High Serum Folate Concentration Is Associated with Better Lung Function in Male Chronic Obstructive Pulmonary Disease Patients Who Are Current Smokers: Analysis of Nationwide Population-Based Survey
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Data Collection and Measurements
2.3. Statistical Analysis
2.4. Ethical Approval
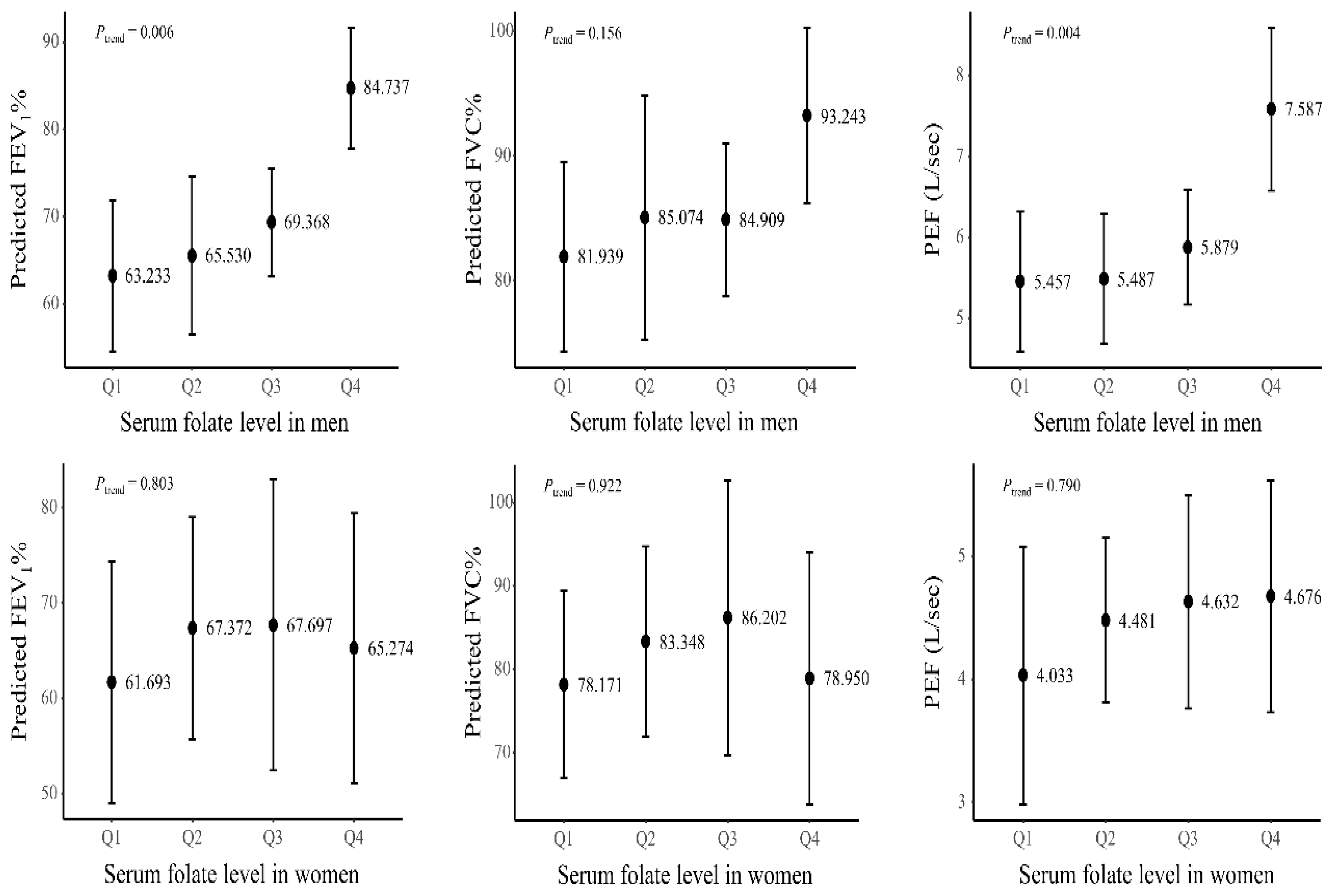
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Decramer, M.; Rennard, S.; Troosters, T.; Mapel, D.W.; Giardino, N.; Mannino, D.; Wouters, E.; Sethi, S.; Cooper, C.B. COPD as a lung disease with systemic consequences–clinical impact, mechanisms, and potential for early intervention. COPD J. Chronic Obstr. Pulm. Dis. 2008, 5, 235–256. [Google Scholar] [CrossRef] [PubMed]
- Soriano, J.B.; Ancochea, J.; Celli, B.R. The most beautiful COPD chart in the world: All together to end COPD! Eur. Respir. J. 2019, 54. [Google Scholar] [CrossRef] [PubMed]
- López-Campos, J.L.; Tan, W.; Soriano, J.B. Global burden of COPD. Respirology 2016, 21, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Lozano, R.; Naghavi, M.; Foreman, K.; Lim, S.; Shibuya, K.; Aboyans, V.; Abraham, J.; Adair, T.; Aggarwal, R.; Ahn, S.Y.; et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2095–2128. [Google Scholar] [CrossRef]
- Lopez, A.D.; Shibuya, K.; Rao, C.; Mathers, C.D.; Hansell, A.L.; Held, L.S.; Schmid, V.; Buist, S. Chronic obstructive pulmonary disease: Current burden and future projections. Eur. Respir. J. 2006, 27, 397–412. [Google Scholar] [CrossRef]
- Kim, T.; Choi, H.; Kim, J. Association between Dietary Nutrient Intake and Chronic Obstructive Pulmonary Disease Severity: A Nationwide Population-Based Representative Sample. COPD J. Chronic Obstr. Pulm. Dis. 2020, 17, 49–58. [Google Scholar] [CrossRef]
- Khan, N.A.; Saini, H.; Mawari, G.; Kumar, S.; Hira, H.S.; Daga, M.K. The Effect of Folic Acid Supplementation on Hyperhomocysteinemia and Pulmonary Function Parameters in Chronic Obstructive Pulmonary Disease: A Pilot Study. J. Clin. Diagn. Res. 2016, 10, OC17–OC21. [Google Scholar] [CrossRef]
- Leng, S.; Picchi, M.A.; Tesfaigzi, Y.; Wu, G.; Gauderman, W.J.; Xu, F.; Gilliland, F.D.; Belinsky, S.A. Dietary nutrients associated with preservation of lung function in Hispanic and non-Hispanic white smokers from New Mexico. Int. J Chron. Obstruct. Pulmon. Dis. 2017, 12, 3171–3181. [Google Scholar] [CrossRef]
- Hirayama, F.; Lee, A.H.; Terasawa, K.; Kagawa, Y. Folate intake associated with lung function, breathlessness and the prevalence of chronic obstructive pulmonary disease. Asia Pac. J. Clin. Nutr. 2010, 19, 103–109. [Google Scholar]
- Naderi, N.; House, J.D. Recent Developments in Folate Nutrition. Adv. Food Nutr. Res. 2018, 83, 195–213. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, E.; Hyun, T. Dietary folate intake and food sources of children and adolescents in Chungcheong area-using nutrient database revised by measured folate in selected foods. J. Nutr. Health 2015, 48, 94–104. [Google Scholar] [CrossRef]
- Li, Y.; Huang, T.; Zheng, Y.; Muka, T.; Troup, J.; Hu, F.B. Folic Acid Supplementation and the Risk of Cardiovascular Diseases: A Meta-Analysis of Randomized Controlled Trials. J. Am. Heart Assoc. 2016, 5, e003768. [Google Scholar] [CrossRef] [PubMed]
- Matsui, E.C.; Matsui, W. Higher serum folate levels are associated with a lower risk of atopy and wheeze. J. Allergy Clin. Immunol. 2009, 123, 1253–1259. [Google Scholar] [CrossRef][Green Version]
- Seemungal, T.A.; Lun, J.C.; Davis, G.; Neblett, C.; Chinyepi, N.; Dookhan, C.; Drakes, S.; Mandeville, E.; Nana, F.; Setlhake, S.; et al. Plasma homocysteine is elevated in COPD patients and is related to COPD severity. Int. J. Chron. Obstruct. Pulmon. Dis. 2007, 2, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, D.; Sharma, N.; Senapati, S. Serum Homocysteine Could Be Used as a Predictive Marker for Chronic Obstructive Pulmonary Disease: A Meta-Analysis. Front. Public Health 2019, 7, 69. [Google Scholar] [CrossRef] [PubMed]
- Kweon, S.; Kim, Y.; Jang, M.-j.; Kim, Y.; Kim, K.; Choi, S.; Chun, C.; Khang, Y.-H.; Oh, K. Data resource profile: The Korea national health and nutrition examination survey (KNHANES). Int. J. Epidemiol. 2014, 43, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Hoesein, F.A.M.; Zanen, P.; Lammers, J.-W.J. Lower limit of normal or FEV1/FVC<0.70 in diagnosing COPD: An evidence-based review. Respir. Med. 2011, 105, 907–915. [Google Scholar]
- Miller, M.R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Crapo, R.; Enright, P.v.; Van der Grinten, C.; Gustafsson, P. Standardisation of spirometry. Eur. Respir. J. 2005, 26, 319–338. [Google Scholar] [CrossRef]
- Schwettmann, L.; Berbu, S. Reference Interval and Status for Serum Folate and Serum Vitamin B12 in a Norwegian Population. Clin. Lab. 2015, 61, 1095–1100. [Google Scholar] [CrossRef]
- Durga, J.; van Boxtel, M.P.; Schouten, E.G.; Kok, F.J.; Jolles, J.; Katan, M.B.; Verhoef, P. Effect of 3-year folic acid supplementation on cognitive function in older adults in the FACIT trial: A randomised, double blind, controlled trial. Lancet 2007, 369, 208–216. [Google Scholar] [CrossRef]
- Vardavas, C.I.; Linardakis, M.K.; Hatzis, C.M.; Malliaraki, N.; Saris, W.H.; Kafatos, A.G. Smoking status in relation to serum folate and dietary vitamin intake. Tob. Induc. Dis. 2008, 4, 8. [Google Scholar] [CrossRef] [PubMed]
- Abu Khaled, M.; Watkins, C.L.; Krumdieck, C.L. Inactivation of B12 and folate coenzymes by butyl nitrite as observed by NMR: Implications on one-carbon transfer mechanism. Biochem. Biophys. Res. Commun. 1986, 135, 201–207. [Google Scholar] [CrossRef]
- Bailey, L.B.; Stover, P.J.; McNulty, H.; Fenech, M.F.; Gregory, J.F., 3rd; Mills, J.L.; Pfeiffer, C.M.; Fazili, Z.; Zhang, M.; Ueland, P.M.; et al. Biomarkers of Nutrition for Development-Folate Review. J. Nutr. 2015, 145, 1636S–1680S. [Google Scholar] [CrossRef] [PubMed]
- Winkels, R.M.; Brouwer, I.A.; Verhoef, P.; van Oort, F.V.; Durga, J.; Katan, M.B. Gender and body size affect the response of erythrocyte folate to folic acid treatment. J. Nutr. 2008, 138, 1456–1461. [Google Scholar] [CrossRef] [PubMed]
- Shvetsov, Y.B.; Hernandez, B.Y.; Wong, S.H.; Wilkens, L.R.; Franke, A.A.; Goodman, M.T. Intraindividual variability in serum micronutrients: Effects on reliability of estimated parameters. Epidemiology 2009, 20, 36–43. [Google Scholar] [CrossRef]
- Grandjean, A.C. Dietary intake data collection: Challenges and limitations. Nutr. Rev. 2012, 70 (Suppl. 2), S101–S104. [Google Scholar] [CrossRef]
- Song, Y.; Joung, H. A traditional Korean dietary pattern and metabolic syndrome abnormalities. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 456–462. [Google Scholar] [CrossRef]
- Jung, S.-J.; Park, S.-H.; Choi, E.-K.; Cha, Y.-S.; Cho, B.-H.; Kim, Y.-G.; Kim, M.-G.; Song, W.O.; Park, T.-S.; Ko, J.-K.; et al. Beneficial effects of Korean traditional diets in hypertensive and type 2 diabetic patients. J. Med. Food 2014, 17, 161–171. [Google Scholar] [CrossRef]
- Kim, Y.-N.; Cho, Y.-O. Folate food source, usual intake, and folate status in Korean adults. Nutr. Res. Pract. 2018, 12, 47–51. [Google Scholar] [CrossRef]
- Gliszczyńska-Świgło, A. Antioxidant activity of water soluble vitamins in the TEAC (trolox equivalent antioxidant capacity) and the FRAP (ferric reducing antioxidant power) assays. Food Chem. 2006, 96, 131–136. [Google Scholar] [CrossRef]
- Nakano, E.; Higgins, J.A.; Powers, H.J. Folate protects against oxidative modification of human LDL. Br. J. Nutr. 2001, 86, 637–639. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Lv, X.; Li, W.; Li, Z.; Liu, H.; Gao, Y.; Huang, G. Folic acid modulates VPO1 DNA methylation levels and alleviates oxidative stress-induced apoptosis in vivo and in vitro. Redox Biol. 2018, 19, 81–91. [Google Scholar] [CrossRef]
- Angelis, N.; Porpodis, K.; Zarogoulidis, P.; Spyratos, D.; Kioumis, I.; Papaiwannou, A.; Pitsiou, G.; Tsakiridis, K.; Mpakas, A.; Arikas, S.; et al. Airway inflammation in chronic obstructive pulmonary disease. J. Thorac. Dis. 2014, 6, S167–S172. [Google Scholar] [CrossRef] [PubMed]
- Simpson, J.L. Airway inflammation in COPD: Is it worth measuring and is it clinically meaningful? Respirology 2020, 25, 47–48. [Google Scholar] [CrossRef] [PubMed]
- Nunomiya, K.; Shibata, Y.; Abe, S.; Inoue, S.; Igarashi, A.; Yamauchi, K.; Aida, Y.; Kishi, H.; Sato, M.; Watanabe, T.; et al. Hyperhomocysteinaemia predicts the decline in pulmonary function in healthy male smokers. Eur. Respir. J. 2013, 42, 18–27. [Google Scholar] [CrossRef] [PubMed]
| Mild (n = 125) | Moderate (n = 163) | Severe (n = 23) | p-Value | |
|---|---|---|---|---|
| Population size | 1,012,555 | 1,237,035 | 164,374 | |
| Sex | 0.166 | |||
| Men | 59.4 (4.6) | 65.4 (4.3) | 81.3 (8.3) | |
| Women | 40.6 (4.6) | 34.6 (4.3) | 18.7 (8.3) | |
| Age groups | <0.001 *,† | |||
| 40–49 | 41.6 (4.9) | 20.2 (4.0) | 6.2 (6.0) | |
| 50–59 | 24.6 (4.6) | 27.8 (4.3) | 13.4 (7.8) | |
| 60–69 | 18.3 (3.5) | 26.4 (4.1) | 18.5 (9.6) | |
| ≤70 | 15.4 (3.5) | 25.6 (3.9) | 61.8 (12.0) | |
| Height (m) | 1.65 (0.0) | 1.63 (0.0) | 1.64 (0.0) | 0.313 |
| Body mass index (kg/m2) | 23.6 (0.3) | 23.4 (0.4) | 22.7 (0.9) | 0.660 |
| Residence | 0.030 | |||
| Rural | 15.4 (3.9) | 24.7 (5.4) | 35.4 (11.7) | |
| Urban | 84.6 (3.9) | 75.3 (5.4) | 64.6 (11.7) | |
| Smoking status | 0.196 | |||
| Never | 47.2 (4.8) | 33.6 (4.2) | 22.4 (9.0) | |
| Former | 26.7 (4.4) | 31.3 (4.4) | 35.5 (11.6) | |
| Current | 26.1 (5.6) | 35.1 (4.7) | 42.1 (12.9) | |
| Smoking Pack–Years § | 26.2 (2.1) | 31.1 (3.4) | 34.0 (5.2) | 0.250 |
| Education | 0.085 | |||
| Middle school or less | 32.7 (4.7) | 38.3 (4.9) | 66.8 (10.9) | |
| High school | 37.7 (4.7) | 32.3 (4.7) | 23.1 (9.6) | |
| College or more | 29.6 (4.1) | 29.4 (4.3) | 10.1 (6.1) | |
| Household income | 0.002 †, ‡ | |||
| Lowest | 18.4 (3.7) | 24.9 (4.2) | 59.8 (11.9) | |
| Lower middle | 19.0 (4.2) | 27.7 (5.1) | 26.6 (11.1) | |
| Higher middle | 26.0 (4.6) | 18.6 (3.9) | 3.5 (3.5) | |
| Highest | 36.7 (5.3) | 28.8 (4.6) | 10.1 (6.1) | |
| Hs-CRP (mg/L) | ||||
| mean (SE) | 0.94 (0.2) | 1.72 (0.4) | 1.68 (0.4) | 0.073 |
| median (IQR) | 0.46 (0.30–0.74) | 0.80 (0.49–1.62) | 1.50 (0.54–2.30) | |
| Total calorie intake (kcal/day) | 0.485 | |||
| mean (SE) | 1925.4 (90.4) | 2004.8 (76.3) | 1769.6 (229.8) | |
| median (IQR) | 1775.3 (1298.2–2476.5) | 1970.5 (1466.7–2525.7) | 1527.4 (861.8–2153.8) | |
| Serum Folate level (ng/mL) | 0.124 | |||
| mean (SE) | 6.86 (0.5) | 6.37 (0.5) | 4.86 (0.8) | |
| median (IQR) | 6.65 (4.58–9.43) | 6.00 (4.40–9.13) | 3.50 (3.10–4.70) |
| Age (Years) | Serum Folate (ng/mL) | Hs-CRP (mg/L) | Predicted FEV1% | FEV1 Value (L) | Predicted FVC% | FVC Value (L) | PEF (L/s) | |
|---|---|---|---|---|---|---|---|---|
| Age (years) | 1 | 0.077 (0.378) | 0.138 (0.017) | −0.345 (<0.001) | −0.640 (<0.001) | −0.400 (<0.001) | −0.464 (<0.001) | −0.476 (<0.001) |
| Serum folate (ng/mL) | 0.077 (0.378) | 1 | 0.032 (0.719) | 0.172 (0.048) | −0.022 (0.800) | 0.138 (0.113) | −0.064 (0.467) | −0.001 (0.990) |
| Hs-CRP (mg/L) | 0.138 (0.017) | 0.032 (0.719) | 1 | −0.153 (0.007) | −0.147 (0.011) | −0.113 (0.050) | −0.091 (0.115) | −0.171 (0.003) |
| Predicted FEV1% | −0.345 (<0.001) | 0.172 (0.048) | −0.153 (0.007) | 1 | 0.713 (<0.001) | 0.881 (<0.001) | 0.539 (<0.001) | 0.572 (<0.001) |
| FEV1 value (L) | −0.640 (<0.001) | −0.022 (0.800) | −0.147 (0.011) | 0.713 (<0.001) | 1 | 0.696 (<0.001) | 0.920 (<0.001) | 0.850 (<0.001) |
| Predicted FVC% | −0.400 (<0.001) | 0.138 (0.113) | −0.113 (0.050) | 0.881 (<0.001) | 0.696 (<0.001) | 1 | 0.657 (<0.001) | 0.512 (<0.001) |
| FVC value (L) | −0.464 (<0.001) | −0.064 (0.467) | −0.091 (0.115) | 0.539 (<0.001) | 0.920 (<0.001) | 0.657 (<0.001) | 1 | 0.790 (<0.001) |
| PEF (L/s) | −0.476 (<0.001) | −0.001 (0.990) | −0.171 (0.003) | 0.572 (<0.001) | 0.850 (<0.001) | 0.512 (<0.001) | 0.790 (<0.001) | 1 |
| Crude | Age and (Sex) Adjusted | Multivariable-Adjusted | ||||
|---|---|---|---|---|---|---|
| β (95% CI) | p-Value | β (95% CI) | p-Value | β (95% CI) | p-Value | |
| Total (n = 311) | ||||||
| Predicted FEV1% | 13.315 (−0.487, 27.118)) | 0.058 | 12.134 (−1.556, 25.824) | 0.081 | 33.260 (17.051, 49.469) | <0.001 |
| FEV1 value (L) | −0.153 (−0.789, 0.482) | 0.631 | 0.466 (0.039, 0.893) | 0.033 | 0.875 (0.320, 1.430) | 0.004 |
| Predicted FVC% | 7.944 (−2.757, 18.644) | 0.143 | 9.289 (−0.932, 19.510) | 0.074 | 22.918 (6.874, 38.961) | 0.007 |
| FVC value (L) | −0.559 (−1.371, 0.252) | 0.173 | 0.562 (0.052, 1.073) | 0.031 | 0.893 (0.200, 1.587) | 0.014 |
| PEF (L/s) | −0.341 (−2.002, 1.321) | 0.683 | 1.358 (−0.081, 2.796) | 0.064 | 3.216 (1.751, 4.681) | <0.001 |
| Men (n = 192) | ||||||
| Predicted FEV1% | 17.590 (−4.043, 39.224) | 0.108 | 21.260 (2.318, 40.023) | 0.029 | 33.560 (14.986, 52.135) | 0.001 |
| FEV1 value (L) | 0.514 (−0.510, 1.537) | 0.315 | 0.774 (0.152, 1.395) | 0.016 | 0.855 (0.175, 1.535) | 0.017 |
| Predicted FVC% | 12.050 (−3.519, 27.618) | 0.125 | 17.506 (5.406, 29.606) | 0.006 | 21.766 (2.790, 40.741) | 0.027 |
| FVC value (L) | 0.597 (−0.472, 1.667) | 0.264 | 0.932 (0.230, 1.634) | 0.011 | 0.872 (0.0005, 1.744) | 0.050 |
| PEF (L/s) | 1.720 (−0.854, 4.294) | 0.184 | 2.019 (−0.143, 4.181) | 0.066 | 3.040 (1.277, 4.804) | 0.002 |
| Women (n = 119) | ||||||
| Predicted FEV1% | −4.154 (−23.515, 15.207) | 0.662 | −2.732 (−17.499, 12.035) | 0.706 | 8.422 (−6.523, 23.366) | 0.255 |
| FEV1 value (L) | −0.446 (−1.264, 0.372) | 0.272 | −0.062 (−0.611, 0.487) | 0.819 | 0.264 (−0.123, 0.652) | 0.171 |
| Predicted FVC% | −4.957 (−26.443, 16.529) | 0.639 | −3.156 (−20.043, 13.730) | 0.704 | 9.877 (−6.439, 26.193) | 0.222 |
| FVC value (L) | −0.505 (−1.597, 0.586) | 0.349 | 0.009 (−0.795, 0.813) | 0.982 | 0.458 (−0.065, 0.980) | 0.083 |
| PEF (L/s) | −0.771 (−2.674, 1.132) | 0.412 | −0.118 (−1.839, 1.604) | 0.889 | 1.000 (−0.600, 2.599) | 0.208 |
| Age and Height Adjusted | Multivariable-Adjusted | |||
|---|---|---|---|---|
| β (95% CI) | p-Value | β (95% CI) | p-Value | |
| Current smokers (n = 72) | ||||
| Predicted FEV1% | 24.174 (2.230, 46.118) | 0.032 | 27.481 (5.971, 48.992) | 0.014 |
| FEV1 value (L) | 0.726 (0.107, 1.345) | 0.023 | 0.879 (0.287, 1.472) | 0.005 |
| Predicted FVC% | 16.842 (3.153, 30.531) | 0.017 | 26.347 (12.030, 40.665) | 0.001 |
| FVC value (L) | 0.801 (0.186, 1.416) | 0.012 | 1.149 (0.544, 1.753) | <0.001 |
| PEF (L/s) | 3.297 (0.764, 5.829) | 0.012 | 4.443 (2.067, 6.819) | 0.001 |
| Former smokers (n = 97) | ||||
| Predicted FEV1% | 19.148 (−15.692, 53.987) | 0.272 | −3.142 (−28.970, 22.685) | 0.804 |
| FEV1 value (L) | 1.062 (−0.224, 2.347) | 0.103 | −0.062 (−0.922, 0.798) | 0.883 |
| Predicted FVC% | 14.371 (−15.869, 44.611) | 0.341 | −7.326 (−31.575, 16.922) | 0.539 |
| FVC value (L) | 1.215 (−0.332, 2.763) | 0.120 | −0.396 (−1.553, 0.760) | 0.486 |
| PEF (L/s) | 1.940 (−1.948, 5.828) | 0.318 | −0.316 (−2.806, 2.174) | 0.796 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, T.; Oak, C.-H.; Jung, M.-H.; Jang, T.-W.; Kim, J. High Serum Folate Concentration Is Associated with Better Lung Function in Male Chronic Obstructive Pulmonary Disease Patients Who Are Current Smokers: Analysis of Nationwide Population-Based Survey. Nutrients 2020, 12, 2219. https://doi.org/10.3390/nu12082219
Kim T, Oak C-H, Jung M-H, Jang T-W, Kim J. High Serum Folate Concentration Is Associated with Better Lung Function in Male Chronic Obstructive Pulmonary Disease Patients Who Are Current Smokers: Analysis of Nationwide Population-Based Survey. Nutrients. 2020; 12(8):2219. https://doi.org/10.3390/nu12082219
Chicago/Turabian StyleKim, Taeyun, Chul-Ho Oak, Mann-Hong Jung, Tae-Won Jang, and Jehun Kim. 2020. "High Serum Folate Concentration Is Associated with Better Lung Function in Male Chronic Obstructive Pulmonary Disease Patients Who Are Current Smokers: Analysis of Nationwide Population-Based Survey" Nutrients 12, no. 8: 2219. https://doi.org/10.3390/nu12082219
APA StyleKim, T., Oak, C.-H., Jung, M.-H., Jang, T.-W., & Kim, J. (2020). High Serum Folate Concentration Is Associated with Better Lung Function in Male Chronic Obstructive Pulmonary Disease Patients Who Are Current Smokers: Analysis of Nationwide Population-Based Survey. Nutrients, 12(8), 2219. https://doi.org/10.3390/nu12082219





