Urinary Excretion of N1-Methylnicotinamide and N1-Methyl-2-Pyridone-5-Carboxamide and Mortality in Kidney Transplant Recipients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Data Collection
2.3. Assessment of N1-MN and 2Py Excretion
2.4. Clinical Endpoints
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics and Cross-Sectional Analyses
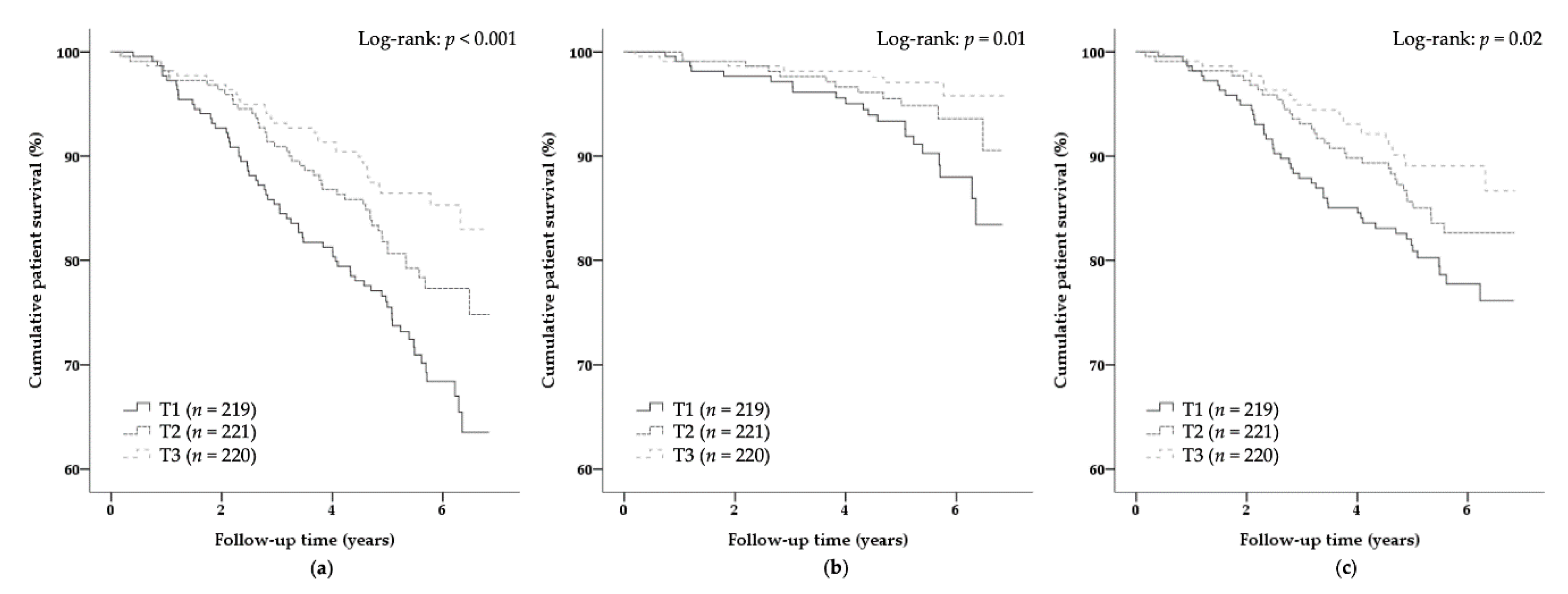
3.2. Primary Prospective Analyses
3.3. Secondary Prospective Analyses
3.4. Sensitivity Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Briggs, J.D. Causes of death after renal transplantation. Nephrol. Dial. Transplant. 2001, 16, 1545–1549. [Google Scholar] [CrossRef] [PubMed]
- Noble, J.; Jouve, T.; Malvezzi, P.; Susal, C.; Rostaing, L. Transplantation of Marginal Organs: Immunological Aspects and Therapeutic Perspectives in Kidney Transplantation. Front. Immunol. 2020, 10, 3142. [Google Scholar] [CrossRef] [PubMed]
- Tong, A.; Budde, K.; Gill, J.; Josephson, M.A.; Marson, L.; Pruett, T.L.; Reese, P.P.; Rosenbloom, D.; Rostaing, L.; Warrens, A.N.; et al. Standardized Outcomes in Nephrology-Transplantation: A Global Initiative to Develop a Core Outcome Set for Trials in Kidney Transplantation. Transplant Direct 2016, 2, e79. [Google Scholar] [CrossRef] [PubMed]
- Neuberger, J.M.; Bechstein, W.O.; Kuypers, D.R.; Burra, P.; Citterio, F.; De Geest, S.; Duvoux, C.; Jardine, A.G.; Kamar, N.; Kramer, B.K.; et al. Practical Recommendations for Long-term Management of Modifiable Risks in Kidney and Liver Transplant Recipients: A Guidance Report and Clinical Checklist by the Consensus on Managing Modifiable Risk in Transplantation (COMMIT) Group. Transplantation 2017, 101, S1–S56. [Google Scholar] [CrossRef] [PubMed]
- Nolte Fong, J.V.; Moore, L.W. Nutrition Trends in Kidney Transplant Recipients: The Importance of Dietary Monitoring and Need for Evidence-Based Recommendations. Front. Med. (Lausanne) 2018, 5, 302. [Google Scholar] [CrossRef] [PubMed]
- Du Plessis, A.S.; Randall, H.; Escreet, E.; Holl, M.; Conradie, M.; Moosa, M.R.; Labadarios, D.; Herselman, M.G. Nutritional status of renal transplant patients. S. Afr. Med. J. 2002, 92, 68–74. [Google Scholar]
- Teplan, V.; Valkovsky, I.; Teplan, V., Jr.; Stollova, M.; Vyhnanek, F.; Andel, M. Nutritional consequences of renal transplantation. J. Ren. Nutr. 2009, 19, 95–100. [Google Scholar] [CrossRef]
- Veroux, M.; Corona, D.; Sinagra, N.; Tallarita, T.; Ekser, B.; Giaquinta, A.; Zerbo, D.; Veroux, P. Nutrition in kidney transplantation. Int. J. Artif. Organs 2013, 36, 677–686. [Google Scholar] [CrossRef]
- Ter Wee, P.M. Protein energy wasting and transplantation. J. Ren. Nutr. 2013, 23, 246–249. [Google Scholar] [CrossRef]
- Hanna, R.M.; Ghobry, L.; Wassef, O.; Rhee, C.M.; Kalantar-Zadeh, K. A Practical Approach to Nutrition, Protein-Energy Wasting, Sarcopenia, and Cachexia in Patients with Chronic Kidney Disease. Blood Purif. 2019, 49, 202–211. [Google Scholar] [CrossRef]
- Eisenga, M.F.; Kieneker, L.M.; Soedamah-Muthu, S.S.; van den Berg, E.; Deetman, P.E.; Navis, G.J.; Gans, R.O.; Gaillard, C.A.; Bakker, S.J.; Joosten, M.M. Urinary potassium excretion, renal ammoniagenesis, and risk of graft failure and mortality in renal transplant recipients. Am. J. Clin. Nutr. 2016, 104, 1703–1711. [Google Scholar] [CrossRef] [PubMed]
- Minovic, I.; Riphagen, I.J.; van den Berg, E.; Kootstra-Ros, J.E.; van Faassen, M.; Gomes Neto, A.W.; Geleijnse, J.M.; Gans, R.O.; Eggersdorfer, M.; Navis, G.J.; et al. Vitamin B-6 deficiency is common and associated with poor long-term outcome in renal transplant recipients. Am. J. Clin. Nutr. 2017, 105, 1344–1350. [Google Scholar] [CrossRef] [PubMed]
- Minovic, I.; van der Veen, A.; van Faassen, M.; Riphagen, I.J.; van den Berg, E.; van der Ley, C.; Gomes-Neto, A.W.; Geleijnse, J.M.; Eggersdorfer, M.; Navis, G.J.; et al. Functional vitamin B-6 status and long-term mortality in renal transplant recipients. Am. J. Clin. Nutr. 2017, 106, 1366–1374. [Google Scholar] [CrossRef] [PubMed]
- Gomes Neto, A.W.; Sotomayor, C.G.; Pranger, I.G.; van den Berg, E.; Gans, R.O.; Soedamah-Muthu, S.S.; Navis, G.J.; Bakker, S.J. Intake of Marine-Derived Omega-3 Polyunsaturated Fatty Acids and Mortality in Renal Transplant Recipients. Nutrients 2017, 9, 363. [Google Scholar] [CrossRef]
- Sotomayor, C.G.; Gomes-Neto, A.W.; Eisenga, M.F.; Nolte, I.M.; Anderson, J.L.C.; de Borst, M.H.; Oste, M.C.J.; Rodrigo, R.; Gans, R.O.B.; Berger, S.P.; et al. Consumption of fruits and vegetables and cardiovascular mortality in renal transplant recipients: A prospective cohort study. Nephrol. Dial. Transplant. 2020, 35, 357–365. [Google Scholar] [CrossRef]
- Oste, M.C.J.; Gomes-Neto, A.W.; Corpeleijn, E.; Gans, R.O.B.; de Borst, M.H.; van den Berg, E.; Soedamah-Muthu, S.S.; Kromhout, D.; Navis, G.J.; Bakker, S.J.L. Dietary Approach to Stop Hypertension (DASH) diet and risk of renal function decline and all-cause mortality in renal transplant recipients. Am. J. Transplant. 2018, 18, 2523–2533. [Google Scholar] [CrossRef]
- Gacitua, T.A.; Sotomayor, C.G.; Groothof, D.; Eisenga, M.F.; Pol, R.A.; Borst, M.H.; Gans, R.O.B.; Berger, S.P.; Rodrigo, R.; Navis, G.J.; et al. Plasma Vitamin C and Cancer Mortality in Kidney Transplant Recipients. J. Clin. Med. 2019, 8, 2064. [Google Scholar] [CrossRef]
- Bogan, K.L.; Brenner, C. Nicotinic acid, nicotinamide, and nicotinamide riboside: A molecular evaluation of NAD+ precursor vitamins in human nutrition. Annu. Rev. Nutr. 2008, 28, 115–130. [Google Scholar] [CrossRef]
- Badawy, A.A. Kynurenine Pathway of Tryptophan Metabolism: Regulatory and Functional Aspects. Int. J. Tryptophan Res. 2017, 10, 1–20. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on Dietary Reference Values for niacin. EFSA J. 2014, 12, 3759. [Google Scholar] [CrossRef]
- World Health Organization and United Nations High Commissions for Refugees. Pellagra and Its Prevention and Control in Major Emergencies; WHO: Geneva, Switzerland, 2000; Available online: http://apps.who.int/iris/bitstream/handle/10665/66704/WHO_NHD_00.10.pdf?sequence=1&isAllowed=y (accessed on 26 March 2018).
- Deen, C.P.J.; van der Veen, A.; van Faassen, M.; Minovic, I.; Gomes-Neto, A.W.; Geleijnse, J.M.; Borgonjen-van den Berg, K.J.; Kema, I.P.; Bakker, S.J.L. Urinary Excretion of N(1)-Methylnicotinamide, as a Biomarker of Niacin Status, and Mortality in Renal Transplant Recipients. J. Clin. Med. 2019, 8, 1948. [Google Scholar] [CrossRef] [PubMed]
- Lal, S.M.; Hewett, J.E.; Petroski, G.F.; Van Stone, J.C.; Ross, G., Jr. Effects of nicotinic acid and lovastatin in renal transplant patients: A prospective, randomized, open-labeled crossover trial. Am. J. Kidney Dis. 1995, 25, 616–622. [Google Scholar] [CrossRef]
- Ahmed, M.H. Niacin as potential treatment for dyslipidemia and hyperphosphatemia associated with chronic renal failure: The need for clinical trials. Ren. Fail. 2010, 32, 642–646. [Google Scholar] [CrossRef] [PubMed]
- Park, C.W. Niacin in patients with chronic kidney disease: Is it effective and safe? Kidney Res. Clin. Pract. 2013, 32, 1–2. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rennick, A.; Kalakeche, R.; Seel, L.; Shepler, B. Nicotinic acid and nicotinamide: A review of their use for hyperphosphatemia in dialysis patients. Pharmacotherapy 2013, 33, 683–690. [Google Scholar] [CrossRef]
- Streja, E.; Kovesdy, C.P.; Streja, D.A.; Moradi, H.; Kalantar-Zadeh, K.; Kashyap, M.L. Niacin and progression of CKD. Am. J. Kidney Dis. 2015, 65, 785–798. [Google Scholar] [CrossRef]
- Taketani, Y.; Masuda, M.; Yamanaka-Okumura, H.; Tatsumi, S.; Segawa, H.; Miyamoto, K.; Takeda, E.; Yamamoto, H. Niacin and Chronic Kidney Disease. J. Nutr. Sci. Vitaminol. (Tokyo) 2015, 61, S173–S175. [Google Scholar] [CrossRef]
- Huang, Y.C.; Lee, M.S.; Wahlqvist, M.L. Prediction of all-cause mortality by B group vitamin status in the elderly. Clin. Nutr. 2012, 31, 191–198. [Google Scholar] [CrossRef]
- Deen, C.P.J.; van der Veen, A.; Gomes-Neto, A.W.; Geleijnse, J.M.; Borgonjen-van den Berg, K.J.; Heiner-Fokkema, M.R.; Kema, I.P.; Bakker, S.J.L. Urinary Excretion of N(1)-methyl-2-pyridone-5-carboxamide and N(1)-methylnicotinamide in Renal Transplant Recipients and Donors. J. Clin. Med. 2020, 9, 437. [Google Scholar] [CrossRef]
- Canto, C.; Menzies, K.J.; Auwerx, J. NAD+ Metabolism and the Control of Energy Homeostasis: A Balancing Act between Mitochondria and the Nucleus. Cell Metab. 2015, 22, 31–53. [Google Scholar] [CrossRef]
- Kapoor, A.; Thiemermann, C. Niacin as a novel therapy for septic shock? Crit. Care Med. 2011, 39, 410–411. [Google Scholar] [CrossRef] [PubMed]
- Kwon, W.Y.; Suh, G.J.; Kim, K.S.; Jung, Y.S.; Kim, S.H.; Kim, J.S.; You, K.M. Niacin and Selenium Attenuate Sepsis-Induced Lung Injury by Up-Regulating Nuclear Factor Erythroid 2-Related Factor 2 Signaling. Crit. Care Med. 2016, 44, e370–e382. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Thiemermann, C. Selenium and Niacin for Sepsis Therapy: The Sum Is Greater Than Its Parts. Crit. Care Med. 2016, 44, 1256–1257. [Google Scholar] [CrossRef] [PubMed]
- Van den Berg, E.; Engberink, M.F.; Brink, E.J.; van Baak, M.A.; Joosten, M.M.; Gans, R.O.; Navis, G.; Bakker, S.J. Dietary acid load and metabolic acidosis in renal transplant recipients. Clin. J. Am. Soc. Nephrol. 2012, 7, 1811–1818. [Google Scholar] [CrossRef]
- Van den Berg, E.; Engberink, M.F.; Brink, E.J.; van Baak, M.A.; Gans, R.O.; Navis, G.; Bakker, S.J. Dietary protein, blood pressure and renal function in renal transplant recipients. Br. J. Nutr. 2013, 109, 1463–1470. [Google Scholar] [CrossRef]
- Van den Berg, E.; Pasch, A.; Westendorp, W.H.; Navis, G.; Brink, E.J.; Gans, R.O.; van Goor, H.; Bakker, S.J. Urinary sulfur metabolites associate with a favorable cardiovascular risk profile and survival benefit in renal transplant recipients. J. Am. Soc. Nephrol. 2014, 25, 1303–1312. [Google Scholar] [CrossRef]
- Combs, G.F.; McClungh, J.P. Vitamin B6. In The Vitamins, 5th ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 350–371. [Google Scholar]
- Siebelink, E.; Geelen, A.; de Vries, J.H. Self-reported energy intake by FFQ compared with actual energy intake to maintain body weight in 516 adults. Br. J. Nutr. 2011, 106, 274–281. [Google Scholar] [CrossRef]
- Streppel, M.T.; de Vries, J.H.; Meijboom, S.; Beekman, M.; de Craen, A.J.; Slagboom, P.E.; Feskens, E.J. Relative validity of the food frequency questionnaire used to assess dietary intake in the Leiden Longevity Study. Nutr. J. 2013, 12, 75. [Google Scholar] [CrossRef]
- Dutch Nutrient Databank. NEVO Table 2006; Voorlichtingsbureau Voor de Voeding: The Hague, The Netherlands, 2006. [Google Scholar]
- Inker, L.A.; Schmid, C.H.; Tighiouart, H.; Eckfeldt, J.H.; Feldman, H.I.; Greene, T.; Kusek, J.W.; Manzi, J.; Van Lente, F.; Zhang, Y.L.; et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N. Engl. J. Med. 2012, 367, 20–29. [Google Scholar] [CrossRef]
- Salvador, C.L.; Hartmann, A.; Asberg, A.; Bergan, S.; Rowe, A.D.; Morkrid, L. Estimating Glomerular Filtration Rate in Kidney Transplant Recipients: Comparing a Novel Equation with Commonly Used Equations in this Population. Transplant. Direct. 2017, 3, e332. [Google Scholar] [CrossRef]
- Bouma, G.; van Faassen, M.; Kats-Ugurlu, G.; de Vries, E.G.; Kema, I.P.; Walenkamp, A.M. Niacin (Vitamin B3) Supplementation in Patients with Serotonin-Producing Neuroendocrine Tumor. Neuroendocrinology 2016, 103, 489–494. [Google Scholar] [CrossRef]
- Peduzzi, P.; Concato, J.; Feinstein, A.R.; Holford, T.R. Importance of events per independent variable in proportional hazards regression analysis. II. Accuracy and precision of regression estimates. J. Clin. Epidemiol. 1995, 48, 1503–1510. [Google Scholar] [CrossRef]
- Harrell, F.E., Jr.; Lee, K.L.; Mark, D.B. Multivariable prognostic models: Issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat. Med. 1996, 15, 361–387. [Google Scholar] [CrossRef]
- Ogundimu, E.O.; Altman, D.G.; Collins, G.S. Adequate sample size for developing prediction models is not simply related to events per variable. J. Clin. Epidemiol. 2016, 76, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Li, H.; Su, P.; Yu, Y.; Sun, X.; Liu, Y.; Yuan, Z.; Xue, F. Sensitivity analysis for mistakenly adjusting for mediators in estimating total effect in observational studies. BMJ Open 2017, 7. [Google Scholar] [CrossRef]
- Strohm, D.; Bechthold, A.; Isik, N.; Leschik-Bonnet, E.; Heseker, H. Revised reference values for the intake of thiamin (vitamin B1), riboflavin (vitamin B2), and niacin. NFS J. 2016, 3, 20–24. [Google Scholar] [CrossRef][Green Version]
- Pumpo, R.; Sarnelli, G.; Spinella, A.; Budillon, G.; Cuomo, R. The metabolism of nicotinamide in human liver cirrhosis: A study on N-methylnicotinamide and 2-pyridone-5-carboxamide production. Am. J. Gastroenterol. 2001, 96, 1183–1187. [Google Scholar] [CrossRef]
- Okamoto, H.; Ishikawa, A.; Nishimuta, M.; Kodama, N.; Yoshitake, Y.; Fukuwatari, T.; Shibata, K. Effects of stress on the urinary excretory pattern of niacin catabolites, the most reliable index of niacin status, in humans. J. Nutr. Sci. Vitaminol. (Tokyo) 2002, 48, 417–419. [Google Scholar] [CrossRef]
- Monteiro, J.P.; da Cunha, D.F.; Filho, D.C.; Silva-Vergara, M.L.; dos Santos, V.M.; da Costa, J.C., Jr.; Etchebehere, R.M.; Goncalves, J.; de Carvalho da Cunha, S.F.; Jordao, A.A.; et al. Niacin metabolite excretion in alcoholic pellagra and AIDS patients with and without diarrhea. Nutrition 2004, 20, 778–782. [Google Scholar] [CrossRef]
- Bergagnini-Kolev, M.C.; Hebert, M.F.; Easterling, T.R.; Lin, Y.S. Pregnancy Increases the Renal Secretion of N(1)-methylnicotinamide, an Endogenous Probe for Renal Cation Transporters, in Patients Prescribed Metformin. Drug Metab. Dispos. 2017, 45, 325–329. [Google Scholar] [CrossRef]
- Mierzejewska, P.; Gawlik-Jakubczak, T.; Jablonska, P.; Czajkowski, M.; Kutryb-Zajac, B.; Smolenski, R.T.; Matuszewski, M.; Slominska, E.M. Nicotinamide metabolism alterations in bladder cancer: Preliminary studies. Nucleosides Nucleotides Nucleic Acids 2018, 37, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Muller, F.; Sharma, A.; Konig, J.; Fromm, M.F. Biomarkers for In Vivo Assessment of Transporter Function. Pharmacol. Rev. 2018, 70, 246–277. [Google Scholar] [CrossRef] [PubMed]
- Maiza, A.; Waldek, S.; Ballardie, F.W.; Daley-Yates, P.T. Estimation of renal tubular secretion in man, in health and disease, using endogenous N-1-methylnicotinamide. Nephron 1992, 60, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Shlipak, M.G.; Katz, R.; Kestenbaum, B.; Fried, L.F.; Newman, A.B.; Siscovick, D.S.; Stevens, L.; Sarnak, M.J. Rate of kidney function decline in older adults: A comparison using creatinine and cystatin C. Am. J. Nephrol. 2009, 30, 171–178. [Google Scholar] [CrossRef]
- Weinstein, J.R.; Anderson, S. The aging kidney: Physiological changes. Adv. Chronic Kidney Dis. 2010, 17, 302–307. [Google Scholar] [CrossRef]
- Troesch, B.; Hoeft, B.; McBurney, M.; Eggersdorfer, M.; Weber, P. Dietary surveys indicate vitamin intakes below recommendations are common in representative Western countries. Br. J. Nutr. 2012, 108, 692–698. [Google Scholar] [CrossRef]
- Yang, Y.; Sauve, A.A. NAD+ metabolism: Bioenergetics, signaling and manipulation for therapy. Biochim. Biophys. Acta 2016, 1864, 1787–1800. [Google Scholar] [CrossRef]
- Awan, A.A.; Niu, J.; Pan, J.S.; Erickson, K.F.; Mandayam, S.; Winkelmayer, W.C.; Navaneethan, S.D.; Ramanathan, V. Trends in the Causes of Death among Kidney Transplant Recipients in the United States (1996–2014). Am. J. Nephrol. 2018, 48, 472–481. [Google Scholar] [CrossRef]
- Pryde, D.C.; Dalvie, D.; Hu, Q.; Jones, P.; Obach, R.S.; Tran, T.D. Aldehyde oxidase: An enzyme of emerging importance in drug discovery. J. Med. Chem. 2010, 53, 8441–8460. [Google Scholar] [CrossRef]
- Chen, C.H.; Ferreira, J.C.; Gross, E.R.; Mochly-Rosen, D. Targeting aldehyde dehydrogenase 2: New therapeutic opportunities. Physiol. Rev. 2014, 94, 1–34. [Google Scholar] [CrossRef]
- Wakino, S.; Hasegawa, K.; Itoh, H. Sirtuin and metabolic kidney disease. Kidney Int. 2015, 88, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Frenay, A.S.; de Borst, M.H.; Bachtler, M.; Tschopp, N.; Keyzer, C.A.; van den Berg, E.; Bakker, S.J.L.; Feelisch, M.; Pasch, A.; van Goor, H. Serum free sulfhydryl status is associated with patient and graft survival in renal transplant recipients. Free Radic. Biol. Med. 2016, 99, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Yeung, F.; Hoberg, J.E.; Ramsey, C.S.; Keller, M.D.; Jones, D.R.; Frye, R.A.; Mayo, M.W. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004, 23, 2369–2380. [Google Scholar] [CrossRef] [PubMed]
- Hassa, P.O.; Haenni, S.S.; Buerki, C.; Meier, N.I.; Lane, W.S.; Owen, H.; Gersbach, M.; Imhof, R.; Hottiger, M.O. Acetylation of poly(ADP-ribose) polymerase-1 by p300/CREB-binding protein regulates coactivation of NF-kappaB-dependent transcription. J. Biol. Chem. 2005, 280, 40450–40464. [Google Scholar] [CrossRef]
- Shibata, K.; Matsuo, H. Effect of dietary tryptophan levels on the urinary excretion of nicotinamide and its metabolites in rats fed a niacin-free diet or a constant total protein level. J. Nutr. 1990, 120, 1191–1197. [Google Scholar] [CrossRef]
- Okamoto, H.; Ishikawa, A.; Yoshitake, Y.; Kodama, N.; Nishimuta, M.; Fukuwatari, T.; Shibata, K. Diurnal variations in human urinary excretion of nicotinamide catabolites: Effects of stress on the metabolism of nicotinamide. Am. J. Clin. Nutr. 2003, 77, 406–410. [Google Scholar] [CrossRef]
- Maitre, L.; Lau, C.E.; Vizcaino, E.; Robinson, O.; Casas, M.; Siskos, A.P.; Want, E.J.; Athersuch, T.; Slama, R.; Vrijheid, M.; et al. Assessment of metabolic phenotypic variability in children’s urine using (1)H NMR spectroscopy. Sci. Rep. 2017, 7, 46082. [Google Scholar] [CrossRef]

| Variable | Sex-Stratified Tertiles of N1-MN + 2Py Excretion | Std. β | p-Value | ||
|---|---|---|---|---|---|
| T1 n = 219 | T2 n = 221 | T3 n = 220 | |||
| Males, μmol/day | <181.3 | 181.3–261.2 | >261.2 | - | - |
| Females, μmol/day | <147.7 | 147.7–216.9 | >216.9 | - | - |
| Demographics | |||||
| Male, n (%) | 126 (58) | 127 (58) | 126 (57) | - | - |
| Age, years | 54.3 ± 12.6 | 52.3 ± 13.4 | 52.4 ± 12.1 | −0.10 | 0.01 |
| BMI, kg/m2 | 25.4 ± 4.5 | 26.7 ± 4.5 | 27.8 ± 5.1 | 0.19 | <0.001 |
| Body surface area, m2 | 1.9 ± 0.2 | 1.9 ± 0.2 | 2.0 ± 0.2 | 0.28 | <0.001 |
| Current smoker, n (%) | 24 (12) | 24 (12) | 30 (15) | 0.04 | 0.34 |
| Alcohol consumption, g/day | 1.0 (0.0–7.8) | 3.2 (0.1–12.0) | 5.1 (0.2–17.7) | 0.18 | <0.001 |
| Nutrition | |||||
| Energy intake, kcal/day | 2098 ± 619 | 2248 ± 718 | 2198 ± 576 | 0.06 | 0.17 |
| Plasma vitamin B6, nmol/L | 27.0 (15.0–41.0) | 26.0 (17.0–42.0) | 41.0 (22.0–66.0) | 0.30 | <0.001 |
| Glucose homeostasis | |||||
| Glucose, mmol/L | 5.2 (4.8–5.7) | 5.3 (4.8–6.0) | 5.3 (4.8–6.2) | 0.08 | 0.05 |
| HbA1c, (%) | 5.8 (5.5–6.1) | 5.8 (5.5–6.2) | 5.8 (5.5–6.3) | −0.003 | 0.95 |
| Diabetes, n (%) | 46 (21) | 51 (23) | 55 (25) | 0.05 | 0.23 |
| Antidiabetic, n (%) | 32 (15) | 34 (15) | 30 (14) | 0.007 | 0.86 |
| Lipid homeostasis | |||||
| Total cholesterol, mmol/L | 5.2 ± 1.2 | 5.1 ± 1.1 | 5.0 ± 1.1 | −0.03 | 0.40 |
| LDL, mmol/L | 3.0 ± 1.0 | 3.0 ± 0.9 | 3.0 ± 0.9 | 0.009 | 0.82 |
| HDL, mmol/L | 1.3 (1.1–1.7) | 1.3 (1.1–1.6) | 1.3 (1.1–1.6) | 0.05 | 0.23 |
| Triglycerides, mmol/L | 1.6 (1.2–2.3) | 1.7 (1.3–2.3) | 1.6 (1.2–2.2) | −0.03 | 0.39 |
| Statin, n (%) | 111 (51) | 122 (55) | 116 (53) | −0.02 | 0.61 |
| Hemodynamic | |||||
| Systolic blood pressure, mmHg | 138 ± 18 | 135 ± 16 | 135 ±17 | −0.08 | 0.05 |
| Diastolic blood pressure, mmHg | 82 ± 12 | 82 ± 11 | 83 ± 11 | 0.01 | 0.72 |
| Mean arterial pressure, mmHg | 108 ± 16 | 107 ± 14 | 107 ± 15 | −0.05 | 0.22 |
| Heart rate, beats per minute | 68 ± 12 | 69 ± 13 | 68 ± 12 | −0.006 | 0.87 |
| Antihypertensive use, n (%) | 196 (90) | 193 (87) | 192 (87) | −0.04 | 0.25 |
| Inflammation | |||||
| Hs-CRP, mg/L | 1.3 (0.5–3.5) | 1.6 (0.7–4.4) | 1.9 (0.9–5.6) | 0.10 | 0.007 |
| Renal function | |||||
| eGFR, ml/min/1.73 m2 | 44.7 ± 20.0 | 46.0 ± 18.5 | 46.6 ± 17.7 | 0.09 | 0.03 |
| Proteinuria, n (%) | 47 (22) | 47 (21) | 38 (17) | −0.06 | 0.14 |
| Immunosuppressive medication | |||||
| Prednisolon dose, mg/day | 7.5 (7.5–10) | 7.5 (7.5–10) | 7.5 (7.5–10) | 0.02 | 0.54 |
| Calcineurin inhibitor, n (%) | 131 (60) | 127 (58) | 115 (48) | −0.05 | 0.23 |
| Tacrolimus, n (%) | 38 (17) | 49 (22) | 33 (15) | −0.02 | 0.54 |
| Cyclosporine, n (%) | 93 (43) | 78 (35) | 82 (37) | −0.03 | 0.46 |
| Proliferation inhibitor, n (%) | 172 (79) | 183 (83) | 193 (88) | 0.10 | 0.01 |
| Azathioprine, n (%) | 35 (16) | 32 (15) | 45 (21) | 0.04 | 0.36 |
| Mycophenolic acid, n (%) | 137 (63) | 151 (68) | 148 (67) | 0.05 | 0.21 |
| Nonimmunosuppressive medication | |||||
| Acetylsalicylic acid, n (%) | 55 (25) | 34 (15) | 38 (17) | −0.08 | 0.03 |
| Anticonvulsant, n (%) | 9 (4) | 4 (2) | 6 (3) | −0.02 | 0.59 |
| Proton pump inhibitor, n (%) | 122 (56) | 99 (45) | 105 (48) | −0.09 | 0.03 |
| Diuretic, n (%) | 95 (43) | 76 (34) | 90 (41) | −0.05 | 0.21 |
| Renal transplantation | |||||
| Time since transplantation, years | 5.9 (2.6–13.4) | 5.1 (1.4–10.7) | 5.8 (2.4–12.2) | −0.02 | 0.65 |
| Donor | |||||
| Age, years | 44 (28–53) | 47 (33–56) | 44 (31–54) | 0.002 | 0.97 |
| Male, n (%) | 108 (50) | 114 (52) | 104 (50) | −0.04 | 0.31 |
| Post mortem status, n (%) | 150 (69) | 133 (61) | 142 (66) | 0.04 | 0.36 |
| Primary renal disease | |||||
| Primary glomerular disease, n (%) | 57 (26) | 68 (31) | 61 (28) | 0.01 | 0.81 |
| Glomerulonephritis, n (%) | 15 (7) | 17 (8) | 18 (8) | 0.06 | 0.14 |
| Tubulointerstitial disease, n (%) | 26 (12) | 28 (13) | 23 (11) | −0.02 | 0.54 |
| Polycystic renal disease, n (%) | 41 (19) | 42 (19) | 54 (25) | 0.02 | 0.59 |
| Dysplasia and hypoplasia, n (%) | 10 (5) | 10 (5) | 8 (4) | −0.01 | 0.79 |
| Renovascular disease, n (%) | 15 (7) | 8 (4) | 13 (6) | −0.04 | 0.29 |
| Diabetic nephropathy, n (%) | 14 (6) | 13 (6) | 8 (4) | −0.03 | 0.46 |
| Other or unknown cause, n (%) | 40 (18) | 35 (16) | 35 (16) | −0.005 | 0.90 |
| Model | N1-MN + 2Py Excretion (log2) As Continuous Variable n = 660 | Sex-Stratified Tertiles of N1-MN + 2Py Excretion 2 | |||||
|---|---|---|---|---|---|---|---|
| T1 n = 219 | T2 n = 221 | T3 n = 220 | |||||
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | HR (95% CI) | p-Value | Reference HR | |
| 1 3 | 0.55 (0.43–0.71) | <0.001 | 2.28 (1.49–3.49) | <0.001 | 1.52 (0.96–2.39) | 0.07 | 1.00 |
| 2 4 | 0.61 (0.47–0.79) | <0.001 | 2.03 (1.31–3.15) | 0.002 | 1.45 (0.92–2.30) | 0.11 | 1.00 |
| 3 5 | 0.60 (0.46–0.78) | <0.001 | 2.13 (1.37–3.33) | 0.001 | 1.51 (0.95–2.40) | 0.08 | 1.00 |
| 4 6 | 0.65 (0.49–0.86) | 0.003 | 1.85 (1.17–2.94) | 0.009 | 1.36 (0.84–2.18) | 0.21 | 1.00 |
| 5 7 | 0.67 (0.52–0.87) | 0.003 | 1.93 (1.23–3.02) | 0.004 | 1.32 (0.82–2.12) | 0.25 | 1.00 |
| 6 8 | 0.69 (0.53–0.90) | 0.006 | 1.74 (1.12–2.72) | 0.02 | 1.42 (0.90–2.25) | 0.13 | 1.00 |
| 7 9 | 0.70 (0.52–0.94) | 0.02 | 1.71 (1.05–2.79) | 0.03 | 1.39 (0.84–2.29) | 0.20 | 1.00 |
| Events (n) | 143 | 66 | 46 | 31 | |||
| Model | N1-MN + 2Py Excretion (log2) As Continuous Variable n = 660 | |
|---|---|---|
| HR (95% CI) | p-Value | |
| Infectious Mortality | ||
| 1 2 | 0.42 (0.27–0.66) | <0.001 |
| 2 3 | 0.47 (0.29–0.75) | 0.002 |
| 3 4 | 0.47 (0.29–0.75) | 0.002 |
| 4 5 | 0.51 (0.31–0.86) | 0.01 |
| 5 6 | 0.54 (0.34–0.86) | 0.009 |
| 6 7 | 0.54 (0.33–0.88) | 0.01 |
| 7 8 | 0.54 (0.32–0.91) | 0.02 |
| Events (n) | 40 | |
| Noninfectious Mortality | ||
| 1 2 | 0.62 (0.46–0.83) | 0.001 |
| 2 3 | 0.68 (0.50–0.93) | 0.02 |
| 3 4 | 0.67 (0.49–0.92) | 0.01 |
| 4 5 | 0.72 (0.51–1.00) | 0.05 |
| 5 6 | 0.74 (0.54–1.01) | 0.06 |
| 6 7 | 0.75 (0.55–1.03) | 0.08 |
| 7 8 | 0.79 (0.55–1.12) | 0.18 |
| Events (n) | 103 | |
| Model | Hs-CRP ≤ 3 mg/L | Hs-CRP > 3 mg/L | ||
|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| All-Cause Mortality | ||||
| 1 2 | 0.46 (0.34–0.64) | <0.001 | 0.64 (0.44–0.95) | 0.03 |
| 2 3 | 0.48 (0.34–0.68) | <0.001 | 0.79 (0.52–1.20) | 0.27 |
| 3 4 | 0.49 (0.35–0.68) | <0.001 | 0.80 (0.53–1.21) | 0.29 |
| 4 5 | 0.50 (0.35–0.72) | <0.001 | 0.89 (0.57–1.39) | 0.61 |
| 5 6 | 0.58 (0.42–0.82) | 0.002 | 0.83 (0.54–1.26) | 0.37 |
| 6 7 | 0.57 (0.41–0.81) | 0.002 | 0.83 (0.54–1.27) | 0.38 |
| 7 8 | 0.56 (0.38–0.83) | 0.003 | 0.90 (0.57–1.42) | 0.64 |
| Events (n) | 81 | 62 | ||
| Infectious Mortality | ||||
| 1 2 | 0.35 (0.20–0.60) | <0.001 | 0.60 (0.28–1.32) | 0.21 |
| 2 3 | 0.38 (0.21–0.67) | 0.001 | 0.71 (0.30–1.65) | 0.43 |
| 3 4 | 0.39 (0.22–0.67) | 0.001 | 0.70 (0.30–1.64) | 0.41 |
| 4 5 | 0.38 (0.21–0.70) | 0.002 | 0.97 (0.39–2.43) | 0.95 |
| 5 6 | 0.47 (0.27–0.83) | 0.009 | 0.79 (0.34–1.84) | 0.58 |
| 6 7 | 0.45 (0.25–0.81) | 0.008 | 0.77 (0.31–1.89) | 0.56 |
| 7 8 | 0.40 (0.20–0.78) | 0.008 | 0.79 (0.34–1.81) | 0.58 |
| Events (n) | 25 | 15 | ||
| Noninfectious Mortality | ||||
| 1 2 | 0.53 (0.36–0.78) | 0.001 | 0.66 (0.42–1.02) | 0.06 |
| 2 3 | 0.54 (0.36–0.83) | 0.005 | 0.82 (0.51–1.31) | 0.41 |
| 3 4 | 0.54 (0.36–0.83) | 0.005 | 0.84 (0.52–1.34) | 0.46 |
| 4 5 | 0.57 (0.37–0.90) | 0.02 | 0.87 (0.52–1.44) | 0.58 |
| 5 6 | 0.65 (0.42–0.98) | 0.04 | 0.84 (0.51–1.37) | 0.48 |
| 6 7 | 0.64 (0.42–0.97) | 0.04 | 0.82 (0.51–1.31) | 0.41 |
| 7 8 | 0.66 (0.42–1.05) | 0.08 | 0.95 (0.55–1.65) | 0.87 |
| Events (n) | 56 | 47 | ||
| Variable | Tertiles of Sex-Stratified N1-MN + 2Py Excretion | Std. β | p-Value | ||
|---|---|---|---|---|---|
| T1 n = 219 | T2 n = 221 | T3 n = 220 | |||
| Urinary excretion | |||||
| N1-MN + 2Py, μmol/day | 131.5 (110.5–150.9) | 203.6 (181.5–225.6) | 313.8 (274.2–382.8) | - | - |
| N1-MN, μmol/day | 14.7 (10.9–19.4) | 21.5 (17.6–27.7) | 34.7 (26.1–45.3) | 0.74 | <0.001 |
| 2Py, μmol/day | 114.5 (94.0–131.6) | 178.2 (155.6–198.3) | 280.0 (242.1–340.4) | 0.99 | <0.001 |
| 2Py/N1-MN | 7.8 (6.0–9.7) | 8.3 (6.5–10.4) | 8.8 (6.4 –11.5) | −0.16 | <0.001 |
| Dietary intake | |||||
| Niacin equivalents intake, mg/day | 33.1 ± 8.5 | 36.6 ± 9.7 | 36.9 ± 9.1 | 0.18 | <0.001 |
| Model | Urinary Excretion | Dietary Intake Niacin Equivalents, mg/day 2 | ||||||
|---|---|---|---|---|---|---|---|---|
| N1-MN, µmol/day | 2Py, µmol/day | 2Py/N1-MN | ||||||
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| 1 3 | 0.53 (0.43–0.65) | <0.001 | 0.59 (0.47–0.75) | <0.001 | 1.06 (1.02–1.10) | 0.003 | 0.58 (0.42–0.81) | 0.001 |
| 2 4 | 0.57 (0.46–0.72) | <0.001 | 0.65 (0.51–0.84) | 0.001 | 1.06 (1.02–1.10) | 0.005 | 0.61 (0.43–0.87) | 0.006 |
| 3 5 | 0.58 (0.47–0.73) | <0.001 | 0.64 (0.49–0.82) | 0.001 | 1.05 (1.01–1.10) | 0.02 | 0.65 (0.46–0.93) | 0.02 |
| 4 6 | 0.61 (0.48–0.77) | <0.001 | 0.69 (0.53–0.91) | 0.009 | 1.06 (1.01–1.10) | 0.01 | 0.65 (0.45–0.93) | 0.02 |
| 5 7 | 0.73 (0.57–0.92) | 0.009 | 0.69 (0.53–0.89) | 0.004 | 1.00 (0.95–1.04) | 0.85 | 0.69 (0.48–0.98) | 0.04 |
| 6 8 | 0.63 (0.50–0.78) | <0.001 | 0.73 (0.57–0.94) | 0.02 | 1.06 (1.02–1.10) | 0.007 | 0.64 (0.45–0.91) | 0.02 |
| 7 9 | 0.64 (0.50–0.82) | <0.001 | 0.74 (0.56–0.98) | 0.04 | 1.07 (1.02–1.12) | 0.006 | 0.77 (0.43–1.38) | 0.38 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deen, C.P.J.; Veen, A.v.d.; Gomes-Neto, A.W.; Geleijnse, J.M.; Berg, K.J.B.-v.d.; Heiner-Fokkema, M.R.; Kema, I.P.; Bakker, S.J.L. Urinary Excretion of N1-Methylnicotinamide and N1-Methyl-2-Pyridone-5-Carboxamide and Mortality in Kidney Transplant Recipients. Nutrients 2020, 12, 2059. https://doi.org/10.3390/nu12072059
Deen CPJ, Veen Avd, Gomes-Neto AW, Geleijnse JM, Berg KJB-vd, Heiner-Fokkema MR, Kema IP, Bakker SJL. Urinary Excretion of N1-Methylnicotinamide and N1-Methyl-2-Pyridone-5-Carboxamide and Mortality in Kidney Transplant Recipients. Nutrients. 2020; 12(7):2059. https://doi.org/10.3390/nu12072059
Chicago/Turabian StyleDeen, Carolien P.J., Anna van der Veen, António W. Gomes-Neto, Johanna M. Geleijnse, Karin J. Borgonjen-van den Berg, M. Rebecca Heiner-Fokkema, Ido P. Kema, and Stephan J.L. Bakker. 2020. "Urinary Excretion of N1-Methylnicotinamide and N1-Methyl-2-Pyridone-5-Carboxamide and Mortality in Kidney Transplant Recipients" Nutrients 12, no. 7: 2059. https://doi.org/10.3390/nu12072059
APA StyleDeen, C. P. J., Veen, A. v. d., Gomes-Neto, A. W., Geleijnse, J. M., Berg, K. J. B.-v. d., Heiner-Fokkema, M. R., Kema, I. P., & Bakker, S. J. L. (2020). Urinary Excretion of N1-Methylnicotinamide and N1-Methyl-2-Pyridone-5-Carboxamide and Mortality in Kidney Transplant Recipients. Nutrients, 12(7), 2059. https://doi.org/10.3390/nu12072059





