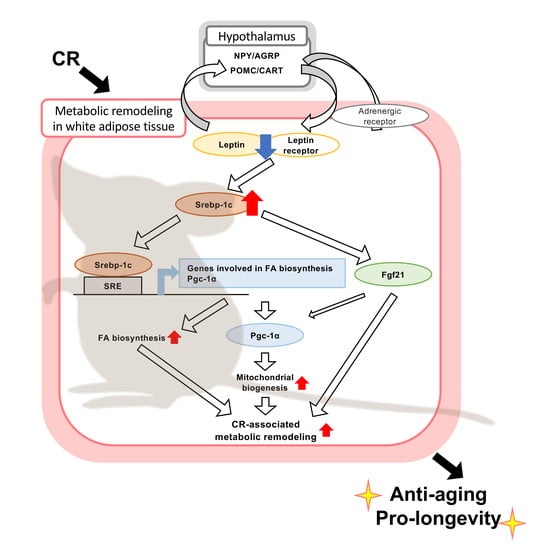
Srebp-1c/Fgf21/Pgc-1α Axis Regulated by Leptin Signaling in Adipocytes—Possible Mechanism of Caloric Restriction-Associated Metabolic Remodeling of White Adipose Tissue
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and the Collection of Mice Embryonic Fibroblasts (MEFs)
2.2. Cell Culture and Reagent Treatment
2.3. Retrovirus Plasmid Construction
2.4. Retrovirus Vector Preparation
2.5. RT-PCR and Semi-Quantitative RT-PCR
2.6. Western Blotting
2.7. Statistical Analysis
3. Results
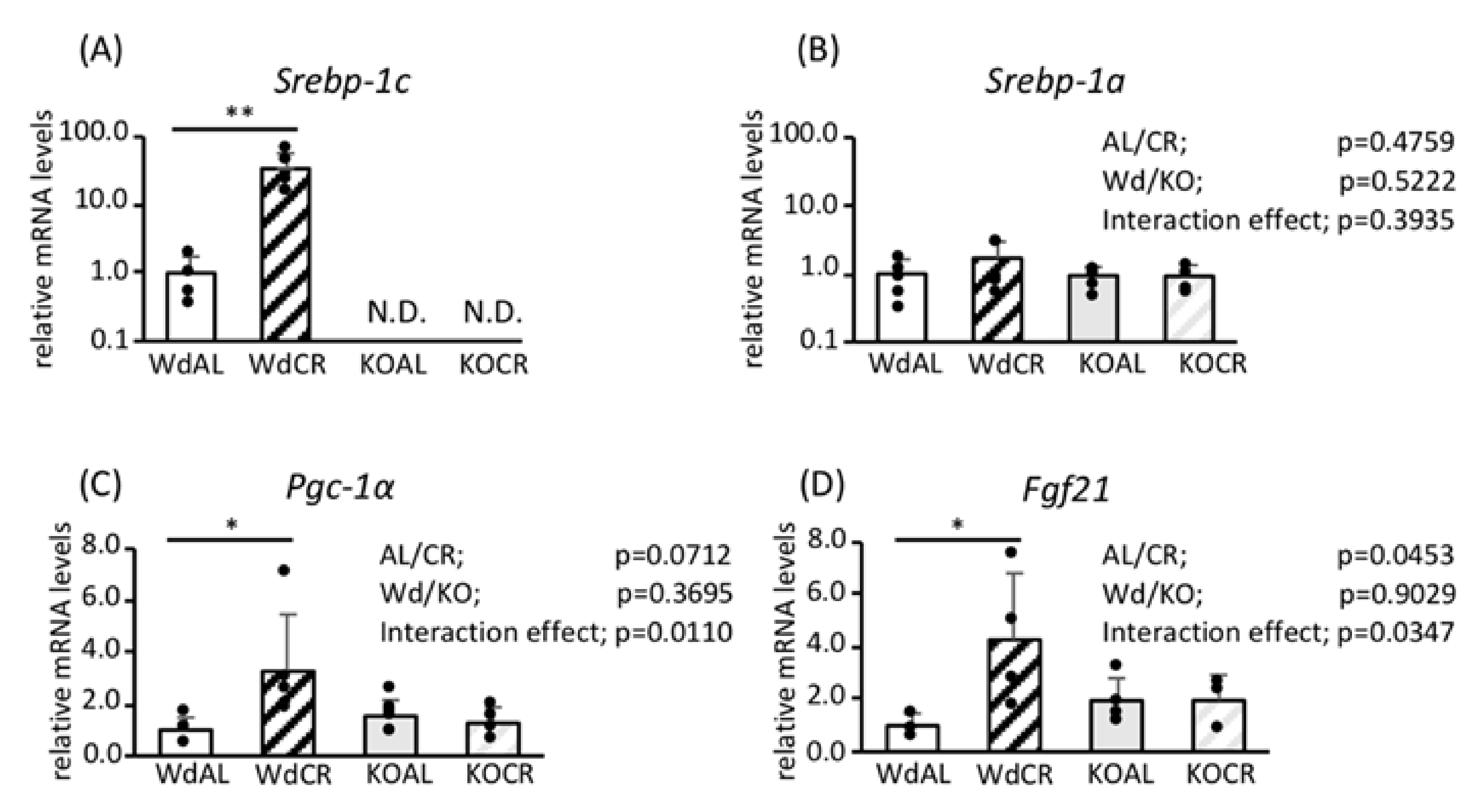
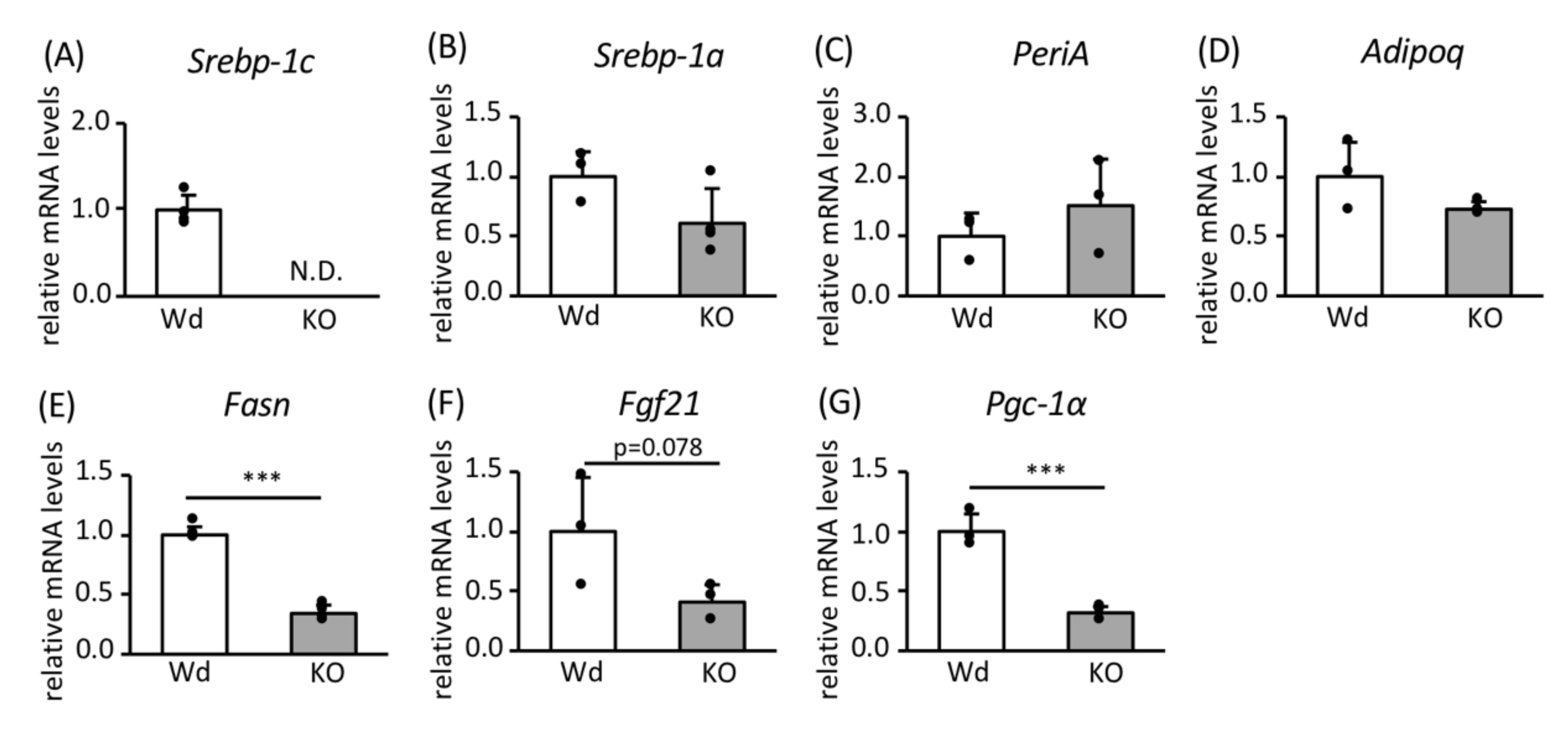
3.1. Role of SREBP-1c in the Effects of CR on Gene Expression in WAT
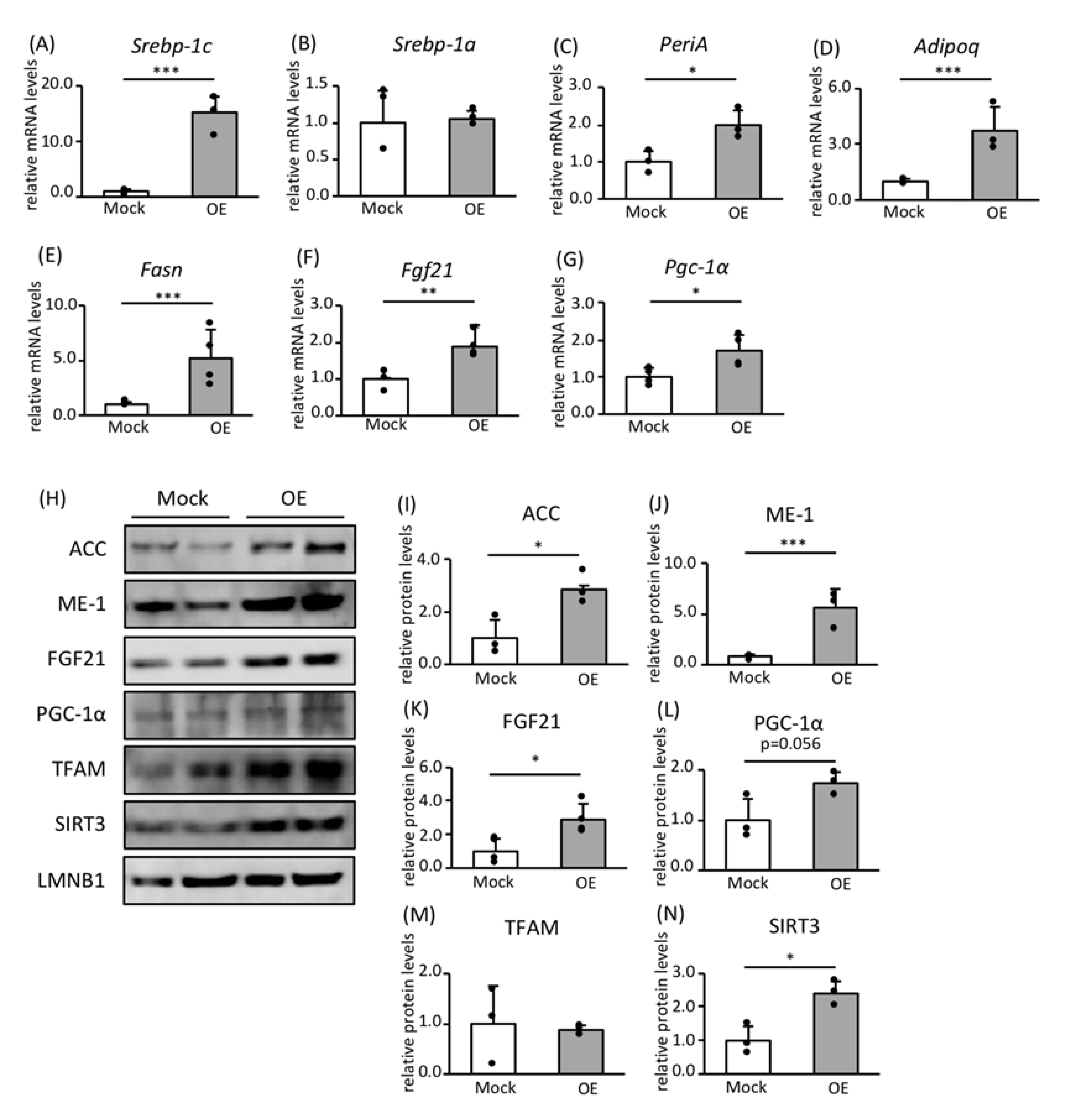
3.2. Effects of SREBP-1c on the Expression of Genes and Proteins Involved in FA Biosynthesis and Mitochondrial Biogenesis
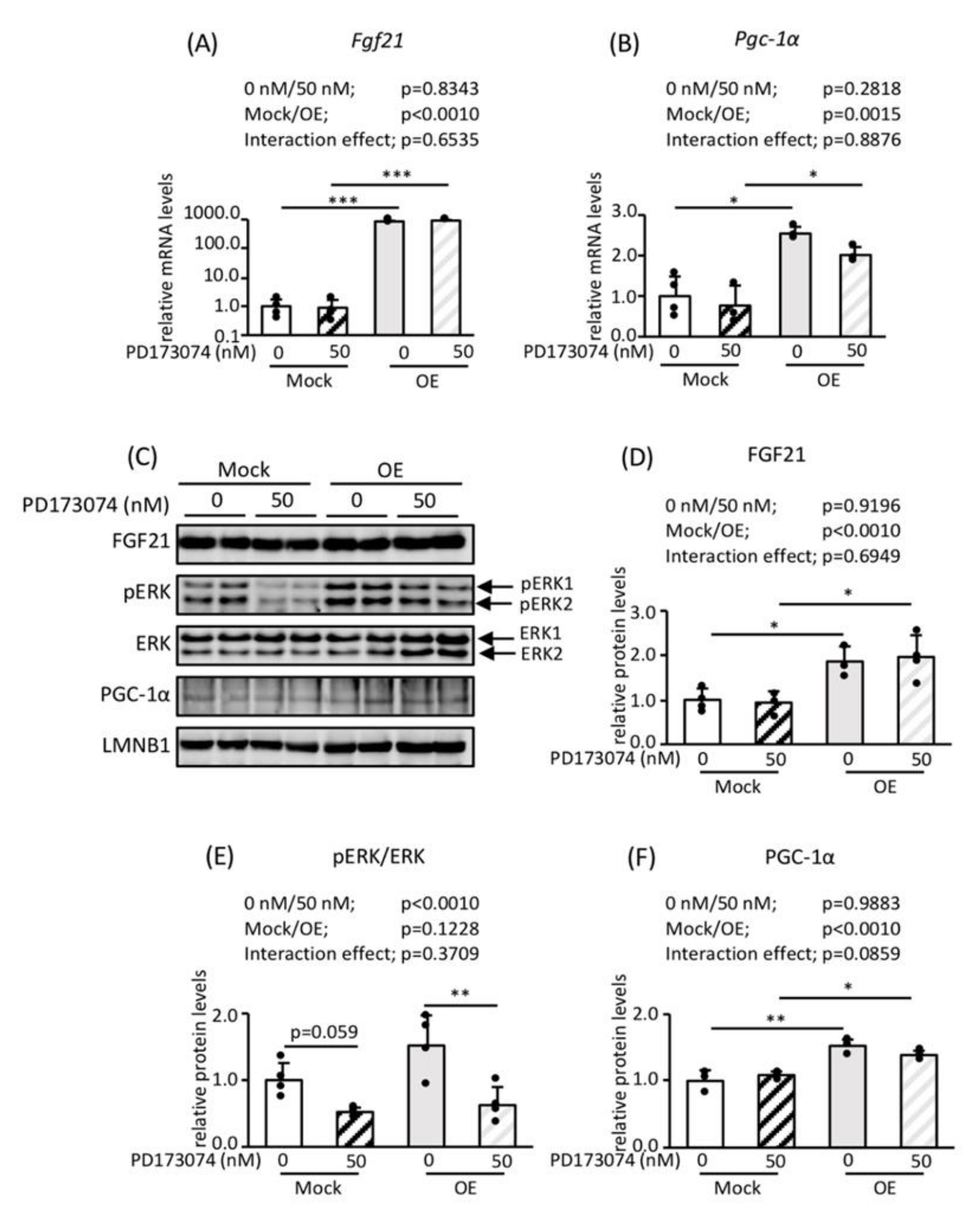
3.3. Roles of FGF21 and PGC-1α in Mitochondrial Biogenesis
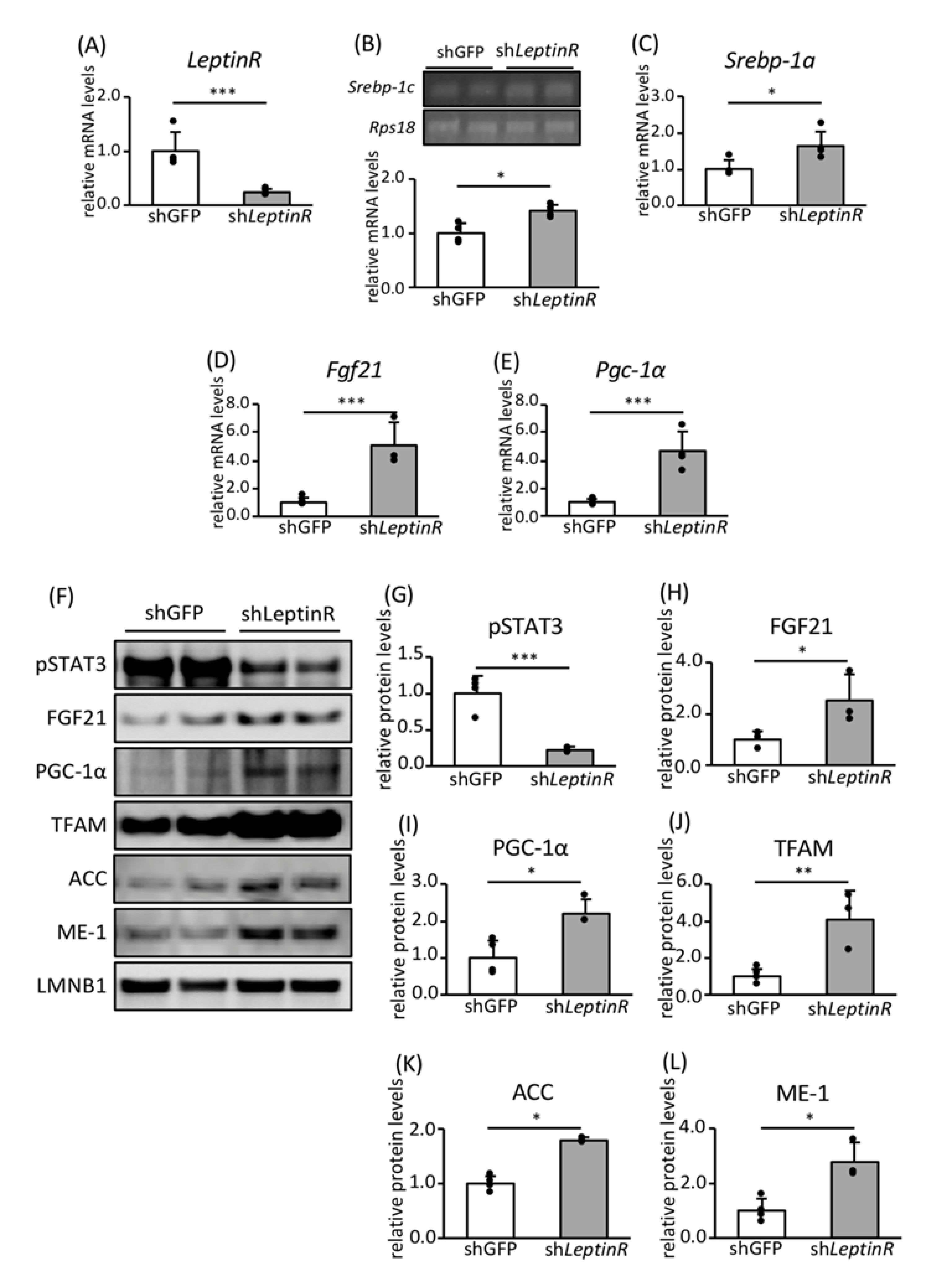
3.4. Effect of Leptin Signaling on the Expression of Genes and Proteins Involved in FA Biosynthesis and Mitochondrial Biogenesis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ouchi, N.; Parker, J.L.; Lugus, J.J.; Walsh, K. Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 2011, 11, 85–97. [Google Scholar] [CrossRef]
- Blüher, M.; Kahn, B.B.; Kahn, C.R. Extended longevity in mice lacking the insulin receptor in adipose tissue. Science 2003, 299, 572–574. [Google Scholar] [CrossRef] [PubMed]
- Otabe, S.; Yuan, X.; Fukutani, T.; Wada, N.; Hashinaga, T.; Nakayama, H.; Hirota, N.; Kojima, M.; Yamada, K. Overexpression of human adiponectin in transgenic mice results in suppression of fat accumulation and prevention of premature death by high-calorie diet. Am. J. Physiol. Endocrinol. Metab. 2007, 293, 2102–2118. [Google Scholar] [CrossRef] [PubMed]
- Farmer, S.R. Transcriptional control of adipocyte formation. Cell Metab. 2006, 4, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.H.; Lin, W.D.; Huang, S.Y.; Lee, Y.H. Effect of a C/EBP gene replacement on mitochondrial biogenesis in fat cells. Genes Dev. 2004, 18, 19701–19975. [Google Scholar] [CrossRef] [PubMed]
- Argmann, C.; Dobrin, R.; Hekkinen, S.; Auburtin, A.; Pouilly, L.; Cock, T.; Koutnikova, H.; Zhu, J.; Schadt, E.E.; Auwerx, J. Pparγ2 is a key driver of longevity in the mouse. PLoS Genet. 2009, 5, e1000752. [Google Scholar] [CrossRef] [PubMed]
- Masoro, E.J. Overview of caloric restriction and ageing. Mech. Ageing Dev. 2005, 126, 913–922. [Google Scholar] [CrossRef]
- Sohal, R.S.; Weindruch, R. Oxidative stress, caloric restriction, and aging. Science 1996, 273, 59–63. [Google Scholar] [CrossRef]
- Bartke, A.; Quainoo, N. Impact of growth hormone-related mutations on mammalian aging. Front. Genet. 2018, 9, 586. [Google Scholar] [CrossRef]
- Bonkowski, M.S.; Rocha, J.S.; Masternak, M.M.; Al-Regaiey, K.A.; Bartke, A. Targeted disruption of growth hormone receptor interferes with the beneficial actions of calorie restriction. Proc. Natl. Acad. Sci. USA 2006, 103, 7901–7905. [Google Scholar] [CrossRef]
- Sun, L.Y.; Spong, A.; Swindell, W.R.; Fang, Y.; Hill, C.; Huber, J.A.; Boehm, J.D.; Westbrook, R.; Salvatori, R.; Bartke, A. Growth hormone-releasing hormone disruption extends lifespan and regulates response to caloric restriction in mice. Elife 2013, 2, e01098. [Google Scholar] [CrossRef] [PubMed]
- Shimokawa, I.; Higami, Y.; Tsuchiya, T.; Otani, H.; Komatsu, T.; Chiba, T.; Yamaza, H. Life span extension by reduction of the growth hormone-insulin-like growth factor-1 axis: Relation to caloric restriction. FASEB J. 2003, 17, 1108–1109. [Google Scholar] [CrossRef] [PubMed]
- Chujo, Y.; Fujii, N.; Okita, N.; Konishi, T.; Narita, T.; Yamada, A.; Haruyama, Y.; Tashiro, K.; Chiba, T.; Shimokawa, I.; et al. Caloric restriction-associated remodeling of rat white adipose tissue: Effects on the growth hormone/insulin-like growth factor-1 axis, sterol regulatory element binding protein-1, and macrophage infiltration. Age 2013, 35, 1143–1156. [Google Scholar] [CrossRef] [PubMed]
- Osborne, T.F.; Espenshade, P.J. Evolutionary conservation and adaptation in the mechanism that regulates SREBP action: What a long, strange tRIP it’s been. Genes Dev. 2009, 23, 2578–2591. [Google Scholar] [CrossRef] [PubMed]
- Shimomura, I.; Shimano, H.; Horton, J.D.; Goldstein, J.L.; Brown, M.S. Differential expression of exons 1a and 1c in mRNAs for sterol regulatory element binding protein-1 in human and mouse organs and cultured cells. J. Clin. Investig. 1997, 99, 838–845. [Google Scholar] [CrossRef]
- Fujii, N.; Narita, T.; Okita, N.; Kobayashi, M.; Furuta, Y.; Chujo, Y.; Sakai, M.; Yamada, A.; Takeda, K.; Konishi, T.; et al. Sterol regulatory element-binding protein-1c orchestrates metabolic remodeling of white adipose tissue by caloric restriction. Aging Cell 2017, 16, 508–517. [Google Scholar] [CrossRef]
- Scarpulla, R.C. Metabolic control of mitochondrial biogenesis through the PGC-1 family regulatory network. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2011, 1813, 1269–1278. [Google Scholar] [CrossRef]
- Anderson, R.M.; Barger, J.L.; Edwards, M.G.; Braun, K.H.; O’Connor, C.E.; Prolla, T.A.; Weindruch, R. Dynamic regulation of PGC-1alpha localization and turnover implicates mitochondrial adaptation in calorie restriction and the stress response. Aging Cell 2008, 7, 101–111. [Google Scholar] [CrossRef]
- Nishimura, T.; Nakatake, Y.; Konishi, M.; Itoh, N. Identification of a novel FGF, FGF-21, preferentially expressed in the liver. Biochim. Biophys. Acta (BBA) Gene Struct. Expr. 2000, 1492, 203–206. [Google Scholar] [CrossRef]
- Ding, X.; Boney-Montoya, J.; Owen, B.M.; Bookout, A.L.; Coate, K.C.; Mangelsdorf, D.J.; Kliewer, S.A. βKlotho is required for fibroblast growth factor 21 effects on growth and metabolism. Cell Metab. 2012, 16, 387–393. [Google Scholar] [CrossRef]
- Ogawa, Y.; Kurosu, H.; Yamamoto, M.; Nandi, A.; Rosenblatt, K.P.; Goetz, R.; Eliseenkova, A.V.; Mohammadi, M.; Kuro-o, M. BetaKlotho is required for metabolic activity of fibroblast growth factor 21. Proc. Natl. Acad. Sci. USA 2007, 104, 7432–7437. [Google Scholar] [CrossRef] [PubMed]
- Markan, K.R.; Naber, M.C.; Ameka, M.K.; Anderegg, M.D.; Mangelsdorf, D.J.; Kliewer, S.A.; Mohammadi, M.; Potthoff, M.J. Circulating FGF21 is liver derived and enhances glucose uptake during refeeding and overfeeding. Diabetes 2014, 63, 4057–4063. [Google Scholar] [CrossRef]
- Zhang, Y.; Lei, T.; Huang, J.F.; Wang, S.B.; Zhou, L.L.; Yang, Z.Q.; Chen, X.D. The link between fibroblast growth factor 21 and sterol regulatory element binding protein 1c during lipogenesis in hepatocytes. Mol. Cell Endocrinol. 2011, 342, 414–417. [Google Scholar] [CrossRef] [PubMed]
- Véniant, M.M.; Hale, C.; Helmering, J.; Chen, M.M.; Stanislaus, S.; Busby, J.; Vonderfecht, S.; Xu, J.; Lloyd, D.J. FGF21 promotes metabolic homeostasis via white adipose and leptin in mice. PLoS ONE 2012, 7, e40164. [Google Scholar] [CrossRef]
- Potthoff, M.J.; Inagaki, T.; Satapati, S.; Ding, X.; He, T.; Goetz, R.; Mohammadi, M.; Finck, B.N.; Mangelsdorf, D.J.; Kliewer, S.A.; et al. FGF21 induces PGC-1alpha and regulates carbohydrate and fatty acid metabolism during the adaptive starvation response. Proc. Natl. Acad. Sci. USA 2009, 106, 10853–10858. [Google Scholar] [CrossRef] [PubMed]
- Fisher, F.M.; Kleiner, S.; Douris, N.; Fox, E.C.; Mepani, R.J.; Verdeguer, F.; Wu, J.; Kharitonenkov, A.; Flier, J.S.; Maratos-Flier, E.; et al. FGF21 regulates PGC-1α and browning of white adipose tissues in adaptive thermogenesis. Genes Dev. 2012, 26, 2712–2781. [Google Scholar] [CrossRef] [PubMed]
- Dutchak, P.A.; Katafuchi, T.; Bookout, A.L.; Choi, J.H.; Yu, R.T.; Mangelsdorf, D.J.; Kliewer, S.A. Fibroblast growth factor-21 regulates PPARγ activity and the antidiabetic actions of thiazolidinediones. Cell 2012, 148, 556–567. [Google Scholar] [CrossRef]
- Zhang, Y.; Xie, Y.; Berglund, E.D.; Coate, K.C.; He, T.T.; Katafuchi, T.; Xiao, G.; Potthoff, M.J.; Wei, W.; Wan, Y.; et al. The starvation hormone, fibroblast growth factor-21, extends lifespan in mice. Elife 2012, 1, e00065. [Google Scholar] [CrossRef]
- Fujii, N.; Uta, S.; Kobayashi, M.; Sato, T.; Okita, N.; Higami, Y. Impact of aging and caloric restriction on fibroblast growth factor 21 signaling in rat white adipose tissue. Exp. Gerontol. 2019, 118, 55–64. [Google Scholar] [CrossRef]
- Wilson-Fritch, L.; Nicoloro, S.; Chouinard, M.; Lazar, M.A.; Chui, P.C.; Leszyk, J.; Straubhaar, J.; Czech, M.P.; Corvera, S. Mitochondrial remodeling in adipose tissue associated with obesity and treatment with rosiglitazone. J. Clin. Investig. 2004, 114, 1281–1289. [Google Scholar] [CrossRef]
- Zhang, Y.; Proenca, R.; Maffei, M.; Barone, M.; Leopold, L.; Friedman, J.M. Positional cloning of the mouse obese gene and its human homologue. Nature 1994, 372, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Ahima, R.S.; Flier, J.S. Leptin. Annu. Rev. Physiol. 2000, 62, 4134–4137. [Google Scholar] [CrossRef]
- Campfield, L.A.; Smith, F.J.; Burn, P. The OB protein (leptin) pathway—A link between adipose tissue mass and central neural networks. Horm. Metab. Res. 1996, 28, 619–632. [Google Scholar] [CrossRef] [PubMed]
- Soukas, A.; Cohen, P.; Socci, N.D.; Friedman, J.M. Leptin-specific patterns of gene expression in white adipose tissue. Genes Dev. 2000, 14, 9639–9680. [Google Scholar]
- Shimokawa, I.; Higami, Y. A role for leptin in the antiaging action of dietary restriction: A hypothesis. Aging 1999, 11, 3803–3882. [Google Scholar] [CrossRef] [PubMed]
- Hotta, Y.; Nakamura, H.; Konishi, M.; Murata, Y.; Takagi, H.; Matsumura, S.; Inoue, K.; Fushiki, T.; Itoh, N. Fibroblast growth factor 21 regulates lipolysis in white adipose tissue but is not required for ketogenesis and triglyceride clearance in liver. Endocrinology 2009, 150, 46254–46633. [Google Scholar] [CrossRef]
- Mikami, K.; Okita, N.; Tokunaga, Y.; Ichikawa, T.; Okazaki, T.; Takemoto, K.; Nagai, W.; Matsushima, S.; Higami, Y. Autophagosomes accumulate in differentiated and hypertrophic adipocytes in a p53-independent manner. Biochem. Biophys. Res. Commun. 2012, 427, 758–763. [Google Scholar] [CrossRef]
- Kim, Y.B.; Uotani, S.; Pierroz, D.D.; Flier, J.S.; Kahn, B.B. In vivo administration of leptin activates signal transduction directly in insulin-sensitive tissues: Overlapping but distinct pathways from insulin. Endocrinology 2000, 141, 23282–23339. [Google Scholar] [CrossRef]
- Ferré, P.; Foufelle, F. Hepatic steatosis: A role for de novo lipogenesis and the transcription factor SREBP-1c. Diabetes Obes. Metab. 2010, 12, 83–92. [Google Scholar] [CrossRef]
- Kammoun, H.L.; Chabanon, H.; Hainault, I.; Luquet, S.; Magnan, C.; Koike, T.; Ferré, P.; Foufelle, F. GRP78 expression inhibits insulin and ER stress-induced SREBP-1c activation and reduces hepatic steatosis in mice. J. Clin. Investig. 2009, 119, 1201–1215. [Google Scholar] [CrossRef]
- Knebel, B.; Haas, J.; Hartwig, S.; Jacob, S.; Köllmer, C.; Nitzgen, U.; Muller-Wieland, D.; Kotzka, J. Liver-specific expression of transcriptionally active srebp-1c is associated with fatty liver and increased visceral fat mass. PLoS ONE 2012, 7, e31812. [Google Scholar] [CrossRef] [PubMed]
- Horton, J.D.; Bashmakov, Y.; Shimomura, I.; Shimano, H. Regulation of sterol regulatory element binding proteins in livers of fasted and refed mice. Proc. Natl. Acad. Sci. USA 1998, 95, 59875–59992. [Google Scholar] [CrossRef] [PubMed]
- Sekiya, M.; Yahagi, N.; Matsuzaka, T.; Takeuchi, Y.; Nakagawa, Y.; Takahashi, H.; Okazaki, H.; Iizuka, Y.; Ohashi, K.; Gotoda, T.; et al. SREBP-1-independent regulation of lipogenic gene expression in adipocytes. J. Lipid Res. 2007, 48, 1581–1591. [Google Scholar] [CrossRef]
- Nisoli, E.; Tonello, C.; Cardile, A.; Cozzi, V.; Bracale, R.; Tedesco, L.; Falcone, S.; Valerio, A.; Cantoni, O.; Clementi, E.; et al. Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science 2005, 310, 314–317. [Google Scholar] [CrossRef] [PubMed]
- López-Lluch, G.; Hunt, N.; Jones, B.; Zhu, M.; Jamieson, H.; Hilmer, S.; Cascajo, M.V.; Allard, J.; Ingram, D.K.; Navas, P.; et al. Calorie restriction induces mitochondrial biogenesis and bioenergetic efficiency. Proc. Natl. Acad. Sci. USA 2006, 103, 1768–1773. [Google Scholar] [CrossRef] [PubMed]
- Okita, N.; Hayashida, Y.; Kojima, Y.; Fukushima, M.; Yuguchi, K.; Mikami, K.; Yamauchi, A.; Watanabe, K.; Noguchi, M.; Nakamura, M.; et al. Differential responses of white adipose tissue and brown adipose tissue to caloric restriction in rats. Mech. Ageing Dev. 2012, 133, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Bruss, M.D.; Khambatta, C.F.; Ruby, M.A.; Aggarwal, I.; Hellerstein, M.K. Calorie restriction increases fatty acid synthesis and whole body fat oxidation rates. Am. J. Physiol. Endocrinol. Metab. 2010, 298, 108–116. [Google Scholar] [CrossRef]
- Hao, Q.; Hansen, J.B.; Petersen, R.K.; Hallenborg, P.; Jørgensen, C.; Cinti, S.; Larsen, P.J.; Steffensen, K.R.; Wang, H.; Collins, S.; et al. ADD1/SREBP1c activates the PGC1-alpha promoter in brown adipocytes. Biochim. Biophys. Acta 2010, 1801, 4214–4229. [Google Scholar]
- Okita, N.; Tsuchiya, T.; Fukushima, M.; Itakura, K.; Yuguchi, K.; Narita, T.; Hashizume, Y.; Sudo, Y.; China, T.; Shimokawa, I.; et al. Chronological analysis of caloric restriction-induced alteration of fatty acid biosynthesis in white adipose tissue of rats. Exp. Gerontol. 2015, 63, 59–66. [Google Scholar] [CrossRef]

| Forward | Reverse | |
|---|---|---|
| Adipoq | 5′-TGC CGA AGA TGA CGT TAC AAC-3′ | 5′-CTT CAG CTC CTG TCA TTC CAA C-3′ |
| Fasn | 5′-AGC AGG CAC ACA CAA TGG AC-3′ | 5′-GAA GAA AGA GAG CCG GTT G-3′ |
| Fgf21 | 5′-GAA GCC CAC CTG GAG ATC AG-3′ | 5′-CAA AGT GAG GCG ATC CAT AGA G-3′ |
| LeptinR | 5′- CAG TCT TCGG GGA TGT GAA TG-3′ | 5′- CAT TGT TTG GCT GTC CCA AG-3′ |
| PeriA | 5′-TGG GAA GCA TCG AGA AGG TG-3′ | 5′-ATG GTG TGT CGA GAA AGA GTG TTG-3′ |
| Pgc-1α | 5′-AGA CGG ATT GCC CTC ATT TG-3′ | 5′-CAG GGT TTG TTC TGA TCC TGT G-3′ |
| Rps18 | 5′-TGC GAG TAC TCA ACA CCA ACA T-3′ | 5′-CTT TCC TCA ACA CCA CAT GAG C-3′ |
| Srebp-1a | 5′-GGC CGA GAT GTG CGA ACT-3′ | 5′-TTG TTG ATG AGC TGG AGC ATG T-3′ |
| Srebp-1c | 5′-GGA GCC ATG GAT TGC ACA TT-3′ | 5′-GGC CCG GGA AGT CAC TGT-3′ |
| Tbp | 5′-CCC TCA CAC TCA GAT CAT CTT CTC-3′ | 5′-GCC TTG TCC CTT GAA GAG AAC C-3′ |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kobayashi, M.; Uta, S.; Otsubo, M.; Deguchi, Y.; Tagawa, R.; Mizunoe, Y.; Nakagawa, Y.; Shimano, H.; Higami, Y. Srebp-1c/Fgf21/Pgc-1α Axis Regulated by Leptin Signaling in Adipocytes—Possible Mechanism of Caloric Restriction-Associated Metabolic Remodeling of White Adipose Tissue. Nutrients 2020, 12, 2054. https://doi.org/10.3390/nu12072054
Kobayashi M, Uta S, Otsubo M, Deguchi Y, Tagawa R, Mizunoe Y, Nakagawa Y, Shimano H, Higami Y. Srebp-1c/Fgf21/Pgc-1α Axis Regulated by Leptin Signaling in Adipocytes—Possible Mechanism of Caloric Restriction-Associated Metabolic Remodeling of White Adipose Tissue. Nutrients. 2020; 12(7):2054. https://doi.org/10.3390/nu12072054
Chicago/Turabian StyleKobayashi, Masaki, Seira Uta, Minami Otsubo, Yusuke Deguchi, Ryoma Tagawa, Yuhei Mizunoe, Yoshimi Nakagawa, Hitoshi Shimano, and Yoshikazu Higami. 2020. "Srebp-1c/Fgf21/Pgc-1α Axis Regulated by Leptin Signaling in Adipocytes—Possible Mechanism of Caloric Restriction-Associated Metabolic Remodeling of White Adipose Tissue" Nutrients 12, no. 7: 2054. https://doi.org/10.3390/nu12072054
APA StyleKobayashi, M., Uta, S., Otsubo, M., Deguchi, Y., Tagawa, R., Mizunoe, Y., Nakagawa, Y., Shimano, H., & Higami, Y. (2020). Srebp-1c/Fgf21/Pgc-1α Axis Regulated by Leptin Signaling in Adipocytes—Possible Mechanism of Caloric Restriction-Associated Metabolic Remodeling of White Adipose Tissue. Nutrients, 12(7), 2054. https://doi.org/10.3390/nu12072054