshima, S.; Sakamoto, H.; Ishiguro, Y.; Terao, J. Accumulation and clearance of capsanthin in blood plasma after the ingestion of paprika juice in men. J. Nutr. 1997, 127, 1475–1479. [Google Scholar] [CrossRef]
- Aizawa, K.; Inakuma, T. Quantitation of carotenoids in commonly consumed vegetables in Japan. Food Sci. Technol. Res. 2007, 13, 247–252. [Google Scholar] [CrossRef][Green Version]
- Kawai, T.; Ohishi, M.; Ito, N.; Takeya, Y.; Maekawa, Y.; Rakugi, H. Cut-off Value of Brachial-Ankle Pulse Wave Velocity to Predict Cardiovascular Disease in Hypertensive Patients: A Cohort Study. J. Atheroscler. Thromb. 2013, 20, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Takashima, N.; Turin, T.C.; Matsui, K.; Rumana, N.; Nakamura, Y.; Kadota, A.; Saito, Y.; Sugihara, H.; Morita, Y.; Ichikawa, M.; et al. The Relationship of Brachial-Ankle Pulse Wave Velocity to Future Cardiovascular Disease Events in the General Japanese Population: The Takashima Study. J. Hum. Hypertens. 2014, 28, 323–327. [Google Scholar] [CrossRef] [PubMed]
- The Japanese Society of Hypertension. Guidelines for the Treatment of Hypertension; The Japanese Society of Hypertension: Tokyo, Japan, 2019; pp. 17–18. [Google Scholar]
- Ministry of Health, Labour and Welfare in Japan. National Health and Nutrition Survey; Ministry of Health, Labour and Welfare: Tokyo, Japan, 2017. [Google Scholar]
- Ito, Y.; Sasaki, R.; Ochiai, J.; Suzuki, S. Serum levels of fat-soluble vitamins in healthy pearsons. Med. Biol. 1990, 121, 303–308. [Google Scholar]
- Pennant, M.; Steur, M.; Moore, C.; Butterworth, A.; Johnson, L. Comparative validity of vitamin C and carotenoids as indicators of fruit and vegetable intake: A systematic review and meta-analysis of randomised controlled trials. Br. J. Nutr. 2015, 114, 1331–1340. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Suganuma, H.; Shimizu, S.; Hayashi, H.; Sawada, K.; Tokuda, I.; Ihara, K.; Nakaji, S. Skin Carotenoid Level as an Alternative Marker of Serum Total Carotenoid Concentration and Vegetable Intake Correlates with Biomarkers of Circulatory Diseases and Metabolic Syndrome. Nutrients 2020, 12, 1825. [Google Scholar] [CrossRef]
- Wang, X.; Ouyang, Y.; Liu, J.; Zhu, M.; Zhao, G.; Bao, W.; Hu, F.B. Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: Systematic review and dose-response meta-analysis of prospective cohort studies. BMJ 2014, 349, g4490. [Google Scholar] [CrossRef]
- Hu, D.; Huang, J.; Wang, Y.; Zhang, D.; Qu, Y. Fruits and vegetables consumption and risk of stroke: A meta-analysis of prospective cohort studies. Stroke 2014, 45, 1613–1619. [Google Scholar] [CrossRef]
- Maoka, T. Biological Functions of Carotenoids. Food Clin. Nutr. 2007, 2, 3–14. [Google Scholar]
- Wang, Y.; Chun, O.K.; Song, W.O. Plasma and dietary antioxidant status as cardiovascular disease risk factors: A review of human studies. Nutrients 2013, 5, 2969–3004. [Google Scholar] [CrossRef]
- Di Mascio, P.; Devasagayam, T.P.; Kaiser, S.; Sies, H. Carotenoids, tocopherols and thiols as biological singlet molecular oxygen quenchers. Biochem. Soc. Trans. 1990, 18, 1054–1056. [Google Scholar] [CrossRef]
- Liu, Z.; Ren, Z.; Zhang, J.; Chuang, C.C.; Kandaswamy, E.; Zhou, T.; Zuo, L. Role of ROS and Nutritional Antioxidants in Human Diseases. Front. Physiol. 2018, 9, 477. [Google Scholar] [CrossRef] [PubMed]
- Goncharov, N.V.; Nadeev, A.D.; Jenkins, R.O.; Avdonin, P.V. Markers and Biomarkers of Endothelium: When Something Is Rotten in the State. Oxid. Med. Cell Longev. 2017, 2017, 9759735. [Google Scholar] [CrossRef] [PubMed]
- Suganuma, H.; Inakuma, T. Protective effect of dietary tomato against endothelial dysfunction in hypercholesterolemic mice. Biosci. Biotechnol. Biochem. 1999, 63, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Tsitsimpikou, C.; Tsarouhas, K.; Kioukia-Fougia, N.; Skondra, C.; Fragkiadaki, P.; Papalexis, P.; Stamatopoulos, P.; Kaplanis, I.; Hayes, A.W.; Tsatsakis, A.; et al. Dietary supplementation with tomato-juice in patients with metabolic syndrome: A suggestion to alleviate detrimental clinical factors. Food Chem. Toxicol. 2014, 74, 9–13. [Google Scholar] [CrossRef]
- Ucci, M.; Di Tomo, P.; Tritschler, F.; Cordone, V.G.P.; Lanuti, P.; Bologna, G.; Di Silvestre, S.; Di Pietro, N.; Pipino, C.; Mandatori, D.; et al. Anti-inflammatory Role of Carotenoids in Endothelial Cells Derived from Umbilical Cord of Women Affected by Gestational Diabetes Mellitus. Oxid. Med. Cell Longev. 2019, 2019, 8184656. [Google Scholar] [CrossRef]
- Lu, M.; Gursky, O. Aggregation and fusion of low-density lipoproteins In Vivo and In Vitro. Biomol. Concepts 2013, 4, 501–518. [Google Scholar] [CrossRef]
- Cocate, P.G.; Natali, A.J.; Alfenas, R.C.; de Oliveira, A.; dos Santos, E.C.; Hermsdorff, H.H. Carotenoid consumption is related to lower lipid oxidation and DNA damage in middle-aged men. Br. J. Nutr. 2015, 114, 257–264. [Google Scholar] [CrossRef]
- Oshima, S.; Ojima, F.; Sakamoto, H.; Ishiguro, Y.; Terao, J. Supplementation with carotenoids inhibits singlet oxygen-mediated oxidation of human plasma low-density lipoprotein. J. Agric. Food Chem. 1996, 44, 2306–2309. [Google Scholar] [CrossRef]
- Ceriello, A.; Motz, E. Is oxidative stress the pathogenic mechanism underlying insulin resistance, diabetes, and cardiovascular disease? The common soil hypothesis revisited. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 816–823. [Google Scholar] [CrossRef]
- Ebihara, K. Studies of Nutritional and Physiological Effects of Dietary Fiber. J. Jpn. Soc. Nutr. Food Sci. 2008, 61, 3–9. [Google Scholar] [CrossRef]
- Nonaka, S.; Arai, C.; Takayama, M.; Matsukura, C.; Ezura, H. Efficient increase of ɣ-aminobutyric acid (GABA) content in tomato fruits by targeted mutagenesis. Sci. Rep. 2017, 7, 7057. [Google Scholar] [CrossRef] [PubMed]
- Utsugi, M.T.; Ohkubo, T.; Kikuya, M.; Kurimoto, A.; Sato, R.I.; Suzuki, K.; Metoki, H.; Hara, A.; Tsubono, Y.; Imai, Y. Fruit and vegetable consumption and the risk of hypertension determined by self measurement of blood pressure at home: The Ohasama study. Hypertens. Res. 2008, 31, 1435–1443. [Google Scholar] [CrossRef] [PubMed]
- Ha, S.; Choi, H.R.; Lee, Y.H. Clustering of four major lifestyle risk factors among Korean adults with metabolic syndrome. PLoS ONE 2017, 12, e0174567. [Google Scholar] [CrossRef] [PubMed]
- Van Gaal, L.F.; Mertens, I.L.; De Block, C.E. Mechanisms linking obesity with cardiovascular disease. Nature 2006, 444, 875–880. [Google Scholar] [CrossRef]
- Rebuffé-Scrive, M.; Eldh, J.; Hafström, L.O.; Björntorp, P. Metabolism of mammary, abdominal, and femoral adipocytes in female before and after menopause. Metabolism 1986, 35, 792–797. [Google Scholar] [CrossRef]
- Heinonen, M.I.; Ollilainen, V.; Linkola, E.K.; Varo, P.T.; Koivistoinen, P.E. Carotenoids in Finnish Foods: Vegetables, Fruits, and Berries. J. Agric. Food Chem. 1989, 37, 655–659. [Google Scholar] [CrossRef]
- Romanchik, J.E.; Morel, D.W.; Harrison, E.H. Distributions of carotenoids and alpha-tocopherol among lipoproteins do not change when human plasma is incubated in vitro. J. Nutr. 1995, 125, 2610–2617. [Google Scholar] [CrossRef]
- McEneny, J.; Wade, L.; Young, I.S.; Masson, L.; Duthie, G.; McGinty, A.; McMaster, C.; Thies, F. Lycopene intervention reduces inflammation and improves HDL functionality in moderately overweight middle-aged individuals. J. Nutr. Biochem. 2013, 24, 163–168. [Google Scholar] [CrossRef]
- Navarro-González, I.; Pérez-Sánchez, H.; Martín-Pozuelo, G.; García-Alonso, J.; Periago, M.J. The inhibitory effects of bioactive compounds of tomato juice binding to hepatic HMGCR: In vivo study and molecular modelling. PLoS ONE 2014, 9, e83968. [Google Scholar] [CrossRef]
- Blum, A.; Merei, M.; Karem, A.; Blum, N.; Ben-Arzi, S.; Wirsansky, I.; Khazim, K. Effects of tomatoes on the lipid profile. Clin. Investig. Med. 2006, 29, 298–300. [Google Scholar]
- Sugiura, M.; Nakamura, M.; Ikoma, Y.; Yano, M.; Ogawa, K.; Matsumoto, H.; Kato, M.; Ohshima, M.; Nagao, A. The homeostasis model assessment-insulin resistance index is inversely associated with serum carotenoids in non-diabetic subjects. J. Epidemiol. 2006, 16, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Sugiura, M.; Ogawa, K.; Ikoma, Y.; Yano, M. Serum β-cryptoxanthin and β-carotene derived from Satsuma mandarin and brachial-ankle pulse wave velocity: The Mikkabi cohort study. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 808–814. [Google Scholar] [CrossRef] [PubMed]
- Sahin, K.; Orhan, C.; Akdemir, F.; Tuzcu, M.; Sahin, N.; Yılmaz, I.; Juturu, V. β-Cryptoxanthin ameliorates metabolic risk factors by regulating NF-κB and Nrf2 pathways in insulin resistance induced by high-fat diet in rodents. Food Chem. Toxicol. 2017, 107, 270–279. [Google Scholar] [CrossRef]
- Shawky, N.M.; Pichavaram, P.; Shehatou, G.S.; Suddek, G.M.; Gameil, N.M.; Jun, J.Y.; Segar, L. Sulforaphane improves dysregulated metabolic profile and inhibits leptin-induced VSMC proliferation: Implications toward suppression of neointima formation after arterial injury in western diet-fed obese mice. J. Nutr. Biochem. 2016, 32, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Kamon, J.; Yamauchi, T.; Terauchi, Y.; Kubota, N.; Kadowaki, T. The mechanisms by which PPARgamma and adiponectin regulate glucose and lipid metabolism. Nihon Yakurigaku Zasshi 2003, 122, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Shirakura, Y.; Takayanagi, K.; Mukai, K.; Tanabe, H.; Inoue, M. β-cryptoxanthin suppresses the adipogenesis of 3T3-L1 cells via RAR activation. J. Nutr. Sci. Vitaminol. 2011, 57, 426–431. [Google Scholar] [CrossRef]
- Miyaki, K.; Song, Y.; Taneichi, S.; Tsutsumi, A.; Hashimoto, H.; Kawakami, N.; Takahashi, M.; Shimazu, A.; Inoue, A.; Kurioka, S.; et al. Socioeconomic Status Is Significantly Associated with the Dietary Intakes of Folate and Depression Scales in Japanese Workers (J-HOPE Study). Nutrients 2013, 5, 565–578. [Google Scholar] [CrossRef]
| Measurement Item | Male | Female | |||||||
|---|---|---|---|---|---|---|---|---|---|
| All | Young (20–39 Years) | Middle-aged (40–59 Years) | Old (≥ 60 Years) | All | Young (20–39 Years) | Middle-aged (40–59 Years) | Old (≥ 60 years) | ||
| Number of samples | N | 538 | 195 | 192 | 151 | 812 | 254 | 292 | 263 |
| Basic markers | Age, year | 48.2 ± 15.5 | 32 ± 5.1 | 48.8 ± 5.7 a | 68.2 ± 6.2 a, b | 49.8 ± 15.9 | 31.3 ± 5.3 | 49.6 ± 5.9 a | 68.1 ± 6.2 a, b |
| Current smoking, % | 34 | 42.6 | 38 | 17.9 a, b | 11.8 *** | 12.6 *** | 17.8 *** | 4.6 a, b *** | |
| Habitual exercise, % | 11 | 12.8 | 9.9 | 9.9 | 7.02 * | 6.3 * | 6.5 | 8.4 | |
| Alcohol intake, g/day | 23.5 ± 25.9 | 21 ± 25.06 | 26.07 ± 27.36 | 23.41 ± 24.72 | 4.91 ± 11.4 *** | 5.17 ± 12.49 *** | 6.76 ± 12.63 *** | 2.51 ± 7.85 a, b *** | |
| Antihypertensive use, % | 17.1 | 0.5 | 13.0 a | 43.7 a, b | 18.0 | 0 | 9.6 a | 44.9 a, b | |
| Biomarkers | BMI, kg/m2 | 23.6 ± 3.37 | 23.39 ± 4 | 24.01 ± 2.9 a | 23.45 ± 3 | 22.00 ± 3.51 *** | 20.83 ± 3.46 *** | 22.6 ± 3.38 a *** | 22.91 ± 3.39 a, b * |
| baPWV, cm/s | 1460.00 ± 353.00 | 1239.86 ± 167.98 | 1386.56 ± 195.81 a | 1837.81 ± 385.63 a, b | 1350.00 ± 346.00 *** | 1068.73 ± 122.56 *** | 1294.01 ± 248.90 a *** | 1685.72 ± 306.02 a, b *** | |
| SBP, mmHg | 126.00 ± 16.80 | 119.96 ± 13.37 | 124.68 ± 17.2 a | 133.74 ± 16.96 a, b | 118.00 ± 18.50 *** | 107.86 ± 12.66 *** | 117.31 ± 18.49 a *** | 128.57 ± 17.57 a, b ** | |
| DBP, mmHg | 78.3 ± 12.5 | 74.41 ± 11.18 | 80.4 ± 13.22 a | 80.6 ± 11.83 a | 71.6 ± 11.3 *** | 66.61 ± 9.33 *** | 73.34 ± 12.35 a *** | 74.55 ± 10.27 a *** | |
| HOMA-IR | 1.05 ± 1.04 | 1.07 ± 1.03 | 1.13 ± 1.31 | 0.93 ± 0.55 | 0.98 ± 0.63 | 0.93 ± 0.53 | 0.93 ± 0.63 * | 1.08 ± 0.7 a, b * | |
| Blood insulin, µU/mL | 4.79 ± 3.32 | 5.12 ± 4.21 | 4.97 ± 3 | 4.13 ± 2.14 b | 4.73 ± 2.53 | 4.79 ± 2.57 | 4.46 ± 2.41 | 4.93 ± 2.53 b *** | |
| FBG, mg/dL | 85.8 ± 17.4 | 82.53 ± 15.4 | 86.02 ± 20.02 a | 89.86 ± 15.21 a, b | 82.1 ± 11.2 *** | 77.48 ± 8.31 *** | 82.02 ± 11.77 a ** | 86.57 ± 11.25 a, b * | |
| Triglyceride, mg/dL | 121 ± 91.7 | 110.28 ± 93.59 | 135.17 ± 82.58 a | 115.36 ± 98.35 b | 76.5 ± 42.8 *** | 62.93 ± 38.96 *** | 77.46 ± 40.5 a *** | 88.47 ± 45.18 a, b ** | |
| HDL-cholesterol, mg/dL | 59.9 ± 16.5 | 58.3 ± 15.42 | 60.18 ± 17.15 | 61.62 ± 16.87 | 70.3 ± 16.3 *** | 69.7 ± 15.14 *** | 72.61 ± 17.8 *** | 68.46 ± 15.37 b *** | |
| BDHQ | Total vegetable, g/day | 170.00 ± 108.00 | 150.74 ± 97.36 | 169.03 ± 99.15 | 196.3 ± 126.91 a | 180.00 ± 111.00 * | 153.64 ± 94.40 | 178.26 ± 100.42 a | 209.83 ± 129.2 a, b |
| Carotenoids | Total carotenoid, µg/mL | 1.1 ± 0.529 | 1.016 ± 0.448 | 1.131 ± 0.558 | 1.182 ± 0.572 a | 1.57 ± 0.713 *** | 1.363 ± 0.607 *** | 1.573 ± 0.684 a *** | 1.777 ± 0.781 a, b *** |
| Lutein, µg/mL | 0.287 ± 0.136 | 0.236 ± 0.096 | 0.294 ± 0.129 a | 0.345 ± 0.16 a, b | 0.333 ± 0.15 *** | 0.265 ± 0.108 ** | 0.332 ± 0.144 a ** | 0.403 ± 0.16 a, b *** | |
| Zeaxanthin, µg/mL | 0.061 ± 0.022 | 0.059 ± 0.021 | 0.064 ± 0.022 | 0.059 ± 0.023 | 0.0618 ± 0.0239 | 0.06 ± 0.023 | 0.063 ± 0.022 | 0.063 ± 0.026 | |
| β-Cryptoxanthin, µg/mL | 0.104 ± 0.059 | 0.093 ± 0.044 | 0.099 ± 0.056 | 0.123 ± 0.073 a, b | 0.163 ± 0.102 *** | 0.129 ± 0.062 *** | 0.158 ± 0.099 a *** | 0.203 ± 0.121 a, b *** | |
| α-Carotene, µg/mL | 0.127 ± 0.147 | 0.120 ± 0.125 | 0.138 ± 0.178 | 0.122 ± 0.127 | 0.183 ± 0.149 *** | 0.17 ± 0.153 *** | 0.183 ± 0.136 a *** | 0.194 ± 0.157 a *** | |
| β-Carotene, µg/mL | 0.279 ± 0.258 | 0.232 ± 0.207 | 0.278 ± 0.279 | 0.343 ± 0.277 a, b | 0.561 ± 0.404 *** | 0.434 ± 0.317 *** | 0.544 ± 0.362 a *** | 0.705 ± 0.475 a, b *** | |
| Lycopene, µg/mL | 0.247 ± 0.142 | 0.276 ± 0.142 | 0.258 ± 0.137 | 0.194 ± 0.134 a, b | 0.27 ± 0.147 *** | 0.304 ± 0.137 ** | 0.292 ± 0.15 ** | 0.21 ± 0.135 a, b | |
| Biomarkers | Pattern 1 | Pattern 2 | Pattern 3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All | 20–39 | 40–59 | 60– | All | 20–39 | 40–59 | 60– | All | 20–39 | 40–59 | 60– | |
| BMI | −0.060 | −0.037 | −0.179 * | 0.008 | −0.054 | −0.003 | −0.218 * | 0.032 | ||||
| baPWV | −0.114 *** | −0.259 ** | −0.169 * | −0.100 | −0.121 *** | −0.248 ** | −0.168 * | −0.092 | −0.119 *** | −0.248 ** | −0.162 * | −0.091 |
| SBP | −0.177 *** | −0.140 | −0.197 * | −0.167 | −0.178 *** | −0.158 | −0.206 * | −0.155 | −0.163 *** | −0.157 | −0.148 | −0.157 |
| DBP | −0.157 ** | −0.073 | −0.147 | −0.237 ** | −0.135 ** | −0.056 | −0.130 | −0.232 ** | −0.119 * | −0.055 | −0.077 | −0.235 ** |
| HOMA−IR | −0.129 ** | −0.164 | −0.047 | −0.095 | −0.135 ** | −0.139 | −0.052 | −0.098 | −0.102 * | −0.186 ** | 0.039 | −0.117 |
| Insulin | −0.104 * | −0.158 | −0.072 | −0.096 | −0.109 * | −0.156 | −0.089 | −0.096 | −0.080 * | −0.139 * | 0.014 | −0.123 |
| FBG | −0.055 | −0.202 ** | 0.004 | −0.025 | −0.025 | −0.182 * | 0.006 | −0.006 | −0.007 | −0.182 * | 0.033 | 0.017 |
| TG | −0.212 *** | −0.252 ** | −0.182 * | −0.262 ** | −0.201 *** | −0.230 * | −0.201 * | −0.257 ** | −0.187 *** | −0.229 ** | −0.158 | −0.262 ** |
| HDL cholesterol | 0.172 *** | 0.191 * | 0.212 * | 0.169 | 0.220 *** | 0.180 * | 0.297 *** | 0.200 * | 0.198 *** | 0.179 * | 0.264 ** | 0.191 * |
| Biomarkers | Pattern 1 | Pattern 2 | Pattern 3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All | 20–39 | 40–59 | 60– | All | 20–39 | 40–59 | 60– | All | 20–39 | 40–59 | 60– | |
| BMI | −0.235 *** | −0.233 *** | −0.268 *** | −0.243 *** | −0.238 *** | −0.254 *** | −0.266 *** | −0.239 *** | ||||
| baPWV | −0.065 ** | −0.137 * | −0.090 | −0.077 | −0.088 *** | −0.135 * | −0.128 * | −0.101 | −0.091 *** | −0.101 | −0.112 | −0.134 * |
| SBP | −0.119 *** | −0.186 ** | −0.176 ** | −0.047 | −0.134 *** | −0.190 ** | −0.195 ** | −0.075 | −0.071 * | −0.067 | −0.126 * | −0.048 |
| DBP | −0.069 | −0.124 | −0.122 * | −0.034 | −0.066 | −0.112 | −0.159 * | −0.032 | −0.006 | −0.038 | −0.102 | 0.017 |
| HOMA−IR | −0.228 *** | −0.231 *** | −0.254 *** | −0.184 ** | −0.252 *** | −0.260 *** | −0.280 *** | −0.203 ** | −0.163 *** | −0.155 * | −0.171 ** | −0.102 |
| Insulin | −0.228 *** | −0.221 *** | −0.275 *** | −0.172 * | −0.259 *** | −0.249 *** | −0.292 *** | −0.199 ** | −0.158 *** | −0.141 * | −0.151 ** | −0.096 |
| FBG | −0.134 *** | −0.142 * | −0.200 *** | −0.075 | −0.132 *** | −0.142 * | −0.189 *** | −0.098 | −0.102 ** | −0.102 | −0.159 ** | −0.054 |
| TG | −0.162 *** | −0.033 | −0.234 *** | −0.242 *** | −0.150 *** | −0.028 | −0.229 *** | −0.258 *** | −0.096 * | 0.016 | −0.156 * | −0.218 ** |
| HDL cholesterol | 0.303 *** | 0.243 *** | 0.281 *** | 0.329 *** | 0.319 *** | 0.252 *** | 0.291 *** | 0.348 *** | 0.247 *** | 0.162 * | 0.208 *** | 0.283 *** |
| Biomarkers | Pattern 1 | Pattern 2 | Pattern 3 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total Carotenoid (Repeated) | Lutein | Zeaxanthin | β-Cryptoxanthin | β-Carotene | Lycopene | Total Carotenoid (Repeated) | Lutein | Zeaxanthin | β-Cryptoxanthin | β-Carotene | Lycopene | Total Carotenoid (Repeated) | Lutein | Zeaxanthin | β−Cryptoxanthin | β−Carotene | Lycopene | |
| BMI | −0.06 | −0.087 | 0.012 | −0.044 | −0.05 | 0.026 | −0.054 | −0.083 | 0.014 | −0.046 | −0.037 | 0.042 | ||||||
| baPWV | −0.114 *** | −0.002 | −0.013 | −0.05 | −0.112 *** | −0.079 ** | −0.121 *** | 0.002 | −0.012 | −0.056 | −0.124 *** | −0.081 ** | −0.119 *** | 0.004 | −0.011 | −0.055 | −0.123 *** | −0.081 ** |
| SBP | −0.177 *** | −0.072 | −0.012 | −0.137 *** | −0.204 *** | −0.117 ** | −0.178 *** | −0.075 | −0.011 | −0.159 *** | −0.214 *** | −0.119 ** | −0.163 *** | −0.055 | −0.011 | −0.148 *** | −0.206 *** | −0.128 ** |
| DBP | −0.157 ** | −0.003 | 0.045 | −0.180 *** | −0.224 *** | −0.078 | −0.135 ** | −0.010 | 0.042 | −0.165 *** | −0.185 *** | −0.063 | −0.119 * | 0.014 | 0.044 | −0.153 *** | −0.176 *** | −0.072 |
| HOMA−IR | −0.129 ** | −0.166 *** | −0.043 | −0.094 * | −0.137 ** | 0.001 | −0.135 ** | −0.158 *** | −0.035 | −0.121 * | −0.173 *** | 0.001 | −0.102 * | −0.148 *** | −0.054 | −0.122 ** | −0.142 *** | −0.074 * |
| Insulin | −0.104 * | −0.191 *** | −0.06 | −0.096 * | −0.126 ** | −0.024 | −0.109 * | −0.176 *** | −0.046 | −0.129 ** | −0.171 *** | −0.028 | −0.080 * | −0.129 *** | −0.057 | −0.105 ** | −0.150 *** | −0.054 |
| FBG | −0.055 | −0.047 | 0.028 | −0.041 | −0.062 | −0.01 | −0.025 | −0.054 | 0.021 | −0.027 | −0.042 | 0.002 | −0.007 | −0.033 | 0.021 | −0.014 | −0.032 | −0.006 |
| TG | −0.212 *** | −0.021 | 0.079 | −0.118 ** | −0.219 *** | 0.01 | −0.201 *** | −0.022 | 0.063 | −0.075 | −0.169 *** | 0.019 | −0.187 *** | 0 | 0.062 | −0.063 | −0.160 *** | 0.009 |
| HDL cholesterol | 0.172 *** | 0.319 *** | 0.233 *** | 0.155 *** | 0.041 | 0.161 *** | 0.220 *** | 0.273 *** | 0.191 *** | 0.202 *** | 0.091 * | 0.183 *** | 0.198 *** | 0.248 *** | 0.190 *** | 0.186 *** | 0.078 | 0.194 *** |
| Biomarker | Pattern 1 | Pattern 2 | Pattern 3 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total Carotenoid (Repeated) | Lutein | Zeaxanthin | β-Cryptoxanthin | β-Carotene | Lycopene | Total Carotenoid (repeated) | Lutein | Zeaxanthin | β-Cryptoxanthin | β-Carotene | Lycopene | Total Carotenoid (Repeated) | Lutein | Zeaxanthin | β-Cryptoxanthin | β-Carotene | Lycopene | |
| BMI | −0.235 *** | −0.250 *** | −0.125 *** | −0.137 *** | −0.229 *** | −0.049 | −0.238 *** | −0.251 *** | −0.121 *** | −0.144 *** | −0.238 *** | −0.053 | ||||||
| baPWV | −0.065 ** | −0.018 | 0.001 | −0.047 * | −0.090 *** | −0.054 * | −0.088 *** | −0.039 | −0.015 | −0.055 * | −0.110 *** | −0.068 ** | −0.091 *** | −0.040 | −0.015 | −0.057 * | −0.115 *** | −0.067 ** |
| SBP | −0.119 *** | −0.090 ** | −0.019 | −0.080 * | −0.152 *** | −0.064 * | −0.134 *** | −0.102 ** | −0.024 | −0.082 * | −0.168 *** | −0.056 | −0.071 * | −0.034 | 0.01 | −0.040 | −0.106 ** | −0.039 |
| DBP | −0.069 | −0.032 | 0.064 | −0.038 | −0.131 *** | 0.031 | −0.066 | −0.044 | 0.045 | −0.029 | −0.119 ** | 0.034 | −0.006 | 0.022 | 0.076 * | 0.01 | −0.063 | 0.05 |
| HOMA−IR | −0.228 *** | −0.243 *** | −0.134 *** | −0.077 * | −0.249 *** | −0.032 | −0.252 *** | −0.246 *** | −0.132 *** | −0.087 * | −0.278 *** | −0.043 | −0.163 *** | −0.141 *** | −0.080 * | −0.024 | −0.183 *** | −0.021 |
| Insulin | −0.228 *** | −0.234 *** | −0.128 *** | −0.080 * | −0.247 *** | −0.029 | −0.259 *** | −0.236 *** | −0.123 *** | −0.092 * | −0.282 *** | −0.041 | −0.158 *** | −0.118 *** | −0.065 * | −0.024 | −0.176 *** | −0.017 |
| FBG | −0.134 *** | −0.192 *** | −0.105 ** | −0.063 | −0.180 *** | −0.017 | −0.132 *** | −0.214 *** | −0.111 *** | −0.047 | −0.164 *** | −0.025 | −0.102 ** | −0.180 *** | −0.095 ** | −0.018 | −0.128 *** | −0.012 |
| TG | −0.162 *** | −0.123 *** | −0.057 | −0.092 * | −0.196 *** | 0.025 | −0.150 *** | −0.103 ** | −0.049 | −0.049 | −0.182 *** | 0.023 | −0.096 * | −0.036 | −0.019 | −0.010 | −0.128 *** | 0.029 |
| HDL cholesterol | 0.303 *** | 0.462 *** | 0.376 *** | 0.159 *** | 0.207 *** | 0.189 *** | 0.319 *** | 0.435 *** | 0.345 *** | 0.170 *** | 0.240 *** | 0.191 *** | 0.247 *** | 0.365 *** | 0.307 *** | 0.119 *** | 0.162 *** | 0.170 *** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsumoto, M.; Waki, N.; Suganuma, H.; Takahashi, I.; Kurauchi, S.; Sawada, K.; Tokuda, I.; Misawa, M.; Ando, M.; Itoh, K.; et al. Association between Biomarkers of Cardiovascular Diseases and the Blood Concentration of Carotenoids among the General Population without Apparent Illness. Nutrients 2020, 12, 2310. https://doi.org/10.3390/nu12082310
Matsumoto M, Waki N, Suganuma H, Takahashi I, Kurauchi S, Sawada K, Tokuda I, Misawa M, Ando M, Itoh K, et al. Association between Biomarkers of Cardiovascular Diseases and the Blood Concentration of Carotenoids among the General Population without Apparent Illness. Nutrients. 2020; 12(8):2310. https://doi.org/10.3390/nu12082310
Chicago/Turabian StyleMatsumoto, Mai, Naoko Waki, Hiroyuki Suganuma, Ippei Takahashi, Sizuka Kurauchi, Kahori Sawada, Itoyo Tokuda, Mina Misawa, Masataka Ando, Ken Itoh, and et al. 2020. "Association between Biomarkers of Cardiovascular Diseases and the Blood Concentration of Carotenoids among the General Population without Apparent Illness" Nutrients 12, no. 8: 2310. https://doi.org/10.3390/nu12082310
APA StyleMatsumoto, M., Waki, N., Suganuma, H., Takahashi, I., Kurauchi, S., Sawada, K., Tokuda, I., Misawa, M., Ando, M., Itoh, K., Ihara, K., & Nakaji, S. (2020). Association between Biomarkers of Cardiovascular Diseases and the Blood Concentration of Carotenoids among the General Population without Apparent Illness. Nutrients, 12(8), 2310. https://doi.org/10.3390/nu12082310





