Abstract
Autism spectrum disorder (ASD) is a group of dysfunctions in social interaction, communication, and behaviors. The etiology of ASD is not yet fully understood; however, it consists of the interaction between genetics and the environment. An increasing amount of evidence points to the possibility that gestational and early-childhood vitamin D deficiency may be involved in the etiology of some cases of ASD. Herein, we systematically review the literature for studies on vitamin D status during pregnancy and ASD outcomes. Forty-three studies in the PubMed and 124 studies in EMBASE databases were initially found. After screening, 26 were identified as candidate studies for inclusion. Finally, 14 articles met the inclusion criteria, which originated from nine countries. The studies included 10 original research studies and four review studies conducted between 2012 and 2020. The strength of evidence that vitamin D levels during pregnancy increase the risk of developing autism is very low. This is because the evidence relies exclusively on observational studies that did not equally consider all important confounders and that assessed the indirect relationship between vitamin D as a surrogate for sunlight exposure and autism risk. The findings of this systematic review are consistent with the hypothesis that low vitamin D levels might contribute to the development of autism. However, we must also recognize the possible confusion bias and therefore experimental studies with very large sample sizes, given incidence of autism, that allow us to detect blood levels in pregnant women would be helpful to clarify this point.
1. Introduction
As described in the DSM-V, autism spectrum disorder (ASD) is a heterogeneous neurodevelopmental condition affecting an individual’s ability to socialize and communicate [1]. ASD often presents with repetitive movements or behaviors, restricted interests, and abnormality in sensory processing [2]. The prevalence of ASD has risen dramatically over the last several decades, from 1 in 5000 children in 1975 to 1 in 45 children in 2014 in the United States. That trend shows a 700% increase within 40 years [3]. However, the cause of that rapid growth is not fully known. True increase in incidence of autism cannot be ruled out and constitutes an “urgent public health concern” [4,5]. Despite considerable effort to determine the etiological factors of ASD, no single or definitive cause has been identified [6]. The etiology of ASD is unknown, although the interaction of genetic and environmental factors is believed to play a fundamental role in the process [7]. Biological factors possibly associated with autism risk include hereditary risk factors [8]. Despite early evidence of heredity of autism, the largest population-based study of twins with autism is that adaptation rates for fraternal twins are higher than previously reported and that the shared prenatal environment constitutes a greater proportion of the risk of autism in twins [9,10,11]. ASD has been tentatively associated with >440 identified gene variants. Of those cases that can be clearly linked to genetic causes, 7–20% can be accounted for by copy number variants, 5–7% are attributed to single-nucleotide polymorphisms, and <5% are linked to genes involved in rare metabolic disorders [12]. Thus, nearly 70% of cases have a cause that has not been linked to genetics [10]. Lifestyle and environmental factors such as nutrition [13,14], toxic substances [15,16], medications [17,18], maternal infections during pregnancy [19,20], vaccine immunization [21], and stress [22] have been extensively studied. In particular, the factors that cause autism—air pollution, cloudy weather, seasonal factors, migration of dark-skinned immigrants to poleward latitudes, birth order, gestational diabetes, preeclampsia, cesarean delivery, autoimmune disease in the family, and nutrition—are all associated with vitamin D [23,24]. To give an example through studies, gestational vitamin D deficiency was associated with a 2.66-fold higher risk in developing gestational diabetes [25] and in utero exposure to gestational diabetes mellitus, giving the child a 4.4 adjusted odds ratio (aOR) for ASD [26]. Birth via cesarean delivery increases the risk of autism [27], and low maternal vitamin D increases the risk of having to give birth via C-sections up to fourfold [28]. A 2009 study in Dermato-Endocrinology examined whether maternal vitamin D deficiency is a risk factor for infantile autism disease (IAD) [29]. In the study using epidemiological data on seasonal variation of birth rates and prevalence of IAD for cohorts born before 1985, the researchers found a strong effective latitudinal (related to wintertime solar UVB radiation) increase in IAD prevalence. Those findings are consistent with the hypothesis that maternal vitamin D deficiency is a risk factor for IAD. Another study examined ASD trends by considering birth data in Denmark, Finland, Norway, Sweden, and Western Australia [30]. The data showed a modest increase in risk for children born in the fall and a modest decrease for children born in the spring. The researchers concluded that the risk was highest for fall births and lowest for spring births, and sunlight levels during critical neurodevelopmental periods explained much of the seasonal trends and are consistent with the hypothesis that a seasonally fluctuating risk factor may influence risk of ASD. In line with several previous studies, an association was apparent between ASD and season of births [31,32,33,34,35]. Moreover, a 2013 ecological study reported that autism prevalence among those aged 6–17 y in 2010 was significantly inversely correlated with solar UVB doses [36]. Those results add to the evidence that vitamin D deficiency may be an important risk factor for autism. In 2008, Cannell proposed the autism–vitamin D theory [37], in which the primary environmental trigger for autism is the lack of vitamin D in pregnancy and early childhood.
Vitamin D metabolism and homeostasis during pregnancy is significantly different than that found outside of pregnancy in order to meet the demands of an optimal intrauterine environment for correct embryonic development and a successful, uncomplicated delivery as well as the immune regulation of the pregnancy [38,39,40]. Pregnancy is characterized by three major adaptations in vitamin D homeostasis that can be seen at both the systemic circulation and placental level; an increase in maternal calcitriol levels, changes in maternal 25(OH)D availability which an affect neonatal 25(OH)D status, and an increase of maternal vitamin D-binding protein concentrations [39]. Calcitriol increases by 2/3 times in the first weeks of pregnancy whereas maternal 25(OH)D crosses the placental barrier and represents the main pool of vitamin D in the fetus and maternal hypovitaminosis D during pregnancy may result in impaired neonatal 25(OH)D status at birth [39]. A limited number of studies have shown changes of vitamin D-binding protein concentrations during pregnancy, with highest concentrations reaching a 40–50% increase compared to non-pregnant women and with levels peaking at the beginning of the third trimester before starting to decrease at term [39]. In regard to immune regulation, the need for immunological tolerance of the mother towards the fetus is a key factor in the changes in vitamin D metabolism that appear during pregnancy [39]. During pregnancy, vitamin D receptor and regulatory metabolic enzymes are expressed in the placenta and decidua, indicating a potential critical point in the immunomodulation at the maternal–fetal interface [39]. Considering these effects, maternal hypovitaminosis D during pregnancy has been associated with pregnancy related disorders and adverse pregnancy outcomes [39].
In recent years, evidence has linked a lack of vitamin D not only to its known effects on calcium and bone metabolism but also to neurocognitive decline [41]. Adequate intake of vitamin D appears essential to global physical and mental health, as suggested by evidence that vitamin D deficiency can be associated with several diseases, such as infections, asthma, inflammatory bowel diseases, obesity, metabolic syndrome, and neuropsychiatric manifestations, including ASD [42]. About 90% of vitamin D is produced in the epidermis from 7-dehydrocholesterol as a reaction to sunlight (solar UVB radiation; 290–315 nm) [43]. Factors that limit the cutaneous production of vitamin D3 include higher latitude, covering of skin, lack of outdoor activities, sunscreen use, old age, female sex, and darker skin pigmentation [44]. In an assessment derived from published studies, Holick and colleagues [43] estimated that due mainly to lack of sunlight exposure, approximately 1 billion people worldwide have inadequate vitamin D levels (as defined by level of 25-hydroxyvitamin D [25(OH)D], the primary circulating form of vitamin D in the serum, of <75 nmol/L). In addition to sunlight, another important source of vitamin D is nutrition. People living in regions where sunlight is reduced, such as in northern Europe, need to include in their diets vitamin D-rich foods such as fatty fish or vitamin D-fortified foods [44].
Because it comes from solar UV radiation and exerts endocrine and autoendocrine effects, vitamin D is known as the sunshine hormone [45]. Therefore, the possible mechanisms of action of vitamin D, which is thought to have an important role in preventing and treating ASD, have been reviewed [46,47]. A 2015 study by Huang and colleagues [48] concluded that calcitriol (the active form of vitamin D3) can be used to alleviate neuroinflammation (caused mainly by oxidants and toxins). Another mechanism is vitamin D’s effect on serotonin through direct genetic regulation of serotonin rate-limiting enzymes, such as both peripheral tryptophan hydroxylase 1 (TPH1) and central TPH2 [49]. Activated vitamin D hormone (calcitriol) downregulates peripheral TPH1 while upregulating central TPH2, thus explaining the serotonin paradox in ASD in which peripheral serotonin is increased but central serotonin is reduced in those patients [49,50].
Calcitriol protects brain tissue by reducing inflammatory cytokine levels [51]. That increase is strongly associated with cognitive impairment in ASD [52]. Calcitriol also protects the brain by stimulating the production of neurotrophins from several sources. Neurotrophins are chemicals that fight toxic effects [53]. In fact, vitamin D taken orally is pro-prehormone. After ingestion, vitamin D is metabolized by the liver and converted to the 25(OH)D form and then forms calcitriol [(1,25(OH)2D]. Calcitriol is a powerful neurosteroid that assists in controlling brain cell growth and acts on vitamin D receptor molecules that appear in most brain cells during the first days of embryo formation [54].
Autistic individuals have abnormalities in immune functions similar to those affected by vitamin D deficiency, such as increased inflammatory cytokine levels [23]. Also, much of the ongoing inflammation in autistic brains is the result of oxidative stress [55,56]. That effect also can be related to vitamin D, which regulates the production of dendritic lymphocytes, in turn reducing the intensity of autoimmune attacks by increasing levels of interleukin 10, an anti-inflammatory cytokine [57].
Herein, we review the recent advances in studying the relationship between vitamin D status during the pregnancy and ASD in the outcome to try to identify the potential role of vitamin D in the etiology of ASD.
2. Materials and Methods
To report this systematic review, we followed the guidelines specified in PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) [58,59].
2.1. Literature Search
We searched the literature to identify publications eligible for inclusion in the review in the PubMed and EMBASE databases. The literature search strategy used included the keywords “pregnancy,” “gestation,” “vitamin D,” “autism,” and “autism spectrum disorder.” The search was limited to human subjects and English language articles published between 2010 and January 2020. Forty-three studies came from PubMed and 124 studies from EMBASE, for a total of 167 articles identified and retrieved. An initial screening identified 26 candidate studies. Figure 1 shows a PRISMA flowchart indicating the search strategy followed. After articles were assessed for eligibility, they were then identified and the first screening of the articles was performed using the information available in the abstract and result section of each study.
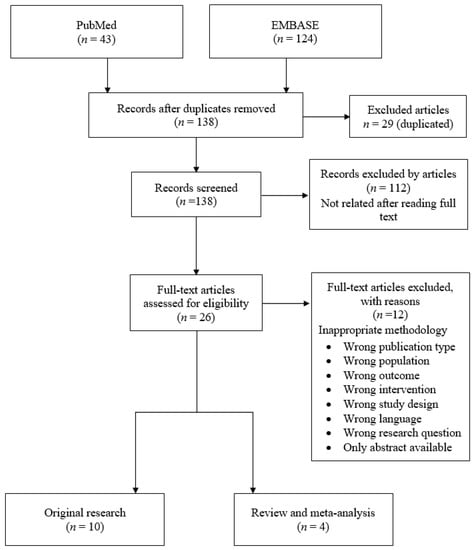
Figure 1.
Search strategy.
2.2. Study Inclusion/Exclusion Criteria and Data Extraction
We included randomized and nonrandomized controlled trials, prospective cohort studies, case-control studies, and systematic reviews on longitudinal studies that investigated how vitamin D levels during pregnancy affected the prevalence of autism in children. The selection criteria included the following:
- Human study of vitamin D supplementation during pregnancy or vitamin D status of pregnancy or vitamin D status of ASD children;
- Available information of circulation concentration of 25(OH)D in maternal blood during pregnancy or in newborn blood at birth;
- Original research article and review in humans (abstracts, case reports, ecological studies, and comments were excluded);
- Outcomes including incidence of autism, measures of autism, severity, measures of neurophysiological aspects of autism, or expression of autism-related genes and vitamin D during pregnancy; and
- Article written in English
- Any study between 2010 and January 2020.
Any study that involved children with any other disease that could affect serum vitamin D levels was excluded. Only publications meeting all criteria were included in this analysis. After a full assessment of the potentially relevant studies, 14 were proposed to be included in this systematic review.
2.3. Study Quality
We evaluated the methodological quality (risk of bias) of studies by using the Newcastle–Ottawa Scale (NOS) for observational studies [60,61]. The NOS criteria included (1) subject selection, 0–4; (2) comparability of subject, 0–2; and (3) clinical outcome, 0–3. Total NOS scores range from 0 (lowest) to 9 (highest). On the basis of NOS scores, studies can be divided into low quality (0–6) and high quality (7–9). Two evaluators conducted the NOS assessment independently. Any discrepancy on NOS scores of potential studies was resolved through discussion or consultation with a third evaluator.
For a more detailed evaluation of the selected articles, the scale proposed by the Scottish Intercollegiate Guidelines Network (SIGN) was used, establishing levels of evidence (Table 1) and recommendations (Table 2) [62]. The SIGN system yields a detailed assessment of the degree of scientific evidence. Epistemological strength is the way the articles are classified. Only the strongest evidence gives way to strong recommendations, whereas weaker evidence can give rise only to weak recommendations. The SIGN system emphasizes that studies with a high rate of bias may lead to biased results, and the bias level of the studies is determined with that system. The risk of bias and study design are used to allow assessment of the level of evidence and the quality of scientific evidence provided. When the numbers 1, 2, 3, and 4 are used to grade the study, ++, +, and −- signs are used to indicate the risk of bias. On the basis of that assessment of the quality of evidence in the articles, they are classified best to worst according to grades A, B, C, and D. That scale rating is constructed on the principles of evidence-based medicine—an approach that ensures the use of the most up-to-date, reliable, and scientifically solid evidence available in making decisions about a particular situation being studied [63].

Table 1.
Levels of evidence.

Table 2.
Grades of recommendation.
We also focused on the internal validity of the studies analyzed. The factors that influence internal validity that were considered are a sample size that was large enough and that inclusion and assessment criteria were specified.
3. Results
3.1. Study Characteristics
The chosen studies were analyzed according to study design, location, participant, sample size, target population, and major findings. Table 3 and Table 4 summarize the characteristics of the studies.

Table 3.
Original research studies on 25-hydroxyvitamin D (25(OH)D) during pregnancy and autism.

Table 4.
Review and meta-analysis studies on vitamin D during pregnancy and autism 3.2. Study Design and Population.
In total, 167 articles were initially assessed in this study. After removing 29 duplicated articles, we screened 138 by their abstracts. Twenty-six articles were retrieved for full-text assessment. Finally, 14 articles from nine countries met the inclusion criteria [30,31,32,64,65,66,67,68,69,70,71,72,73,74]. The studies included 10 original research and 4 review studies conducted between 2012 and 2020.
The original research studies used data from five countries: China [31,32], Sweden [30,64,65], the Netherlands [66,67], Spain [68], and Australia [69]. Data from one study were obtained from neighboring states, across the United States, Canada, and Israel [70]. The review research studies included data from the United States, Australia, Sweden, China, United Kingdom, Spain, Denmark, the Netherlands, Vietnam, the Republic of Seychelles, India, Greece, Egypt, Brazil, Turkey, Qatar, Saudi Arabia, Asia, and Europe [71,72,73,74].
3.2. Original Research Studies
Chen and colleagues [31] have shown that mothers in the autistic group had significantly lower maternal serum levels of 25(OH)D than in neurotypical groups, with 55.9% and 29.4% being vitamin D deficient, respectively. Lower first-trimester maternal serum levels of 25(OH)D were associated with a significantly increased risk of developing autism in offspring (p < 0.001). Fernell and colleagues [65] analyzed 25(OH)D in 58 Sweden-born sibling pairs, in which one child had ASD and the other did not. The collapsed group of children with ASD had significantly lower vitamin D levels than their siblings. The findings suggest that low prenatal vitamin D may act as a risk factor for ASD (p = 0.013). Stubb and colleagues [70] published an open-label prospective study in 2016, prescribing vitamin D during pregnancy to the expectant mothers of children with autism at a dose of 5000 IU/day. The newborn children were monitored for 3 years, during which they were assessed for autism at 18 and 36 months of age. The final outcome was that 1 of 19 (5%) developed autism in contrast to the overall recurrence rate of approximately 20%, which is evident across the literature (n = 20).
Vinkhuyzen and colleagues [66] in their study based on a Rotterdam birth cohort (n = 4334), found that individuals in the 25(OH)D-deficient group at midgestation had more than a twofold increased risk of ASD than the 25(OH)D-sufficient group (p = 0.03). Also, in another study conducted by Vinkhuyzen and colleagues [67] in 2018, they aimed to determine the relationship between vitamin D deficiency in pregnancy and Social Responsiveness Scale (SRS) status of their children (n = 4229). 25(OH)D concentration was measured in serum and was measured from two samples with the first sample taken at 20.6 weeks and the second sample collected at birth, from neonatal cord blood. The 25(OH)D-deficient group had significantly higher (p < 0.001) (more abnormal) SRS scores than the 25(OH)D-sufficient group [25(OH)D > 50 nmol/L]. Those study results serve to clarify the relationship between maternal vitamin D deficiency and offspring risk of ASD with and without intellectual disability.
The Stockholm Youth Cohort is a registry-based total population study (N = 509,639) [64] in which maternal vitamin D deficiency, defined as a lifetime-recorded diagnosis of unspecified vitamin D deficiency (ICD-10
E55.9 or ICD-9 268.9) corresponding to a serum 25-hydroxyvitamin D level of less than 25 nmol/L, was associated with offspring risk of ASD with, but not without, intellectual disability. In a 2019 study, Lee and colleagues [30] found that neonatal 25(OH)D <25 nmol/L was associated with 1.33 times higher odds of ASD (95% confidence interval (CI), 1.02 to 1.75) than for levels of 25(OH)D ≥50 nmol/L. In Nordic-born mothers, maternal 25(OH)D insufficiency (25 to <50 nmol/L) at ~11 weeks’ gestation was associated with 1.58 times higher odds of ASD (95% CI, 1.00 to 2.49) than for 25(OH)D sufficiency (≥50 nmol/L). Wu and colleagues [32] analyzed the concentration of 25(OH)D3 in children with ASD, and controls were assessed from neonatal dried blood samples. The median 25(OH)D3 level was significantly (p < 0.0001) lower in children with ASD than for controls. As a result, neonatal vitamin D status was significantly associated with the risk of ASDs and intellectual disability.
A 2019 study [68] clearly showed that vitamin D deficiency during critical periods of pregnancy development could lead to persistent brain alterations in the fetus. Maternal plasma vitamin D3 was measured in pregnancy through a single maternal blood sample at a mean of 13.3 weeks of gestation, with findings that per each 10-ng/mL increment of maternal vitamin D3, children obtained higher social competence scores at 5 years old (coefficient = 0.77; 95% CI, 0.19 to 1.35). Also, in a cohort study by Whitehouse and colleagues [69], offspring of mothers with low 25(OH)D concentrations (<49 nmol/L) at 18 weeks of gestation showed increased risk for “high” scores (≥2 standard deviations above the mean) on the Attention Switching subscale.
3.3. Review and Meta-Analysis Studies
Diverse studies have investigated the impact of prenatal exposure to vitamin D levels on brain development and autism. A systematic review and meta-analysis published in 2019 [71] summarized evidence of the association between 25(OH)D levels in maternal blood in pregnancy or newborn blood at birth and neurodevelopmental outcomes. Those outcomes included cognition, psychomotor performance, language development, behavioral difficulties, attention deficit–hyperactivity disorder (ADHD), and autistic traits. That meta-analysis offers supporting evidence that increased prenatal exposure to 25(OH)D levels is associated with improved cognitive development and reduced risk of ADHD and autism-related traits later in life. Furthermore, an alternative review [72] showed that depending on the timing of the exposure to low vitamin D status, different brain areas might be affected, possibly causing different neurodevelopment and cognitive outcomes in infants. In agreement with other studies, Kočovská and colleagues and Wang and colleagues [73,74] showed that decreased vitamin D levels during pregnancy and decreased exposure to solar UVB might increase the risk for ASD.
3.4. Summary of Findings
Vitamin D plays many roles in various processes in the human body. Supporting findings from the past 15 years have come from animal studies, human molecular, cellular and physiological, genetic, and neurodevelopmental studies. And vitamin D deficiency during pregnancy or early childhood has been suggested as a possible environmental risk factor for ASD.
Our literature review identified many observational studies, but few intervention trials have been conducted that investigate the relationship between vitamin D level and ASD during pregnancy. The strength of evidence that vitamin D levels during pregnancy increase the risk of developing autism is very low. That evidence exclusively relies on observational studies that did not equally consider all important confounders and that assessed the indirect relationship between vitamin D as a surrogate for sunlight exposure and autism risk.
Despite the limited and inconclusive results of meta-analyses, research has established that early exposure to inadequate vitamin D may interact with other factors and contribute to the etiology of autism, and individuals with ASD may be a population at risk of vitamin D deficiency/inadequacy. Therefore, evidence-based clinical recommendations are needed to prevent ASD and manage ASD symptoms. Until better data are available, vitamin D is worth considering as a potential preventive measure for ASD.
4. Discussion
ASD, a complex, heterogeneous neurodevelopmental disorder, had an estimated prevalence of ~1% in children in 2017 [75]. ASD is associated with biological and environmental factors. Epigenetic mechanisms have detected that gene–environment interactions are important in mediating risk [76,77].
ASD and low vitamin D are commonly interrelated, and children with ASD also have vitamin D deficiencies. Vitamin D deficiency affects ~1 billion people globally and is a potential environmental risk factor for ASD [43]. Epidemiological studies and data obtained in humans have yielded evidence that a mother’s diet during pregnancy plays a vital role in the development of the neural circuitry that regulates behavior, thus determining persistent behavioral effects in the offspring [78]. Generally, vitamin D concentration during pregnancy is associated with a higher or lower incidence of ASD or autistic traits in offspring [79]. A 2013 report [36] emphasized that people with low activity of vitamin D enzymes and people with maternal or early childhood vitamin D deficiency would have low activity in the vitamin D system, which is important for brain development. In addition, through several feasible mechanisms, vitamin D can help children with autism.
Considering whether prenatal vitamin D deficiency is associated with the development of ASD seems reasonable. The findings suggest that autistic children have lover 25(OH) D levels than their unaffected siblings [67,80]. That finding emphasizes that more randomized controlled trials are needed. But until new studies are completed, it seems prudent to maximize vitamin D levels in pregnant and lactating women, infants, and young children, as the Endocrine Society recommends, aiming for levels found in humans living in a sun-rich environment (46 ng/mL) [67,80].
Furthermore, nutritional quality control in the mother’s diet during pregnancy is a potentially effective strategy to prevent autism in the offspring. Nutritional deficiencies can be prevented by using safe and easily accessible supplements. That effect is seen in the results of our review, which show that using the right amount of vitamin D during pregnancy may reduce the risk of ASD [81]. Currently it is suggested that all pregnant women maintain a circulating 25(OH)D concentration of at least 40 ng/mL for the first 16 weeks of gestation [82] and to achieve this, intakes of at least 4000 IU/d vitamin D3 would be necessary [83,84]. Supplementation could be especially important in women with children diagnosed with ASD as previous studies have suggested that supplementation could reduce the recurrence rate of autism in siblings [65,70,85]. However, while the results of these studies are promising, these are preliminary studies with small sample sizes. Therefore, in order to adequately inform recommendations for mothers with children diagnosed with ASD, further research in larger samples is needed to confirm the results as well as to determine appropriate dosage.
By contrast, in a study that included maternal vitamin D levels, no relationship was apparent between maternal vitamin D level and autism. That study reported that no relationship existed between maternal vitamin D levels and autism in children of both white and Somali mothers [86], but fewer than 20 mothers were included in each group and analyses were performed several years after birth. A review in January 2020 concluded that vitamin D deficiency in the data available now does not adequately support the hypothesis that it may contribute to the etiology of ASD [87].
Future epidemiological studies should include prenatal or neonatal vitamin D levels and have enough statistical power to show convincing evidence of a relationship between low neonatal vitamin D status and the risk of autism.
Strengths and Limitations of This Review
The clinical trials included in our analysis show significant differences in sample size, use of reinforcements, and follow-up. In addition, researchers may not be able to access all publications on the relationship between vitamin D and autism during pregnancy because the area of analysis is limited to studies published in English and available through the PubMed and EMBASE databases. However, the main outcome measured was common in all the studies and included the risk/incidence of autism/ASD in offspring.
5. Conclusions
The findings of this systematic review are consistent with the hypothesis that low vitamin D levels might contribute to the development of autism. However, we also must recognize the possibility of confusion bias because a diet with adequate levels of vitamin D is usually also adequate in other micronutrients. Experimental studies that allow us to detect blood levels in pregnant women would therefore be helpful to clarify that point. However, we must bear in mind that doing so would require a design with very large sample sizes given the incidence of autism. Further studies examining the relationship between vitamin D and autism risk are needed.
Author Contributions
Conceptualization, N.U., I.P.-C., M.M.S.-V.; methodology, N.U., W.B.G., I.P.-C., M.M.S.-V.; formal analysis, N.U., W.B.G., I.P.-C., M.M.S.-V.; investigation, N.U., W.B.G., I.P.-C., M.M.S.-V.; data curation, N.U., I.P.-C.; writing—original draft preparation, N.U., W.B.G., I.P.-C., M.M.S.-V.; writing—review and editing, N.U., W.B.G., I.P.-C., M.M.S.-V.; supervision, M.M.S.-V. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders; DSM-V: Washington, DC, USA, 2013. [Google Scholar]
- Bakken, T.E.; Miller, J.A.; Ding, S.; Sunkin, S.M.; Smith, K.A.; Ng, L.; Szafer, A.; Dalley, R.A.; Royall, J.J.; Lemon, T. A Comprehensive Transcriptional Map of Primate Brain Development. Nature 2016, 535, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Zablotsky, B.; Black, L.I.; Maenner, M.J.; Schieve, L.A.; Blumberg, S.J. Estimated Prevalence of Autism and Other Developmental Disabilities Following Questionnaire Changes in the 2014 National Health Interview Survey. Natl. Health. Stat. Rep. 2015, 87, 1–20. [Google Scholar]
- Centers for Disease Control and Prevention. Prevalence of Autism Spectrum Disorders-Autism and Developmental Disabilities Monitoring Network, United State, 2006. MMWR Surveill. Summ. 2009, 58, 1–20. [Google Scholar]
- Centers for Disease Control and Prevention. Prevalence of Autism Spectrum Disorder among Children Aged 8 Years—Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2014. MMWR Surveill. Summ. 2018, 67, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Sandin, S.; Lichtenstein, P.; Kuja-Halkola, R.; Hultman, C.; Larsson, H.; Reichenberg, A. The Heritability of Autism Spectrum Disorder. JAMA 2017, 318, 1182–1184. [Google Scholar] [CrossRef]
- Lai, M.; Lombardo, M.; Baron-Cohen, S. Autism. Lancet 2014, 383, 896–910. [Google Scholar] [CrossRef]
- Magnusson, C.; Rai, D.; Goodman, A.; Lundberg, M.; Idring, S.; Svensson, A.; Koupil, I.; Serlachius, E.; Dalman, C. Migration and Autism Spectrum Disorder: Population-Based Study. Br. J. Psychiatry 2012, 201, 109–115. [Google Scholar] [CrossRef]
- Hallmayer, J.; Cleveland, S.; Torres, A.; Phillips, J.; Cohen, B.; Torigoe, T.; Miller, J.; Fedele, A.; Collins, J.; Smith, K. Genetic Heritability and Shared Environmental Factors among Twin Pairs with Autism. Arch. Gen. Psychiatry 2011, 68, 1095–1102. [Google Scholar] [CrossRef]
- Folstein, S.; Rutter, M. Infantile Autism: A Genetic Study of 21 Twin Pairs. J. Child Psychol. Psychiatry 1977, 18, 297–321. [Google Scholar] [CrossRef]
- Bailey, A.; Le Couteur, A.; Gottesman, I.; Bolton, P.; Simonoff, E.; Yuzda, E.; Rutter, M. Autism as a Strongly Genetic Disorder: Evidence from a British Twin Study. Psychol. Med. 1995, 25, 63–77. [Google Scholar] [CrossRef]
- Schaaf, C.P.; Zoghbi, H.Y. Solving the Autism Puzzle a Few Pieces at a Time. Neuron 2011, 70, 806–808. [Google Scholar] [CrossRef] [PubMed]
- DeVilbiss, E.A.; Gardner, R.M.; Newschaffer, C.J.; Lee, B.K. Maternal Folate Status as a Risk Factor for Autism Spectrum Disorders: A Review of Existing Evidence. Br. J. Nutr. 2015, 114, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Bala, K.A.; Doğan, M.; Kaba, S.; Mutluer, T.; Aslan, O.; Doğan, S.Z. Hormone Disorder and Vitamin Deficiency in Attention Deficit Hyperactivity Disorder (ADHD) and Autism Spectrum Disorders (ASDs). J. Pediatric Endocrinol. Metab. 2016, 29, 1077–1082. [Google Scholar] [CrossRef] [PubMed]
- Kardas, F.; Bayram, A.K.; Demirci, E.; Akin, L.; Ozmen, S.; Kendirci, M.; Canpolat, M.; Oztop, D.B.; Narin, F.; Gumus, H. Increased Serum Phthalates (MEHP, DEHP) and Bisphenol a Concentrations in Children with Autism Spectrum Disorder: The Role of Endocrine Disruptors in Autism Etiopathogenesis. J. Child Neurol. 2016, 31, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Skalny, A.V.; Skalnaya, M.G.; Bjørklund, G.; Nikonorov, A.A.; Tinkov, A.A. Mercury as a Possible Link between Maternal Obesity and Autism Spectrum Disorder. Med. Hypotheses 2016, 91, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Bromley, R. The Treatment of Epilepsy in Pregnancy: The Neurodevelopmental Risks Associated with Exposure to Antiepileptic Drugs. Reprod. Toxicol. 2016, 64, 203–210. [Google Scholar] [CrossRef]
- Kaplan, Y.C.; Keskin-Arslan, E.; Acar, S.; Sozmen, K. Prenatal Selective Serotonin Reuptake Inhibitor use and the Risk of Autism Spectrum Disorder in Children: A Systematic Review and Meta-Analysis. Reprod. Toxicol. 2016, 66, 31–43. [Google Scholar] [CrossRef]
- Estes, M.L.; McAllister, A.K. Maternal Immune Activation: Implications for Neuropsychiatric Disorders. Science 2016, 353, 772–777. [Google Scholar] [CrossRef]
- Jiang, H.; Xu, L.; Shao, L.; Xia, R.; Yu, Z.; Ling, Z.; Yang, F.; Deng, M.; Ruan, B. Maternal Infection during Pregnancy and Risk of Autism Spectrum Disorders: A Systematic Review and Meta-Analysis. Brain Behav. Immun. 2016, 58, 165–172. [Google Scholar] [CrossRef]
- Offit, P.A. Vaccines and Autism in Primate Model. Proc. Natl. Acad. Sci. USA 2015, 112, 12236–12237. [Google Scholar] [CrossRef]
- Rodgers, A.B.; Morgan, C.P.; Bronson, S.L.; Revello, S.; Bale, T.L. Paternal Stress Exposure Alters Sperm microRNA Content and Reprograms Offspring HPA Stress Axis Regulation. J. Neurosci. 2013, 33, 9003–9012. [Google Scholar] [CrossRef] [PubMed]
- Szachta, P.; Skonieczna-Żydecka, K.; Adler, G.; Karakua-Juchnowicz, H.; Madlani, H.; Ignyś, I. Immune Related Factors in Pathogenesis of Autism Spectrum Disorders. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 3060–3072. [Google Scholar] [PubMed]
- Liu, L.; Zhang, D.; Rodzinka-Pasko, J.; Li, Y. Environmental Risk Factors for Autism Spectrum Disorders. Nervenarzt 2016, 87, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Qiu, C.; Hu, F.B.; David, R.M.; van Dam, R.M.; Bralley, A.; Williams, M.A. Maternal Plasma 25-Hydroxyvitamin D Concentrations and the Risk for Gestational Diabetes Mellitus. PLoS ONE 2008, 3, e3753. [Google Scholar] [CrossRef]
- Sacks, K.N.; Friger, M.; Shoham-Vardi, I.; Abokaf, H.; Spiegel, E.; Sergienko, R.; Landau, D.; Sheiner, E. Prenatal Exposure to Gestational Diabetes Mellitus as an Independent Risk Factor for Long-Term Neuropsychiatric Morbidity of the Offspring. Obstet. Gynecol. 2016, 215, 380. [Google Scholar]
- Curran, E.A.; O’Neill, S.M.; Cryan, J.F.; Kenny, L.C.; Dinan, T.G.; Khashan, A.S.; Kearney, P.M. Research Review: Birth by Caesarean Section and Development of Autism Spectrum Disorder and Attention-deficit/Hyperactivity Disorder: A Systematic Review and Meta-analysis. J. Child Psychol. Psychiatry 2015, 56, 500–508. [Google Scholar] [CrossRef]
- Merewood, A.; Mehta, S.D.; Chen, T.C.; Bauchner, H.; Holick, M.F. Association between Vitamin D Deficiency and Primary Cesarean Section. J. Clin. Endocrinol. Metab. 2009, 94, 940–945. [Google Scholar] [CrossRef]
- Grant, W.B.; Soles, C.M. Epidemiologic Evidence for Supporting the Role of Maternal Vitamin D Deficiency as a Risk Factor for the Development of Infantile Autism. Derm. Endocrinol. 2009, 1, 223–228. [Google Scholar] [CrossRef]
- Lee, B.K.; Eyles, D.W.; Magnusson, C.; Newschaffer, C.J.; McGrath, J.J.; Kvaskoff, D.; Ko, P.; Dalman, C.; Karlsson, H.; Gardner, R.M. Developmental Vitamin D and Autism Spectrum Disorders: Findings from the Stockholm Youth Cohort. Mol. Psychiatry 2019, 1–11. [Google Scholar] [CrossRef]
- Chen, J.; Xin, K.; Wei, J.; Zhang, K.; Xiao, H. Lower Maternal Serum 25 (OH) D in First Trimester Associated with Higher Autism Risk in Chinese Offspring. J. Psychosom. Res. 2016, 89, 98–101. [Google Scholar] [CrossRef]
- Wu, D.; Wen, X.; Han, X.; Wang, S.; Wang, Y.; Shen, M.; Fan, S.; Zhuang, J.; Li, M.; Hu, B. Relationship between Neonatal Vitamin D at Birth and Risk of Autism Spectrum Disorders: The NBSIB Study. J. Bone Miner. Res. 2018, 33, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Brook, J.S.; Leukefeld, C.G.; De La Rosa, M.; Brook, D.W. Season of Birth: A Predictor of ADHD Symptoms in Early Midlife. Psychiatry Res. 2018, 267, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Boland, M.R.; Parhi, P.; Li, L.; Miotto, R.; Carroll, R.; Iqbal, U.; Nguyen, P.; Schuemie, M.; You, S.C.; Smith, D. Uncovering Exposures Responsible for Birth Season–disease Effects: A Global Study. J. Am. Med Inform. Assoc. 2018, 25, 275–288. [Google Scholar] [CrossRef] [PubMed]
- Shalev, H.; Solt, I.; Chodick, G. Month of Birth and Risk of Autism Spectrum Disorder: A Retrospective Cohort of Male Children Born in Israel. BMJ Open 2017, 7, 014606. [Google Scholar] [CrossRef]
- Cannell, J.J.; Grant, W.B. What is the Role of Vitamin D in Autism. Derm. Endocrinol. 2013, 5, 199–204. [Google Scholar] [CrossRef]
- Cannell, J.J. Autism and Vitamin D. Med. Hypotheses 2008, 70, 750–759. [Google Scholar] [CrossRef]
- Wagner, C.L.; Hollis, B.W.; Kotsa, K.; Fakhoury, H.; Karras, S.N. Vitamin D Administration during Pregnancy as Prevention for Pregnancy, Neonatal and Postnatal Complications. Rev. Endocr. Metab. Disord. 2017, 18, 307–322. [Google Scholar] [CrossRef]
- Karras, S.N.; Wagner, C.L.; Castracane, V.D. Understanding Vitamin D Metabolism in Pregnancy: From Physiology to Pathophysiology and Clinical Outcomes. Metabolism 2018, 86, 112–123. [Google Scholar] [CrossRef]
- Karras, S.N.; Fakhoury, H.; Muscogiuri, G.; Grant, W.B.; van den Ouweland, J.M.; Colao, A.M.; Kotsa, K. Maternal Vitamin D Levels during Pregnancy and Neonatal Health: Evidence to Date and Clinical Implications. Ther. Adv. Musculoskelet. Dis. 2016, 8, 124–135. [Google Scholar] [CrossRef]
- Spiro, A.; Buttriss, J. Vitamin D: An Overview of Vitamin D Status and Intake in E Urope. Nutr. Bull. 2014, 39, 322–350. [Google Scholar] [CrossRef]
- Saggese, G.; Vierucci, F.; Prodam, F.; Cardinale, F.; Cetin, I.; Chiappini, E.; de’Angelis, G.L.; Massari, M.; Del Giudice, E.M.; Del Giudice, M.M. Vitamin D in Pediatric Age: Consensus of the Italian Pediatric Society and the Italian Society of Preventive and Social Pediatrics, Jointly with the Italian Federation of Pediatricians. Ital. J. Pediatrics 2018, 44, 51. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin D Deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Mithal, A.; Wahl, D.A.; Bonjour, J.; Burckhardt, P.; Dawson-Hughes, B.; Eisman, J.A.; Fuleihan, G.E.; Josse, R.G.; Lips, P.; Morales-Torres, J. Global Vitamin D Status and Determinants of Hypovitaminosis D. Osteoporos. Int. 2009, 20, 1807–1820. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.H.; Shin, H.J.; Lee, Y.J. Vitamin D Status and Childhood Health. Korean J. Pediatr. 2013, 56, 417–423. [Google Scholar] [CrossRef]
- Ali, A.; Cui, X.; Eyles, D. Developmental Vitamin D Deficiency and Autism: Putative Pathogenic Mechanisms. J. Steroid Biochem. Mol. Biol. 2018, 175, 108–118. [Google Scholar] [CrossRef]
- DeLuca, G.; Kimball, S.; Kolasinski, J.; Ramagopalan, S.; Ebers, G. The Role of Vitamin D in Nervous System Health and Disease. Neuropathol. Appl. Neurobiol. 2013, 39, 458–484. [Google Scholar] [CrossRef]
- Huang, Y.; Ho, Y.; Lai, C.; Chiu, C.; Wang, J. 1, 25-Dihydroxyvitamin D 3 Attenuates Endotoxin-Induced Production of Inflammatory Mediators by Inhibiting MAPK Activation in Primary Cortical Neuron-Glia Cultures. J. Neuroinflamm. 2015, 12, 147. [Google Scholar] [CrossRef]
- Cannell, J.J. Vitamin D and Autism, What’s New. Rev. Endocr. Metab. Disord. 2017, 18, 183–193. [Google Scholar] [CrossRef]
- Patrick, R.P.; Ames, B.N. Vitamin D Hormone Regulates Serotonin Synthesis. Part 1: Relevance for Autism. FASEB J. 2014, 28, 2398–2413. [Google Scholar] [CrossRef]
- Arnson, Y.; Itzhaky, D.; Mosseri, M.; Barak, V.; Tzur, B.; Agmon-Levin, N.; Amital, H. Vitamin D Inflammatory Cytokines and Coronary Events: A Comprehensive Review. Clin. Rev. Allergy Immunol. 2013, 45, 236–247. [Google Scholar] [CrossRef]
- Krakowiak, P.; Goines, P.E.; Tancredi, D.J.; Ashwood, P.; Hansen, R.L.; Hertz-Picciotto, I.; Van de Water, J. Neonatal Cytokine Profiles Associated with Autism Spectrum Disorder. Biol. Psychiatry 2017, 81, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Wrzosek, M.; Łukaszkiewicz, J.; Wrzosek, M.; Jakubczyk, A.; Matsumoto, H.; Piątkiewicz, P.; Radziwoń-Zaleska, M.; Wojnar, M.; Nowicka, G. Vitamin D and the Central Nervous System. Pharmacol. Rep. 2013, 65, 271–278. [Google Scholar] [CrossRef]
- Kesby, J.P.; Eyles, D.W.; Burne, T.H.; McGrath, J.J. The Effects of Vitamin D on Brain Development and Adult Brain Function. Mol. Cell. Endocrinol. 2011, 347, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Smaga, I.; Niedzielska, E.; Gawlik, M.; Moniczewski, A.; Krzek, J.; Przegaliński, E.; Pera, J.; Filip, M. Oxidative Stress as an Etiological Factor and a Potential Treatment Target of Psychiatric Disorders. Part 2. Depression, Anxiety, Schizophrenia and Autism. Pharmacol. Rep. 2015, 67, 569–580. [Google Scholar] [CrossRef] [PubMed]
- Cortelazzo, A.; De Felice, C.; Guerranti, R.; Signorini, C.; Leoncini, S.; Zollo, G.; Leoncini, R.; Timperio, A.M.; Zolla, L.; Ciccoli, L. Expression and Oxidative Modifications of Plasma Proteins in Autism Spectrum Disorders: Interplay between Inflammatory Response and Lipid Peroxidation. Proteom. Clin. Appl. 2016, 10, 1103–1112. [Google Scholar] [CrossRef] [PubMed]
- Bakdash, G.; van Capel, T.M.; Mason, L.M.; Kapsenberg, M.L.; de Jong, E.C. Vitamin D3 Metabolite Calcidiol Primes Human Dendritic Cells to Promote the Development of Immunomodulatory IL-10-Producing T Cells. Vaccine 2014, 32, 6294–6302. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015 Statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef]
- Stang, A. Critical Evaluation of the Newcastle-Ottawa Scale for the Assessment of the Quality of Nonrandomized Studies in Meta-Analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef]
- Wells, G.A.; Tugwell, P.; O’Connell, D.; Welch, V.; Peterson, J.; Shea, B.; Losos, M. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomized Studies in Meta-Analyses; University of Ottawa: Ottawa, ON, Canada, 2015. [Google Scholar]
- Scottish Intercollegiate Guidelines Network (SIGN). SIGN 50: A Guideline Developer’s Handbook; Scottish Intercollegiate Guidelines Network: Edinburgh, UK, 2015. [Google Scholar]
- Sackett, D.L. Evidence-Based Medicine. Semin. Perinatol. 1997, 21, 3–5. [Google Scholar] [CrossRef]
- Magnusson, C.; Kosidou, K.; Dalman, C.; Lundberg, M.; Lee, B.K.; Rai, D.; Karlsson, H.; Gardner, R.; Arver, S. Maternal Vitamin D Deficiency and the Risk of Autism Spectrum Disorders: Population-Based Study. BJPsych Open 2016, 2, 170–172. [Google Scholar] [CrossRef] [PubMed]
- Fernell, E.; Bejerot, S.; Westerlund, J.; Miniscalco, C.; Simila, H.; Eyles, D.; Gillberg, C.; Humble, M.B. Autism Spectrum Disorder and Low Vitamin D at Birth: A Sibling Control Study. Mol. Autism 2015, 6, 3. [Google Scholar] [CrossRef] [PubMed]
- Vinkhuyzen, A.A.; Eyles, D.W.; Burne, T.H.; Blanken, L.M.; Kruithof, C.J.; Verhulst, F.; White, T.; Jaddoe, V.W.; Tiemeier, H.; McGrath, J.J. Gestational Vitamin D Deficiency and Autism Spectrum Disorder. BJPsych Open 2017, 3, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Vinkhuyzen, A.A.; Eyles, D.W.; Burne, T.H.; Blanken, L.M.; Kruithof, C.J.; Verhulst, F.; Jaddoe, V.W.; Tiemeier, H.; McGrath, J.J. Gestational Vitamin D Deficiency and Autism-Related Traits: The Generation R Study. Mol. Psychiatry 2018, 23, 240–246. [Google Scholar] [CrossRef]
- López-Vicente, M.; Sunyer, J.; Lertxundi, N.; González, L.; Rodríguez-Dehli, C.; Sáenz-Torre, M.E.; Vrijheid, M.; Tardón, A.; Llop, S.; Torrent, M. Maternal Circulating Vitamin D 3 Levels during Pregnancy and Behaviour Across Childhood. Sci. Rep. 2019, 9, 1–8. [Google Scholar] [CrossRef]
- Whitehouse, A.J.; Holt, B.J.; Serralha, M.; Holt, P.G.; Hart, P.H.; Kusel, M.M. Maternal Vitamin D Levels and the Autism Phenotype among Offspring. J. Autism Dev. Disord. 2013, 43, 1495–1504. [Google Scholar] [CrossRef]
- Stubbs, G.; Henley, K.; Green, J. Autism: Will Vitamin D Supplementation during Pregnancy and Early Childhood Reduce the Recurrence Rate of Autism in Newborn Siblings. Med. Hypotheses 2016, 88, 74–78. [Google Scholar] [CrossRef]
- García-Serna, A.M.; Morales, E. Neurodevelopmental Effects of Prenatal Vitamin D in Humans: Systematic Review and Meta-Analysis. Mol. Psychiatry 2019, 1–14. [Google Scholar] [CrossRef]
- Mazahery, H.; Camargo, C.A.; Conlon, C.; Beck, K.L.; Kruger, M.C.; Von Hurst, P.R. Vitamin D and Autism Spectrum Disorder: A Literature Review. Nutrients 2016, 8, 236. [Google Scholar] [CrossRef]
- Kočovská, E.; Fernell, E.; Billstedt, E.; Minnis, H.; Gillberg, C. Vitamin D and Autism: Clinical Review. Res. Dev. Disabil. 2012, 33, 1541–1550. [Google Scholar] [CrossRef]
- Wang, T.; Shan, L.; Du, L.; Feng, J.; Xu, Z.; Staal, W.G.; Jia, F. Serum Concentration of 25-Hydroxyvitamin D in Autism Spectrum Disorder: A Systematic Review and Meta-Analysis. Eur. Child Adolesc. Psychiatry 2016, 25, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Kerley, C.P.; Power, C.; Gallagher, L.; Coghlan, D. Lack of Effect of Vitamin D3 Supplementation in Autism: A 20-Week, Placebo-Controlled RCT. Arch. Dis. Child. 2017, 102, 1030–1036. [Google Scholar] [CrossRef] [PubMed]
- Newschaffer, C.J.; Falb, M.D.; Gurney, J.G. National Autism Prevalence Trends from United States Special Education Data. Pediatrics 2005, 115, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Kinney, D.K.; Barch, D.H.; Chayka, B.; Napoleon, S.; Munir, K.M. Environmental Risk Factors for Autism: Do they Help Cause De Novo Genetic Mutations that Contribute to the Disorder. Med. Hypotheses 2010, 74, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Wiens, D.; DeSoto, M.C. Is High Folic Acid Intake a Risk Factor for Autism—A Review. Brain Sci. 2017, 7, 149. [Google Scholar] [CrossRef]
- Peretti, S.; Mariano, M.; Mazzocchetti, C.; Mazza, M.; Pino, M.; Verrotti Di Pianella, A.; Valenti, M. Diet: The Keystone of Autism Spectrum Disorder. Nutr. Neurosci. 2019, 22, 825–839. [Google Scholar] [CrossRef]
- Luxwolda, M.F.; Kuipers, R.S.; Kema, I.P.; Dijck-Brouwer, D.J.; Muskiet, F.A. Traditionally Living Populations in East Africa have a Mean Serum 25-Hydroxyvitamin D Concentration of 115 Nmol/L. Br. J. Nutr. 2012, 108, 1557–1561. [Google Scholar] [CrossRef]
- DeVilbiss, E.A.; Magnusson, C.; Gardner, R.M.; Rai, D.; Newschaffer, C.J.; Lyall, K.; Dalman, C.; Lee, B.K. Antenatal Nutritional Supplementation and Autism Spectrum Disorders in the Stockholm Youth Cohort: Population Based Cohort Study. BMJ 2017, 359, 4273. [Google Scholar] [CrossRef]
- Wagner, C.; Baggerly, C.; McDonnell, S.; Baggerly, K.A.; French, C.; Baggerly, L.; Hamilton, S.; Hollis, B. Post-Hoc Analysis of Vitamin D Status and Reduced Risk of Preterm Birth in Two Vitamin D Pregnancy Cohorts Compared with South Carolina March of Dimes 2009–2011 Rates. J. Steroid Biochem. Mol. Biol. 2016, 155, 245–251. [Google Scholar] [CrossRef]
- Hollis, B.W.; Johnson, D.; Hulsey, T.C.; Ebeling, M.; Wagner, C.L. Vitamin D Supplementation during Pregnancy: Double-blind, Randomized Clinical Trial of Safety and Effectiveness. J. Bone Miner. Res. 2011, 26, 2341–2357. [Google Scholar] [CrossRef]
- Heaney, R.P.; Davies, K.M.; Chen, T.C.; Holick, M.F.; Barger-Lux, M.J. Human Serum 25-Hydroxycholecalciferol Response to Extended Oral Dosing with Cholecalciferol. Am. J. Clin. Nutr. 2003, 77, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, R.J.; Iosif, A.; Angel, E.G.; Ozonoff, S. Association of Maternal Prenatal Vitamin use with Risk for Autism Spectrum Disorder Recurrence in Young Siblings. JAMA Psychiatry 2019, 76, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Fernell, E.; Barnevik-Olsson, M.; Bågenholm, G.; Gillberg, C.; Gustafsson, S.; Sääf, M. Serum Levels of 25-hydroxyvitamin D in Mothers of Swedish and of Somali Origin Who have Children with and without Autism. Acta Paediatr. 2010, 99, 743–747. [Google Scholar] [CrossRef] [PubMed]
- Principi, N.; Esposito, S. Vitamin D Deficiency during Pregnancy and Autism Spectrum Disorders Development. Front. Psychiatry. 2020, 10, 987. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).