Efficacy of a High-Iron Dietary Intervention in Women with Celiac Disease and Iron Deficiency without Anemia: A Clinical Trial
Abstract
1. Introduction
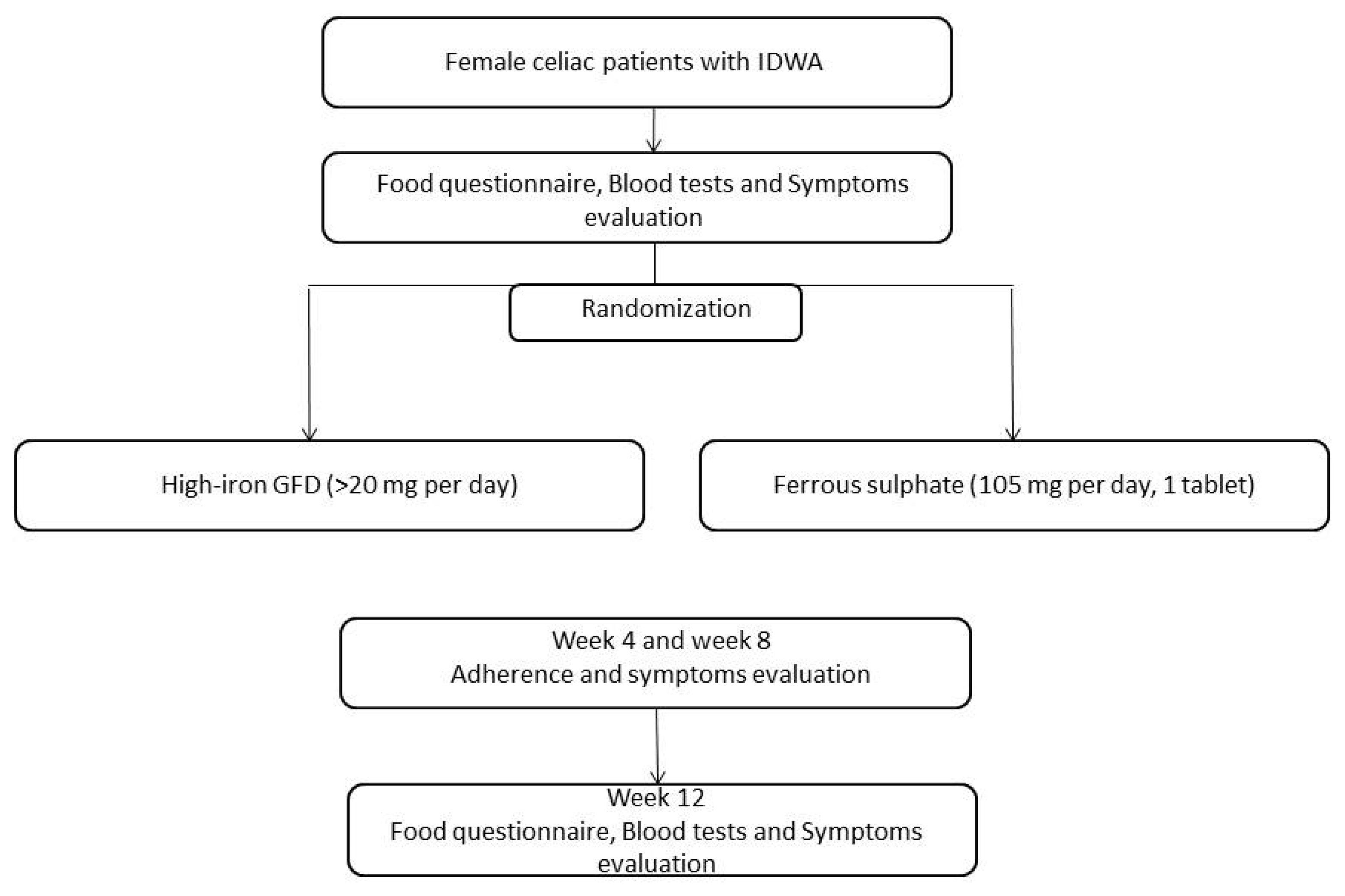
2. Patients and Design
High-Iron Gluten-Free Diet
3. Statistical Analysis
4. Results
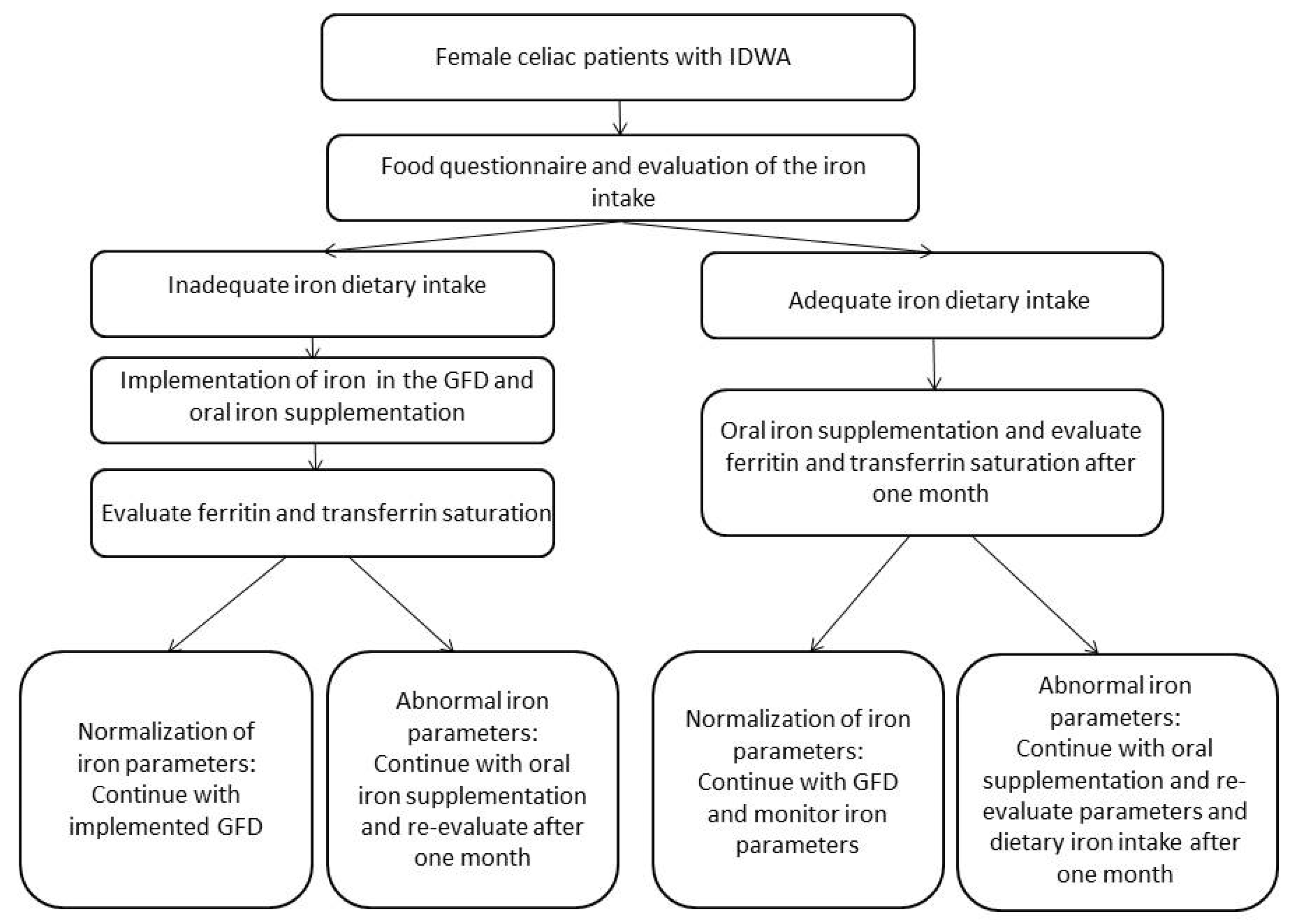
5. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lebwohl, B.; Sanders, D.S.; Green, P.H.R. Coeliac disease. Lancet 2018, 391, 70–81. [Google Scholar] [CrossRef]
- Van Berge-Henegouwen, G.P.; Mulder, C.J. Pioneer in the gluten free diet: Willem-Karel Dicke 1905–1962, over 50 years of gluten free diet. Gut 1993, 34, 1473–1475. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Tapia, A.; Murray, J.A. Celiac disease. Curr. Opin. Gastroenterol. 2010, 26, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Gujral, N.; Freeman, H.J.; Thomson, A.B. Celiac disease: Prevalence, diagnosis, pathogenesis and treatment. World J. Gastroenterol. 2012, 18, 6036–6059. [Google Scholar] [CrossRef]
- Schuppan, D.; Junker, Y.; Barisani, N. Celiac Disease: From Pathogenesis to Novel Therapies. Gastroenterology 2009, 137, 1912–1933. [Google Scholar] [CrossRef]
- Bardella, M.T.; Elli, L.; Velio, P.; Fredella, C.; Prampolini, L.; Cesana, B. Silent Celiac Disease Is Frequent in the Siblings of Newly Diagnosed Celiac Patients. Digestion 2007, 75, 182–187. [Google Scholar] [CrossRef]
- Rubio-Tapia, A.; Hill, I.D.; Kelly, C.P.; Calderwood, A.H.; Murray, J.A. ACG Clinical Guidelines: Diagnosis and Management of Celiac Disease. Am. J. Gastroenterol. 2013, 108, 656–676. [Google Scholar] [CrossRef]
- Ludvigsson, J.F.; Bai, J.C.; Biagi, F.; Card, T.; Ciacci, C.; Ciclitira, P.J.; Green, P.H.R.; Hadjivassiliou, M.; Holdoway, A.; Van Heel, D.A.; et al. Diagnosis and management of adult coeliac disease: Guidelines from the British Society of Gastroenterology. Gut 2014, 63, 1210–1228. [Google Scholar] [CrossRef]
- Corazza, G.R.; Valentini, R.A.; Andreani, M.L.; D’Anchino, M.; Leva, M.T.; Ginaldi, L.; De Feudis, L.; Quaglino, D.; Gasbarrini, G. Subclinical Coeliac Disease is a Frequent Cause of Iron-Deficiency Anaemia. Scand. J. Gastroenterol. 1995, 30, 153–156. [Google Scholar] [CrossRef]
- Carroccio, A.; Iannitto, E.; Cavataio, F.; Montalto, G.; Tumminello, M.; Campagna, P.; Lipari, M.G.; Notarbartolo, A.; Iacono, G. Sideropenic anemia and celiac disease: One study, two points of view. Dig. Dis. Sci. 1998, 43, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Annibale, B.; Severi, C.; Chistolini, A.; Antonelli, G.; Lahner, E.; Marcheggiano, A.; Iannoni, C.; Monarca, B.; Fave, G.D. Efficacy of gluten-free diet alone on recovery from iron deficiency anemia in adult celiac patients. Am. J. Gastroenterol. 2001, 96, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Halfdanarson, T.R.; Litzow, M.R.; Murray, J.A. Hematologic manifestations of celiac disease. Blood 2007, 109, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yu, L.; Ding, J.; Chen, Y. Iron Metabolism in Cancer. Int. J. Mol. Sci. 2018, 20, 95. [Google Scholar] [CrossRef] [PubMed]
- Dev, S.; Babitt, J.L. Overview of iron metabolism in health and disease. Hemodial. Int. 2017, 21, S6–S20. [Google Scholar] [CrossRef] [PubMed]
- Gulec, S.; Anderson, G.J.; Collins, J.F. Mechanistic and regulatory aspects of intestinal iron absorption. Am. J. Physiol. Gastrointest. Liver Physiol. 2014, 307, G397–G409. [Google Scholar] [CrossRef] [PubMed]
- Patterson, A.J.; Brown, W.J.; Roberts, D.C.; Seldon, M.R. Dietary treatment of iron deficiency in women of childbearing age. Am. J. Clin. Nutr. 2001, 74, 650–656. [Google Scholar] [CrossRef]
- CREA. Centro di Ricerca Alimenti e Nutrizione—Tabelle di Composizione Degli Alimenti. Available online: https://www.crea.gov.it/alimenti-e-nutrizione (accessed on 17 October 2016).
- Lane, D.J.; Richardson, D.R. The active role of vitamin C in mammalian iron metabolism: Much more than just enhanced iron absorption! Free Radic. Boil. Med. 2014, 75, 69–83. [Google Scholar] [CrossRef]
- Samman, S.; Sandström, B.; Toft, M.B.; Bukhave, K.; Jensen, M.; Sørensen, S.S.; Hansen, M. Green tea or rosemary extract added to foods reduces nonheme-iron absorption. Am. J. Clin. Nutr. 2001, 73, 607–612. [Google Scholar] [CrossRef]
- Kristensen, M.B.; Tetens, I.; Jørgensen, A.B.A.; Thomsen, A.D.; Milman, N.; Hels, O.; Sandström†, B.; Hansen, M. A decrease in iron status in young healthy women after long-term daily consumption of the recommended intake of fibre-rich wheat bread. Eur. J. Nutr. 2005, 44, 334–340. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Iron deficiency—United States, 1999–2000. Morb. Mortal. Wkly. Rep. 2002, 51, 897–899. [Google Scholar]
- Elli, L.; Poggiali, E.; Tomba, C.; Andreozzi, F.; Nava, I.; Bardella, M.T.; Campostrini, N.; Girelli, D.; Conte, D.; Cappellini, M.D. Does TMPRSS6 RS855791 Polymorphism Contribute to Iron Deficiency in Treated Celiac Disease? Am. J. Gastroenterol. 2015, 110, 200–202. [Google Scholar] [CrossRef] [PubMed]
- Vici, G.; Belli, L.; Biondi, M.; Polzonetti, V. Gluten free diet and nutrient deficiencies: A review. Clin. Nutr. 2016, 35, 1236–1241. [Google Scholar] [CrossRef] [PubMed]
- Wild, D.; Robins, G.G.; Burley, V.; Howdle, P.D. Evidence of high sugar intake, and low fibre and mineral intake, in the gluten-free diet. Aliment. Pharmacol. Ther. 2010, 32, 573–581. [Google Scholar] [CrossRef]
- Lopez, A.; Cacoub, P.; MacDougall, I.C.; Peyrin-Biroulet, L. Iron deficiency anaemia. Lancet 2016, 387, 907–916. [Google Scholar] [CrossRef]
- Waldvogel-Abramowski, S.; Waeber, G.; Gassner, C.; Buser, A.; Frey, B.M.; Favrat, B.; Tissot, J.-D. Physiology of iron metabolism. Transfus. Med. Hemother. 2014, 41, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Tetens, I.; Bendtsen, K.M.; Henriksen, M.; Ersbøll, A.K.; Milman, N. The impact of a meat-versus a vegetable-based diet on iron status in women of childbearing age with small iron stores. Eur. J. Nutr. 2007, 46, 439–445. [Google Scholar] [CrossRef]
- Weinborn, V.; Pizarro, F.; Olivares, M.; Brito, A.; Arredondo, M.; Flores, S.; Valenzuela, C. The Effect of Plant Proteins Derived from Cereals and Legumes on Heme Iron Absorption. Nutrients 2015, 7, 5446. [Google Scholar] [CrossRef]
- Hurrell, R.F.; Egli, I. Iron bioavailability and dietary reference values. Am. J. Clin. Nutr. 2010, 91, 1461S–1467S. [Google Scholar] [CrossRef]
- Hallert, C.; Grännö, C.; Grant, C.; Hultén, S.; Midhagen, G.; Ström, M.; Svensson, H.; Valdimarsson, T.; Wickström, T. Quality of life of adult coeliac patients treated for 10 years. Scand. J. Gastroenterol. 1998, 33, 933–938. [Google Scholar] [CrossRef]
| GFD-HI Group (n = 10) | FS Group (n = 12) | |||||
|---|---|---|---|---|---|---|
| t0 | t3 | p | t0 | t3 | p | |
| Ferritin (ng/mL) | 9 (4) | 9 (5.2) | 0.26 | 8.5 (5) | 34 (30.8) | 0.002 |
| Hemoglobin (g/dL) | 12.9 (0.4) | 12.9 (1.2) | 0.72 | 12.9 (0.6) | 13.8 (1.0) | 0.03 |
| Iron (mcg/dL) | 59 (53) | 61 (58) | 0.46 | 51 (37) | 98 (27.5) | 0.03 |
| Transferrin (mg/dL) | 314 (51) | 300 (72) | 0.06 | 304 (75.5) | 256.5 (32.5) | 0.002 |
| Transferrin saturation (%) | 14 (6) | 10 (13) | 0.14 | 12 (9.5) | 24.5 (11) | 0.007 |
| GFD-HI Group (n = 10) | FS Group (n = 12) | ||||||
|---|---|---|---|---|---|---|---|
| t0 | t3 | P * | t0 | t3 | P * | P †GFD-HI vs. FS at t3 | |
| Abdominal pain | 0 (3) | 0 (1) | 0.85 | 0 (1.5) | 0.5 (2) | 0.35 | 0.581 |
| Epigastric burning | 0 (-) | 0 (-) | - | 0.5 (4) | 0 (2.5) | 0.58 | 0.056 |
| Abdominal bloating | 4 (5) | 1 (4) | 0.22 | 2.5 (5.5) | 0.5 (4) | 0.10 | 0.964 |
| Diarrhea | 0 (0) | 0 (0) | 0.31 | 0 (0) | 0 (2) | 0.04 | 0.300 |
| Constipation | 0 (0) | 1 (5) | 0.28 | 0 (4) | 0 (5) | 1.0 | 0.547 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scricciolo, A.; Elli, L.; Doneda, L.; Bascunan, K.A.; Branchi, F.; Ferretti, F.; Vecchi, M.; Roncoroni, L. Efficacy of a High-Iron Dietary Intervention in Women with Celiac Disease and Iron Deficiency without Anemia: A Clinical Trial. Nutrients 2020, 12, 2122. https://doi.org/10.3390/nu12072122
Scricciolo A, Elli L, Doneda L, Bascunan KA, Branchi F, Ferretti F, Vecchi M, Roncoroni L. Efficacy of a High-Iron Dietary Intervention in Women with Celiac Disease and Iron Deficiency without Anemia: A Clinical Trial. Nutrients. 2020; 12(7):2122. https://doi.org/10.3390/nu12072122
Chicago/Turabian StyleScricciolo, Alice, Luca Elli, Luisa Doneda, Karla A Bascunan, Federica Branchi, Francesca Ferretti, Maurizio Vecchi, and Leda Roncoroni. 2020. "Efficacy of a High-Iron Dietary Intervention in Women with Celiac Disease and Iron Deficiency without Anemia: A Clinical Trial" Nutrients 12, no. 7: 2122. https://doi.org/10.3390/nu12072122
APA StyleScricciolo, A., Elli, L., Doneda, L., Bascunan, K. A., Branchi, F., Ferretti, F., Vecchi, M., & Roncoroni, L. (2020). Efficacy of a High-Iron Dietary Intervention in Women with Celiac Disease and Iron Deficiency without Anemia: A Clinical Trial. Nutrients, 12(7), 2122. https://doi.org/10.3390/nu12072122






