Nutritional Status Plays More Important Role in Determining Functional State in Older People Living in the Community than in Nursing Home Residents
Abstract
:1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Transparency Declaration
References
- Kaiser, M.J.; Bauer, J.M.; Rämsch, C.; Uter, W.; Guigoz, Y.; Cederholm, T.; Thomas, D.R.; Anthony, S.; Charlton, K.E.; Maggio, M.; et al. Frequency of malnutrition in older adults: A multinational perspective using the mini nutritional assessment. J. Am. Geriatr. Soc. 2010, 58, 1734–1738. [Google Scholar] [CrossRef] [PubMed]
- Adams, N.E.; Bowie, A.J.; Simmance, N.; Murray, M.; Crowe, T.C. Recognition by medical and nursing professionals of malnutrition and risk of malnutrition in elderly hospitalised patients. Nutr. Diet. 2008, 65, 144–150. [Google Scholar] [CrossRef]
- Chapman, I.M. Nutritional disorders in the elderly. Med. Clin. N. Am. 2006, 90, 887–907. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Reig, M.; Aranda-Reneo, I.; Peña-Longobardo, L.M.; Oliva-Moreno, J.; Barcons-Vilardell, N.; Hoogendijk, E.O.; Abizanda, P. Use of health resources and healthcare costs associated with nutritional risk: The FRADEA study. Clin. Nutr. 2018, 37, 1299–1305. [Google Scholar] [CrossRef]
- Zhang, X.S.; Liu, Y.H.; Zhang, Y.; Xu, Q.; Yu, X.M.; Yang, X.Y.; Liu, Z.; Li, H.Z.; Li, F.; Xue, C.Y. Handgrip strength as a predictor of nutritional status in Chinese elderly inpatients at hospital admission. Biomed. Environ. Sci. 2017, 30, 802–810. [Google Scholar] [PubMed]
- Stange, I.; Poeschl, K.; Stehle, P.; Sieber, C.C.; Volkert, D. Screening for malnutrition in nursing home residents: Comparison of different risk markers and their association to functional impairment. J. Nutr. Health Aging 2013, 17, 357–363. [Google Scholar] [CrossRef]
- Cobo, C.M. The influence of institutionalization on the perception of autonomy and quality of life in old people. Rev. Esc. Enferm. USP 2014, 48, 1013–1019. [Google Scholar] [CrossRef] [Green Version]
- Jerez-Roig, J.; de Brito Macedo Ferreira, L.M.; Torres de Araújo, J.R.; Costa Lima, K. Functional decline in nursing home residents: A prognostic study. PLoS ONE 2017, 12, e0177353. [Google Scholar] [CrossRef]
- Jerez-Roig, J.; de Brito Macedo Ferreira, L.M.; Torres de Araújo, J.R.; Costa Lima, K. Dynamics of activities of daily living performance in institutionalized older adults: A two-year longitudinal study. Disabil. Health J. 2017, 10, 279–285. [Google Scholar] [CrossRef]
- Carpenter, G.I.; Hastie, C.L.; Morris, J.N.; Fries, B.; Ankri, J. Measuring change in activities of daily living in nursing home residents with moderate to severe cognitive impairment. BMC Geriat. 2006, 6, 7. [Google Scholar] [CrossRef]
- Wirth, R.; Smoliner, C.; Sieber, C.C.; Volkert, D. Cognitive function is associated with body composition and nutritional risk of geriatric patients. J. Nutr. Health Aging 2011, 15, 706–710. [Google Scholar] [CrossRef] [PubMed]
- Conzade, R.; Phu, S.; Vogrin, S.; Bani Hassan, E.; Sepúlveda-Loyola, W.; Thorand, B.; Duque, G. Changes in nutritional status and musculoskeletal health in a geriatric post-fall care plan setting. Nutrients 2019, 11, 1551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katz, S.; Ford, A.B.; Moskowitz, R.W.; Jackson, B.A.; Jaffe, M.W. Studies of illness in the aged: The index of ADL, a standardized measure of biogical and psychosocial function. JAMA 1963, 185, 914–919. [Google Scholar] [CrossRef] [PubMed]
- Lawton, M.; Brody, E.M. Instrumental Activities of Daily Living (IADL) Scale: Original observer-rated version. Psychopharmacol. Bull. 1988, 24, 785–792. [Google Scholar]
- Podsiadlo, D.; Richardson, S. The timed “up & go”: A test of basic functional mobility for frail elderly persons. J. Am. Geriatr. Soc. 1991, 39, 142–148. [Google Scholar] [PubMed]
- Folstein, M.F.; Folstein, S.E.; McHugh, R. “Minimental state” a practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Yesavage, J.A.; Brink, T.L.; Rose, T.L.; Lum, O.; Huang, V.; Adey, M.; Leirer, V.O. Development and validation of a geriatric depression screening scale: A preliminary report. J. Psychiatr. Res. 1983, 17, 37–49. [Google Scholar] [CrossRef]
- Rantanen, T.; Guralnik, J.M.; Foley, D.; Masaki, K.; Leveille, S.; Curb, J.D.; White, L. Midlife hand grip strength as a predictor of old age disability. JAMA 1999, 281, 558–560. [Google Scholar] [CrossRef] [Green Version]
- Guigoz, Y.B.; Vellas, B.; Garry, J. Mini Nutritional Assessment: A practical assessment tool for grading the nutritional state of elderly patients. Facts Res. Gerontol. 1994, 2, 15–59. [Google Scholar]
- WHO. Waist Circumference and Waist-Hip Ratio: Report of a WHO Expert Consultation; WHO Document Production Services: Geneva, Switzerland, 2008. [Google Scholar]
- Maltron International Ltd. Operating and Service Manual BioScan 920−2; Maltron International Ltd.: Essex, UK, 2010. [Google Scholar]
- Janssen, I.; Heymsfield, S.B.; Baumgartner, R.N.; Ross, R.J. Estimation of skeletal muscle mass by bioelectrical impedance analysis. Appl. Physiol. 2000, 89, 465–471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janssen, I.; Baumgartner, R.N.; Ross, R.; Rosenberg, I.H.; Roubenoff, R. Skeletal muscle cutpoints associated with elevated physical disability risk in older men and women. Am. J. Epidemiol. 2004, 159, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Hyer, K.; Thomas, K.S.; Branch, L.G.; Harman, J.S.; Johnson, C.E.; Weech-Maldonado, R. The influence of nurse staffing levels on quality of care in nursing homes. Gerontologist 2011, 51, 610–616. [Google Scholar] [CrossRef] [PubMed]
- González-Colaço Harmand, M.; Meillon, C.; Rullier, L.; Avila-Funes, J.A.; Bergua, V.; Dartigues, J.F.; Amieva, H. Cognitive decline after entering a nursing home: A 22-year follow-up study of institutionalized and noninstitutionalized elderly people. J. Am. Med. Dir. Assoc. 2014, 15, 504–508. [Google Scholar] [CrossRef] [PubMed]
- Amarya, S.; Singh, K.; Sabharwal, M. Changes during aging and their association with malnutrition. J. Clin. Gerontol. Geriatr. 2015, 6, 78–84. [Google Scholar] [CrossRef] [Green Version]
- Winter, J.E.; MacInnis, R.J.; Wattanapenpaiboon, N.; Nowson, C.A. BMI and all-cause mortality in older adults: A meta-analysis. Am. J. Clin. Nutr. 2014, 99, 875–890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kvamme, J.M.; Holmen, J.; Wilsgaard, T.; Florholmen, J.; Midthjell, K.; Jacobsen, B.K. Body mass index and mortality in elderly men and women: The Tromsø and HUNT studies. J. Epidemiol. Community Health 2012, 66, 611–617. [Google Scholar] [CrossRef] [Green Version]
- Volpato, S.; Romagnoni, F.; Soattin, L.; Blè, A.; Leoci, V.; Bollini, C.; Fellin, R.; Zuliani, G. Body mass index, body cell mass, and 4-year all-cause mortality risk in older nursing home residents. J. Am. Geriatr. Soc. 2004, 52, 886–891. [Google Scholar] [CrossRef]
- Lee, Y.; Kwon, O.; Shin, C.S.; Lee, S.M. Use of bioelectrical impedance analysis for the assessment of nutritional status in critically ill patients. Clin. Nutr. Res. 2015, 4, 32–40. [Google Scholar] [CrossRef] [Green Version]
- Wirth, R.; Volkert, D.; Rosler, A.; Sieber, C.C.; Bauer, J.M. Bioelectric impedance phase angle is associated with hospital mortality of geriatric patients. Arch. Gerontol. Geriatr. 2010, 51, 290–294. [Google Scholar] [CrossRef]
- Kyle, U.G.; Genton, L.; Pichard, C. Low phase angle determined by bioelectrical impedance analysis is associated with malnutrition and nutritional risk at hospital admission. Clin. Nutr. 2013, 32, 294–299. [Google Scholar] [CrossRef] [Green Version]
- Smoliner, C.; Norman, K.; Wagner, K.H.; Hartig, W.; Lochs, H.; Pirlich, M. Malnutrition and depression in the institutionalised elderly. Br. J. Nutr. 2009, 102, 1663–1667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naidoo, I.; Charlton, K.E.; Esterhuizen, T.M.; Cassim, B. High risk of malnutrition associated with depressive symptoms in older South. Africans living in KwaZulu-Natal, South. Africa: A cross-sectional survey. J. Health Popul. Nutr. 2015, 33, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schrader, E.; Grosch, E.; Bertsch, T.; Sieber, C.C.; Volkert, D. Nutritional and Functional Status in Geriatric Day Hospital Patients—MNA Short Form. Versus Full MNA. J. Nutr. Health Aging 2016, 20, 918–926. [Google Scholar] [CrossRef] [PubMed]
- Kiesswetter, E.; Pohlhausen, S.; Uhlig, K.; Diekmann, R.; Lesser, S.; Heseker, H.; Stehle, P.; Sieber, C.C.; Volkert, D. Malnutrition is related to functional impairment in older adults receiving home care. J. Nutr. Health Aging 2013, 17, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Akbar, F.; Setiati, S. Correlation between hand grip strength and nutritional status in elderly patients. In Journal of Physics: Conference Series; IOP Publishing Ltd.: Bristol, UK, 2018; Volume 1073, p. 042032. [Google Scholar]
- Norman, K.; Stobäus, N.; Smoliner, C.; Zocher, D.; Scheufele, R.; Valentini, L.; Lochs, H.; Pirlich, M. Determinants of hand grip strength, knee extension strength and functional status in cancer patients. Clin. Nutr. 2010, 29, 586–591. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, F.; Cristi-Montero, C.; González-Ruíz, K.; Correa-Bautista, J.E.; Ramírez-Vélez, R. Bioelectrical impedance vector analysis and muscular fitness in healthy men. Nutrients 2016, 8, 407. [Google Scholar] [CrossRef] [Green Version]
- de Wit, L.M.; van Straten, A.; van Herten, M.; Penninx, B.W.; Cuijpers, P. Depression and body mass index, a u-shaped association. BMC Public Health 2009, 9, 14. [Google Scholar] [CrossRef] [Green Version]
- Lorenzo, I.; Serra-Prat, M.; Yébenes, J.C. The role of water homeostasis in muscle function and frailty: A Review. Nutrients 2019, 11, 1857. [Google Scholar] [CrossRef] [Green Version]
- Yamada, Y.; Yoshida, T.; Yokoyama, K.; Watanabe, Y.; Miyake, M.; Yamagata, E.; Yamada, M.; Kimura, M.; Study, K.-K. The extracellular to intracellular water ratio in upper legs is negatively associated with skeletal muscle strength and gait speed in older people. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 72, 293–298. [Google Scholar] [CrossRef] [Green Version]
- Serra-Prat, M.; Lorenzo, I.; Palomera, E.; Ramírez, S.; Yébenes, J.C. Total body water and intracellular water relationships with muscle strength, frailty and functional performance in an elderly population. J. Nutr. Health Aging 2019, 23, 96–101. [Google Scholar] [CrossRef]
- Serra-Prat, M.; Lorenzo, I.; Palomera, E.; Yébenes, J.C.; Campins, L.; Cabré, M. Intracellular water content in lean mass is associated with muscle strength, functional capacity, and frailty in community-dwelling elderly individuals. A cross-sectional study. Nutrients 2019, 11, 661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norman, K.; Stobäus, N.; Pirlich, M.; Bosy-Westphal, A. Bioelectrical phase angle and impedance vector analysis--clinical relevance and applicability of impedance parameters. Clin. Nutr. 2012, 31, 854–861. [Google Scholar] [CrossRef] [PubMed]
- Mundstock, E.; Amaral, M.A.; Baptista, R.R.; Sarria, E.E.; Dos Santos, R.R.G.; Filho, A.D.; Rodrigues, C.A.S.; Forte, G.C.; Castro, L.; Padoin, A.V.; et al. Association between phase angle from bioelectrical impedance analysis and level of physical activity: Systematic review and meta-analysis. Clin. Nutr. 2019, 38, 1504–1510. [Google Scholar] [CrossRef] [PubMed]
- Souza, M.F.; Tomeleri, C.M.; Ribeiro, A.S.; Schoenfeld, B.J.; Silva, A.M.; Sardinha, L.B.; Cyrino, E.S. Effect of resistance training on phase angle in older women: A randomized controlled trial. Scand. J. Med. Sci. Sports 2017, 27, 1308–1316. [Google Scholar] [CrossRef]
- Pigłowska, M.; Kostka, T.; Drygas, W.; Jegier, A.; Leszczyńska, J.; Bill-Bielecka, M.; Kwaśniewska, M. Body composition, nutritional status, and endothelial function in physically active men without metabolic syndrome e a 25 year cohort study. Lipids Health Dis. 2016, 15, 84. [Google Scholar] [CrossRef] [Green Version]
- Dos Santos, L.; Cyrino, E.S.; Antunes, M.; Santos, D.A.; Sardinha, L.B. Changes in phase angle and body composition induced by resistance training in older women. Eur. J. Clin. Nutr. 2016, 70, 1408–1413. [Google Scholar] [CrossRef]
- Douma, J.G.; Volkers, K.M.; Engels, G.; Marieke, H.; Sonneveld, M.H.; Goossens, R.H.M.; Scherder, E.J.A. Setting-related influences on physical inactivity of older adults in residential care settings: A review. BMC Geriatr. 2017, 17, 97. [Google Scholar] [CrossRef] [Green Version]
- Lindwall, M.; Larsman, P.; Hagger, M.S. The reciprocal relationship between physical activity and depression in older European adults: A prospective cross-lagged panel design using SHARE data. Health Psychol. 2011, 30, 453–462. [Google Scholar] [CrossRef]
- Almeida, O.; Khan, K.M.; Hankey, G.J.; Yeap, B.B.; Golledge, J.; Flicker, L. 150 minutes of vigorous physical activity per week predicts survival and successful ageing: A population-based 11-year longitudinal study of 12 201 older Australian men. Br. J. Sports Med. 2014, 48, 220–225. [Google Scholar] [CrossRef] [Green Version]
- Maresova, P.; Javanmardi, E.; Barakovic, S.; Barakovic Husic, J.; Tomsone, S.; Krejcar, O.; Kuca, K. Consequences of chronic diseases and other limitations associated with old age—A scoping review. BMC Public Health 2019, 19, 1431. [Google Scholar] [CrossRef]
- Boscart, V.; Crutchlow, L.E.; Sheiban Taucar, L.; Johnson, K.; Heyer, M.; Davey, M.; Costa, A.; Heckman, G. Chronic disease management models in nursing homes: A scoping review. BMJ Open 2020, 10, e032316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calderón-Larrañaga, A.; Vetrano, D.L.; Ferrucci, L.; Mercer, S.W.; Marengoni, A.; Onder, G.; Eriksdotter, M.; Fratiglioni, L. Multimorbidity and functional impairment-bidirectional interplay, synergistic effects and common pathways. J. Intern. Med. 2019, 285, 255–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harsányiová, M.; Prokop, P. Living condition, weight loss and cognitive decline among people with dementia. Nurs. Open 2018, 5, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Buchman, A.S.; Boyle, A.; Wilson, R.S.; James, B.D.; Leurgans, S.E.; Arnold, S.E.; Bennett, D.A. Loneliness and the rate of motor decline in old age: The rush memory and aging project, a community-based cohort study. BMC Geriatr. 2010, 10, 77. [Google Scholar] [CrossRef] [Green Version]
- de Sire, A.; Baricich, A.; Renò, F.; Cisari, C.; Fusco, N.; Invernizzi, M. Myostatin as a potential biomarker to monitor sarcopenia in hip fracture patients undergoing a multidisciplinary rehabilitation and nutritional treatment: A preliminary study. Aging Clin. Exp. Res. 2020, 32, 959–962. [Google Scholar] [CrossRef]
- Iolascon, G.; Gimigliano, R.; Bianco, M.; De Sire, A.; Moretti, A.; Giusti, A.; Malavolta, N.; Migliaccio, S.; Migliore, A.; Napoli, N.; et al. Are dietary supplements and nutraceuticals effective for musculoskeletal health and cognitive function? A scoping review. J. Nutr. Health Aging 2017, 21, 527–538. [Google Scholar] [CrossRef]
- Marshall, R.N.; Smeuninx, B.; Morgan, T.; Breen, L. Nutritional strategies to offset disuse-induced skeletal muscle atrophy and anabolic resistance in older adults: From whole-foods to isolated ingredients. Nutrients 2020, 12, 1533. [Google Scholar] [CrossRef]
- de Sire, A.; de Sire, R.; Petito, V.; Masi, L.; Cisari, C.; Gasbarrini, A.; Scaldaferri, F.; Invernizzi, M. Gut-Joint Axis: The role of physical exercise on gut microbiota modulation in older people with osteoarthritis. Nutrients 2020, 12, 574. [Google Scholar] [CrossRef] [Green Version]
- Landi, F.; Calvani, R.; Tosato, M.; Martone, A.M.; Ortolani, E.; Savera, G.; D’Angelo, E.; Sisto, A.; Marzetti, E. Protein intake and muscle health in old age: From biological plausibility to clinical evidence. Nutrients 2016, 8, 295. [Google Scholar] [CrossRef]
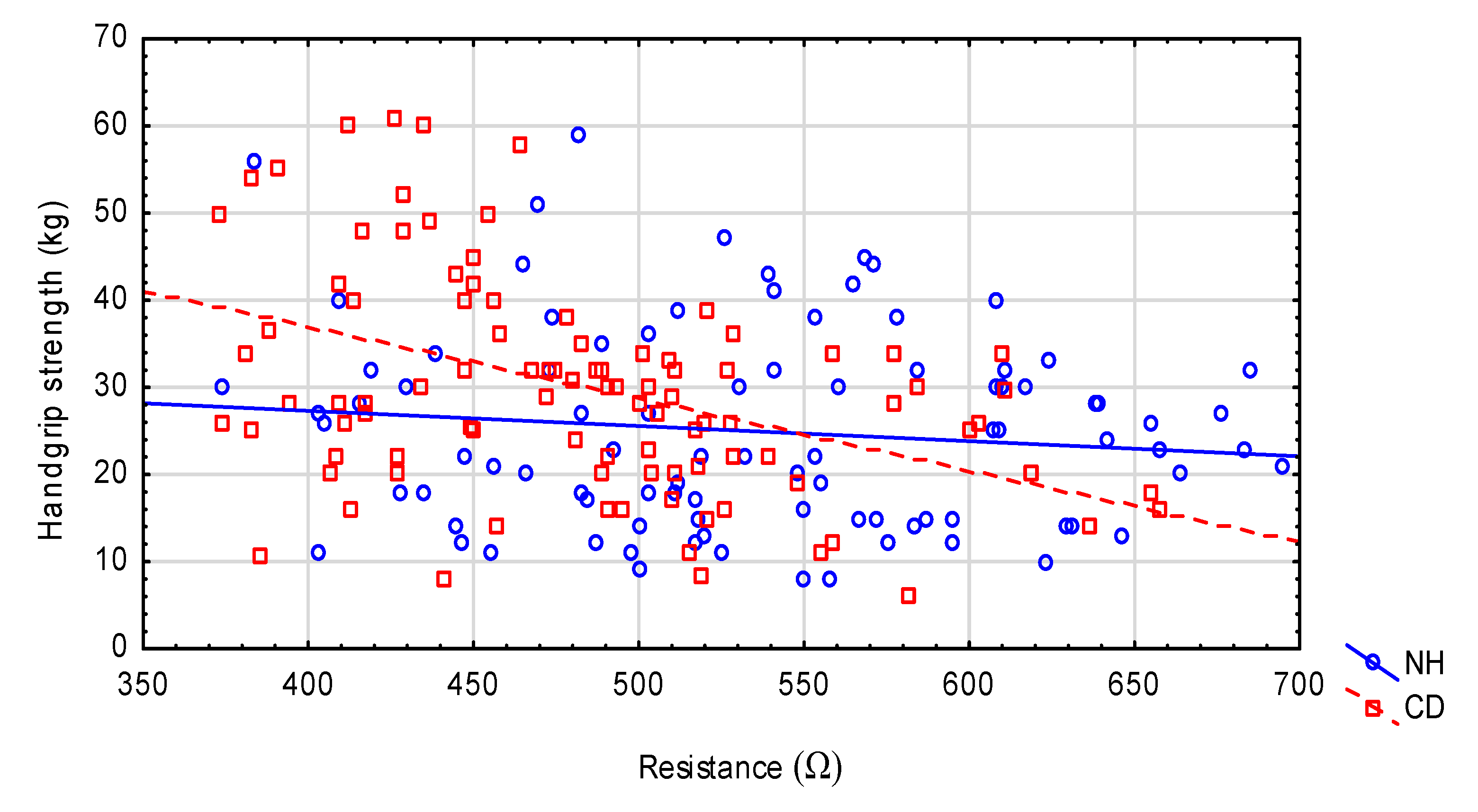
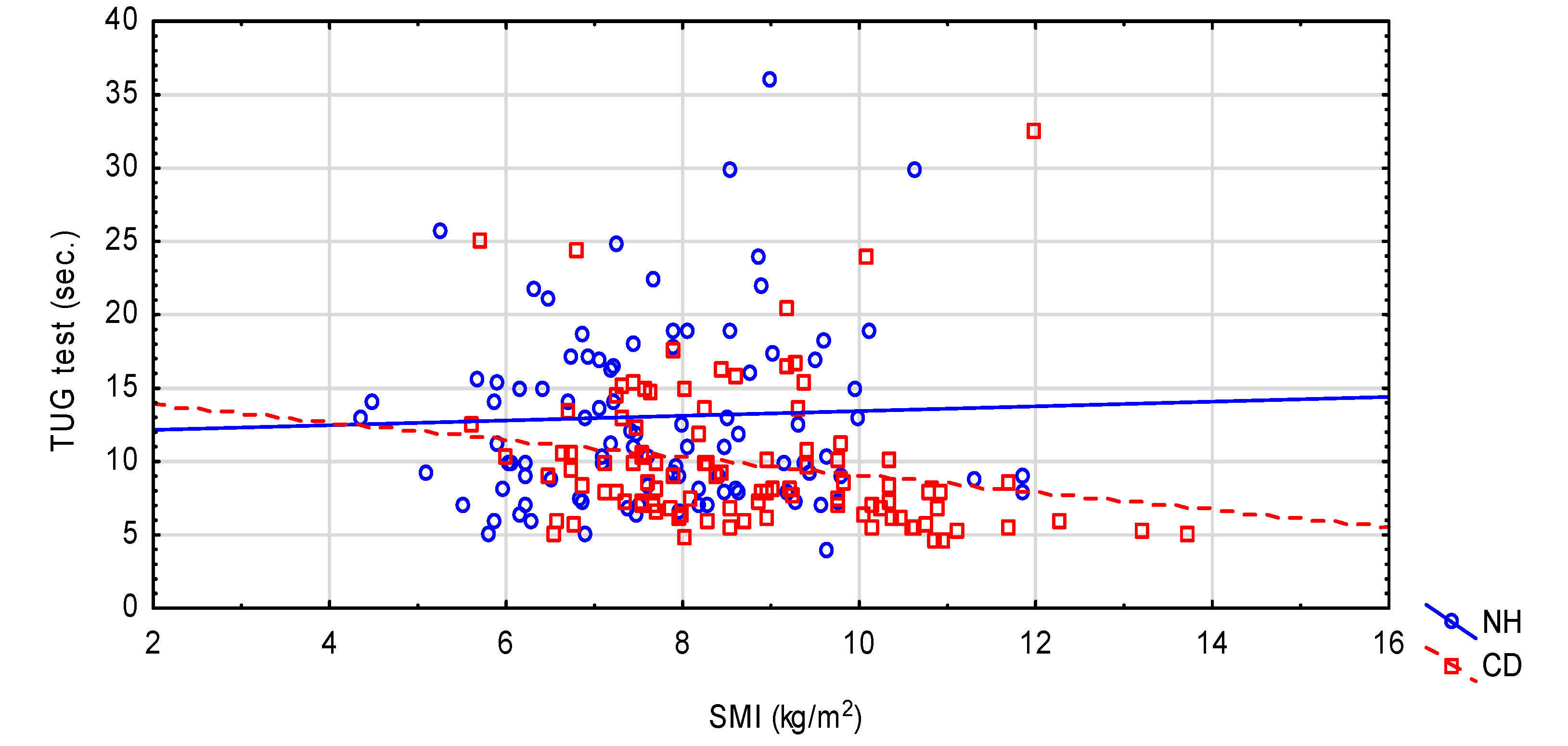

| Variables | NH n = 100 | CD n = 100 | p-Value |
|---|---|---|---|
| Age (years) | 74.6 ± 9.74 73 (67.0; 82.0) | 74.9 ± 8.50 75 (66.5; 83.0) | NS |
| Women (%) | 75 | 75 | NS |
| ADL | 5.54 ± 0.55 5.50 (5.50; 6.00) | 5.73 ± 0.42 6.0 (5.50; 6.00) | 0.015 |
| IADL | 4.65 ± 2.26 5.0 (3.0; 7.0) | 7.13 ± 1.71 8.0 (7.0; 8.0) | <0.001 |
| TUG | 13.0 ± 7.07 10.98 (8.16; 16.38) | 9.88 ± 4.89 8.3 (6.66; 11.0) | <0.001 |
| MMSE | 23.7 ± 4.69 24.0 (22.0; 27.0) | 26.8 ± 3.84 28.0 (25.0; 30.0) | <0.001 |
| GDS | 5.76 ± 3.28 5.50 (3.00; 8.00) | 4.65 ± 3.53 4.0 (2.0; 6.5) | 0.01 |
| Handgrip strength (kg) | 24.7 ± 11.2 (23.0 15.0; 32.0) | 30.2 ± 12.7 29.0 (21.5; 36.25) | 0.002 |
| Variable | NH n = 100 | CD n = 100 | p-Value |
|---|---|---|---|
| Body mass (kg) | 67.1 ± 15.7 65.0 (56.55; 78.00) | 72.6 ± 14.0 71.05 (63.45; 80.55) | 0.014 |
| BMI (kg/m2) | 27.2 ± 5.66 26.7 (22.6; 31.7) | 28.7± 4.65 28.2 (25.3; 30.6) | 0.043 |
| Waist circumference (cm) | 94.3 ± 13.4 | 93.8 ±11.2 | NS |
| WHtR | 0.602 ± 0.088 | 0.591 ± 0.070 | NS |
| Calf circumference (cm) | 35.1 ± 3.90 34.7 (32.0; 37.0) | 37.1 ± 4.04 37.0 (35.0; 39.0) | <0.001 |
| MNA | 21.1 ± 3.42 21.3 (18.5; 23.5) | 25.4 ± 3.29 26.0 (23.5; 28.0) | <0.001 |
| FM (%) | 37.6 ± 10.2 | 37.1 ± 8.15 | NS |
| FFM (%) | 62.4 ± 10.2 | 62.9 ± 8.15 | NS |
| TBW (%) | 50.5 ± 6.28 50.0 (45.8; 53.3) | 51.2 ± 5.41 50.9 (47.7; 54.4) | NS |
| ECW (%) | 47.3 ± 2.50 47.6 (45.9; 48.8) | 46.4 ± 2.13 47.1 (45.0; 47.9) | 0.007 |
| ICW (%) | 52.7 ± 2.50 52.4 (51.2; 54.1) | 53.6 ± 2.14 52.9 (52.1; 55.0) | 0.008 |
| ECW/ICW | 0.90 ± 0.09 0.91 (0.85; 0.95) | 0.87 ± 0.07 0.89 (0.82; 0.92) | 0.007 |
| BCM (%) | 34.8 ± 5.26 34.0 (31.2; 37.5) | 35.6 ± 4.72 34.6 (32.3; 38.2) | NS |
| ECM/BCM | 0.80 ± 0.09 0.80 (0.75; 0.84) | 0.77 ± 0.07 0.78 (0.75; 0.81) | 0.037 |
| RMR/kg (kcal/kg) | 19.1 ± 3.57 18.8 (16.2; 21.3) | 18.2 ± 2.42 18.0 (16.7; 20.0) | NS |
| Body volume (Lt) | 66.5 ± 15.9 64.7 (55.2; 77.4) | 71.3 ± 14.2 69.6 (62.0; 78.4) | 0.030 |
| Body density(kg/Lt) | 1.02 ± 0.02 1.01 (1.00; 1.03) | 1.02 ± 0.02 1.01 (1.00;1.03) | NS |
| SMM (kg) | 19.4 ±5.77 18.4 (15.6; 22.9) | 22.4 ± 6.14 21.1 (17.6; 25.4) | <0.001 |
| SMI (kg/m2) | 7.75 ± 1.52 7.60 (6.72; 8.80) | 8.74 ± 1.64 8.44 (7.54; 9.93) | <0.001 |
| Z (Ω) | 552.7 ± 93.2 | 485.0 ± 69.6 | <0.001 |
| R (Ω) | 549.0 ± 92.4 | 481.3 ± 69.4 | <0.001 |
| Xc (Ω) | 66.7 ± 15.7 66.5 (56.3; 74.5) | 63.5 ± 10.26 63.8 (55.7; 70.5) | NS |
| PhA (degrees) | 6.90 ± 1.005 | 7.55 ± 1.03 | <0.001 |
| Variable | Handgrip Strength | ADL | IADL | TUG Test | MNA | MMSE | GDS |
|---|---|---|---|---|---|---|---|
| Age | −0.378 *** | −0.288 ** | |||||
| Body mass (kg) | 0.362 *** | ||||||
| BMI (kg/m2) | |||||||
| Waist circ. (cm) | 0.243 * | ||||||
| WHtR | −0.266 ** | ||||||
| Calf circ. (cm) | 0.245 * | ||||||
| MNA | 0.292 ** | 0.272 ** | −0.206 * | ||||
| FM (%) | −0.438*** | ||||||
| FFM (%) | 0.438 *** | ||||||
| TBW (%) | 0.305 ** | −0.219 * | |||||
| ECW (%) | −0.530 *** | −0.305 ** | |||||
| ICW (%) | 0.530*** | 0.305 ** | |||||
| ECW/ICW | −0.535 *** | −0.303 ** | |||||
| BCM (%) | 0.421 *** | ||||||
| ECM (%) | 0.395 *** | ||||||
| ECM/BCM | |||||||
| RMR/kg (kcal/kg) | 0.247 * | ||||||
| Body volume (Lt) | 0.333 *** | ||||||
| Body density | 0.438 *** | ||||||
| SMM (kg) | 0.533 *** | 0.214 * | |||||
| SMI (kg/m2) | 0.396 *** | ||||||
| Z (Ω) | |||||||
| R (Ω) | |||||||
| Xc (Ω) | 0.226 * | ||||||
| PhA (degrees) | 0.414 *** | −0.214 | 0.212 * |
| Variable | Handgrip Strength | ADL | IADL | TUG | MNA | MMSE | GDS |
|---|---|---|---|---|---|---|---|
| Age (years) | −0.438 *** | −0.238 * | 0.481 *** | −0.207 * | −0.533 *** | 0.201 * | |
| Body mass (kg) | 0.377 *** | 0.0734 | −0.202 * | 0.1488 | 0.317 ** | ||
| BMI (kg/m2) | |||||||
| Waist circ. (cm) | |||||||
| WHtR | |||||||
| Calf circ (cm) | 0.213 * | ||||||
| MNA | 0.318 ** | 0.272 ** | 0.438 *** | −0.464 *** | 0.364 *** | −0.527 *** | |
| FM (%) | −0.540 *** | −0.231 * | 0.322 ** | −0.225 * | 0.275 ** | ||
| FFM (%) | 0.540 *** | 0.231 * | −0.322 ** | 0.225 * | −0.275 ** | ||
| TBW (%) | 0.420 *** | 0.219 * | −0.229 * | −0.238 * | |||
| ECW (%) | −0.599 *** | 0.341 *** | −0.245 * | −0.395 *** | 0.204 * | ||
| ICW (%) | 0.600 *** | −0.341 *** | 0.246 * | 0.396 *** | −0.204 * | ||
| ECW/ICW | −0.600 *** | 0.344 *** | −0.246 * | −0.398 *** | 0.205 * | ||
| BCM (%) | 0.496 *** | −0.281 ** | −0.226 * | ||||
| ECM (%) | 0.496 *** | 0.199 * | −0.315 ** | −0.258 * | |||
| ECM/BCM | |||||||
| RMR/kg (kcal/kg) | 0.321 ** | −0.267 ** | |||||
| Body volume (Lt) | 0.322 ** | 0.317 ** | |||||
| Body density (kcal/Lt) | 0.540 *** | 0.230 * | −0.326 ** | 0.228 * | −0.276 ** | ||
| SMM (kg) | 0.681 *** | −0.399 *** | 0.285 ** | 0.329 ** | −0.204 * | ||
| SMI (kg/m2) | 0.605 *** | −0.345 ** | 0.262 ** | 0.266 ** | −0.226 * | ||
| Z (Ω) | −0.395 *** | 0.2154 * | |||||
| R (Ω) | −0.402 *** | 0.220 * | |||||
| Xc (Ω) | |||||||
| PhA (degrees) | 0.457 *** | 0.271 ** | −0.453 *** | 0.324 ** | 0.413 *** | −0.307 ** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pigłowska, M.; Guligowska, A.; Kostka, T. Nutritional Status Plays More Important Role in Determining Functional State in Older People Living in the Community than in Nursing Home Residents. Nutrients 2020, 12, 2042. https://doi.org/10.3390/nu12072042
Pigłowska M, Guligowska A, Kostka T. Nutritional Status Plays More Important Role in Determining Functional State in Older People Living in the Community than in Nursing Home Residents. Nutrients. 2020; 12(7):2042. https://doi.org/10.3390/nu12072042
Chicago/Turabian StylePigłowska, Małgorzata, Agnieszka Guligowska, and Tomasz Kostka. 2020. "Nutritional Status Plays More Important Role in Determining Functional State in Older People Living in the Community than in Nursing Home Residents" Nutrients 12, no. 7: 2042. https://doi.org/10.3390/nu12072042
APA StylePigłowska, M., Guligowska, A., & Kostka, T. (2020). Nutritional Status Plays More Important Role in Determining Functional State in Older People Living in the Community than in Nursing Home Residents. Nutrients, 12(7), 2042. https://doi.org/10.3390/nu12072042






