Noodles Made from High Amylose Wheat Flour Attenuate Postprandial Glycaemia in Healthy Adults
Abstract
1. Introduction
2. Materials and Methods
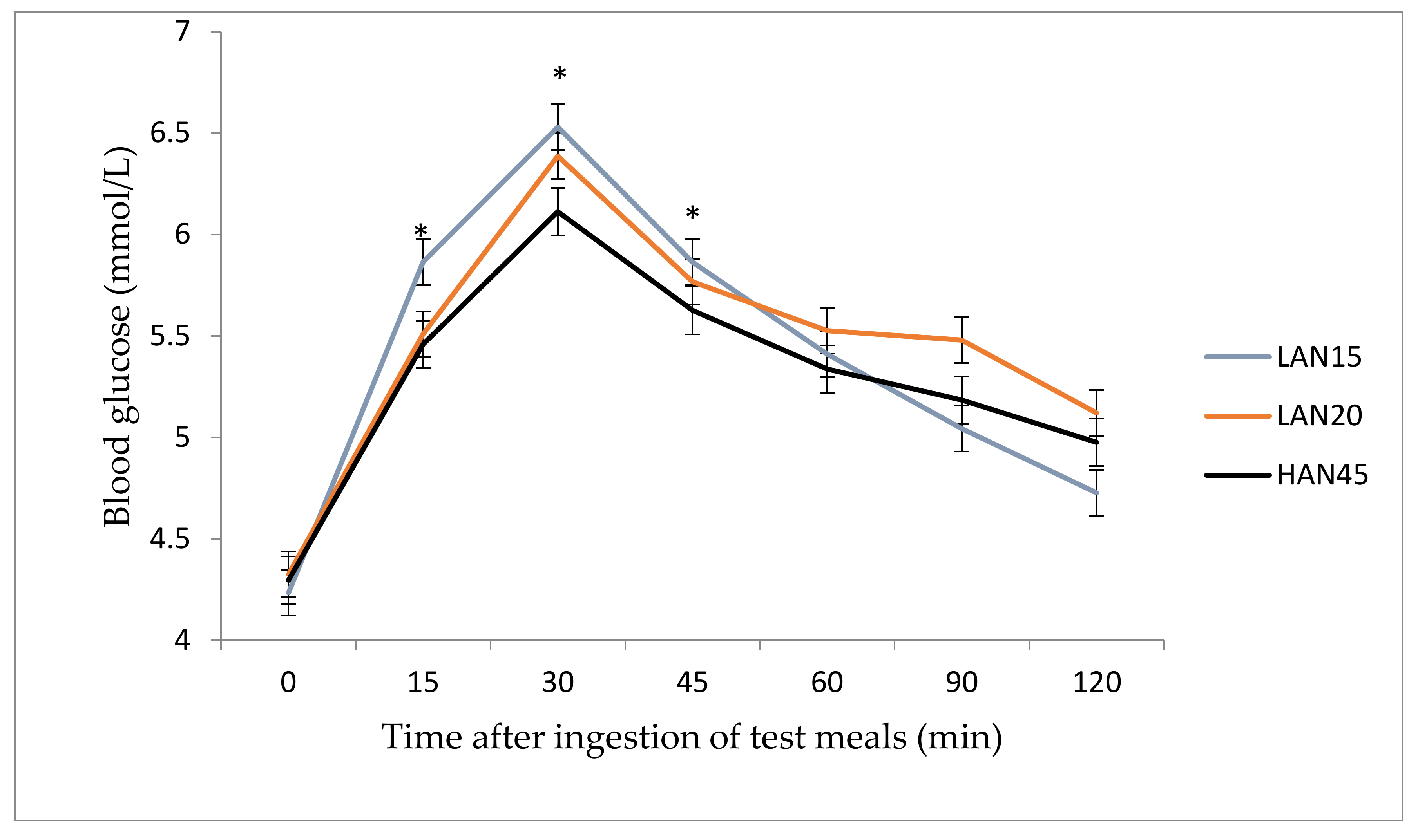
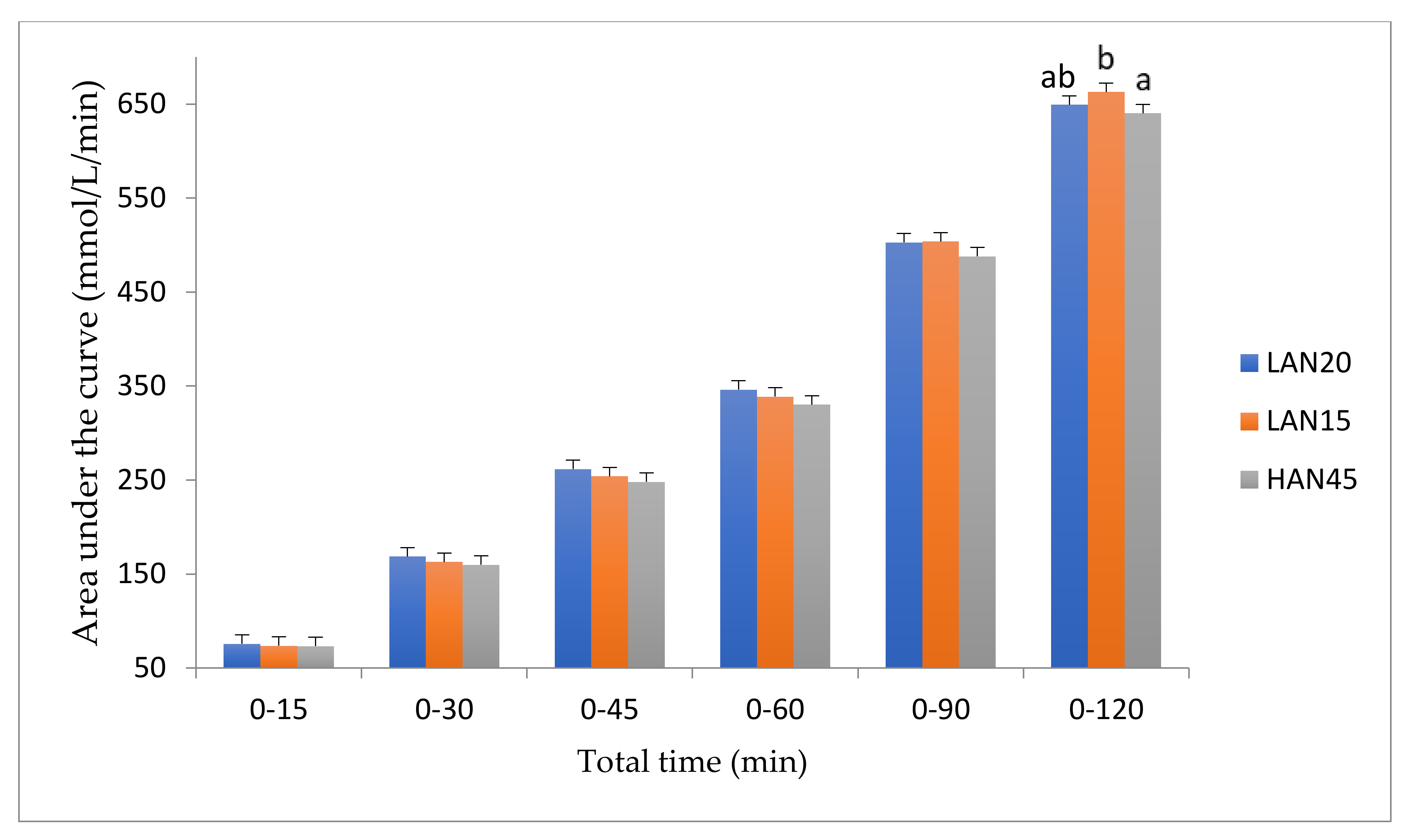
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Frequency of breakfast consumption | Never | Less than once a week | 1–2 days a week | 3–4 days a week | 5–6 days a week | 7 days a week |
| Frequency of exercise | Never | Less than once a week | 1–2 days a week | 3–4 days a week | 5–6 days a week | 7 days a week |
References
- Zuñiga, Y.L.M.; Rebello, S.A.; Oi, P.L.; Zheng, H.; Lee, J.; Tai, E.S.; Van Dam, R.M. Rice and noodle consumption is associated with insulin resistance and hyperglycaemia in an Asian population. Br. J. Nutr. 2013, 111, 1118–1128. [Google Scholar] [CrossRef]
- Niu, M.; Hou, G.G. Whole wheat noodle: Processing, quality improvement, and nutritional and health benefits. Cereal Chem. J. 2018, 96, 23–33. [Google Scholar] [CrossRef]
- Solah, V.A.; Fenton, H.K.; Crosbie, G.B. Wheat: Grain Structure of Wheat and Wheat-based Products. In Encyclopedia of Food and Health; Oxford Academic Press: Oxford, UK, 2016; pp. 470–477. [Google Scholar]
- Huh, I.S.; Kim, H.; Jo, H.K.; Lim, C.S.; Kim, J.S.; Kim, S.J.; Kwon, O.; Oh, B.; Chang, N. Instant noodle consumption is associated with cardiometabolic risk factors among college students in Seoul. Nutr. Res. Pract. 2017, 11, 232–239. [Google Scholar] [CrossRef]
- Guariguata, L.; Whiting, D.R.; Hambleton, I.R.; Beagley, J.; Linnenkamp, U.; Shaw, J.E. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res. Clin. Pract. 2014, 103, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Aeberli, I.; Zimmermann, M. Glycaemic control, insulin resistance and obesity. In Novel Food Ingredients for Weight Control; Henry, C.J.K., Ed.; Woodhead Publishing: Cambridge, UK, 2007; pp. 43–57. [Google Scholar]
- Fan, J.; Song, Y.; Wang, Y.; Hui, R.; Zhang, W. Dietary Glycemic Index, Glycemic Load, and Risk of Coronary Heart Disease, Stroke, and Stroke Mortality: A Systematic Review with Meta-Analysis. PLoS ONE 2012, 7, e52182. [Google Scholar] [CrossRef]
- Alssema, M.; Boers, H.M.; Ceriello, A.; Kilpatrick, E.S.; Mela, D.J.; Priebe, M.G.; Schrauwen, P.; Wolffenbuttel, B.H.R.; Pfeiffer, A.F.H. Diet and glycaemia: The markers and their meaning. A report of the Unilever Nutrition Workshop. Br. J. Nutr. 2014, 113, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Barclay, A.W.; Petocz, P.; McMillan-Price, J.; Flood, V.M.; Prvan, T.; Mitchell, P.; Brand-Miller, J.C. Glycemic index, glycemic load, and chronic disease risk—A meta-analysis of observational studies. Am. J. Clin. Nutr. 2008, 87, 627–637. [Google Scholar] [CrossRef]
- Blaak, E.E.; Antoine, J.-M.; Benton, D.; Björck, I.; Bozzetto, L.; Brouns, F.; Diamant, M.; Dye, L.; Hulshof, T.; Holst, J.J.; et al. Impact of postprandial glycaemia on health and prevention of disease. Obes. Rev. 2012, 13, 923–984. [Google Scholar] [CrossRef]
- Brand-Miller, J.C. Glycemic Load and Chronic Disease. Nutr. Rev. 2003, 61, S49–S55. [Google Scholar] [CrossRef]
- Lockyer, S.; Nugent, A.P. Health effects of resistant starch. Nutr. Bull. 2017, 42, 10–41. [Google Scholar] [CrossRef]
- Brand-Miller, J.C.; Atkinson, F.S.; Gahler, R.J.; Kacinik, V.; Lyon, M.R.; Wood, S. Effects of PGX, a novel functional fibre, on acute and delayed postprandial glycaemia. Eur. J. Clin. Nutr. 2010, 64, 1488–1493. [Google Scholar] [CrossRef]
- Jenkins, D.J.; Wolever, T.M.; Jenkins, A.L.; Taylor, R.H. Dietary fibre, carbohydrate metabolism and diabetes. Mol. Asp. Med. 1987, 9, 97–112. [Google Scholar] [CrossRef]
- Yu, K.; Ke, M.-Y.; Li, W.-H.; Zhang, S.-Q.; Fang, X.C. The impact of soluble dietary fibre on gastric emptying, postprandial blood glucose and insulin in patients with type 2 diabetes. Asia Pac. J. Clin. Nutr. 2014, 23, 210–218. [Google Scholar] [PubMed]
- Maziarz, M.P.; Preisendanz, S.; Juma, S.; Imrhan, V.; Prasad, C.; Vijayagopal, P. Resistant starch lowers postprandial glucose and leptin in overweight adults consuming a moderate-to-high-fat diet: A randomized-controlled trial. Nutr. J. 2017, 16, 14. [Google Scholar] [CrossRef] [PubMed]
- Castro-Acosta, M.L.; Lenihan-Geels, G.N.; Corpe, C.P.; Hall, W.L. Berries and anthocyanins: Promising functional food ingredients with postprandial glycaemia-lowering effects. Proc. Nutr. Soc. 2016, 75, 342–355. [Google Scholar] [CrossRef] [PubMed]
- Hallström, E.; Sestili, F.; Lafiandra, D.; Björck, I.; Ostman, E. A novel wheat variety with elevated content of amylose increases resistant starch formation and may beneficially influence glycaemia in healthy subjects. Food Nutr. Res. 2011, 55, 7074. [Google Scholar] [CrossRef]
- Barkeling, B.; Granfelt, Y.; Björck, I.; Rossner, S. Effects of carbohydrates in the form of pasta and bread on food intake and satiety in man. Nutr. Res. 1995, 15, 467–476. [Google Scholar] [CrossRef]
- Pérez, S.; Bertoft, E. The molecular structures of starch components and their contribution to the architecture of starch granules: A comprehensive review. Starch/Stärke 2010, 62, 389–420. [Google Scholar] [CrossRef]
- Wang, S.; Copeland, L. Molecular disassembly of starch granules during gelatinization and its effect on starch digestibility: A review. Food Funct. 2013, 4, 1564. [Google Scholar] [CrossRef]
- Belobrajdic, D.P.; Regina, A.; Klingner, B.; Zajac, I.T.; Chapron, S.; Berbezy, P.; Bird, A.R. High-Amylose Wheat Lowers the Postprandial Glycemic Response to Bread in Healthy Adults: A Randomized Controlled Crossover Trial. J. Nutr. 2019, 149, 1335–1345. [Google Scholar] [CrossRef]
- Ley, S.H.; Hamdy, O.; Mohan, V.; Hu, F.B. Prevention and management of type 2 diabetes: Dietary components and nutritional strategies. Lancet 2014, 383, 1999–2007. [Google Scholar] [CrossRef]
- O’Connor, L.E.; Campbell, W.W. A novel fiber composite ingredient incorporated into a beverage and bar blunts postprandial serum glucose and insulin responses: A randomized controlled trial. Nutr. Res. 2016, 36, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Luhovyy, B.L.; Mollard, R.C.; Yurchenko, S.; Nunez, M.F.; Berengut, S.; Liu, T.T.; Smith, C.E.; Pelkman, C.L.; Anderson, G.H. The Effects of Whole Grain High-Amylose Maize Flour as a Source of Resistant Starch on Blood Glucose, Satiety, and Food Intake in Young Men. J. Food Sci. 2014, 79, H2550–H2556. [Google Scholar] [CrossRef] [PubMed]
- Keogh, J.B.; Lau, C.W.H.; Noakes, M.; Bowen, J.; Clifton, P.M. Effects of meals with high soluble fibre, high amylose barley variant on glucose, insulin, satiety and thermic effect of food in healthy lean women. Eur. J. Clin. Nutr. 2006, 61, 597–604. [Google Scholar] [CrossRef]
- King, R.A.; Noakes, M.; Bird, A.R.; Morell, M.K.; Topping, D.L. An extruded breakfast cereal made from a high amylose barley cultivar has a low glycemic index and lower plasma insulin response than one made from a standard barley. J. Cereal Sci. 2008, 48, 526–530. [Google Scholar] [CrossRef]
- Hasjim, J.; Lee, S.-O.; Hendrich, S.; Setiawan, S.; Ai, Y.; Jane, J.-L. Characterization of a Novel Resistant-Starch and Its Effects on Postprandial Plasma-Glucose and Insulin Responses. Cereal Chem. J. 2010, 87, 257–262. [Google Scholar] [CrossRef]
- Trinidad, T.P.; Mallillin, A.C.; Encabo, R.R.; Sagum, R.S.; Felix, A.D.; Juliano, B.O. The effect of apparent amylose content and dietary fibre on the glycemic response of different varieties of cooked milled and brown rice. Int. J. Food Sci. Nutr. 2012, 64, 89–93. [Google Scholar] [CrossRef]
- Zenel, A.M.; Stewart, M.L. High Amylose White Rice Reduces Post-Prandial Glycemic Response but Not Appetite in Humans. Nutrients 2015, 7, 5362–5374. [Google Scholar] [CrossRef]
- Järvi, A.E.; Karlström, B.E.; Granfeldt, Y.E.; Björck, I.M.; Vessby, B.O.; Asp, N.G. The influence of food structure on postprandial metabolism in patients with non-insulin-dependent diabetes mellitus. Am. J. Clin. Nutr. 1995, 61, 837–842. [Google Scholar] [CrossRef]
- Ek, K.L.; Brand-Miller, J.; Copeland, L. Glycemic effect of potatoes. Food Chem. 2012, 133, 1230–1240. [Google Scholar] [CrossRef]
- Chiu, Y.-T.; Stewart, M.L. Effect of variety and cooking method on resistant starch content of white rice and subsequent postprandial glucose response and appetite in humans. Asia Pac. J. Clin. Nutr. 2013, 22, 372–379. [Google Scholar]
- Stunkard, A.J.; Messick, S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J. Psychosom. Res. 1985, 29, 71–83. [Google Scholar] [CrossRef]
- Solah, V.A.; Brand-Miller, J.C.; Atkinson, F.S.; Gahler, R.J.; Kacinik, V.; Lyon, M.R.; Wood, S. Dose–response effect of a novel functional fibre, PolyGlycopleX, PGX, on satiety. Appetite 2014, 77, 74–78. [Google Scholar] [CrossRef]
- Brouns, F.; Björck, I.; Frayn, K.N.; Gibbs, A.L.; Lång, V.; Slama, G.; Wolever, T.M.S. Glycaemic index methodology. Nutr. Res. Rev. 2005, 18, 145–171. [Google Scholar] [CrossRef] [PubMed]
- Ai, Y.; Jane, J. Starch: Structure, Property, and Determination. In Encyclopedia of Food and Health; Oxford Academic Press: Oxford, UK, 2016; pp. 165–174. [Google Scholar]
- Mir, J.A.; Srikaeo, K.; García, J. Effects of amylose and resistant starch on starch digestibility of rice flours and starches. Int. Food Res. J. 2013, 20, 1329–1335. [Google Scholar]
- Chung, H.-J.; Liu, Q.; Lee, L.; Wei, D. Relationship between the structure, physicochemical properties and in vitro digestibility of rice starches with different amylose contents. Food Hydrocoll. 2011, 25, 968–975. [Google Scholar] [CrossRef]
- Xie, Y.-Y.; Hu, X.-P.; Jin, Z.-Y.; Xu, X.-M.; Chen, H.-Q. Effect of temperature-cycled retrogradation on in vitro digestibility and structural characteristics of waxy potato starch. Int. J. Boil. Macromol. 2014, 67, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Ramdath, D.D. Glycemic index, glycemic load, and their health benefits. In Encyclopedia of Food Grains, 2nd ed.; Academic Press: Oxford, UK, 2016; pp. 241–247. [Google Scholar]
- Htoon, A.; Shrestha, A.K.; Flanagan, B.M.; López-Rubio, A.; Bird, A.R.; Gilbert, E.P.; Gidley, M.J. Effects of processing high amylose maize starches under controlled conditions on structural organisation and amylase digestibility. Carbohydr. Polym. 2009, 75, 236–245. [Google Scholar] [CrossRef]
- Li, E.; Dhital, S.; Hasjim, J. Effects of grain milling on starch structures and flour/starch properties. Starch/Stärke 2013, 66, 15–27. [Google Scholar] [CrossRef]
- Ames, N.; Blewett, H.; Storsley, J.; Thandapilly, S.J.; Zahradka, P.; Taylor, C.G. A double-blind randomised controlled trial testing the effect of a barley product containing varying amounts and types of fibre on the postprandial glucose response of healthy volunteers. Br. J. Nutr. 2015, 113, 1373–1383. [Google Scholar] [CrossRef]
- Banchathanakij, R.; Suphantharika, M. Effect of different β-glucans on the gelatinisation and retrogradation of rice starch. Food Chem. 2009, 114, 5–14. [Google Scholar] [CrossRef]
- Parada, J.; Aguilera, J.M. In vitroDigestibility and Glycemic Response of Potato Starch is Related to Granule Size and Degree of Gelatinization. J. Food Sci. 2009, 74, E34–E38. [Google Scholar] [CrossRef]
- Sun, B.; Tian, Y.; Chen, L.; Jin, Z. Effect of acid-ethanol treatment and debranching on the structural characteristics and digestible properties of maize starches with different amylose contents. Food Hydrocoll. 2017, 69, 229–235. [Google Scholar] [CrossRef]
- Lin, M.; Shyr, C.R.; Lin, J. Bread containing type 3 resistant starch reduced glycaemic index and glycaemic response in healthy young adults. Curr. Top. Nutraceuticals Res. 2012, 10, 143–149. [Google Scholar]
- Karimi, P.; Farhangi, M.A.; Sarmadi, B.; Gargari, B.P.; Javid, A.Z.; Pouraghaei, M.; Dehghan, P. The Therapeutic Potential of Resistant Starch in Modulation of Insulin Resistance, Endotoxemia, Oxidative Stress and Antioxidant Biomarkers in Women with Type 2 Diabetes: A Randomized Controlled Clinical Trial. Ann. Nutr. Metab. 2015, 68, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.; Higbee, D.R.; Donahoo, W.T.; Brown, I.L.; Bell, M.L.; Bessesen, D.H. Resistant starch consumption promotes lipid oxidation. Nutr. Metab. 2004, 1, 8. [Google Scholar] [CrossRef]
- Yamada, Y.; Hosoya, S.; Nishimura, S.; Tanaka, T.; Kajimoto, Y.; Nishimura, A.; Kajimoto, O. Effect of Bread Containing Resistant Starch on Postprandial Blood Glucose Levels in Humans. Biosci. Biotechnol. Biochem. 2005, 69, 559–566. [Google Scholar] [CrossRef]
- Haub, M.D.; Hubach, K.L.; Al-Tamimi, E.K.; Ornelas, S.; Seib, P.A. Different Types of Resistant Starch Elicit Different Glucose Reponses in Humans. J. Nutr. Metab. 2010, 2010, 1–4. [Google Scholar] [CrossRef]
- Woo, J.; Ho, S.C.; Sham, A.; Sea, M.M.; Lam, K.S.L.; Lam, T.H.; Janus, E.D. Diet and glucose tolerance in a Chinese population. Eur. J. Clin. Nutr. 2003, 57, 523–530. [Google Scholar] [CrossRef][Green Version]
- Nanri, A.; Mizoue, T.; Noda, M.; Takahashi, Y.; Kato, M.; Inoue, M.; Tsugane, S. Rice intake and type 2 diabetes in Japanese men and women: The Japan Public Health Center-based Prospective Study. Am. J. Clin. Nutr. 2010, 92, 1468–1477. [Google Scholar] [CrossRef]
- Liu, R.; Solah, V.A.; Wei, Y.; Wu, G.; Wang, X.; Crosbie, G.; Fenton, H.K. Sensory evaluation of Chinese white salted noodles and steamed bread made with Australian and Chinese wheat flour. Cereal Chem. J. 2018, 96, 66–75. [Google Scholar] [CrossRef]
- Jenkins, D.J.; Thorne, M.J.; Wolever, T.M.; Jenkins, A.L.; Rao, A.V.; Thompson, L.U. The effect of starch-protein interaction in wheat on the glycemic response and rate of in vitro digestion. Am. J. Clin. Nutr. 1987, 45, 946–951. [Google Scholar] [CrossRef] [PubMed]
- Paterson, M.A.; King, B.R.; Smart, C.E.; Smith, T.; Rafferty, J.; Lopez, P.E. Impact of dietary protein on postprandial glycaemic control and insulin requirements in Type 1 diabetes: A systematic review. Diabet. Med. 2019, 36, 1585–1599. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, R.R.; Dhital, S.; Mense, A.; Gidley, M.J.; Shi, Y.-C. Intact cellular structure in cereal endosperm limits starch digestion in vitro. Food Hydrocoll. 2018, 81, 139–148. [Google Scholar] [CrossRef]
| Noodle Type | Moisture % | Protein % | Ash % | Starch (RS Not Included) % | Resistant Starch % of Flour | Amylose % of Total Starch |
|---|---|---|---|---|---|---|
| LAN15 | 11.8 (0.2) | 10.1 (0.21) | 0.31 (0.01) | 80.3 (0.7) | - | 15.0 (0.8) |
| LAN20 | 12.6 (0.2) | 13.2 (0.35) | 0.36 (0.03) | 74.0 (1.0) | - | 19.6 (1.5) |
| HAN45 | 12.0 (0.3) | 15.0 (0.12) | 0.48 (0.02) | 66.1 (0.5) | * 8.8 | 45.1 (1.5) |
| Noodle Type | RVA peak Viscosity (cP) | RVA Breakdown (cP) | RVA Final Viscosity (cP) |
|---|---|---|---|
| LAN15 | 3435 | 1635 | 1352 |
| LAN20 | 3409 | 1395 | 1474 |
| HAN45 | 787 | 309 | 866 |
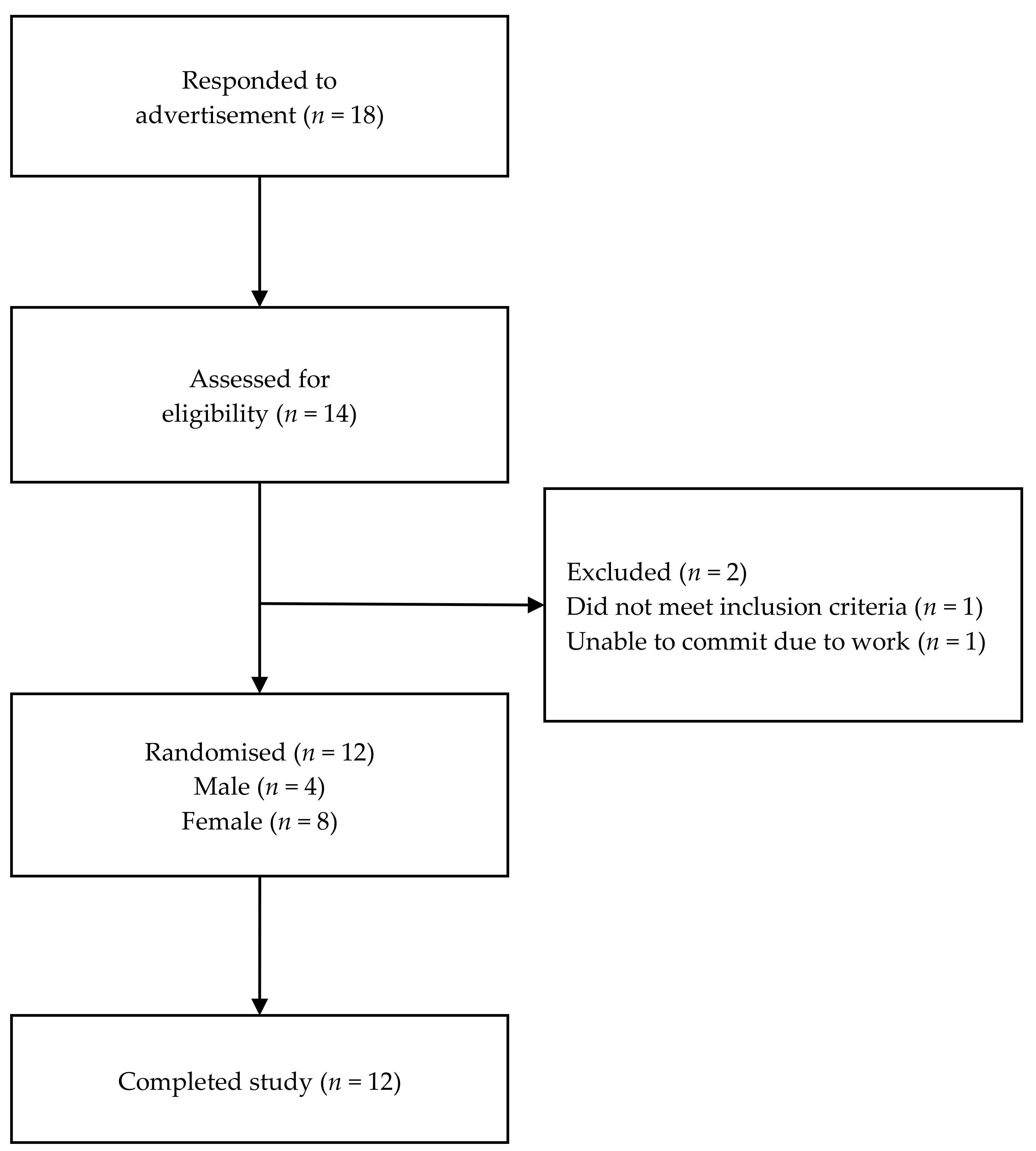
| Characteristic | Total (n = 12) | Women (n = 8) | Men (n = 4) |
|---|---|---|---|
| Age (years) | 22.25 ± 1.86 | 22.25 ± 1.67 | 22.25 ± 2.50 |
| Weight (kg) | 59.04 ± 11.88 | 52.81 ± 5.57 | 71.50 ± 11.62 |
| Height (m) | 1.68 ± 12.91 | 1.61 ± 4.11 | 1.83 ± 11.69 |
| Body mass index (kg/m2) | 20.63 ± 1.64 | 20.32 ± 1.28 | 21.25 ± 2.29 |
| Fasting blood glucose (mmol/L) | 4.30 ± 0.49 | 4.20 ± 0.51 | 4.50 ± 0.39 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ang, K.; Bourgy, C.; Fenton, H.; Regina, A.; Newberry, M.; Diepeveen, D.; Lafiandra, D.; Grafenauer, S.; Hunt, W.; Solah, V. Noodles Made from High Amylose Wheat Flour Attenuate Postprandial Glycaemia in Healthy Adults. Nutrients 2020, 12, 2171. https://doi.org/10.3390/nu12082171
Ang K, Bourgy C, Fenton H, Regina A, Newberry M, Diepeveen D, Lafiandra D, Grafenauer S, Hunt W, Solah V. Noodles Made from High Amylose Wheat Flour Attenuate Postprandial Glycaemia in Healthy Adults. Nutrients. 2020; 12(8):2171. https://doi.org/10.3390/nu12082171
Chicago/Turabian StyleAng, Kim, Carla Bourgy, Haelee Fenton, Ahmed Regina, Marcus Newberry, Dean Diepeveen, Domenico Lafiandra, Sara Grafenauer, Wendy Hunt, and Vicky Solah. 2020. "Noodles Made from High Amylose Wheat Flour Attenuate Postprandial Glycaemia in Healthy Adults" Nutrients 12, no. 8: 2171. https://doi.org/10.3390/nu12082171
APA StyleAng, K., Bourgy, C., Fenton, H., Regina, A., Newberry, M., Diepeveen, D., Lafiandra, D., Grafenauer, S., Hunt, W., & Solah, V. (2020). Noodles Made from High Amylose Wheat Flour Attenuate Postprandial Glycaemia in Healthy Adults. Nutrients, 12(8), 2171. https://doi.org/10.3390/nu12082171






