Ergogenic Effect of BCAAs and L-Alanine Supplementation: Proof-of-Concept Study in a Murine Model of Physiological Exercise
Abstract
1. Introduction
2. Materials and Methods
2.1. Preliminary Pharmacokinetic Study
2.2. Animal Groups, Treatments, and Training Protocol
2.3. In Vivo Monitoring and Functional Tests
2.3.1. Forelimb Grip Strength, Resistance to Exercise, and Isometric Torque
2.3.2. Hind Limb Ultrasonography
2.4. Ex vivo procedures
2.4.1. Sample Collection, Processing, and Storage
2.4.2. Muscle Histology, Histochemistry, and Immunofluorescence
2.4.3. Isolation of Total RNA, Reverse Transcription, and qRT-PCR for Gene Expression Analysis
2.4.4. Determination of pAMPK/AMPK Ratio by Western Blot
2.4.5. Determination of CK and LDH Plasma Levels
2.4.6. Determination of Salivary IgA Levels
2.4.7. Determination of Hepatic and Muscular ALT Activity
2.5. Statistics
3. Results
3.1. Pharmacokinetic Data
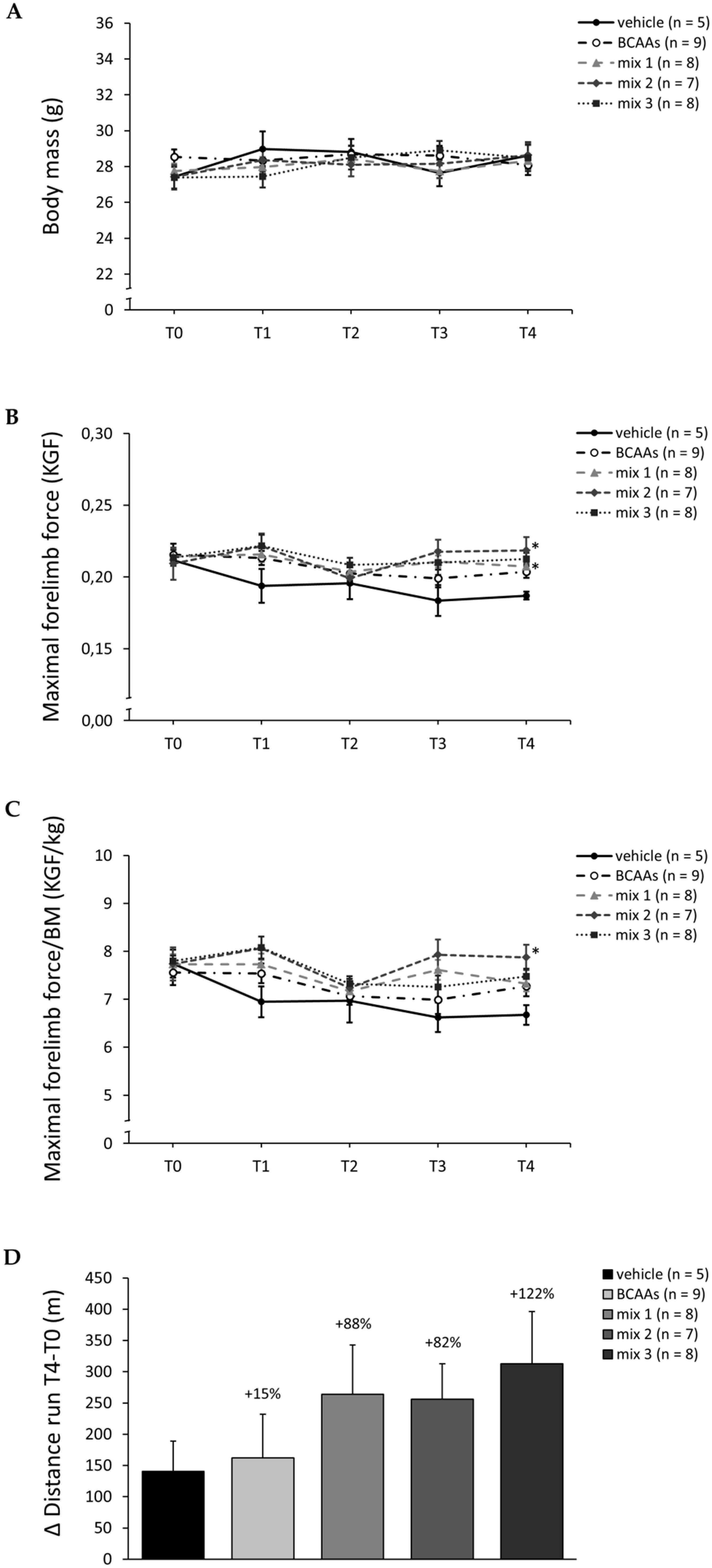
3.2. In Vivo Data (4-Week Study)
3.3. Ex Vivo Data (4-Week Study)
3.3.1. Weights of Vital Organs and Muscles
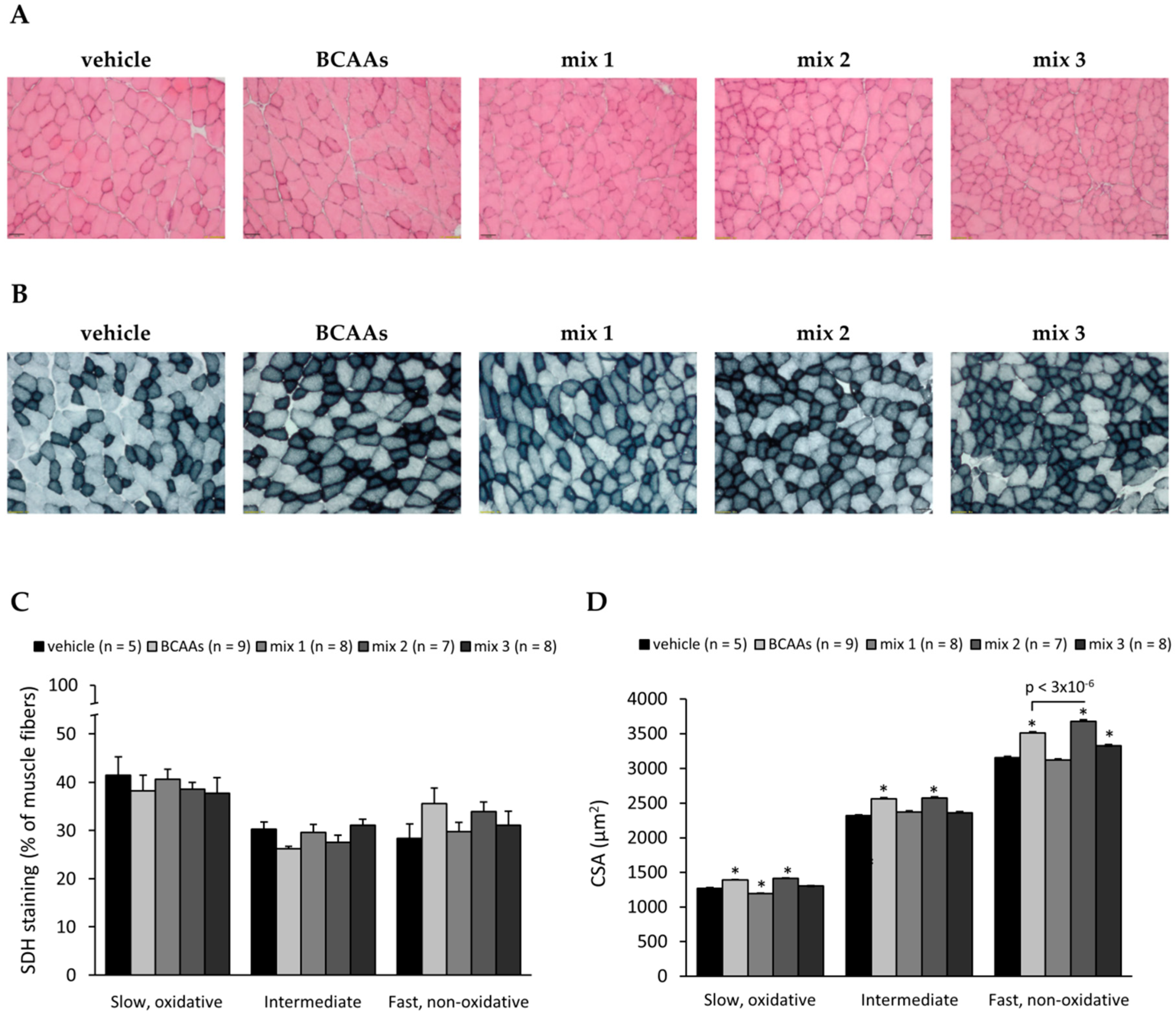
3.3.2. Muscle Histology Characterization and Myofiber Type Classification
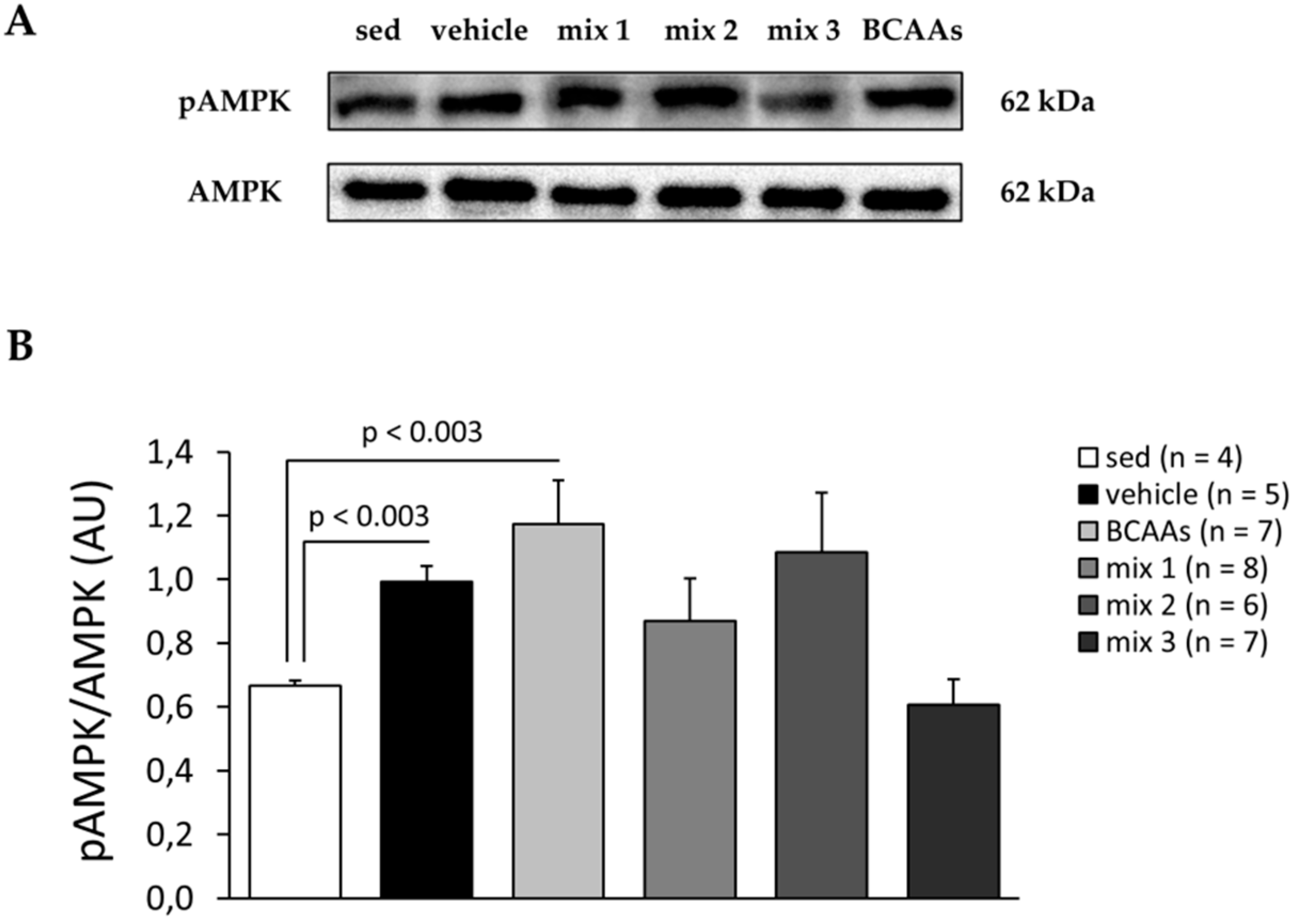
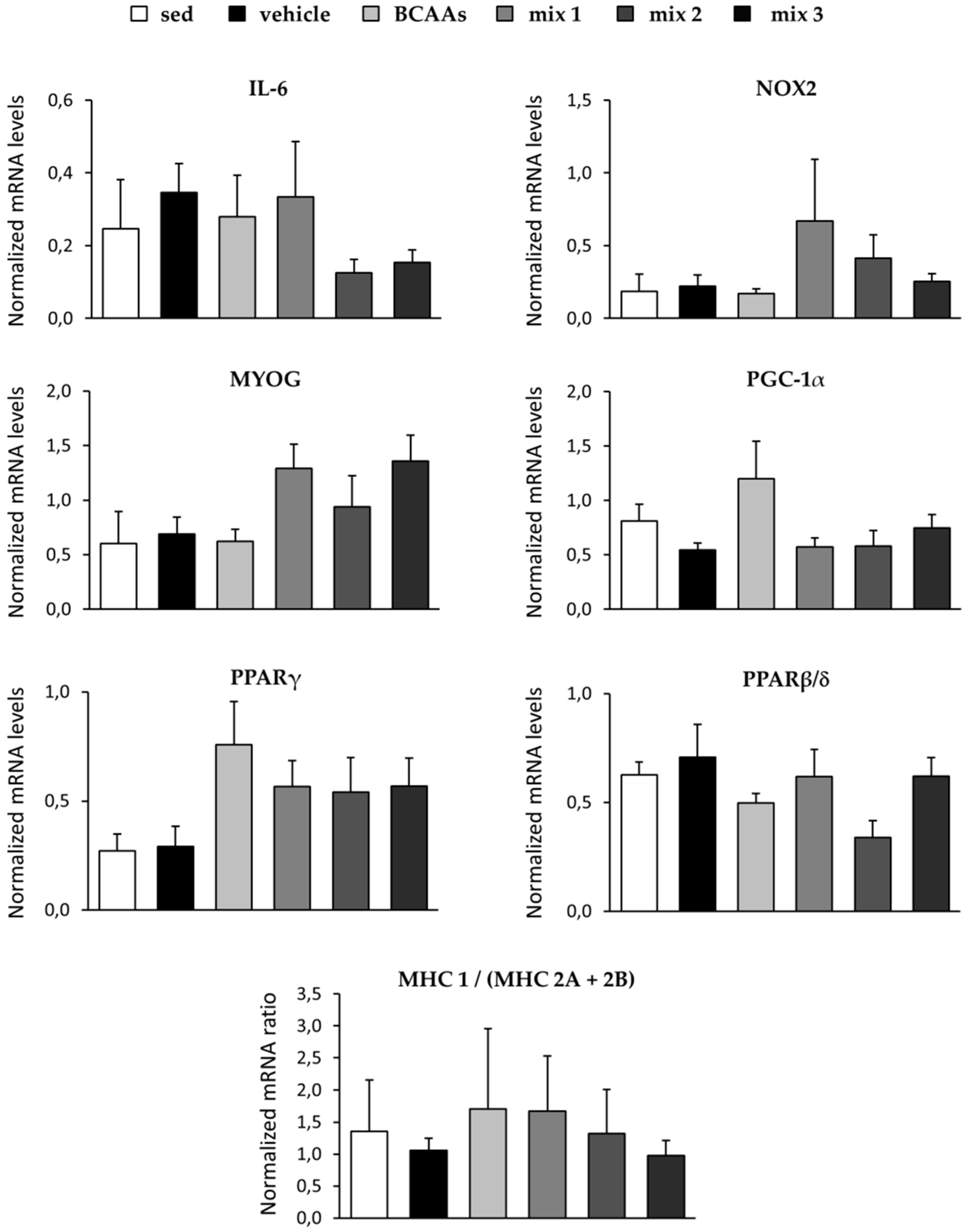
3.3.3. Protein and Gene Expression Analyses
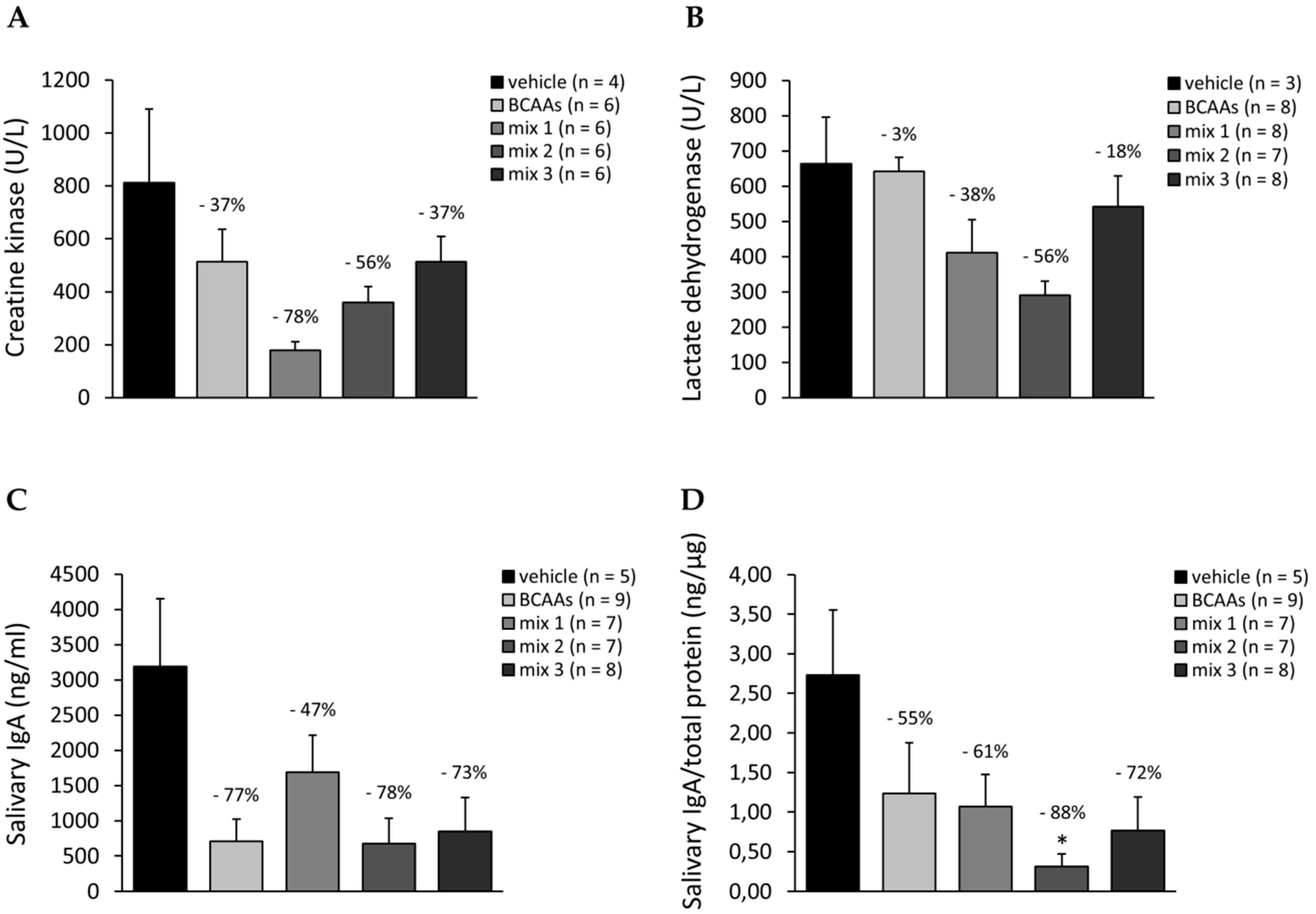
3.3.4. Biomarkers Related to Muscle Damage, Immune Response, and Amino Acid Metabolism
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Harper, A.E.; Miller, R.H.; Block, K.P. Branched-Chain Amino Acid Metabolism. Annu. Rev. Nutr. 1984, 4, 409–454. [Google Scholar] [CrossRef] [PubMed]
- Bifari, F.; Nisoli, E. Branched-chain amino acids differently modulate catabolic and anabolic states in mammals: A pharmacological point of view. Br. J. Pharmacol. 2017, 174, 1366–1377. [Google Scholar] [CrossRef] [PubMed]
- Holeček, M. Branched-chain amino acids in health and disease: Metabolism, alterations in blood plasma, and as supplements. Nutr. Metab. 2018, 15, 33. [Google Scholar] [CrossRef] [PubMed]
- Nair, K.S.; Short, K.R. Hormonal and signaling role of branched-chain amino acids. J. Nutr. 2005, 135, 1547S–1552S. [Google Scholar] [CrossRef]
- Nair, K.S.; Schwartz, R.G.; Welle, S. Leucine as a regulator of whole body and skeletal muscle protein metabolism in humans. Am. J. Physiol. 1992, 263, E928–E934. [Google Scholar] [CrossRef]
- Zhang, S.; Zeng, X.; Ren, M.; Mao, X.; Qiao, S. Novel metabolic and physiological functions of branched chain amino acids: A review. J. Anim. Sci. Biotechnol. 2017, 8, 10. [Google Scholar] [CrossRef]
- Drummond, M.J.; Rasmussen, B.B. Leucine-Enriched Nutrients and the Regulation of mTOR Signalling and Human Skeletal Muscle Protein Synthesis. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 222–226. [Google Scholar] [CrossRef]
- Wilkinson, D.J.; Hossain, T.; Hill, D.S.; Phillips, B.E.; Crossland, H.; Williams, J.; Loughna, P.; Churchward-Venne, T.A.; Breen, L.; Phillips, S.M.; et al. Effects of leucine and its metabolite β-hydroxy-β-methylbutyrate on human skeletal muscle protein metabolism. J. Physiol. 2013, 591, 2911–2923. [Google Scholar] [CrossRef]
- Tischler, M.E.; Desautels, M.; Goldberg, A.L. Does leucine, leucyl-tRNA, or some metabolite of leucine regulate protein synthesis and degradation in skeletal and cardiac muscle? J. Biol. Chem. 1982, 257, 1613–1621. [Google Scholar]
- Ikeda, T.; Aizawa, J.; Nagasawa, H.; Gomi, I.; Kugota, H.; Nanjo, K.; Jinno, T.; Masuda, T.; Morita, S. Effects and feasibility of exercise therapy combined with branched-chain amino acid supplementation on muscle strengthening in frail and pre-frail elderly people requiring long-term care: A crossover trial. Appl. Physiol. Nutr. Metab. Physiol. Appl. Nutr. Metab. 2016, 41, 438–445. [Google Scholar] [CrossRef]
- Fouré, A.; Bendahan, D. Is Branched-Chain Amino Acids Supplementation an Efficient Nutritional Strategy to Alleviate Skeletal Muscle Damage? A Systematic Review. Nutrients 2017, 9, 1047. [Google Scholar] [CrossRef]
- Yuan, H.-X.; Xiong, Y.; Guan, K.-L. Nutrient Sensing, Metabolism, and Cell Growth Control. Mol. Cell 2013, 49, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Shimomura, Y.; Fujii, H.; Suzuki, M.; Murakami, T.; Fujitsuka, N.; Nakai, N. Branched-chain alpha-keto acid dehydrogenase complex in rat skeletal muscle: Regulation of the activity and gene expression by nutrition and physical exercise. J. Nutr. 1995, 125, 1762S–1765S. [Google Scholar] [PubMed]
- Shimomura, Y.; Yamamoto, Y.; Bajotto, G.; Sato, J.; Murakami, T.; Shimomura, N.; Kobayashi, H.; Mawatari, K. Nutraceutical effects of branched-chain amino acids on skeletal muscle. J. Nutr. 2006, 136, 529S–532S. [Google Scholar] [CrossRef] [PubMed]
- Shimomura, Y.; Murakami, T.; Nakai, N.; Nagasaki, M.; Harris, R.A. Exercise Promotes BCAA Catabolism: Effects of BCAA Supplementation on Skeletal Muscle during Exercise. J. Nutr. 2004, 134, 1583S–1587S. [Google Scholar] [CrossRef]
- D’Antona, G.; Ragni, M.; Cardile, A.; Tedesco, L.; Dossena, M.; Bruttini, F.; Caliaro, F.; Corsetti, G.; Bottinelli, R.; Carruba, M.O.; et al. Branched-Chain Amino Acid Supplementation Promotes Survival and Supports Cardiac and Skeletal Muscle Mitochondrial Biogenesis in Middle-Aged Mice. Cell Metab. 2010, 12, 362–372. [Google Scholar] [CrossRef]
- MacLean, D.A.; Graham, T.E.; Saltin, B. Branched-chain amino acids augment ammonia metabolism while attenuating protein breakdown during exercise. Am. J. Physiol. 1994, 267, E1010–E1022. [Google Scholar] [CrossRef]
- Coombes, J.S.; McNaughton, L.R. Effects of branched-chain amino acid supplementation on serum creatine kinase and lactate dehydrogenase after prolonged exercise. J. Sports Med. Phys. Fitness 2000, 40, 240–246. [Google Scholar]
- Wolfe, R.R. Branched-chain amino acids and muscle protein synthesis in humans: Myth or reality? J. Int. Soc. Sports Nutr. 2017, 14, 1–7. [Google Scholar] [CrossRef]
- Columbus, D.A.; Fiorotto, M.L.; Davis, T.A. Leucine is a major regulator of muscle protein synthesis in neonates. Amino Acids 2015, 47, 259–270. [Google Scholar] [CrossRef]
- Spillane, M.; Emerson, C.; Willoughby, D.S. The effects of 8 weeks of heavy resistance training and branched-chain amino acid supplementation on body composition and muscle performance. Nutr. Health 2012, 21, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Watson, P.; Shirreffs, S.M.; Maughan, R.J. The effect of acute branched-chain amino acid supplementation on prolonged exercise capacity in a warm environment. Eur. J. Appl. Physiol. 2004, 93, 306–314. [Google Scholar] [CrossRef]
- Ferrando, A.A.; Williams, B.D.; Stuart, C.A.; Lane, H.W.; Wolfe, R.R. Oral branched-chain amino acids decrease whole-body proteolysis. JPEN J. Parenter. Enteral Nutr. 1995, 19, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Holecek, M.; Siman, P.; Vodenicarovova, M.; Kandar, R. Alterations in protein and amino acid metabolism in rats fed a branched-chain amino acid- or leucine-enriched diet during postprandial and postabsorptive states. Nutr. Metab. 2016, 13, 12. [Google Scholar] [CrossRef] [PubMed]
- Haymond, M.W.; Miles, J.M. Branched chain amino acids as a major source of alanine nitrogen in man. Diabetes 1982, 31, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Holeček, M.; Skopec, F.; Šprongl, L.; Mráz, J.; Skalská, H.; Pecka, M. Effect of alanyl-glutamine on leucine and protein metabolism in irradiated rats. Amino Acids 2002, 22, 95–108. [Google Scholar] [CrossRef]
- Coqueiro, A.Y.; Raizel, R.; Hypólito, T.M.; Tirapegui, J. Effects of supplementation with L-glutamine and L-alanine in the body composition of rats submitted to resistance exercise. Rev. Bras. Ciênc. Esporte 2017, 39, 417–423. [Google Scholar] [CrossRef]
- Minegishi, Y.; Otsuka, A.; Ota, N.; Ishii, K.; Shimotoyodome, A. Combined Supplementation of Pre-Exercise Carbohydrate, Alanine, and Proline and Continuous Intake of Green Tea Catechins Effectively Boost Endurance Performance in Mice. Nutrients 2018, 10, 925. [Google Scholar] [CrossRef]
- Raizel, R.; Leite, J.S.M.; Hypólito, T.M.; Coqueiro, A.Y.; Newsholme, P.; Cruzat, V.F.; Tirapegui, J. Determination of the anti-inflammatory and cytoprotective effects of l-glutamine and l-alanine, or dipeptide, supplementation in rats submitted to resistance exercise. Br. J. Nutr. 2016, 116, 470–479. [Google Scholar] [CrossRef]
- Gervasi, M.; Sisti, D.; Amatori, S.; Zeppa, S.D.; Annibalini, G.; Piccoli, G.; Vallorani, L.; Benelli, P.; Rocchi, M.B.L.; Barbieri, E.; et al. Effects of a commercially available branched-chain amino acid-alanine-carbohydrate-based sports supplement on perceived exertion and performance in high intensity endurance cycling tests. J. Int. Soc. Sports Nutr. 2020, 17, 6. [Google Scholar] [CrossRef]
- Conte, E.; Bresciani, E.; Rizzi, L.; Cappellari, O.; De Luca, A.; Torsello, A.; Liantonio, A. Cisplatin-induced skeletal muscle dysfunction: Mechanisms and counteracting therapeutic strategies. Int. J. Mol. Sci. 2020, 21, 1242. [Google Scholar] [CrossRef] [PubMed]
- Camerino, G.M.; Pierno, S.; Liantonio, A.; Bellis, M.D.; Cannone, M.; Sblendorio, V.; Conte, E.; Mele, A.; Tricarico, D.; Tavella, S.; et al. Effects of Pleiotrophin Overexpression on Mouse Skeletal Muscles in Normal Loading and in Actual and Simulated Microgravity. PLoS ONE 2013, 8, e72028. [Google Scholar] [CrossRef] [PubMed]
- Capogrosso, R.F.; Mantuano, P.; Uaesoontrachoon, K.; Cozzoli, A.; Giustino, A.; Dow, T.; Srinivassane, S.; Filipovic, M.; Bell, C.; Vandermeulen, J.; et al. Ryanodine channel complex stabilizer compound S48168/ARM210 as a disease modifier in dystrophin-deficient mdx mice: Proof-of-concept study and independent validation of efficacy. FASEB J. 2018, 32, 1025–1043. [Google Scholar] [CrossRef] [PubMed]
- Mantuano, P.; Sanarica, F.; Conte, E.; Morgese, M.G.; Capogrosso, R.F.; Cozzoli, A.; Fonzino, A.; Quaranta, A.; Rolland, J.-F.; De Bellis, M.; et al. Effect of a long-term treatment with metformin in dystrophic mdx mice: A reconsideration of its potential clinical interest in Duchenne muscular dystrophy. Biochem. Pharmacol. 2018, 154, 89–103. [Google Scholar] [CrossRef]
- Mele, A.; Mantuano, P.; De Bellis, M.; Rana, F.; Sanarica, F.; Conte, E.; Morgese, M.G.; Bove, M.; Rolland, J.-F.; Capogrosso, R.F.; et al. A long-term treatment with taurine prevents cardiac dysfunction in mdx mice. Transl. Res. J. Lab. Clin. Med. 2019, 204, 82–99. [Google Scholar] [CrossRef] [PubMed]
- Sanarica, F.; Mantuano, P.; Conte, E.; Cozzoli, A.; Capogrosso, R.F.; Giustino, A.; Cutrignelli, A.; Cappellari, O.; Rolland, J.F.; De Bellis, M.; et al. Proof-of-concept validation of the mechanism of action of Src tyrosine kinase inhibitors in dystrophic mdx mouse muscle: In vivo and in vitro studies. Pharmacol. Res. 2019, 145, 104260. [Google Scholar] [CrossRef]
- Capogrosso, R.F.; Cozzoli, A.; Mantuano, P.; Camerino, G.M.; Massari, A.M.; Sblendorio, V.T.; De Bellis, M.; Tamma, R.; Giustino, A.; Nico, B.; et al. Assessment of resveratrol, apocynin and taurine on mechanical-metabolic uncoupling and oxidative stress in a mouse model of duchenne muscular dystrophy: A comparison with the gold standard, α-methyl prednisolone. Pharmacol. Res. 2016, 106, 101–113. [Google Scholar] [CrossRef]
- De Angelis, K.; Wichi, R.B.; Jesus, W.R.A.; Moreira, E.D.; Morris, M.; Krieger, E.M.; Irigoyen, M.C. Exercise training changes autonomic cardiovascular balance in mice. J. Appl. Physiol. 2004, 96, 2174–2178. [Google Scholar] [CrossRef]
- Meier, P.; Renga, M.; Hoppeler, H.; Baum, O. The impact of antioxidant supplements and endurance exercise on genes of the carbohydrate and lipid metabolism in skeletal muscle of mice. Cell Biochem. Funct. 2013, 31, 51–59. [Google Scholar] [CrossRef]
- De Luca, A.; Pierno, S.; Liantonio, A.; Cetrone, M.; Camerino, C.; Fraysse, B.; Mirabella, M.; Servidei, S.; Rüegg, U.T.; Camerino, D.C. Enhanced dystrophic progression in mdx mice by exercise and beneficial effects of taurine and insulin-like growth factor-1. J. Pharmacol. Exp. Ther. 2003, 304, 453–463. [Google Scholar] [CrossRef]
- Capogrosso, R.F.; Mantuano, P.; Cozzoli, A.; Sanarica, F.; Massari, A.M.; Conte, E.; Fonzino, A.; Giustino, A.; Rolland, J.-F.; Quaranta, A.; et al. Contractile efficiency of dystrophic mdx mouse muscle: In vivo and ex vivo assessment of adaptation to exercise of functional end points. J. Appl. Physiol. 2017, 122, 828–843. [Google Scholar] [CrossRef] [PubMed]
- Harish, P.; Malerba, A.; Lu-Nguyen, N.; Forrest, L.; Cappellari, O.; Roth, F.; Trollet, C.; Popplewell, L.; Dickson, G. Inhibition of myostatin improves muscle atrophy in oculopharyngeal muscular dystrophy (OPMD). J. Cachexia Sarcopenia Muscle 2019, 10, 1016–1026. [Google Scholar] [CrossRef] [PubMed]
- Wada, M.; Orihara, K.; Kamagata, M.; Hama, K.; Sasaki, H.; Haraguchi, A.; Miyakawa, H.; Nakao, A.; Shibata, S. Circadian clock-dependent increase in salivary IgA secretion modulated by sympathetic receptor activation in mice. Sci. Rep. 2017, 7, 8802. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Cánoves, P.; Scheele, C.; Pedersen, B.K.; Serrano, A.L. Interleukin-6 myokine signaling in skeletal muscle: A double-edged sword? FEBS J. 2013, 280, 4131–4148. [Google Scholar] [CrossRef]
- Choi, S.; Disilvio, B.; Fernstrom, M.H.; Fernstrom, J.D. Oral branched-chain amino acid supplements that reduce brain serotonin during exercise in rats also lower brain catecholamines. Amino Acids 2013, 45, 1133–1142. [Google Scholar] [CrossRef]
- Coqueiro, A.Y.; Raizel, R.; Bonvini, A.; Hypólito, T.; Godois, A.D.M.; Pereira, J.R.R.; de Oliveira, G.A.B.; Lara, R.D.S.B.; Rogero, M.M.; Tirapegui, J. Effects of Glutamine and Alanine Supplementation on Central Fatigue Markers in Rats Submitted to Resistance Training. Nutrients 2018, 10, 119. [Google Scholar] [CrossRef]
- Kornegay, J.N.; Childers, M.K.; Bogan, D.J.; Bogan, J.R.; Nghiem, P.; Wang, J.; Fan, Z.; Howard, J.F.; Schatzberg, S.J.; Dow, J.L.; et al. The paradox of muscle hypertrophy in muscular dystrophy. Phys. Med. Rehabil. Clin. N. Am. 2012, 23, 149–172. [Google Scholar] [CrossRef]
- Bassit, R.A.; Sawada, L.A.; Bacurau, R.F.P.; Navarro, F.; Martins, E.; Santos, R.V.T.; Caperuto, E.C.; Rogeri, P.; Costa Rosa, L.F.B.P. Branched-chain amino acid supplementation and the immune response of long-distance athletes. Nutr. Burbank Los Angel. Cty. Calif 2002, 18, 376–379. [Google Scholar] [CrossRef]
- Hennigar, S.R.; McClung, J.P.; Pasiakos, S.M. Nutritional interventions and the IL-6 response to exercise. FASEB J. 2017, 31, 3719–3728. [Google Scholar] [CrossRef]
- Burrill, J.S.; Long, E.K.; Reilly, B.; Deng, Y.; Armitage, I.M.; Scherer, P.E.; Bernlohr, D.A. Inflammation and ER Stress Regulate Branched-Chain Amino Acid Uptake and Metabolism in Adipocytes. Mol. Endocrinol. 2015, 29, 411–420. [Google Scholar] [CrossRef]
- Malerba, A.; Roth, F.; Harish, P.; Dhiab, J.; Lu-Nguyen, N.; Cappellari, O.; Jarmin, S.; Mahoudeau, A.; Ythier, V.; Lainé, J.; et al. Pharmacological modulation of the ER stress response ameliorates oculopharyngeal muscular dystrophy. Hum. Mol. Genet. 2019, 28, 1694–1708. [Google Scholar] [CrossRef] [PubMed]
- Peake, J.M.; Neubauer, X.O.; Walsh, N.P.; Simpson, R.J. Recovery of the immune system after exercise. J. Appl. Physiol. 2017, 122, 1077–1087. [Google Scholar] [CrossRef] [PubMed]
- Into, T.; Takigawa, T.; Niida, S.; Shibata, K. MyD88 deficiency alters expression of antimicrobial factors in mouse salivary glands. PLoS ONE 2014, 9, e113333. [Google Scholar] [CrossRef] [PubMed]



| Formulation | Composition: BCAAs + ALA (Weight Ratio of L-Leu: L-Ile: L-Val: L-Ala) | Final Dose (mg/kg) |
|---|---|---|
| BCAAs | 2:1:1 | 656 |
| mix 1 | 2:1:1:1 | 820 |
| mix 2 | 2:1:1:2 | 984 |
| mix 3 | 2:1:1:3 | 1148 |
| PK Parameters—Cmax (µg/mL) | |||
|---|---|---|---|
| Group | L-Valine | L-Leucine | L-Isoleucine |
| BCAAs (n = 6) | 6.54 ± 0.62 | 11.14 ± 3.52 | 5.18 ± 1.59 |
| mix 1 (n = 6) | 21.21 ± 2.77 ° | 23.04 ± 3.19 | 11.19 ± 1.58 |
| mix 2 (n = 6) | 13.88 ± 2.94 | 18.82 ± 2.92 | 10.37 ± 2.03 |
| mix 3 (n = 6) | 13.15 ± 2.13 | 10.93 ± 2.11 | 5.61 ± 0.96 |
| Group | Weight of Hind Limb Muscles/BM (mg/g) | Weight of Vital Organs/BM (mg/g) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| TA | GC | EDL | QUAD | SOL | Liver | Heart | Kidneys | Spleen | |
| vehicle (n = 5) | 1.7 ± 0.02 | 2.2 ± 0.02 | 0.38 ± 0.03 | 6.1 ± 0.57 | 0.25 ± 0.01 | 53.2 ± 3.4 | 5.2 ± 0.10 | 5.9 ± 0.11 | 2.8 ± 0.15 |
| BCAAs (n = 9) | 1.6 ± 0.03 | 2.3 ± 0.03 | 0.32 ± 0.01 | 6.9 ± 0.10 | 0.30 ± 0.02 | 57.8 ± 2.3 | 5.4 ± 0.12 | 5.9 ± 0.17 | 3.3 ± 0.17 |
| mix 1 (n = 8) | 2.1 ± 0.03 *,° | 2.1 ± 0.03 | 0.42 ± 0.02 ° | 7.8 ± 0.18 *,° | 0.36 ± 0.01 | 42.6 ± 2.3 ° | 5.6 ± 0.33 | 6.5 ± 0.12 | 3.3 ± 0.24 |
| mix 2 (n = 7) | 1.9 ± 0.02 *,° | 2.4 ± 0.12 | 0.37 ± 0.02 | 7.9 ± 0.17 *,° | 0.47 ± 0.04 * | 47.2 ± 2.9 | 5.9 ± 0.27 | 6.4 ± 0.21 | 3.9 ± 0.63 |
| mix 3 (n = 8) | 1.8 ± 0.03 ° | 2.1 ± 0.02 ° | 0.44 ± 0.03 ° | 7.1 ± 0.18 | 0.32 ± 0.03 | 52.1 ± 3.3 | 5.4 ± 0.19 | 6.3 ± 0.14 | 3.7 ± 0.36 |
| TA Muscle Histology (H&E Staining) | |||
|---|---|---|---|
| Group | Total Area of Damage (%) | Infiltration (%) | Non-Muscle Area (%) |
| vehicle (n = 5) | 0.62 ± 0.05 | 0.61 ± 0.05 | 0.015 ± 0.012 |
| BCAAs (n = 9) | 0.48 ± 0.11 | 0.44 ± 0.11 | 0.035 ± 0.019 |
| mix 1 (n = 8) | 0.79 ± 0.15 | 0.76 ± 0.15 | 0.023 ± 0.017 |
| mix 2 (n = 7) | 0.52 ± 0.11 | 0.50 ± 0.10 | 0.023 ± 0.015 |
| mix 3 (n = 8) | 0.48 ± 0.09 | 0.48 ± 0.09 | 0.00 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mantuano, P.; Bianchini, G.; Cappellari, O.; Boccanegra, B.; Conte, E.; Sanarica, F.; Mele, A.; Camerino, G.M.; Brandolini, L.; Allegretti, M.; et al. Ergogenic Effect of BCAAs and L-Alanine Supplementation: Proof-of-Concept Study in a Murine Model of Physiological Exercise. Nutrients 2020, 12, 2295. https://doi.org/10.3390/nu12082295
Mantuano P, Bianchini G, Cappellari O, Boccanegra B, Conte E, Sanarica F, Mele A, Camerino GM, Brandolini L, Allegretti M, et al. Ergogenic Effect of BCAAs and L-Alanine Supplementation: Proof-of-Concept Study in a Murine Model of Physiological Exercise. Nutrients. 2020; 12(8):2295. https://doi.org/10.3390/nu12082295
Chicago/Turabian StyleMantuano, Paola, Gianluca Bianchini, Ornella Cappellari, Brigida Boccanegra, Elena Conte, Francesca Sanarica, Antonietta Mele, Giulia M. Camerino, Laura Brandolini, Marcello Allegretti, and et al. 2020. "Ergogenic Effect of BCAAs and L-Alanine Supplementation: Proof-of-Concept Study in a Murine Model of Physiological Exercise" Nutrients 12, no. 8: 2295. https://doi.org/10.3390/nu12082295
APA StyleMantuano, P., Bianchini, G., Cappellari, O., Boccanegra, B., Conte, E., Sanarica, F., Mele, A., Camerino, G. M., Brandolini, L., Allegretti, M., De Bellis, M., Aramini, A., & De Luca, A. (2020). Ergogenic Effect of BCAAs and L-Alanine Supplementation: Proof-of-Concept Study in a Murine Model of Physiological Exercise. Nutrients, 12(8), 2295. https://doi.org/10.3390/nu12082295






