Thymocid®, a Standardized Black Cumin (Nigella sativa) Seed Extract, Modulates Collagen Cross-Linking, Collagenase and Elastase Activities, and Melanogenesis in Murine B16F10 Melanoma Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
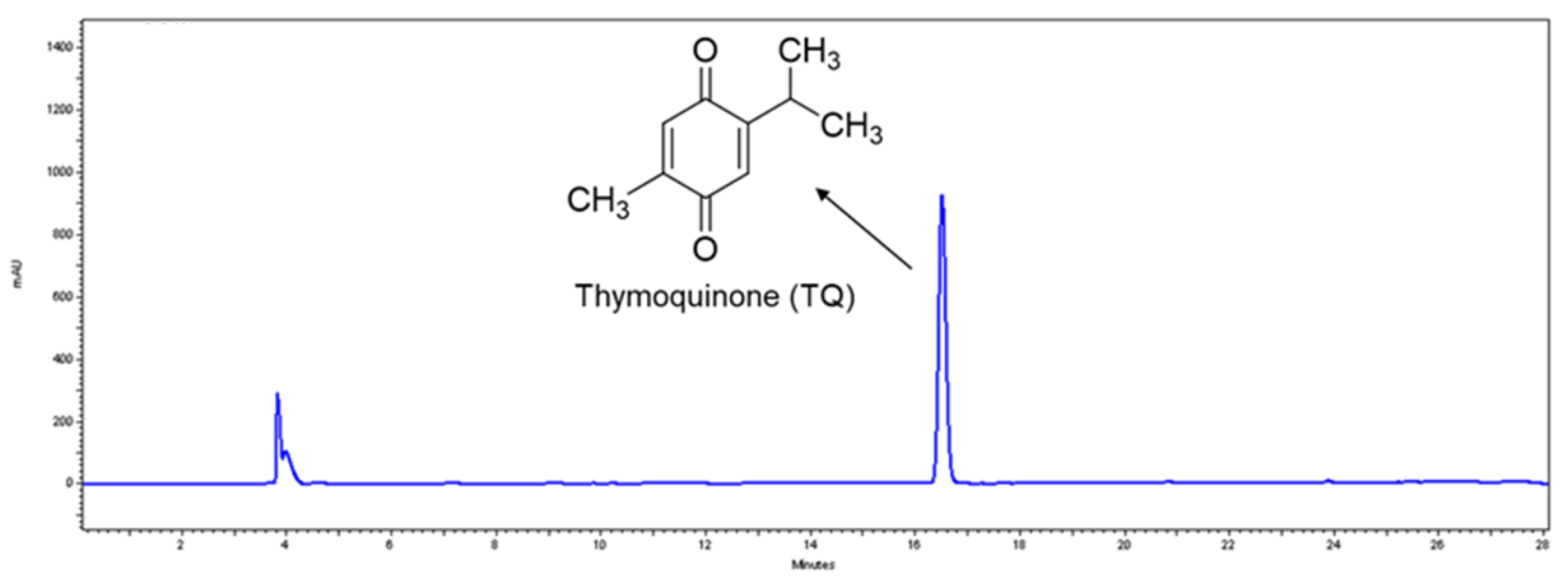
2.2. Quantification of Thymoquinone (TQ) in Thymocid®
2.3. Bovine Serum Albumin (BSA)–Fructose Glycation Assay
2.4. Collagen Cross-Linking Assay
2.5. Collagenase Inhibition Assay
2.6. Elastase Inhibition Assay
2.7. Tyrosinase Inhibition Assay
2.8. Cell Culture
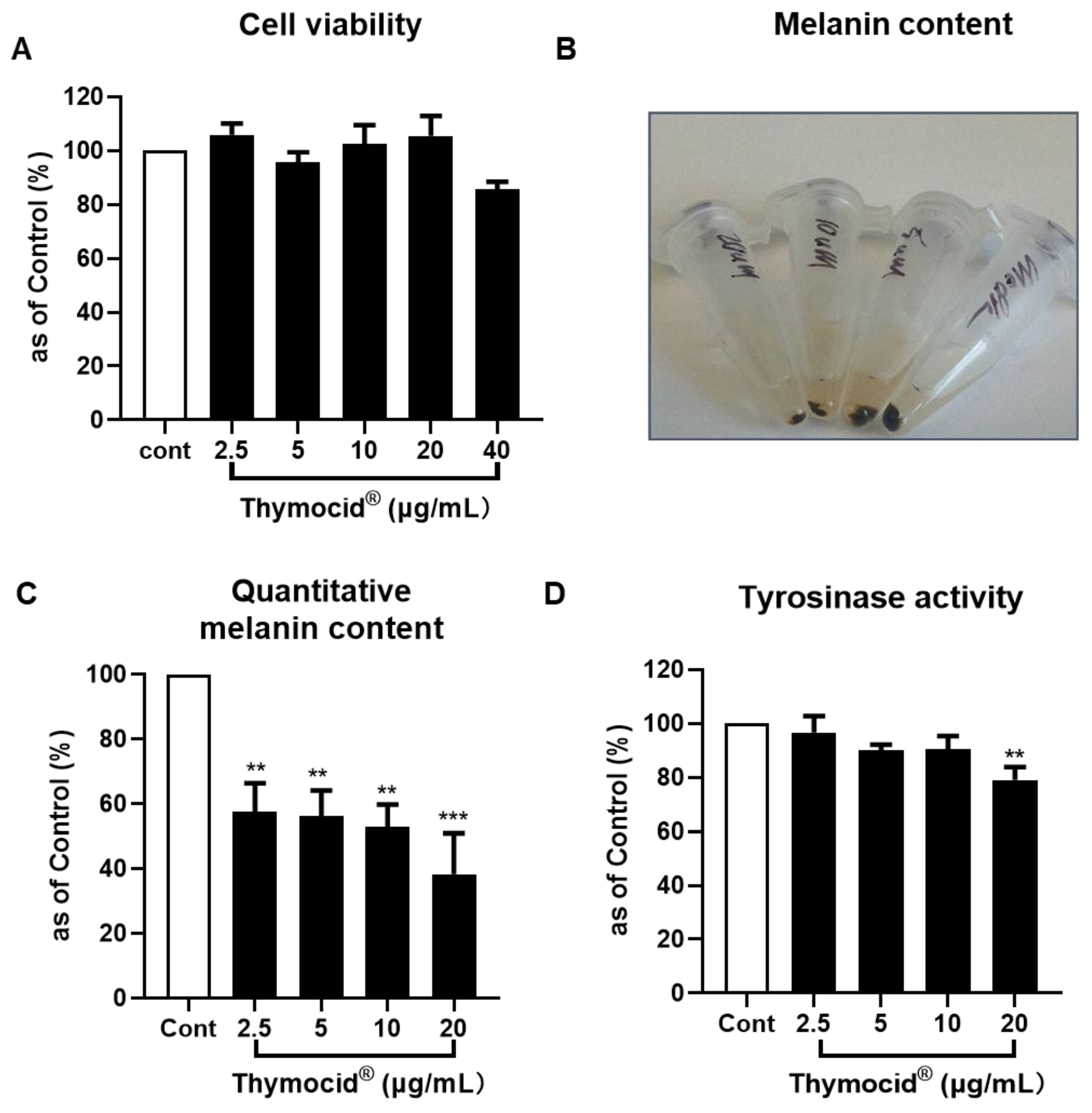
2.9. Cell Viability Assay
2.10. Melanogenesis Assay
2.11. Cellular Tyrosinase Activity Assay
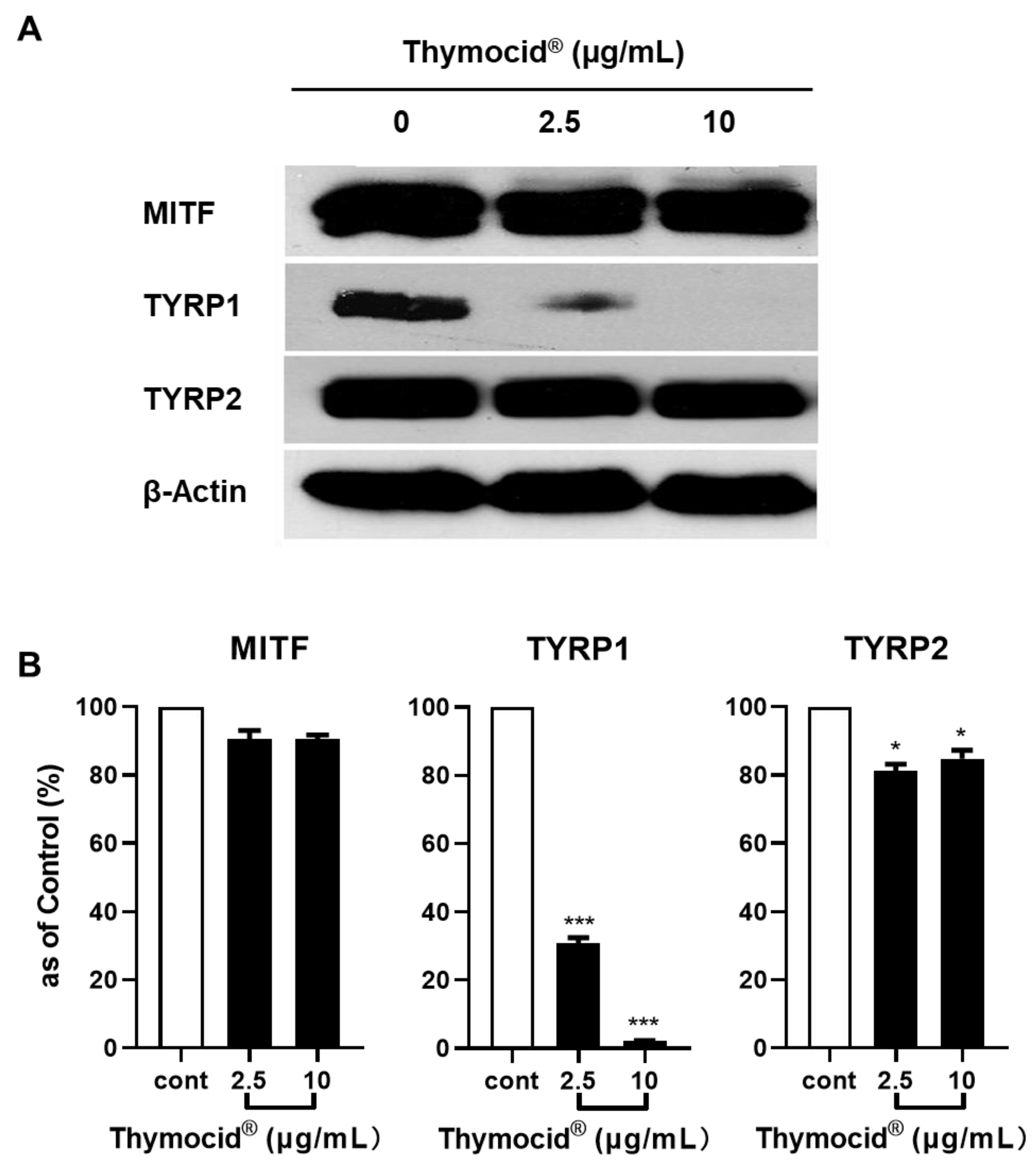
2.12. Real-Time Polymerase Chain Reaction (RT-PCR)
2.13. Preparation of Cellular Protein Lysates and Western Blot Assay
2.14. Statistical Analyses
3. Results
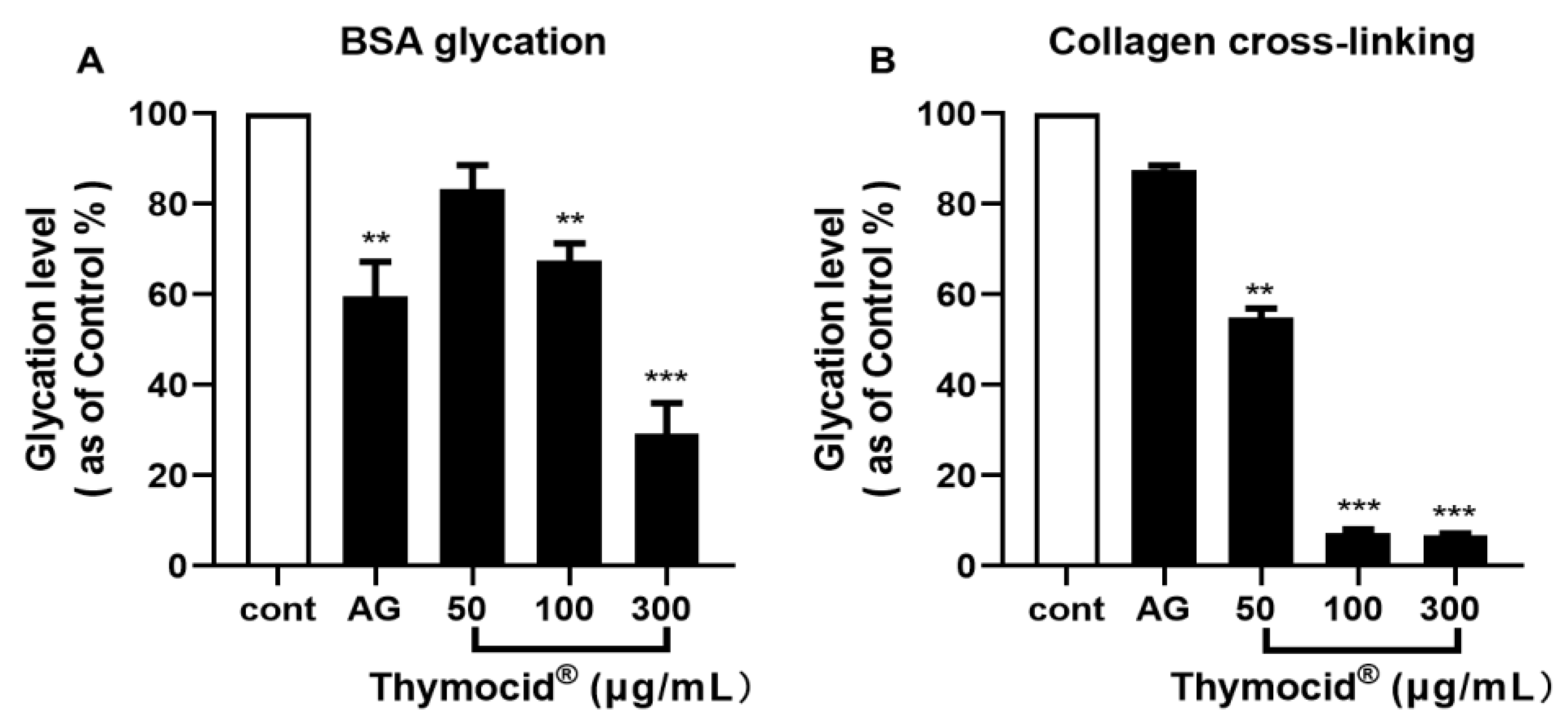
3.1. Thymocid® Inhibits the Formation of Advanced Glycation End-Products (AGEs) and Collagen Cross-Linking
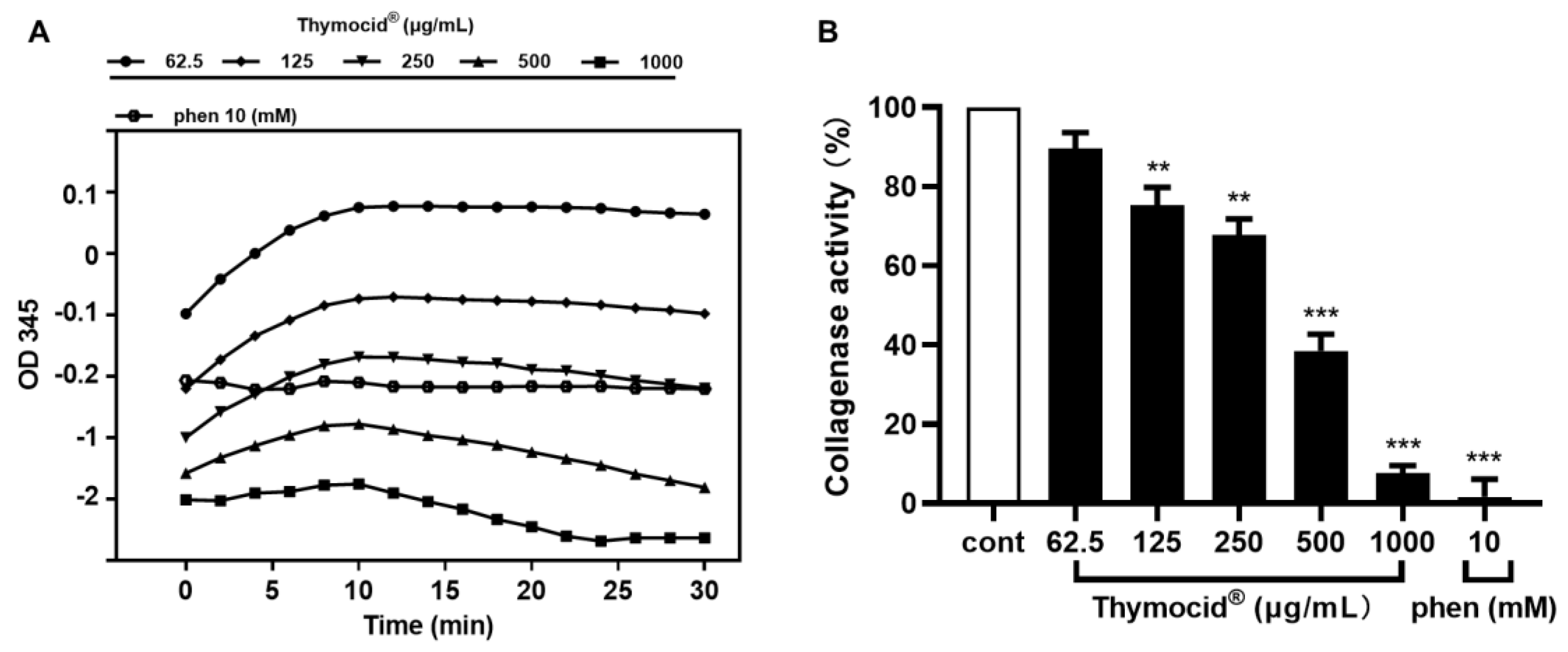
3.2. Thymocid® Inhibits Collagenase Activity
3.3. Thymocid® Inhibits Elastase Activity
3.4. Thymocid® Increases Tyrosinase Activity
3.5. Thymocid® Reduces the Melanin Content in B16F10 Melanoma Cells
3.6. Thymocid® Suppresses the mRNA and Protein Expression Levels of Melanogenesis-Related Markers in B16F10 Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AG | aminoguanidine |
| AGEs | advanced glycation end-products |
| AAAPVN | N-succinyl-Ala-Ala-Ala-p-nitroanilide |
| BCSEs | black cumin seed extracts |
| BSA | bovine serum albumin |
| EGCG | epigallocatechin gallate |
| FALGPA | N-(3-[2-furyl]-acryloyl)-Leu-Gly-Pro-Ala |
| L-DOPA | L-3,4-dihydroxyphenylalanine |
| MGO | methylglyoxal |
| MITF | microphthalmia-associated transcription factor |
| phen | 1,10-phenanthroine |
| TQ | thymoquinone |
| TYR | tyrosinase |
| TYRP1 | tyrosinase-related protein-1 |
| TYRP2 | tyrosinase-related protein-2 |
References
- Bharti, U.; Hamal, I.A.; Jaiswal, A.; Patel, S. Black cumin (Nigella sativa L.)—A review. J. Plant Dev. 2009, 4, 1–43. [Google Scholar]
- Hasan, R.; Javaid, A.; Fatima, S. The effects of short-term administration of weight reducing herbal drug (mehzileen) on serum enzymes in common rabbits. J. Basic Appl. Sci. 2012, 8. [Google Scholar] [CrossRef]
- Takruri, H.R.H.; Dameh, M.A.F. Study of the nutritional value of black cumin seeds (Nigella sativa L.). J. Sci. Food Agric. 1998, 76, 404–410. [Google Scholar] [CrossRef]
- Eid, A.M.; Elmarzugi, N.A.; Abu Ayyash, L.M.; Sawafta, M.N.; Daana, H.I. A review on the cosmeceutical and external applications of Nigella sativa. J. Trop. Med. 2017, 2017. [Google Scholar] [CrossRef]
- Akram Khan, M.; Afzal, M. Chemical composition of Nigella sativa Linn: Part 2 Recent advances. Inflammopharmacology 2016, 24, 67–79. [Google Scholar] [CrossRef]
- Yuan, T.; Nahar, P.; Sharma, M.; Liu, K.; Slitt, A.; Aisa, H.A.; Seeram, N.P. Indazole-type alkaloids from Nigella sativa seeds exhibit antihyperglycemic effects via AMPK activation in vitro. J. Nat. Prod. 2014, 77, 2316–2320. [Google Scholar] [CrossRef]
- Agarwal, R.; Kharya, M.D.; Shrivastava, R. Antimicrobial & anthelmintic activities of the essential oil of Nigella sativa Linn. Indian J. Exp. Biol. 1979, 17, 1264–1265. [Google Scholar]
- Mahmoud, M.R.; El-Abhar, H.S.; Saleh, S. The effect of Nigella sativa oil against the liver damage induced by Schistosoma mansoni infection in mice. J. Ethnopharmacol. 2002, 79, 1–11. [Google Scholar] [CrossRef]
- Aljabre, S.H.M.; Randhawa, M.A.; Akhtar, N.; Alakloby, O.M.; Alqurashi, A.M.; Aldossary, A. Antidermatophyte activity of ether extract of Nigella sativa and its active principle, thymoquinone. J. Ethnopharmacol. 2005, 101, 116–119. [Google Scholar] [CrossRef]
- Ali, B.H.; Blunden, G. Pharmacological and toxicological properties of Nigella sativa. Phytother. Res. 2003, 17, 299–305. [Google Scholar] [CrossRef]
- Aljabre, S.H.M.; Alakloby, O.M.; Randhawa, M.A. Dermatological effects of Nigella sativa. J. Dermatol. Dermatol. Surg. 2015, 19, 92–98. [Google Scholar] [CrossRef]
- Abdul-Ameer, N.; Al-Harchan, H. Treatment of acne vulgaris with Nigella sativa oil lotion. Iraq. Postgrad. Med. J. 2010, 2, 140–143. [Google Scholar]
- Ali, S.A.; Meitei, K.V. Nigella sativa seed extract and its bioactive compound thymoquinone: The new melanogens causing hyperpigmentation in the wall lizard melanophores. J. Pharm. Pharmacol. 2011, 63, 741–746. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Ji, J.; Park, S.H. Antiwrinkle and antimelanogenesis activity of the ethanol extracts of Lespedeza cuneata G. Don for development of the cosmeceutical ingredients. Food Sci. Nutr. 2018, 6, 1307–1316. [Google Scholar] [CrossRef]
- Wang, L.; Oh, J.Y.; Jayawardena, T.U.; Jeon, Y.J.; Ryu, B.M. Anti-inflammatory and anti-melanogenesis activities of sulfated polysaccharides isolated from Hizikia fusiforme: Short communication. Int. J. Biol. Macromol. 2020, 142, 545–550. [Google Scholar] [CrossRef]
- Jung, H.J.; Kyoung Lee, A.; Park, Y.J.; Lee, S.; Kang, D.; Jung, Y.S.; Young Chung, H.; Ryong Moon, H. (2E,5E)-2,5-Bis(3-hydroxy-4-methoxybenzylidene) cyclopentanone exerts anti-melanogenesis and anti-wrinkle activities in B16F10 melanoma and hs27 fibroblast cells. Molecules 2018, 23, 1415. [Google Scholar] [CrossRef]
- Lim, H.Y.; Jeong, D.; Park, S.H.; Shin, K.K.; Hong, Y.H.; Kim, E.; Yu, Y.G.; Kim, T.R.; Kim, H.; Lee, J.; et al. Antiwrinkle and antimelanogenesis effects of tyndallized Lactobacillus acidophilus KCCM12625P. Int. J. Mol. Sci. 2020, 21, 1620. [Google Scholar] [CrossRef]
- Jeong, S.; Yoon, S.; Kim, S.; Jung, J.; Kor, M.; Shin, K.; Lim, C.; Han, H.S.; Lee, H.; Park, K.Y.; et al. Anti-wrinkle benefits of peptides complex stimulating skin basement membrane proteins expression. Int. J. Mol. Sci. 2020, 21, 73. [Google Scholar] [CrossRef]
- Ganceviciene, R.; Liakou, A.I.; Theodoridis, A.; Makrantonaki, E.; Zouboulis, C.C. Skin anti-aging strategies. Dermato Endocrinol. 2012, 4, 308–319. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Maity, N.; Nema, N.K.; Sarkar, B.K. Bioactive compounds from natural resources against skin aging. Phytomedicine 2011, 19, 64–73. [Google Scholar] [CrossRef]
- Liu, C.; Guo, H.; DaSilva, N.A.; Li, D.; Zhang, K.; Wan, Y.; Gao, X.H.; Chen, H.D.; Seeram, N.P.; Ma, H. Pomegranate (Punica granatum) phenolics ameliorate hydrogen peroxide-induced oxidative stress and cytotoxicity in human keratinocytes. J. Funct. Foods 2019, 54, 559–567. [Google Scholar] [CrossRef]
- Sheng, J.; Liu, C.; Petrovas, S.; Wan, Y.; Chen, H.D.; Seeram, N.P.; Ma, H. Phenolic-enriched maple syrup extract protects human keratinocytes against hydrogen peroxide and methylglyoxal induced cytotoxicity. Dermatol. Ther. 2020, 33. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Guo, H.; Dain, J.A.; Wan, Y.; Gao, X.-H.; Chen, H.-D.; Seeram, N.P.; Ma, H. Cytoprotective effects of a proprietary red maple leaf extract and its major polyphenol, ginnalin A, against hydrogen peroxide and methylglyoxal induced oxidative stress in human keratinocytes. Food Funct. 2020. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, H.; Liu, W.; Yuan, T.; Seeram, N.P. New antiglycative compounds from cumin (Cuminum cyminum) spice. J. Agric. Food Chem. 2015, 63, 10097–10102. [Google Scholar] [CrossRef]
- Thring, T.S.A.; Hili, P.; Naughton, D.P. Anti-collagenase, anti-elastase and anti-oxidant activities of extracts from 21 plants. Bmc Complement. Altern. Med. 2009, 9. [Google Scholar] [CrossRef] [PubMed]
- Brotzman, N.; Xu, Y.; Graybill, A.; Cocolas, A.; Ressler, A.; Seeram, N.P.; Ma, H.; Henry, G.E. Synthesis and tyrosinase inhibitory activities of 4-oxobutanoate derivatives of carvacrol and thymol. Bioorg. Med. Chem. Lett. 2019, 29, 56–58. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Xu, J.; DaSilva, N.A.; Wang, L.; Wei, Z.; Guo, L.; Johnson, S.L.; Lu, W.; Xu, J.; Gu, Q.; et al. Cosmetic applications of glucitol-core containing gallotannins from a proprietary phenolic-enriched red maple (Acer rubrum) leaves extract: Inhibition of melanogenesis via down-regulation of tyrosinase and melanogenic gene expression in B16F10 melanoma ce. Arch. Dermatol. Res. 2017, 309, 265–274. [Google Scholar] [CrossRef]
- Chan, Y.Y.; Kim, K.H.; Cheah, S.H. Inhibitory effects of Sargassum polycystum on tyrosinase activity and melanin formation in B16F10 murine melanoma cells. J. Ethnopharmacol. 2011, 137, 1183–1188. [Google Scholar] [CrossRef]
- Amin, B.; Hosseinzadeh, H. Black cumin (Nigella sativa) and its active constituent, thymoquinone: An overview on the analgesic and anti-inflammatory effects. Planta Med. 2016, 82, 8–16. [Google Scholar] [CrossRef]
- Kundu, J.K.; Liu, L.; Shin, J.W.; Surh, Y.J. Thymoquinone inhibits phorbol ester-induced activation of NF-κB and expression of COX-2, and induces expression of cytoprotective enzymes in mouse skin in vivo. Biochem. Biophys. Res. Commun. 2013, 438, 721–727. [Google Scholar] [CrossRef]
- Laothaweerungsawat, N.; Sirithunyalug, J.; Chaiyana, W. Chemical compositions and anti-skin-ageing activities of Origanum vulgare L. essential oil from tropical and mediterranean region. Molecules 2020, 25, 1101. [Google Scholar] [CrossRef] [PubMed]
- Losso, J.N.; Bawadi, H.A.; Chintalapati, M. Inhibition of the formation of advanced glycation end products by thymoquinone. Food Chem. 2011, 128, 55–61. [Google Scholar] [CrossRef]
- Pandey, R.; Kumar, D.; Ali, A. Nigella sativa seed extracts prevent the glycation of protein and DNA. Curr. Perspect. Med. Aromat. Plants 2018, 1, 1–7. [Google Scholar]
- Burits, M.; Bucar, F. Antioxidant activity of Nigella sativa essential oil. Phytother. Res. 2000, 323–328. [Google Scholar] [CrossRef]
- Kacem, R.; Meraihi, Z. Effects of essential oil extracted from Nigella sativa (L.) seeds and its main components on human neutrophil elastase activity. Yakugaku Zasshi 2006, 126, 301–305. [Google Scholar] [CrossRef]
- Schmelzer, C.E.H.; Jung, M.C.; Wohlrab, J.; Neubert, R.H.H.; Heinz, A. Does human leukocyte elastase degrade intact skin elastin? FEBS J. 2012, 279, 4191–4200. [Google Scholar] [CrossRef]
- Regad, T. Molecular and cellular pathogenesis of melanoma initiation and progression. Cell. Mol. Life Sci. 2013, 70, 4055–4065. [Google Scholar] [CrossRef]
- Osborne, R.; Hakozaki, T.; Laughlin, T.; Finlay, D.R. Application of genomics to breakthroughs in the cosmetic treatment of skin ageing and discoloration. Br. J. Dermatol. 2012, 166, 16–19. [Google Scholar] [CrossRef]
- Videira, I.F.D.S.; Moura, D.F.L.; Magina, S. Mechanisms regulating melanogenesis. An. Bras. Dermatol. 2013. [Google Scholar] [CrossRef]
- Del Marmol, V.; Beermann, F. Tyrosinase and related proteins in mammalian pigmentation. FEBS Lett. 1996, 381, 165–168. [Google Scholar] [CrossRef]
- Gilchrest, B.A. A review of skin ageing and its medical therapy. Br. J. Dermatol. 1996, 135, 867–875. [Google Scholar] [CrossRef] [PubMed]
- Iwata, M.; Corn, T.; Iwata, S.; Everett, M.A.; Fuller, B.B. The relationship between tyrosinase activity and skin color in human foreskins. J. Investig. Dermatol. 1990, 95, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Urabe, K.; Winder, A.; Jiménez-Cervantes, C.; Imokawa, G.; Brewington, T.; Solano, F.; García-Borrón, J.C.; Hearing, V.J. Tyrosinase related protein 1 (TRP1) functions as a DHICA oxidase in melanin biosynthesis. EMBO J. 1994, 13, 5818–5825. [Google Scholar] [CrossRef] [PubMed]
- Sulaimon, S.S.; Kitchell, B.E. Review article the biology of melanocytes. Vet. Dermatol. 2003, 14, 57–65. [Google Scholar] [CrossRef]
- Kim, Y.J.; Uyama, H. Tyrosinase inhibitors from natural and synthetic sources: Structure, inhibition mechanism and perspective for the future. Cell. Mol. Life Sci. 2005, 62, 1707–1723. [Google Scholar] [CrossRef]
- Mady, R.F.; El-Hadidy, W.; Elachy, S. Effect of Nigella sativa oil on experimental toxoplasmosis. Parasitol. Res. 2016, 115, 379–390. [Google Scholar] [CrossRef]
- Sarangarajan, R.; Boissy, R.E. Tyrp1 and oculocutaneous albinism type 3. Pigment Cell Res. 2001, 14, 437–444. [Google Scholar] [CrossRef]

| Sample | Concentration (µg/mL) | Inhibition Rate (%) a | |
|---|---|---|---|
| Type-I | Type-III | ||
| Thymocid® | 1000 | 75.4 ± 4.5 | 91.1 ± 1.2 |
| 500 | 69.2 ± 1.3 | 88.6 ± 2.3 | |
| 250 | 56.2 ± 3.2 | 78.4 ± 3.2 | |
| 125 | 35.4 ± 6.9 | 61.3 ± 2.1 | |
| 62.5 | 25.3 ± 5.9 | 36.0 ± 2.7 | |
| EGCG b | 92 | 73.0 ± 1.8 | 75.2 ± 4.0 |
| Sample | Concentration (µg/mL) | Enzyme Activity (%) a | |
|---|---|---|---|
| L-Tyrosine | L-DOPA | ||
| Thymocid® | 1000 | 228.7 ± 9.6 | 146.3 ± 18.6 |
| 500 | 192.0 ± 12.3 | 133.1 ± 6.1 | |
| 250 | 170.0 ± 6.8 | 133.9 ± 15.4 | |
| 125 | 162.5 ± 7.3 | 116.1 ± 6.7 | |
| 62.5 | 153.3 ± 6.6 | 113.7 ± 3.0 | |
| Kojic acid b | 10 | 49.5 ± 3.0 | 66.2 ± 13.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; DaSilva, N.A.; Liu, W.; Xu, J.; Dombi, G.W.; Dain, J.A.; Li, D.; Chamcheu, J.C.; Seeram, N.P.; Ma, H. Thymocid®, a Standardized Black Cumin (Nigella sativa) Seed Extract, Modulates Collagen Cross-Linking, Collagenase and Elastase Activities, and Melanogenesis in Murine B16F10 Melanoma Cells. Nutrients 2020, 12, 2146. https://doi.org/10.3390/nu12072146
Li H, DaSilva NA, Liu W, Xu J, Dombi GW, Dain JA, Li D, Chamcheu JC, Seeram NP, Ma H. Thymocid®, a Standardized Black Cumin (Nigella sativa) Seed Extract, Modulates Collagen Cross-Linking, Collagenase and Elastase Activities, and Melanogenesis in Murine B16F10 Melanoma Cells. Nutrients. 2020; 12(7):2146. https://doi.org/10.3390/nu12072146
Chicago/Turabian StyleLi, Huifang, Nicholas A. DaSilva, Weixi Liu, Jialin Xu, George W. Dombi, Joel A. Dain, Dongli Li, Jean Christopher Chamcheu, Navindra P. Seeram, and Hang Ma. 2020. "Thymocid®, a Standardized Black Cumin (Nigella sativa) Seed Extract, Modulates Collagen Cross-Linking, Collagenase and Elastase Activities, and Melanogenesis in Murine B16F10 Melanoma Cells" Nutrients 12, no. 7: 2146. https://doi.org/10.3390/nu12072146
APA StyleLi, H., DaSilva, N. A., Liu, W., Xu, J., Dombi, G. W., Dain, J. A., Li, D., Chamcheu, J. C., Seeram, N. P., & Ma, H. (2020). Thymocid®, a Standardized Black Cumin (Nigella sativa) Seed Extract, Modulates Collagen Cross-Linking, Collagenase and Elastase Activities, and Melanogenesis in Murine B16F10 Melanoma Cells. Nutrients, 12(7), 2146. https://doi.org/10.3390/nu12072146