Earlier Nutrient Fortification of Breastmilk Fed LBW Infants Improves Jaundice Related Outcomes
Abstract
1. Introduction
2. Materials and Methods
2.1. Population Overview
2.2. Participation
2.3. Data Collection
2.4. Outcomes
2.5. Feeding Protocol
2.6. Data Analysis
2.7. Sample Size Calculation
3. Results
3.1. Overall Characteristics
3.2. Anthropometric Findings
3.3. Jaundice Outcomes
3.4. Clinical Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cohen, R.S.; Wong, R.J.; Stevenson, D.K. Understanding Neonatal Jaundice: A Perspective on Causation. Pediatr. Neonatol. 2010, 5, 143–148. [Google Scholar] [CrossRef]
- Kirk, J.M. Neonatal jaundice: A critical review of the role and practice of bilirubin analysis. Ann. Clin. Biochem. 2008, 45, 452–462. [Google Scholar] [CrossRef] [PubMed]
- American Academy of Pediatrics Subcommittee on Hyperbilirubinemia. Management of Hyperbilirubinemia in the Newborn Infant 35 or More Weeks of Gestation. Pediatrics 2004, 114, 297–316. [Google Scholar] [CrossRef]
- Laurance, B.M.; Smith, B.H.; Wallis, H. The Premature Baby’s Diet. Lancet 1962, 279, 589–590. [Google Scholar] [CrossRef]
- Wennberg, R.P.; Schwartz, R.; Sweet, A.Y. Early versus delayed feeding of low birth weight infants: Effects on physiologic jaundice. J. Pediatr. 1966, 68, 860–866. [Google Scholar] [CrossRef]
- Kuhr, M.; Paneth, N. Feeding practices and early neonatal jaundice. J. Pediatr. Gastroenterol. Nutr. 1982, 1, 485–488. [Google Scholar] [CrossRef]
- Narayanan, I.; Gupta, A.; Mandal, R.N.; Chugh, R.K.; Singh, S. Infant feeding and early neonatal jaundice. Indian J. Pediatr. 1987, 54, 257–260. [Google Scholar] [CrossRef]
- Gourley, G.R.; Kreamer, B.; Cohnen, M.; Kosorok, M.R. Neonatal jaundice and diet. Arch. Pediatr. Adolesc. Med. 1999, 153, 184–188. [Google Scholar] [CrossRef][Green Version]
- Dunn, L.; Hulman, S.; Weiner, J.; Kliegman, R. Beneficial effects of early hypocaloric enteral feeding on neonatal gastrointestinal function: Preliminary report of a randomized trial. J. Pediatr. 1988, 112, 622–629. [Google Scholar] [CrossRef]
- Makay, B.; Duman, N.; Ozer, E.; Kumral, A.; Yeşilirmak, D.; Ozkan, H. Randomized, controlled trial of early intravenous nutrition for prevention of neonatal jaundice in term and near-term neonates. J. Pediatr. Gastroenterol. Nutr. 2007, 44, 354–358. [Google Scholar] [CrossRef]
- Fan, W.Q.; Gan, A.; Crane, O. Commencing Nutrient Supplements before Full Enteral Feed Volume Achievement Is Beneficial for Moderately Preterm to Late Preterm Low Birth Weight Babies: A Prospective, Observational Study. Nutrients 2018, 10, 1340. [Google Scholar] [CrossRef] [PubMed]
- Fenton, T.R.; Kim, J.H. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr. 2013, 13, 59. [Google Scholar] [CrossRef]
- Mabhandi, T.; Ramdin, T.; Ballot, D.E. Growth of extremely low birth weight infants at a tertiary hospital in a middle-income country. BMC Pediatr. 2019, 19, 231. [Google Scholar] [CrossRef] [PubMed]
- Samarani, M.; Restom, G.; Mardini, J.; Abi Fares, G.; Hallit, S.; Fadous Khalife, M.C. Comparative study between Fenton and intergrowth 21 charts in a sample of Lebanese premature babies. BMC Pediatr. 2020, 20, 74. [Google Scholar] [CrossRef] [PubMed]
- Tenório, M.; Mello, C.; Santos, J.; Oliveira, A. Comparison of adequacy of birth weight for gestational age according to different intrauterine growth curves. Rev. Bras. Saúde Matern. Infant. 2019, 19, 935–940. [Google Scholar] [CrossRef]
- Pritchard, N.L.; Hiscock, R.J.; Lockie, E.; Permezel, M.; McGauren, M.F.G.; Kennedy, A.L.; Green, B.; Walker, S.P.; Lindquist, A.C. Identification of the optimal growth chartsfor use in a preterm population: An Australian state-wide retrospective cohort study. PLoS Med. 2019, 16, e1002923. [Google Scholar] [CrossRef] [PubMed]
- Maisels, M.J.; Watchko, J.F. Treatment of jaundice in low birthweight infants. Arch. Dis. Child. Fetal Neonatal Ed. 2003, 88, F459–F463. [Google Scholar] [CrossRef]
- De Carvalho, M.; Klaus, M.H.; Merkatz, R.B. Frequency of breast-feeding and serum bilirubin concentration. Am. J. Dis. Child. 1982, 136, 737–738. [Google Scholar] [CrossRef]
- Bosma, P.J.; Seppen, J.; Goldhoorn, B.; Bakker, C.; Oude Elferink, R.P.; Chowdhury, J.R.; Chowdhury, N.R.; Jansen, P.L. Bilirubin UDP-glucuronosyltransferase 1 is the only relevant bilirubin glucuronidating isoform in man. J. Biol. Chem. 1994, 269, 17960–17964. [Google Scholar]
- Onishi, S.; Kawade, N.; Itoh, S.; Isobe, K.; Sugiyama, S. Postnatal development of uridine diphosphate glucuronyltransferase activity towards bilirubin and 2-aminophenol in human liver. Biochem. J. 1979, 184, 705–707. [Google Scholar] [CrossRef]
- Strassburg, C.P.; Strassburg, A.; Kneip, S.; Barut, A.; Tukey, R.H.; Rodeck, B.; Manns, M.P. Developmental aspects of human hepatic drug glucuronidation in young children and adults. Gut 2002, 50, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, R.; Maruo, Y.; Chen, S.; Tukey, R.H. Role of extrahepatic UDP-glucuronosyltransferase 1A1: Advances in understanding breast milk-induced neonatal hyperbilirubinemia. Toxicol. Appl. Pharmacol. 2015, 289, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, R.; Chen, S.; Karin, M.; Tukey, R.H. Reduced expression of UGT1A1 in intestines of humanized UGT1 mice via inactivation of NF-κB leads to hyperbilirubinemia. Gastroenterology 2012, 142, 109.e2–118.e2. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, M.; Muraca, M.; Hammerman, C.; Rubaltelli, F.F.; Vlei, M.T.; Vreman, H.J.; Stevenson, D.K. Imbalance between production and conjugation of bilirubin: A fundamental concept in the mechanism of neonatal jaundice. Pediatrics 2002, 110, e47. [Google Scholar] [CrossRef]
- Fujiwara, R.; Haag, M.; Schaeffeler, E.; Nies, A.T.; Zanger, U.M.; Schwab, M. Systemic regulation of bilirubin homeostasis: Potential benefits of hyperbilirubinemia. Hepatology 2018, 67, 1609–1619. [Google Scholar] [CrossRef]
- Aoshima, N.; Fujie, Y.; Itoh, T.; Tukey, R.H.; Fujiwara, R. Glucose induces intestinal human UDP-glucuronosyltransferase (UGT) 1A1 to prevent neonatal hyperbilirubinemia. Sci. Rep. 2014, 4, 6343. [Google Scholar] [CrossRef]
- Bertino, E.; Giribaldi, M.; Cester, E.A.; Coscia, A.; Trapani, B.M.; Peila, C.; Arslanoglu, S.; Moro, G.E.; Cavallarin, L. New human milk fortifiers for the preterm infant. J. Pediatr. Neonatal Individ. Med. 2017, 6, e060124. [Google Scholar] [CrossRef]
- Hofman, D.L.; van Buul, V.J.; Brouns, F.J. Nutrition, Health, and Regulatory Aspects of Digestible Maltodextrins. Crit. Rev. Food Sci. Nutr. 2016, 56, 2091–2100. [Google Scholar] [CrossRef]
- Faal, G.; Masjedi, H.K.; Sharifzadeh, G.; Kiani, Z. Efficacy of zinc sulfate on indirect hyperbilirubinemia in premature infants admitted to neonatal intensive care unit: A double-blind, randomized clinical trial. BMC Pediatr. 2020, 20, 130. [Google Scholar] [CrossRef]
- Vítek, L.; Muchová, L.; Zelenka, J.; Zadinová, M.; Malina, J. The effect of zinc salts on serum bilirubin levels in hyperbilirubinemic rats. J. Pediatr. Gastroenterol. Nutr. 2005, 40, 135–140. [Google Scholar] [CrossRef]
- Vítek, L.; Kotal, P.; Jirsa, M.; Malina, J.; Cerná, M.; Chmelar, D.; Fevery, J. Intestinal colonization leading to fecal urobilinoid excretion may play a role in the pathogenesis of neonatal jaundice. J. Pediatr. Gastroenterol. Nutr. 2000, 30, 294–298. [Google Scholar] [CrossRef] [PubMed]
- Verkade, H.J.; Hoving, E.B.; Muskiet, F.A.; Martini, I.A.; Jansen, G.; Okken, A.; Vonk, R.J.; Bijleveld, C.M. Fat absorption in neonates: Comparison of long-chain-fatty-acid and triglyceride compositions of formula, feces, and blood. Am. J. Clin. Nutr. 1991, 53, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Buiter, H.D.; Dijkstra, S.S.; Oude Elferink, R.F.; Bijster, P.; Woltil, H.A.; Verkade, H.J. Neonatal jaundice and stool production in breast- or formula-fed term infants. Eur. J. Pediatr. 2008, 167, 501–507. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Elfarargy, M.S.; Ali, D.A.; Al-Ashmawy, G.M. Study of Vitamin D and melatonin supplementation as adjuvant therapies in neonatal jaundice. Curr. Pediatr. Res. 2019, 23, 101–105. [Google Scholar]
- Bhat, J.A.; Sheikh, S.A.; Ara, R. Correlation of 25-hydroxy vitamin D level with neonatal hyperbilirubinemia in term healthy newborn: A prospective hospital-based observation study. Int. J. Pediatr. Adolesc. Med. 2019, in press. [Google Scholar] [CrossRef]
- Aletayeb, S.M.; Dehdashtiyan, M.; Aminzadeh, M.; Malekyan, A.; Jafrasteh, S. Comparison between maternal and neonatal serum vitamin D levels in term jaundiced and nonjaundiced cases. J. Chin. Med. Assoc. 2016, 79, 614–617. [Google Scholar] [CrossRef]
- Wu, P.Y.; Lim, R.C.; Hodgman, J.E.; Kokosky, M.J.; Teberg, A.J. Effect of phototherapy in preterm infants on growth in the neonatal period. J. Pediatr. 1974, 85, 563–566. [Google Scholar] [CrossRef]
- Terrin, G.; Canani, B.C.; Di Chiara, M.; Pietravalle, A.; Aleandri, V.; Conte, F.; De Curtis, M. Zinc in EarlyLife: A Key Element in the Fetus and Preterm Neonate. Nutrients 2015, 7, 10427–10446. [Google Scholar] [CrossRef]
- Starke, I.C.; Pieper, R.; Neumann, K.; Zentek, J.; Vahjen, W. The impact of high dietary zinc oxide on the development of the intestinal microbiota in weaned piglets. FEMS Microbiol. Ecol. 2014, 87, 416–427. [Google Scholar] [CrossRef]
| F80 (n = 105) | F160 (n = 110) | p Value | |
|---|---|---|---|
| Gestational Age (weeks), mean ± sd. | 34.6 ± 1.1 | 34.3 ± 1.1 | 0.107 |
| Gender (male) | 51 (49) | 57 (52) | 0.683 |
| Birth weight (g), mean ± sd. | 2134 ± 357 | 2076 ± 349 | 0.881 |
| Birth weight (Z-score), mean ± sd. | −0.58 ± 0.93 | −0.55 ± 0.88 | 0.761 |
| Days to start supplement, mean ± sd | 2.6 ± 0.8 | 7.4 ± 1.7 | <0.0001 |
| Reason for prematurity | |||
| PPROM | 32 (30) | 40 (36) | 0.388 |
| Preeclampsia | 13 (12) | 14 (13) | 1 |
| APH | 6 (6) | 9 (8) | 0.595 |
| SGA | 29 (28) | 19 (170) | 0.074 |
| Multiples | 7 (7) | 8 (7) | 1 |
| Non-reassuring CTG | 15 (14) | 11 (10) | 0.405 |
| Mode of delivery | |||
| Normal vaginal birth | 41 (39) | 45 (41) | 0.889 |
| Instrumental delivery | 8 (8) | 5 (5) | 0.345 |
| Elective caesarean | 15 (14) | 11 (10) | 0.225 |
| Emergency caesarean | 41 (39) | 48 (44) | 0.293 |
| F80 (n = 105) | F160 (n = 110) | p Value | |
|---|---|---|---|
| Age (years), mean ± sd | 29.6 ± 5.3 | 30.5 ± 5.5 | 0.237 |
| Region of Birth | 0.394 | ||
| Australia or New Zealand | 59 (56) | 48 (44) | |
| Middle East | 9 (9) | 12 (11) | |
| South Asia | 24 (23) | 36 (33) | |
| Others | 11 (10) | 12 (11) | |
| Language | 0.449 | ||
| English | 92 (88) | 92 (84) | |
| Arabic | 9 (9) | 7 (6) | |
| Others | 4 (4) | 10 (9) | |
| Antenatal Complications | |||
| Pre-eclampsia | 18 (17) | 20 (18) | 0.722 |
| GDM | 30 (29) | 30 (27) | 0.664 |
| GBS positive | 6 (6) | 3 (3) | 0.510 |
| F80 (n = 105) | F160 (n = 110) | p Value | |
|---|---|---|---|
| Maximum weight loss (g), mean ± sd | 108 ± 8 | 118 ± 6 | 0.072 |
| Maximum weight loss as % birth weight | 5% | 6% | 0.034 |
| Days to regain birth weight, mean ± sd | 10.0 ± 0.3 | 11.5 ± 0.3 | <0.0001 |
| Weight gain: Δ Z-score at 10 days, mean ± sd | −0.70 ± 0.03 | −0.79 ± 0.03 | 0.01 |
| Weight gain: Δ Z-score at discharge, mean ± sd | −0.64 ± 0.03 | −0.68 ± 0.04 | 0.14 |
| Discharge weight (g), mean ± sd | 2348 ± 293 | 2359 ± 273 | 0.785 |
| F80 (n = 105) | F160 (n = 110) | p Value | |
|---|---|---|---|
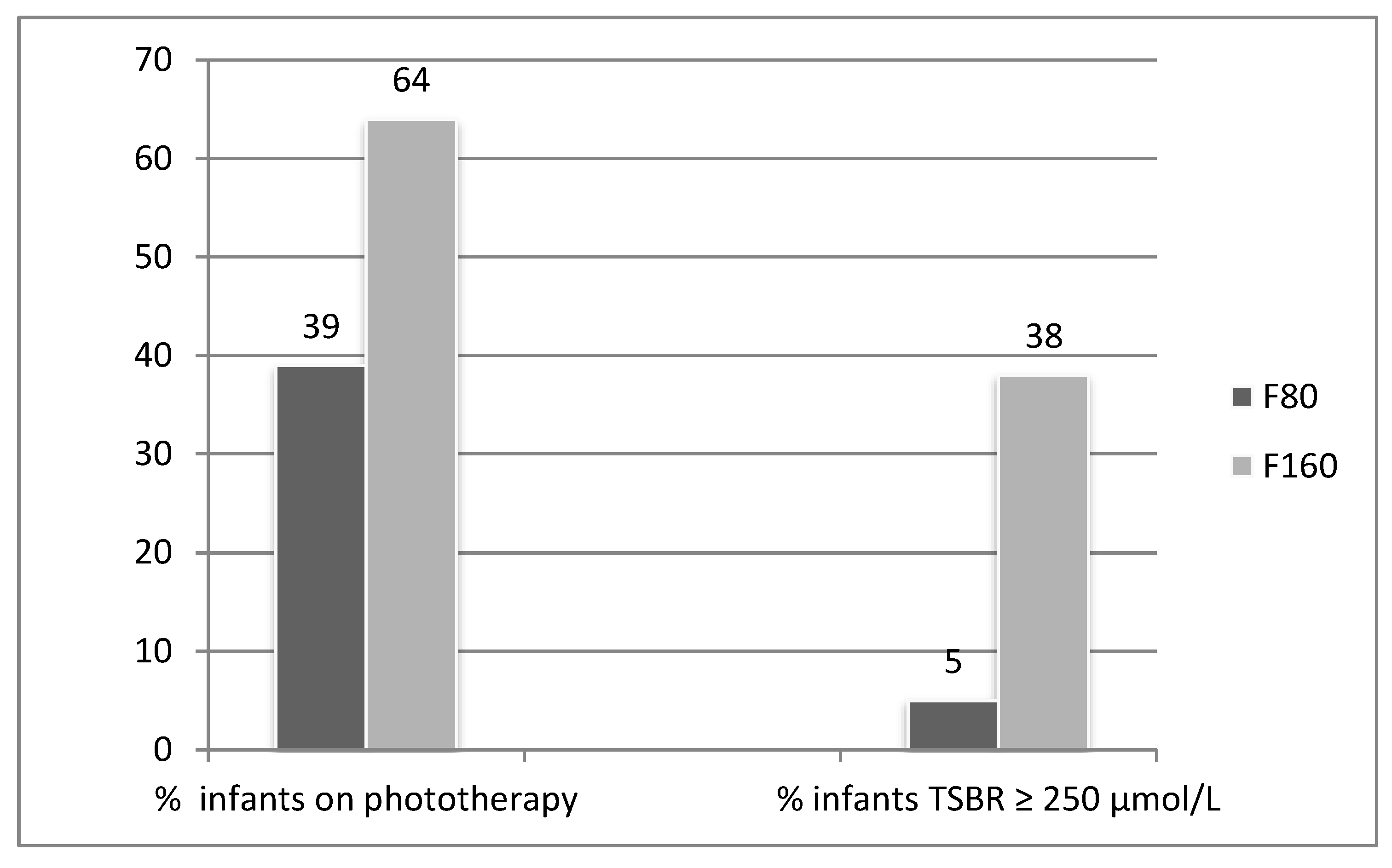
| Phototherapy Extent | |||
| One Light | 9 (9) | 24 (22) | 0.008 |
| Two Lights | 19 (18) | 17 (15) | 0.7153 |
| Three Lights | 13 (12) | 29 (26) | 0.0103 |
| Median Dose (number of lights) | 2 | 2 | |
| Maximum S. Bilrubin Value | |||
| TSBR (Mean ± sd); µmol/l | 196 ± 46 | 228 ± 52 | 0.0003 |
| USBR (Mean ± sd); µmol/l | 184 ± 44 | 212 ± 50 | 0.0008 |
| CSBR (Mean ± sd); µmol/l | 12 ± 4 | 16 ± 5 | <0.0001 |
| % CSBR of TSBR (Mean ± sd) | 6.2 ± 1.6 | 7.0 ± 2.8 | 0.0694 |
| Days to Maximum TSBR | 4.8 ± 1.8 | 5.1 ± 2.0 | 0.4298 |
| F80 (n = 105) | F160 (n = 110) | p Value | |
|---|---|---|---|
| Feeding Intolerance | 28 (26.0) | 49(45.2) | 0.007 |
| Presumed Sepsis | 41 (48) | 67 (60) | 0.693 |
| RDS | 16 (15) | 27 (24) | 0.882 |
| Hypoglycaemia | 27 (25) | 30 (27) | 0.818 |
| NEC | 0 (0) | 0 (0) | 1 |
| LOS in SCN (days), mean ± sd | 16.0 ± 0.6 | 18.8 ± 0.7 | 0.036 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, X.W.; Fan, W.Q. Earlier Nutrient Fortification of Breastmilk Fed LBW Infants Improves Jaundice Related Outcomes. Nutrients 2020, 12, 2116. https://doi.org/10.3390/nu12072116
Ma XW, Fan WQ. Earlier Nutrient Fortification of Breastmilk Fed LBW Infants Improves Jaundice Related Outcomes. Nutrients. 2020; 12(7):2116. https://doi.org/10.3390/nu12072116
Chicago/Turabian StyleMa, Xiao Wei, and Wei Qi Fan. 2020. "Earlier Nutrient Fortification of Breastmilk Fed LBW Infants Improves Jaundice Related Outcomes" Nutrients 12, no. 7: 2116. https://doi.org/10.3390/nu12072116
APA StyleMa, X. W., & Fan, W. Q. (2020). Earlier Nutrient Fortification of Breastmilk Fed LBW Infants Improves Jaundice Related Outcomes. Nutrients, 12(7), 2116. https://doi.org/10.3390/nu12072116






