Early-Life Supplementation of Bovine Milk Osteopontin Supports Neurodevelopment and Influences Exploratory Behavior
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Housing
2.2. Dietary Groups and Feeding Procedures
2.3. Behavioral Testing
2.4. Magnetic Resonance Imaging
2.4.1. Structural MRI Acquisition and Analysis
2.4.2. Diffusion Tensor Imaging Acquisition and Analysis
2.4.3. Voxel-Based Morphometry
2.5. Sample Collection and Assessment
2.5.1. Sample Collection
2.5.2. Volatile Fatty Acid (VFA) Analysis
2.6. Statistical Analysis
3. Results
3.1. Growth Performance and Health
3.2. VFA Concentration
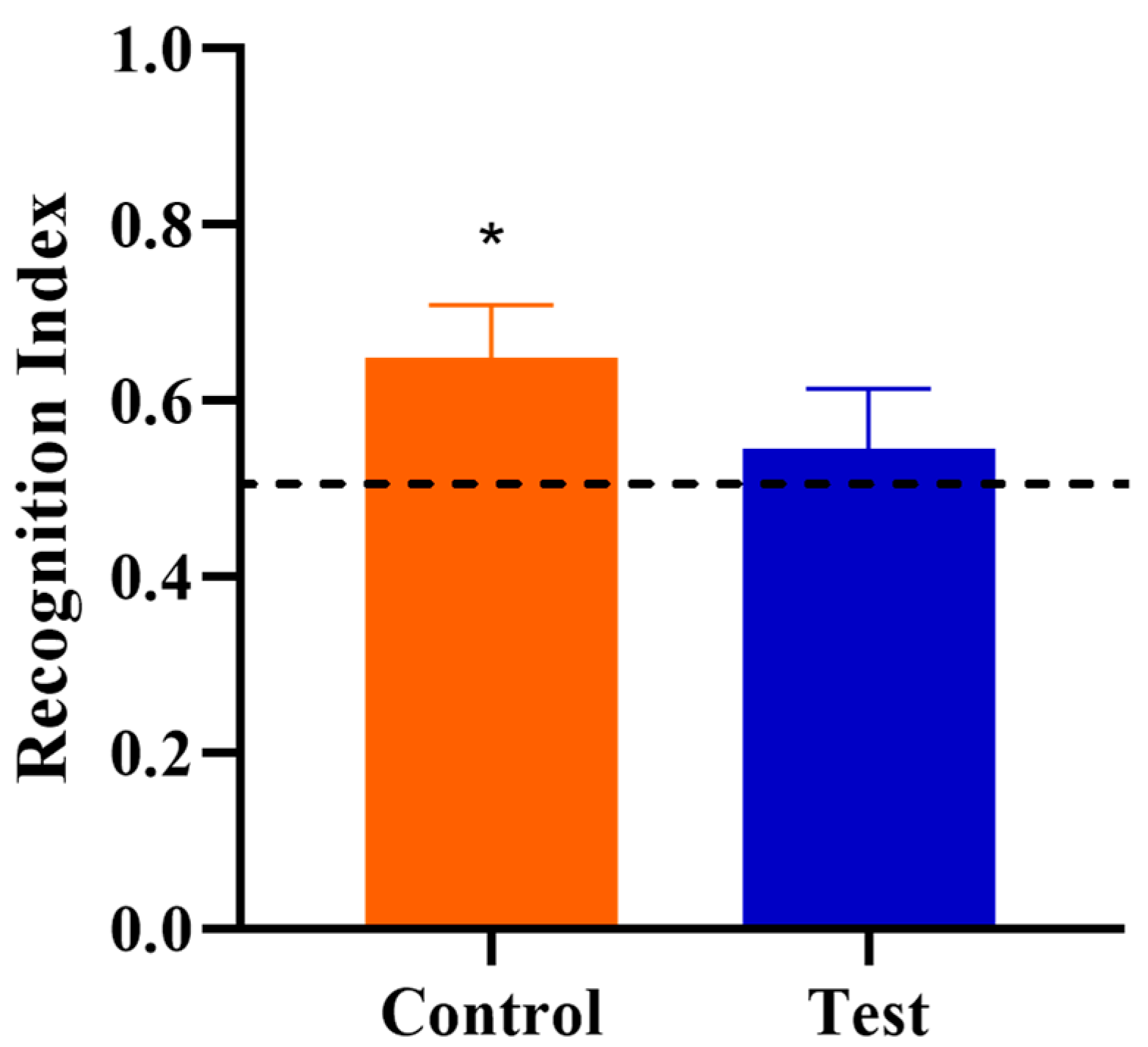
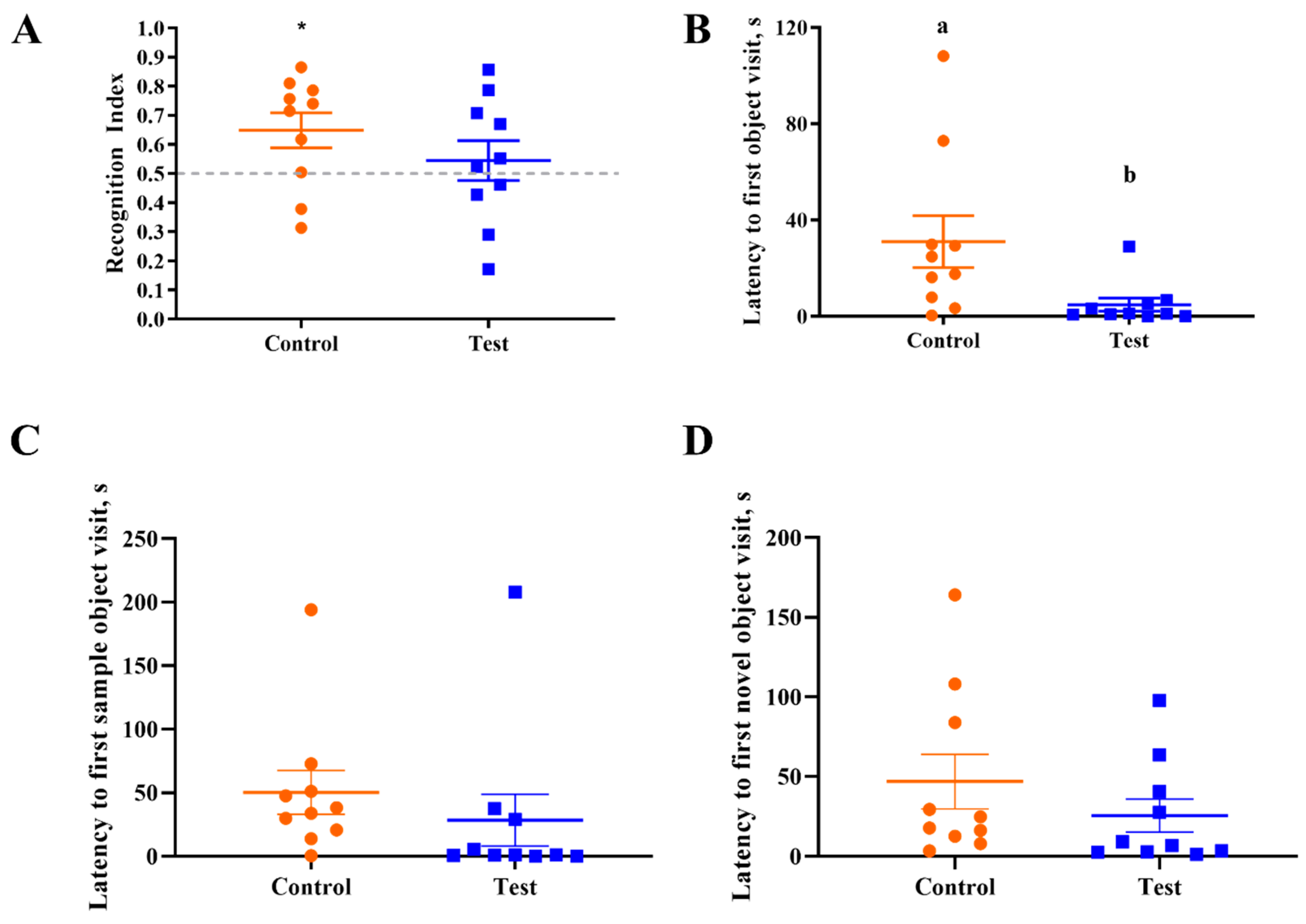
3.3. Novel Object Recognition
3.4. Brain Volume
3.5. Diffusion Tensor Imaging
3.6. Voxel-Based Morphometry
4. Discussion
4.1. Milk Intake and Growth Performance
4.2. VFA
4.3. Magnetic Resonance Imaging
4.4. Behavior
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| OPN | Osteopontin |
| NOR | novel object recognition |
| PND | postnatal day |
| VFA | volatile fatty acid |
References
- Ballard, O.; Morrow, A.L. Human milk composition: Nutrients and bioactive factors. Pediatr. Clin. N. Am. 2013, 60, 49–74. [Google Scholar] [CrossRef] [PubMed]
- Lönnerdal, B. Nutritional and physiologic significance of human milk proteins. Am. J. Clin. Nutr. 2003, 77, 1537S–1543S. [Google Scholar] [CrossRef]
- Agostoni, C.; Braegger, C.; Decsi, T.; Kolacek, S.; Koletzko, B.; Michaelsen, K.F.; Mihatsch, W.; Moreno, L.A.; Puntis, J.; Shamir, R.; et al. Breast-feeding: A commentary by the espghan Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2009, 49, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Lönnerdal, B. Infant formula and infant nutrition: Bioactive proteins of human milk and implications for composition of infant formulas. Am. J. Clin. Nutr. 2014, 99, 712–717. [Google Scholar] [CrossRef]
- Lönnerdal, B. Bioactive Proteins in Human Milk: Health, Nutrition, and Implications for Infant Formulas. J. Pediatr. 2016, 173, S4–S9. [Google Scholar] [CrossRef] [PubMed]
- Lonnerdal, B.; Kvistgaard, A.S.; Peerson, J.M.; Donovan, S.M.; Peng, Y.M. Growth, nutrition, and cytokine response of breast-fed infants and infants fed formula with added bovine osteopontin. J. Pediatr. Gastroenterol. Nutr. 2016, 62, 650–657. [Google Scholar] [CrossRef] [PubMed]
- Schack, L.; Lange, A.; Kelsen, J.; Agnholt, J.; Christensen, B.; Petersen, T.E.; Sørensen, E.S. Considerable variation in the concentration of osteopontin in human milk, bovine milk, and infant formulas. J. Dairy Sci. 2009, 92, 5378–5385. [Google Scholar] [CrossRef] [PubMed]
- Christensen, B.; Sørensen, E.S. Structure, function and nutritional potential of milk osteopontin. Int. Dairy J. 2016, 57, 1–6. [Google Scholar] [CrossRef]
- Sodek, J.; Ganss, B.; Mckeel, D. Osteopontin. Crit. Rev. Oral Biol. Med. 2000, 11, 279–303. [Google Scholar] [CrossRef]
- Jiang, R.; Lönnerdal, B. Biological roles of milk osteopontin. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 214–219. [Google Scholar] [CrossRef]
- Belin, M.T.; Boulanger, P. Involvement of cellular adhesion sequences in the attachment of adenovirus to the HeLa cell surface. J. Gen. Virol. 1993, 74, 1485–1497. [Google Scholar] [CrossRef] [PubMed]
- Pabst, H.F.; Spady, D.W.; Pilarski, L.M.; Carson, M.M.; Beeler, J.A.; Krezolek, M.P. Differential modulation of the immune response by breast- or formula-feeding of intans. Acta Paediatr. 1997, 86, 1291–1297. [Google Scholar] [CrossRef] [PubMed]
- Lönnerdal, B. Biological effects of novel bovine milk fractions. Nestle Nutr. Inst. Workshop Ser. Pediatr. Program 2011, 67, 41–54. [Google Scholar]
- Chatterton, D.E.W.; Rasmussen, J.T.; Heegaard, C.W.; Sørensen, E.S.; Petersen, T.E. In vitro digestion of novel milk protein ingredients for use in infant formulas: Research on biological functions. Trends Food Sci. Technol. 2004, 15, 373–383. [Google Scholar] [CrossRef]
- Donovan, S.M.; Monaco, M.H.; Drnevich, J.; Kvistgaard, A.S.; Hernell, O.; Lönnerdal, B. Bovine Osteopontin Modifies the Intestinal Transcriptome of Formula-Fed Infant Rhesus Monkeys to Be More Similar to Those That Were Breastfed. J. Nutr. 2014, 144, 1910–1919. [Google Scholar] [CrossRef]
- Anderson, J.W.; Johnstone, B.M.; Remley, D.T. Breast-feeding and cognitive development: A meta-analysis. Am. J. Clin. Nutr. 1999, 70, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Deoni, S.C.; Dean, D.C., III; Piryatinsky, I.; O’Muircheartaigh, J.; Waskiewicz, N.; Lehman, K.; Han, M.; Dirks, H. Breastfeeding and early white matter development: A cross-sectional study. Neuroimage 2013, 82, 77–86. [Google Scholar] [CrossRef]
- Jiang, R.; Prell, C.; Lönnerdal, B. Milk osteopontin promotes brain development by up-regulating osteopontin in the brain in early life. FASEB J. 2019, 33, 1681–1694. [Google Scholar] [CrossRef]
- Comi, C.; Carecchio, M.; Chiocchetti, A.; Nicola, S.; Galimberti, D.; Fenoglio, C.; Cappellano, G.; Monaco, F.; Scarpini, E.; Dianzani, U. Osteopontin is increased in the cerebrospinal fluid of patients with Alzheimer’s disease and its levels correlate with cognitive decline. J. Alzheimer’s Dis. 2010, 19, 1143–1148. [Google Scholar] [CrossRef]
- Selvaraju, R.; Bernasconi, L.; Losberger, C.; Graber, P.; Kadi, L.; Avellana-Adalid, V.; Picard-Riera, N.; Van Evercooren, A.B.; Cirillo, R.; Kosco-Vilbois, M.; et al. Osteopontin is upregulated during in vivo demyelination and remyelination and enhances myelin formation in vitro. Mol. Cell. Neurosci. 2004, 25, 707–721. [Google Scholar] [CrossRef]
- Sun, Y.; Yin, X.S.; Guo, H.; Han, R.K.; He, R.D.; Chi, L.J. Elevated osteopontin levels in mild cognitive impairment and Alzheimer’s disease. Mediat. Inflamm. 2013, 2013. [Google Scholar] [CrossRef]
- Van Velthoven, C.T.J.; Heijnen, C.J.; Van Bel, F.; Kavelaars, A. Osteopontin enhances endogenous repair after neonatal hypoxic-ischemic brain injury. Stroke 2011, 42, 2294–2301. [Google Scholar] [CrossRef]
- Jiang, R.; Lönnerdal, B. Evaluation of Bioactivities of Bovine Milk Osteopontin Using a Knockout Mouse Model. J. Pediatr. Gastroenterol. Nutr. 2020, 71, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.R.; Ullrey, D.E. The pig as a model for human nutrition. Annu. Rev. Nutr. 1987, 7, 361–382. [Google Scholar] [CrossRef] [PubMed]
- Dawson, H.D. A Comparative assessment of the pig, mouse and human genomes: structural and functonal analysis of genes involved in immunity and inflammation. In The Minipig in Biomedical Research; McAnulty, P.A., Dayan, A., Hastings, K.H., Ganderup, N.C., Eds.; CRC Press: Boca Raton, FL, USA, 2011; pp. 321–341. [Google Scholar]
- Pang, X.; Hua, X.; Yang, Q.; Ding, D.; Che, C.; Cui, L.; Jia, W.; Bucheli, P.; Zhao, L. Inter-species transplantation of gut microbiota from human to pigs. ISME J. 2007, 1, 156–162. [Google Scholar] [CrossRef]
- Odle, J.; Lin, X.; Jacobi, S.K.; Kim, S.W.; Stahl, C.H. The Suckling Piglet as an Agrimedical Model for the Study of Pediatric Nutrition and Metabolism. Annu. Rev. Anim. Biosci. 2014, 2, 419–444. [Google Scholar] [CrossRef]
- Dobbing, J.; Sands, J. Comparative aspects of the brain growth spurt. Early Hum. Dev. 1979, 3, 79–83. [Google Scholar] [CrossRef]
- Kornum, B.R.; Knudsen, G.M. Cognitive testing of pigs (Sus scrofa) in translational biobehavioral research. Neurosci. Biobehav. Rev. 2011, 35, 437–451. [Google Scholar] [CrossRef] [PubMed]
- Butler, W.T. Structural and functional domains of osteopontin. Ann. N. Y. Acad. Sci. 1995, 760, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Garlow, J.E.; Ka, H.; Johnson, G.A.; Burghardt, R.C.; Jaeger, L.A.; Bazer, F.W. Analysis of osteopontin at the maternal-placental interface in pigs. Biol. Reprod. 2002, 66, 718–725. [Google Scholar] [CrossRef] [PubMed]
- Burkey, T.E.; Skjolaas, K.A.; Dritz, S.S.; Minton, J.E. Expression of Toll-like receptors, interleukin 8, macrophage migration inhibitory factor, and osteopontin in tissues from pigs challenged with Salmonella enterica serovar Typhimurium or serovar Choleraesuis. Vet. Immunol. Immunopathol. 2007, 115, 309–319. [Google Scholar] [CrossRef]
- Ren, S.; Hui, Y.; Goericke-Pesch, S.; Pankratova, S.; Kot, W.; Pan, X.; Thymann, T.; Sangild, P.T.; Nguyen, D.N. Gut and immune effects of bioactive milk factors in preterm pigs exposed to prenatal inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 2019, 317, G67–G77. [Google Scholar] [CrossRef]
- Mudd, A.T.; Alexander, L.S.; Berding, K.; Waworuntu, R.V.; Berg, B.M.; Donovan, S.M.; Dilger, R.N. Dietary Prebiotics, Milk Fat Globule Membrane, and Lactoferrin Affects Structural Neurodevelopment in the Young Piglet. Front. Pediatr. 2016, 4, 1–10. [Google Scholar] [CrossRef]
- National Research Council. Nutrient Requirements of Swine; 11 revised; National Academies Press: Washington, DC, USA, 2012. [Google Scholar]
- Fil, J.E.; Fleming, S.A.; Chichlowski, M.; Gross, G.; Berg, B.M.; Dilger, R.N. Evaluation of Dietary Bovine Milk Fat Globule Membrane Supplementation on Growth, Serum Cholesterol and Lipoproteins, and Neurodevelopment in the Young Pig. Front. Pediatr. 2019, 7, 1–11. [Google Scholar] [CrossRef]
- Fleming, S.A.; Dilger, R.N. Young pigs exhibit differential exploratory behavior during novelty preference tasks in response to age, sex, and delay. Behav. Brain Res. 2017, 321, 50–60. [Google Scholar] [CrossRef]
- Conrad, M.S.; Sutton, B.P.; Dilger, R.N.; Johnson, R.W. An in vivo three-dimensional magnetic resonance imaging-based averaged brain collection of the neonatal piglet (Sus scrofa). PLoS ONE 2014, 9, e107650. [Google Scholar] [CrossRef]
- Mudd, A.T.; Getty, C.M.; Sutton, B.P.; Dilger, R.N. Perinatal choline deficiency delays brain development and alters metabolite concentrations in the young pig. Nutr. Neurosci. 2016, 19, 425–433. [Google Scholar] [CrossRef]
- Kainonen, E.; Rautava, S.; Isolauri, E. Immunological programming by breast milk creates an anti-inflammatory cytokine milieu in breast-fed infants compared to formula-fed infants. Br. J. Nutr. 2013, 109, 1962–1970. [Google Scholar] [CrossRef]
- Kvistgaard, A.S.; Matulka, R.A.; Dolan, L.C.; Ramanujam, K.S. Pre-clinical in vitro and in vivo safety evaluation of bovine whey derived osteopontin, Lacprodan® OPN-10. Food Chem. Toxicol. 2014, 73, 59–70. [Google Scholar] [CrossRef]
- Wang, M.; Smith, B.; Adams, B.; Tran, M.; Dilger, R.; Donovan, S. Osteopontin-Enriched Algae Modulates the Gut Microbiota Composition in Weaning Piglets Infected with Enterotoxigenic Escherichia Coli (P06-069-19). Curr. Dev. Nutr. 2019, 3, 581. [Google Scholar] [CrossRef]
- Sampson, T.R.; Mazmanian, S.K. Control of brain development, function, and behavior by the microbiome. Cell Host Microbe 2015, 17, 565–576. [Google Scholar] [CrossRef]
- Fleming, S.A.; Monaikul, S.; Patsavas, A.J.; Waworuntu, R.V.; Berg, B.M.; Dilger, R.N. Dietary polydextrose and galactooligosaccharide increase exploratory behavior, improve recognition memory, and alter neurochemistry in the young pig. Nutr. Neurosci. 2019, 22, 499–512. [Google Scholar] [CrossRef]
- Greenough, W.T. What’s Special about Development? Thoughts on the Bases of Experience-Sensitive Synaptic Plasticity; Academic Press, Inc.: Cambridge, MA, USA, 1986. [Google Scholar] [CrossRef]
- Giedd, J.N.; Blumenthal, J.; Jeffries, N.O.; Castellanos, F.X.; Liu, H.; Zijdenbos, A.; Paus, T.; Evans, A.C.; Rapoport, J.L. Brain development during childhood and adolescence: A longitudinal MRI study. Nat. Neurosci. 1999, 10, 861–863. [Google Scholar] [CrossRef]
- Conrad, M.S.; Dilger, R.N.; Johnson, R.W. Brain growth of the domestic pig (Sus scrofa) from 2 to 24 weeks of age: A longitudinal MRI study. Dev. Neurosci. 2012, 34, 291–298. [Google Scholar] [CrossRef]
- Sauerwein, H.C.; Lassonde, M.C.; Cardu, B.; Geoffroy, G. Interhemispheric integration of sensory and motor functions in agenesis of the corpus callosum. Neuropsychologia 1981, 19, 445–454. [Google Scholar] [CrossRef]
- Moes, P.; Schilmoeller, K.; Schilmoeller, G. Physical, motor, sensory and developmental features associated with agenesis of the corpus callosum. Child. Care Health Dev. 2009, 35, 656–672. [Google Scholar] [CrossRef]
- Rudge, P.; Warrington, E. Selective Impairment of Memory and. Brain 1991, 114, 349–360. [Google Scholar] [CrossRef]
- Elberger, A.J. The corpus callosum is a critical factor for developing maximum visual acuity. Dev. Brain Res. 1982, 5, 350–353. [Google Scholar] [CrossRef]
- Qiu, D.; Tan, L.H.; Zhou, K.; Khong, P.L. Diffusion tensor imaging of normal white matter maturation from late childhood to young adulthood: Voxel-wise evaluation of mean diffusivity, fractional anisotropy, radial and axial diffusivities, and correlation with reading development. Neuroimage 2008, 41, 223–232. [Google Scholar] [CrossRef]
- Assaf, Y.; Pasternak, O. Diffusion tensor imaging (DTI)-based white matter mapping in brain research: A review. J. Mol. Neurosci. 2008, 34, 51–61. [Google Scholar] [CrossRef]
- Jones, D.K.; Leemans, A. Diffusion Tensor Imaging; Humana Press: Totowa, NJ, USA, 2010. [Google Scholar] [CrossRef]
- Wahl, M.; Lauterbach-Soon, B.; Hattingen, E.; Jung, P.; Singer, O.; Volz, S.; Klein, J.C.; Steinmetz, H.; Ziemann, U. Human motor corpus callosum: Topography, somatotopy, and link between microstructure and function. J. Neurosci. 2007, 27, 12132–12138. [Google Scholar] [CrossRef] [PubMed]
- Jacobi, S.K.; Yatsunenko, T.; Li, D.; Dasgupta, S.; Yu, R.K.; Berg, B.M.; Chichlowski, M.; Odle, J. Dietary Isomers of Sialyllactose Increase Ganglioside Sialic Acid Concentrations in the Corpus Callosum and Cerebellum and Modulate the Colonic Microbiota of Formula-Fed Piglets. J. Nutr. 2016, 146, 200–208. [Google Scholar] [CrossRef]
- Qiu, M.; Li, Q.; Liu, G.; Xie, B.; Wang, J. Voxel-based analysis of white matter during adolescence and young adulthood. Brain Dev. 2010, 32, 531–537. [Google Scholar] [CrossRef]
- Baron, J.C.; Chételat, G.; Desgranges, B.; Perchey, G.; Landeau, B.; De La Sayette, V.; Eustache, F. In vivo mapping of gray matter loss with voxel-based morphometry in mild Alzheimer’s disease. Neuroimage 2001, 14, 298–309. [Google Scholar] [CrossRef]
- Summerfield, C.; Junqué, C.; Tolosa, E.; Salgado-Pineda, P.; Gómez-Ansón, B.; Martí, M.J.; Pastor, P.; Ramírez-Ruíz, B.; Mercader, J. Structural Brain Changes in Parkinson Disease With Dementia. Arch. Neurol. 2005, 62, 281. [Google Scholar] [CrossRef]
- Särkämö, T.; Ripollés, P.; Vepsäläinen, H.; Autti, T.; Silvennoinen, H.M.; Salli, E.; Laitinen, S.; Forsblom, A.; Soinila, S.; Rodríguez-Fornells, A. Structural changes induced by daily music listening in the recovering brain after middle cerebral artery stroke: A voxel-based morphometry study. Front. Hum. Neurosci. 2014, 8, 1–16. [Google Scholar] [CrossRef]
- Martin, S.B.; Covell, D.J.; Joseph, J.E.; Chebrolu, H.; Smith, C.D.; Kelly, T.H.; Jiang, Y.; Gold, B.T. Human experience seeking correlates with hippocampus volume: Convergent evidence from manual tracing and voxel-based morphometry. Neuropsychologia 2007, 45, 2874–2881. [Google Scholar] [CrossRef]
- Cutuli, D.; Pagani, M.; Caporali, P.; Galbusera, A.; Laricchiuta, D.; Foti, F.; Neri, C.; Spalletta, G.; Caltagirone, C.; Petrosini, L.; et al. Effects of omega-3 fatty acid supplementation on cognitive functions and neural substrates: A voxel-based morphometry study in aged mice. Front. Aging Neurosci. 2016, 8, 1–14. [Google Scholar] [CrossRef]
- Mudd, A.T.; Dilger, R.N. Early-Life Nutrition and Neurodevelopment: Use of the Piglet as a Translational Model. Adv. Nutr. An. Int. Rev. J. 2017, 8, 92–104. [Google Scholar] [CrossRef]
- Renner, M.J.; Rosenzweig, M.R. Object interactions in juvenile rats (Rattus norvegicus): Effects of different experiential histories. J. Comp. Psychol. 1986, 100, 229–236. [Google Scholar] [CrossRef]
- Forkman, B.; Boissy, A.; Meunier-Salaün, M.C.; Canali, E.; Jones, R.B. A critical review of fear tests used on cattle, pigs, sheep, poultry and horses. Physiol. Behav. 2007, 92, 340–374. [Google Scholar] [CrossRef]
- Donald, R.D.; Healy, S.D.; Lawrence, A.B.; Rutherford, K.M.D. Emotionality in growing pigs: Is the open field a valid test? Physiol. Behav. 2011, 104, 906–913. [Google Scholar] [CrossRef]
- Prut, L.; Belzung, C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: A review. Eur. J. Pharmacol. 2003, 463, 3–33. [Google Scholar] [CrossRef]
- Murphy, E.; Nordquist, R.E.; van der Staay, F.J. A review of behavioural methods to study emotion and mood in pigs, Sus scrofa. Appl. Anim. Behav. Sci. 2014, 159, 9–28. [Google Scholar] [CrossRef]

| Diet | Pooled SEM | p-Value 2 | ||
|---|---|---|---|---|
| Measure | Control | Test | ||
| ADG, g/day | 195 | 188 | 22.4 | 0.70 |
| ADMI, g liquid milk/day | 1225 | 1183 | 111.8 | 0.63 |
| G:F, g BWG:g liquid milk intake | 0.159 | 0.156 | 0.005 | 0.71 |
| Diet | Pooled SEM | p-Value | ||
|---|---|---|---|---|
| Item | Control | Test | ||
| Ascending Colon | ||||
| DM, % | 23.77 | 23.95 | 0.677 | 0.849 |
| SCFA absolute, μmol/g DM | ||||
| Acetate | 47.34 | 56.40 | 6.043 | 0.304 |
| Propionate | 23.72 | 23.05 | 2.831 | 0.869 |
| Butyrate | 9.82 | 10.35 | 1.442 | 0.798 |
| Total SCFA | 80.88 | 89.80 | 9.800 | 0.528 |
| SCFA relative, % of total | ||||
| Acetate | 59.12 | 63.00 | 2.334 | 0.067 |
| Propionate | 28.92 | 25.75 | 1.660 | 0.018 |
| Butyrate | 11.95 | 11.27 | 0.747 | 0.479 |
| BCFA absolute, μmol/g DM | ||||
| Isovalerate | 5.61 | 4.32 | 0.703 | 0.203 |
| Valerate | 2.48 | 2.47 | 0.298 | 0.976 |
| Isobutyrate | 3.76 | 2.80 | 0.444 | 0.136 |
| Total BCFA | 12.30 | 9.58 | 1.553 | 0.222 |
| BCFA relative, % of total | ||||
| Isovalerate | 45.96 | 44.96 | 1.808 | 0.319 |
| Valerate | 23.12 | 26.18 | 2.547 | 0.046 |
| Isobutyrate | 30.90 | 28.86 | 0.846 | 0.037 |
| Feces | ||||
| DM, % | 30.15 | 32.00 | 1.922 | 0.437 |
| SCFA absolute, μmol/g DM | ||||
| Acetate | 43.09 | 30.33 | 4.953 | 0.080 |
| Propionate | 15.59 | 9.80 | 2.912 | 0.111 |
| Butyrate | 5.91 | 3.83 | 1.586 | 0.154 |
| Total SCFA | 73.39 | 43.95 | 11.30 | 0.051 |
| SCFA relative, % of total | ||||
| Acetate | 68.82 | 70.57 | 3.516 | 0.606 |
| Propionate | 21.40 | 21.43 | 1.362 | 0.986 |
| Butyrate | 9.86 | 7.99 | 2.309 | 0.294 |
| BCFA absolute, μmol/g DM | ||||
| Isovalerate | 5.07 | 3.67 | 0.825 | 0.248 |
| Valerate | 2.31 | 1.48 | 0.466 | 0.116 |
| Isobutyrate | 3.20 | 2.33 | 0.485 | 0.224 |
| Total BCFA | 10.61 | 7.49 | 1.671 | 0.195 |
| BCFA relative, % of total | ||||
| Isovalerate | 48.00 | 48.81 | 0.927 | 0.545 |
| Valerate | 21.56 | 19.79 | 1.349 | 0.305 |
| Isobutyrate | 30.45 | 31.40 | 0.815 | 0.279 |
| Control | Test | Pooled | ||||
|---|---|---|---|---|---|---|
| Measures During the Test Trial | n | Mean | n | Mean | SEM | p-Value 2 |
| Recognition Index | 10 | 0.65 | 10 | 0.55 | 0.064 | 0.269 |
| Exploration of the novel object | ||||||
| Novel object visit time, s | 11 | 36.0 | 10 | 43.8 | 11.19 | 0.617 |
| Number of novel object visits | 11 | 8.3 | 10 | 9.6 | 1.72 | 0.584 |
| Mean novel object visit time, s | 10 | 4.1 | 10 | 4.3 | 0.86 | 0.839 |
| Latency to first novel object visit, s | 10 | 46.8 | 10 | 25.5 | 14.08 | 0.299 |
| Latency to last object visit, s | 10 | 220.5 | 10 | 260.1 | 13.90 | 0.059 |
| Standard error of novel object visit time, s/visit | 9 | 1.5 | 10 | 1.5 | 0.35 | 0.981 |
| Exploration of the sample object | ||||||
| Sample object visit time, s | 11 | 17.0 | 10 | 44.3 | 10.56 | 0.077 |
| Number of sample object visits | 11 | 6.1 | 10 | 7.4 | 1.34 | 0.487 |
| Mean sample object visit time, s/visit | 10 | 2.6 | 10 | 5.6 | 1.20 | 0.093 |
| Latency to first sample object visit, s | 10 | 50.3 | 10 | 28.4 | 18.88 | 0.424 |
| Latency to last sample object visit, s | 10 | 212.4 | 10 | 259.7 | 20.86 | 0.126 |
| Standard error of sample object visit time, s/visit | 9 | 1.0 | 10 | 1.9 | 0.41 | 0.120 |
| Exploration of all objects | ||||||
| Total object visit time, s | 11 | 53.0 | 10 | 88.1 | 17.80 | 0.170 |
| Number of object visits | 11 | 14.4 | 10 | 17.0 | 2.57 | 0.466 |
| Mean object visit time, s/visit | 10 | 3.4 | 10 | 4.9 | 0.830 | 0.234 |
| Latency to first object visit, s | 10 | 31.1 | 10 | 4.9 | 7.84 | 0.029 |
| Latency to last object visit, s | 10 | 249.9 | 10 | 277.3 | 11.45 | 0.108 |
| Standard error of total visit time, s/visit | 9 | 1.5 | 10 | 1.5 | 0.35 | 0.981 |
| Region of Interest | Diet | Pooled SEM | p-Value | |
| Control | Test | |||
| Gray matter | 66.79 | 66.66 | 3.663 | 0.974 |
| White matter | 25.49 | 26.27 | 1.104 | 0.616 |
| Cerebrospinal fluid | 7.34 | 7.06 | 3.574 | 0.931 |
| Cerebellum | 10.16 | 10.09 | 0.561 | 0.827 |
| Cerebral aqueduct | 0.02 | 0.02 | 0.001 | 0.628 |
| Corpus callosum | 0.39 | 0.41 | 0.005 | 0.013 |
| Fourth ventricle | 0.03 | 0.03 | 0.001 | 0.672 |
| Hypothalamus | 0.15 | 0.15 | 0.003 | 0.519 |
| Lateral ventricle | 0.54 | 0.57 | 0.009 | 0.026 |
| Left caudate | 0.35 | 0.36 | 0.007 | 0.105 |
| Left cortex | 26.08 | 27.10 | 0.428 | 0.093 |
| Left hippocampus | 0.47 | 0.48 | 0.007 | 0.115 |
| Left inferior colliculi | 0.11 | 0.11 | 0.004 | 0.932 |
| Left internal capsule | 0.81 | 0.86 | 0.018 | 0.017 |
| Left olfactory bulb | 1.87 | 1.98 | 0.060 | 0.201 |
| Left putamen-globus pallidus | 0.19 | 0.20 | 0.007 | 0.015 |
| Left superior colliculi | 0.27 | 0.27 | 0.005 | 0.869 |
| Medulla | 2.42 | 2.45 | 0.121 | 0.715 |
| Midbrain | 3.39 | 3.39 | 0.069 | 0.974 |
| Pons | 2.04 | 2.04 | 0.079 | 0.971 |
| Right caudate | 0.36 | 0.37 | 0.005 | 0.098 |
| Right cortex | 25.93 | 26.91 | 0.278 | 0.020 |
| Right hippocampus | 0.49 | 0.52 | 0.009 | 0.024 |
| Right inferior colliculi | 0.11 | 0.11 | 0.003 | 0.943 |
| Right internal capsule | 0.78 | 0.84 | 0.011 | 0.002 |
| Right olfactory bulb | 1.84 | 1.95 | 0.056 | 0.171 |
| Right putamen-globus pallidus | 0.18 | 0.19 | 0.003 | 0.012 |
| Right superior colliculi | 0.28 | 0.29 | 0.004 | 0.614 |
| Thalamus | 1.82 | 1.87 | 0.022 | 0.156 |
| Third ventricle | 0.03 | 0.03 | 0.001 | 0.665 |
| Region of Interest | Diet | Pooled SEM | p-Value | |
|---|---|---|---|---|
| Control | Test | |||
| Corpus callosum | 0.36 | 0.31 | 0.036 | 0.020 |
| Cerebellum | 0.23 | 0.24 | 0.005 | 0.359 |
| Left caudate | 0.31 | 0.31 | 0.013 | 0.602 |
| Left hippocampus | 0.35 | 0.34 | 0.014 | 0.655 |
| Left internal capsule | 0.56 | 0.55 | 0.021 | 0.882 |
| Right caudate | 0.29 | 0.30 | 0.011 | 0.813 |
| Right hippocampus | 0.35 | 0.35 | 0.011 | 0.571 |
| Right internal capsule | 0.56 | 0.55 | 0.028 | 0.833 |
| Left side | 0.36 | 0.36 | 0.002 | 0.599 |
| Right side | 0.36 | 0.36 | 0.002 | 0.714 |
| Thalamus | 0.29 | 0.30 | 0.005 | 0.451 |
| T1 white matter | 0.37 | 0.37 | 0.003 | 0.744 |
| Average FA mask | 0.32 | 0.33 | 0.002 | 0.850 |
| Tissue | Comparison 2 | Anatomic Region 3 | Cluster, Number of Voxels 4 | Peak-Level | Local Maxima Coordinates 5 | |||
|---|---|---|---|---|---|---|---|---|
| Pseudo-t | p-Value | X | Y | Z | ||||
| Gray | Control > Test | Right Cortex | 55 | 3.21 | 0.0077 | 16 | 3 | 14 |
| Gray | Test > Control | Right Cortex | 27 | 1.70 | 0.0087 | 22 | −3 | 3 |
| Gray | Test > Control | Brainstem | 23 | 0.49 | 0.0088 | 8 | −9 | −15 |
| White | Control > Test | Brainstem | 21 | 1.48 | 0.0084 | 6 | −8 | −15 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joung, S.; Fil, J.E.; Heckmann, A.B.; Kvistgaard, A.S.; Dilger, R.N. Early-Life Supplementation of Bovine Milk Osteopontin Supports Neurodevelopment and Influences Exploratory Behavior. Nutrients 2020, 12, 2206. https://doi.org/10.3390/nu12082206
Joung S, Fil JE, Heckmann AB, Kvistgaard AS, Dilger RN. Early-Life Supplementation of Bovine Milk Osteopontin Supports Neurodevelopment and Influences Exploratory Behavior. Nutrients. 2020; 12(8):2206. https://doi.org/10.3390/nu12082206
Chicago/Turabian StyleJoung, Sangyun, Joanne E. Fil, Anne B. Heckmann, Anne S. Kvistgaard, and Ryan N. Dilger. 2020. "Early-Life Supplementation of Bovine Milk Osteopontin Supports Neurodevelopment and Influences Exploratory Behavior" Nutrients 12, no. 8: 2206. https://doi.org/10.3390/nu12082206
APA StyleJoung, S., Fil, J. E., Heckmann, A. B., Kvistgaard, A. S., & Dilger, R. N. (2020). Early-Life Supplementation of Bovine Milk Osteopontin Supports Neurodevelopment and Influences Exploratory Behavior. Nutrients, 12(8), 2206. https://doi.org/10.3390/nu12082206






