Home Enteral Nutrition in Adults—Nationwide Multicenter Survey
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Primary Disease
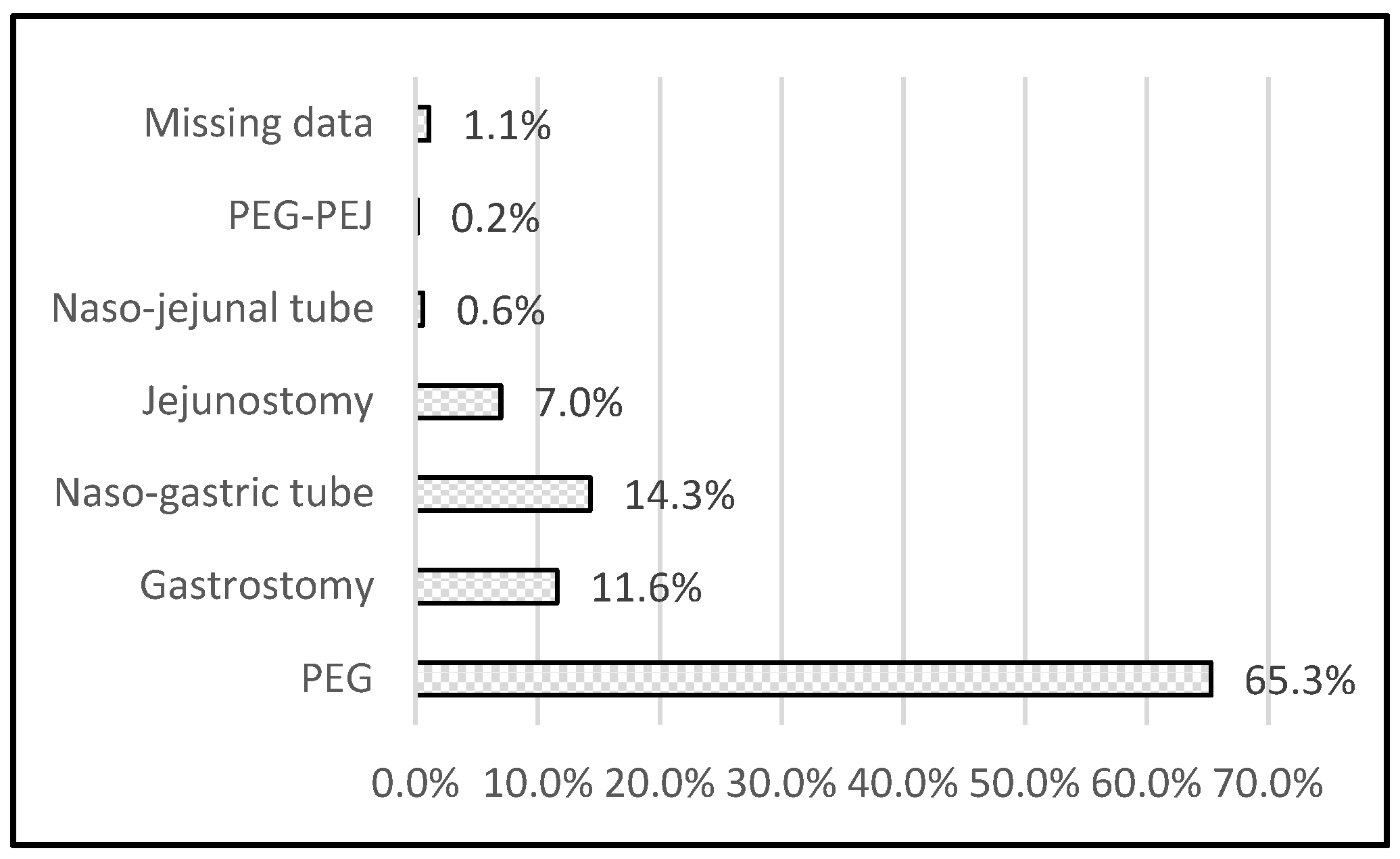
3.2. Technique of EN
3.3. Enteral Diets
3.4. Outcomes
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bischoff, S.C.; Austin, P.; Boeykens, K.; Chourdakis, M.; Cuerda, C.; Jonkers-Schuitema, C.; Lichota, M.; Nyulasi, I.; Schneider, S.M.; Stanga, Z.; et al. ESPEN Guideline ESPEN guideline on home enteral nutrition. Clin. Nutr. 2019. [Google Scholar] [CrossRef]
- Howard, L.; Ament, M.; Richard Fleming, C.; Shike, M.; Steiger, E. Current use and clinical outcome of home parenteral and enteral nutrition therapies in the United States. Gastroenterology 1995, 109, 355–365. [Google Scholar] [CrossRef]
- Mundi, M.S.; Pattinson, A.; McMahon, M.T.; Davidson, J.; Hurt, R.T. Prevalence of Home Parenteral and Enteral Nutrition in the United States. Nutr. Clin. Pract. 2017, 32, 799–805. [Google Scholar] [CrossRef] [PubMed]
- Paccagnella, A.; Marcon, M.L.; Baruffi, C.; Giometto, M.; Mauri, A.; Vigo, C.; Scantamburlo, A.; Sambado, L.; Sambataro, M.; Trevisiol, E.; et al. Enteral nutrition at home and in nursing homes: An 11-year (2002–2012) epidemiological analysis. Minerva Gastroenterol. Dietol. 2016, 62, 1–10. [Google Scholar]
- Kłek, S.; Hermanowicz, A.; Dziwiszek, G.; Matysiak, K.; Szczepanek, K.; Szybinski, P.; Galas, A. Home enteral nutrition reduces complications, length of stay, and health care costs: Results from a multicenter study. Am. J. Clin. Nutr. 2014, 100, 609–615. [Google Scholar] [CrossRef]
- Klek, S.; Chourdakis, M.; Bischoff, S.; Dubrov, S.; Forbes, A.; Galas, A.; Genton, L.; Gundogdu, H.R.; Irtun, O.; Jagmane, I.; et al. Economy matters to fight against malnutrition: Results from a multicenter survey. Clin. Nutr. 2017, 36, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.; Banks, M.D.; Bauer, J.D. A survey of home enteral nutrition practices and reimbursement in the asia pacific region. Nutrients 2018, 10, 214. [Google Scholar] [CrossRef]
- Gavazzi, C.; Colatruglio, S.; Valoriani, F.; Mazzaferro, V.; Sabbatini, A.; Biffi, R.; Mariani, L.; Miceli, R. Impact of home enteral nutrition in malnourished patients with upper gastrointestinal cancer: A multicentre randomised clinical trial. Eur. J. Cancer 2016, 64, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Fearon, K.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN guidelines on nutrition in cancer patients. Clin. Nutr. 2017, 36, 11–48. [Google Scholar] [CrossRef] [PubMed]
- De Luis, D.A.; Izaola, O.; Cuellar, L.A.; Terroba, M.C.; Cabezas, G.; De La Fuente, B. Experience over 12 years with home enteral nutrition in a healthcare area of Spain. J. Hum. Nutr. Diet. 2013, 26, 39–44. [Google Scholar] [CrossRef]
- De Luis, D.A.; Aller, R.; Izaola, O.; Terroba, M.C.; Cabezas, G.; Cuellar, L.A. Experience of 6 years with home enteral nutrition in an area of Spain. Eur J Clin Nutr 2006, 60, 553–557. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hebuterne, X.; Bozzetti, F.; Moreno Villares, J.M.; Pertkiewicz, M.; Shaffer, J.; Staun, M.; Thul, P.; Van Gossum, A. Home enteral nutrition in adults: A European multicentre survey. Clin. Nutr. 2003, 22, 261–266. [Google Scholar] [CrossRef]
- Paccagnella, A.; Baruffi, C.; Pizzolato, D.; Favaro, V.; Marcon, M.L.; Morello, M.; Semenzin, M.; Rebuffi, S.; Fossa, E.; Faronato, P.; et al. Home enteral nutrition in adults: A five-year (2001–2005) epidemiological analysis. Clin. Nutr. 2008, 27, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Gaggiotti, G.; Ambrosi, S.; Spazzafumo, L.; Sgattoni, C.; Orlandoni, P.; Rosati, S. Two-year outcome data from the Italian Home Enteral Nutrition (IHEN) Register. Clin. Nutr. 1995, 14 (Suppl. 1), 2–5. [Google Scholar] [CrossRef]
- Klek, S.; Pawlowska, D.; Dziwiszek, G.; Komoń, H.; Compala, P.; Nawojski, M. Evolución de la nutrición enteral domiciliaria (hen) en polonia durante cinco años después de su implantación: Un estudio multicéntrico. Nutr. Hosp. 2015, 32, 196–201. [Google Scholar] [CrossRef]
- Hubbard, G.P.; Andrews, S.; White, S.; Simpson, G.; Topen, S.; Carnie, L.; Murphy, C.; Collins, R.; Davies, J.; Owen, A.; et al. A survey of bolus tube feeding prevalence and practice in adult patients requiring home enteral tube feeding. Br. J. Nutr. 2019, 122, 1271–1278. [Google Scholar] [CrossRef]
- Smith, T.; Naghibi, M. BANs Report 2016: Artificial Nutrition Support in the UK 2005–2015. Adult Home Parenteral Nutrition & Home Intravenous Fluids. Available online: www.bapen.org.uk/images/pdfs/reports/bans-report-2016.pdf (accessed on 10 July 2020).
- Stratton, R.; Evill, R.; Smith, T. BANS Report (2018) Home Enteral Tube Feeding (HETF) in Adults (2010–2015). A Report by the British Artificial Nutrition Survey (BANS)—A Committee of BAPEN. Available online: https://www.bapen.org.uk/pdfs/reports/bans/bans-report-2018.pdf (accessed on 10 July 2020).
- Cawsey, S.I.; Soo, J.; Gramlich, L.M. Home enteral nutrition: Outcomes relative to indication. Nutr. Clin. Pract. 2010, 25, 296–300. [Google Scholar] [CrossRef]
- Wanden-berghe Lozano, C. Registro del grupo nadya-senpe de nutrición enteral domiciliaria en españa; años 2016 y 2017. Nutr. Hosp. 2018. [Google Scholar] [CrossRef]
- Epp, L.; Lammert, L.; Vallumsetla, N.; Hurt, R.T.; Mundi, M.S. Use of Blenderized Tube Feeding in Adult and Pediatric Home Enteral Nutrition Patients. Nutr. Clin. Pract. 2017, 32, 201–205. [Google Scholar] [CrossRef]
- Klek, S.; Szybinski, P.; Sierzega, M.; Szczepanek, K.; Sumlet, M.; Kupiec, M.; Koczur-Szozda, E.; Steinhoff-Nowak, M.; Figula, K.; Kowalczyk, T.; et al. Commercial enteral formulas and nutrition support teams improve the outcome of home enteral tube feeding. J. Parenter Enter. Nutr. 2011, 35, 380–385. [Google Scholar] [CrossRef]
- Wanden-Berghe, C.; Patino-Alonso, M.C.; Galindo-Villardón, P.; Sanz-Valero, J. Complications associated with enteral nutrition: CAFANE study. Nutrients 2019, 11, 2041. [Google Scholar] [CrossRef]
- Gómez Candela, C.; de Cos, A.I.; Iglesias, C.; Carbonell, M.D.; Camarero, E.; Carrera, J.A.; Celador, A.; Chamorro, J.; Ferrón, F.; Luna, P.G.; et al. Artificial nutrition in the home. Annual information 1996. Group NADYA-SENPE Nutr. Hosp. 1996, 14, 145–152. [Google Scholar]
- Santarpia, L.; Pagano, M.C.; Pasanisi, F.; Contaldo, F. Home artificial nutrition: An update seven years after the regional regulation. Clin. Nutr. 2014, 33, 872–878. [Google Scholar] [CrossRef] [PubMed]
| Overall | January 2018 | New Patients | |
|---|---|---|---|
| N | n = 4586,100% | n = 2620, 57.1% | n = 1966, 42.9% |
| Age (mean ± SD) | 64 ± 19 | 62 ± 21 | 67 ± 16 |
| Male | 53.30% | 51.70% | 55.60% |
| Female | 46.70% | 48.30% | 44.40% |
| BMI (kg m−2) | 20.2 (SD-4.3) | 20 (SD-4.3) | 20.6 (SD-4.3) |
| NRS 2002 | 4.28 (SD-1.17) | 4.23 (SD-1.26) | 4.36 (SD-1) |
| Overall | January 2018 | New Patients | |
|---|---|---|---|
| Neurology | 54.5% | 61.6% | 45.1% |
| Neurovascular | 17.4% | 17.7% | 16.9% |
| Neurodegenerative | 13.7% | 13.4% | 14.0% |
| Cerebral palsy | 4.3% | 6.1% | 1.8% |
| Multiple sclerosis | 3.0% | 4.2% | 1.5% |
| Amyotrophic Lateral Sclerosis | 7.5% | 8.8% | 5.7% |
| Other Encephalopathy | 3.4% | 5.2% | 1.2% |
| Other neurological | 3.8% | 4.4% | 3.0% |
| Cancer | 33.9% | 24.7% | 46.3% |
| Head and neck | 20.2% | 15.7% | 26.2% |
| GI | 11.7% | 7.6% | 17.2% |
| Other (cancer) | 1.8% | 1.2% | 2.6% |
| Gastroenterology1 | 2.5% | 2.6% | 2.3% |
| Inherited diseases2 | 1.5% | 2.3% | 0.5% |
| Other3 | 7.5% | 8.8% | 5.8% |
| Administration Technique | % |
| Pump | 8.0 |
| Bolus | 74.4 |
| Gravity flow | 17.6 |
| Calorie provision | kcal/kg/day (median) |
| All patients | 24 |
| Neurology | 23.1 |
| Cancer | 24.2 |
| Inherited diseases | 27.8 |
| Gastroenterology | 24.4 |
| Other | 25.0 |
| Diet | All (%) | Cancer (%) | Neurology (%) | Other (%) |
|---|---|---|---|---|
| Standard | 28.1 | 24.1 | 30.8 | 26.8 |
| Protein-enriched | 20.0 | 21.3 | 20.2 | 14.7 |
| Oligopeptide, protein enriched | 0.0 | 0.1 | 0.0 | 0.0 |
| Oligopeptide, hypercaloric, protein enriched | 0.1 | 0.2 | 0.1 | 0.0 |
| Oligopeptide | 10.0 | 15.4 | 6.9 | 8.7 |
| Hypercaloric, protein enriched | 12.0 | 13.9 | 10.4 | 14.2 |
| Hypercaloric | 0.8 | 1.3 | 0.5 | 0.8 |
| Fiber-enriched, protein enriched, isocaloric | 5.0 | 4.9 | 4.9 | 5.7 |
| Fiber-enriched, protein enriched, hypercaloric | 0.4 | 0.4 | 0.4 | 0.4 |
| Fiber-enriched, hypercaloric | 0.2 | 0.1 | 0.0 | 0.8 |
| Fiber-enriched | 11.6 | 8.1 | 13.4 | 13.0 |
| Diabetic | 7.8 | 6.8 | 8.5 | 7.0 |
| Diabetic, hypercaloric, protein enriched | 2.2 | 2.2 | 2.0 | 2.8 |
| Diabetic, hypercaloric | 0.3 | 0.2 | 0.2 | 0.9 |
| Missing data | 1.7 | 1.1 | 1.6 | 4.2 |
| Outcome (%) | All | Neurology | Cancer | Inherited | Gastroenterology | Other |
|---|---|---|---|---|---|---|
| Still on HEN | 48.5 | 52.9 | 37.3 | 60.3 | 60.2 | 60.8 |
| Death | 40.2 | 37.3 | 49.2 | 29.4 | 23.0 | 28.3 |
| Resumed sufficient oral nutrition | 5.3 | 4.3 | 7.0 | 5.9 | 5.3 | 4.7 |
| Resignation from HEN | 1.2 | 1.1 | 0.9 | 1.5 | 2.7 | 2.9 |
| Transfer to another unit of HEN | 0.6 | 0.7 | 0.3 | 1.5 | 1.8 | 0.6 |
| Transfer to stationary palliative care unit | 4.1 | 3.6 | 5.2 | 1.5 | 7.1 | 2.7 |
| Duration of HEN (median; interquartile range) | 354; 1108 | 615; 1275 | 209; 534 | 1020; 1517 | 419; 1671 | 943; 1845 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Folwarski, M.; Kłęk, S.; Zoubek-Wójcik, A.; Szafrański, W.; Bartoszewska, L.; Figuła, K.; Jakubczyk, M.; Jurczuk, A.; Kamocki, Z.; Kaźmierczak-Siedlecka, K.; et al. Home Enteral Nutrition in Adults—Nationwide Multicenter Survey. Nutrients 2020, 12, 2087. https://doi.org/10.3390/nu12072087
Folwarski M, Kłęk S, Zoubek-Wójcik A, Szafrański W, Bartoszewska L, Figuła K, Jakubczyk M, Jurczuk A, Kamocki Z, Kaźmierczak-Siedlecka K, et al. Home Enteral Nutrition in Adults—Nationwide Multicenter Survey. Nutrients. 2020; 12(7):2087. https://doi.org/10.3390/nu12072087
Chicago/Turabian StyleFolwarski, Marcin, Stanisław Kłęk, Agata Zoubek-Wójcik, Waldemar Szafrański, Lidia Bartoszewska, Krzysztof Figuła, Marlena Jakubczyk, Anna Jurczuk, Zbigniew Kamocki, Karolina Kaźmierczak-Siedlecka, and et al. 2020. "Home Enteral Nutrition in Adults—Nationwide Multicenter Survey" Nutrients 12, no. 7: 2087. https://doi.org/10.3390/nu12072087
APA StyleFolwarski, M., Kłęk, S., Zoubek-Wójcik, A., Szafrański, W., Bartoszewska, L., Figuła, K., Jakubczyk, M., Jurczuk, A., Kamocki, Z., Kaźmierczak-Siedlecka, K., Kowalczyk, T., Kwella, B., Matras, P., Skonieczna-Żydecka, K., Sonsala-Wołczyk, J., Szopiński, J., Urbanowicz, K., & Zmarzły, A. (2020). Home Enteral Nutrition in Adults—Nationwide Multicenter Survey. Nutrients, 12(7), 2087. https://doi.org/10.3390/nu12072087






