Feeding Interventions for Infants with Growth Failure in the First Six Months of Life: A Systematic Review
Abstract
1. Introduction
- (1)
- to identify and describe feeding interventions with a focus on restoring or improving the volume and quality of breastmilk and breastfeeding when breastfeeding practices are sub-optimal or prematurely stopped, and
- (2)
- to assess the impact of these interventions on feeding practices, anthropometry, morbidity, and mortality status.
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Search Strategy
2.3. Study Selection
2.4. Data Extraction
2.5. Analysis
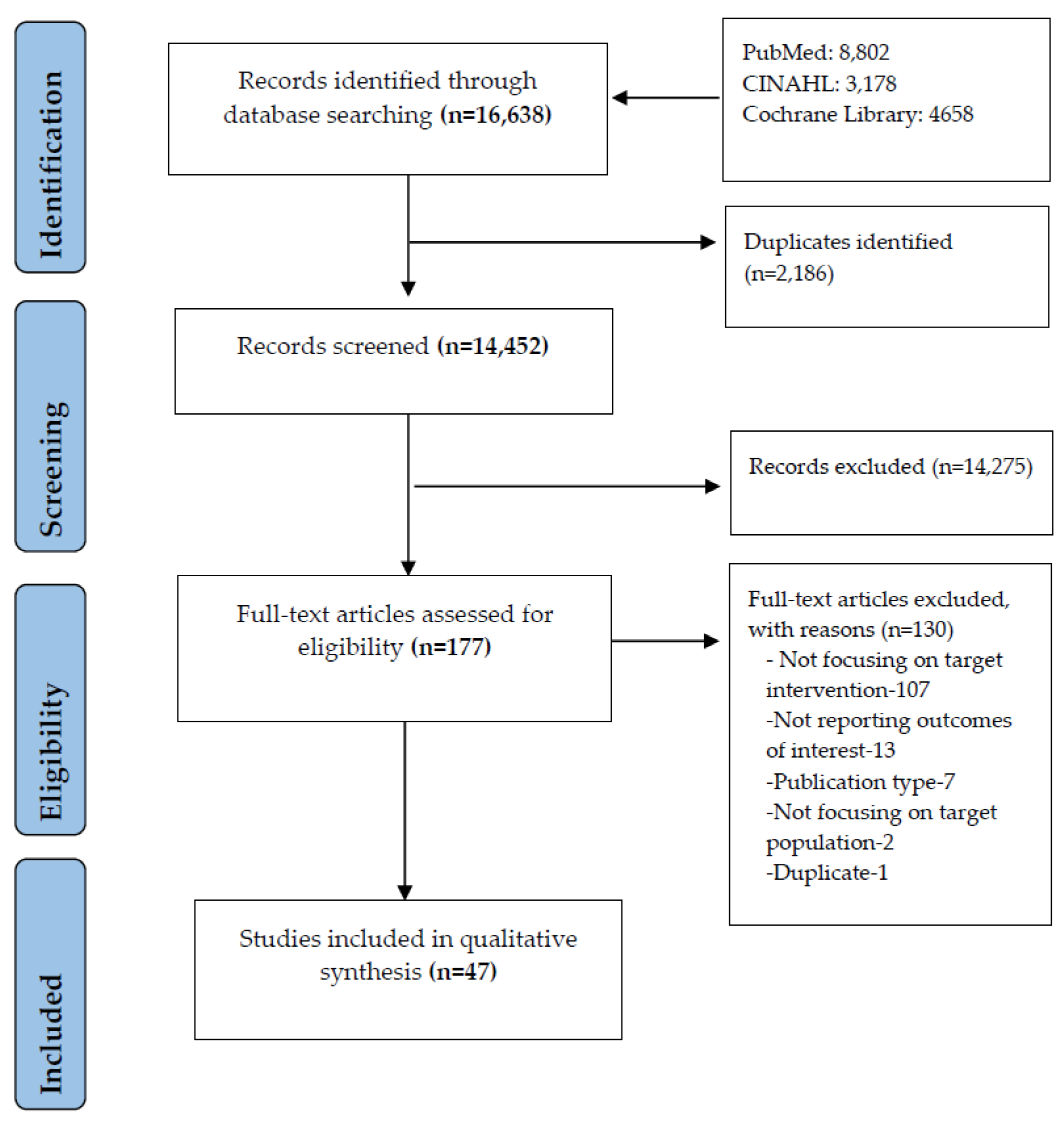
3. Results
3.1. Study Selection
3.2. General Characteristics of the Included Studies
3.3. Synthesis of Results
3.3.1. Cup Feeding
3.3.2. Formula Fortification or Supplementation
Bovine/Cow Milk Based Formula
Protein Supplementation
Lactase Fortification
Fortification with Iron
Nutrient Fortification
Cream Supplementation
Early and Delayed Fortification
Fortified Human Milk
Different Formulas
Continued EBF and Early Limited Formula
3.3.3. Enteral Feed Interventions
3.3.4. Other Interventions
4. Discussion
4.1. Summary of Key Findings
4.2. This Review’s Findings in Context
4.3. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
Appendix A
| Author (Year) | Population Characteristics | Intervention | Outcome | Review Author’s Interpretation |
|---|---|---|---|---|
| Cup Feeding Interventions (n = 2) | ||||
| Abouelfettoh (2008) [24] | Preterm (LBW) (GA: 35.13 wk, Bwt: 2150 g) Recruitment: NICU | Cup feeding IG: Only cup feeding CG: Only bottle feeding | Feeding practices (IG = 30 vs. CG = 30) | |
| 1 wk post-discharge | ||||
| BF (%) (80 vs. 64, p = 0.03) | ↑* | |||
| Yilmaz (2014) [25] | Preterm (VLBW) (GA: 32–35 wk, Bwt: 1543 g) Recruitment: NICU | Cup feeding G1: Cup feeding G2: Bottle feeding | Feeding practices (G1 = 254 vs. G2 = 268) | |
| At discharge, 3 and 6 m (%) | ||||
| EBF at discharge (72 vs. 46, p < 0.0001) | ↑ *** | |||
| EBF at 3 m (77 vs. 47, p < 0.0001) | ↑ *** | |||
| EBF at 6 m (57 vs. 42, p < 0.001) | ↑ *** | |||
| Anthropometry (G1 = 254 vs. G2 = 268) | ||||
| First 7 d | ||||
| Weight gain, mean (g/d) (16.7 vs. 16.8, p = 0.64) |  | |||
| Formula Fortification/Supplementation Interventions (31) | ||||
| Abrams (2014) [26] | Preterm (VLBW) (Bwt: <1250 g) Recruitment: NICU | Cow milk G1: CM (cow milk formula + cow milk based fortifier) G2: HM (HM (mother’s own/donor milk) + HM based fortifier) | Anthropometry (G1 = 93 vs. G2 = 167) | |
| During NICU stay | ||||
| Weight (g/kg/d) (13.6 ± 4.1 vs. 14.9 ± 7.2, p = 0.11) |  | |||
| Length (cm/wk) (0.89 ± 0.45 vs. 0.97 ± 0.35, p = 0.12) |  | |||
| Morbidity (G1 = 93 vs G2 = 167) | ||||
| Sepsis (%) (34 vs. 30, p = 0.46) |  | |||
| NEC (%) (17 vs. 5, p = 0.002) | ↓ ** | |||
| Mortality (G1 = 93 vs. G2 = 167) | ||||
| Death (%) (8 vs. 2, p = 0.04) | ↓ * | |||
| Cristofalo (2013) [33] | Preterm (ELBW) (GA: <27 wk, Bwt: 989 g) Recruitment: NICU | Bovine/cow milk G1: Bovine milk-based preterm formula 1–4 d after birth and continued at 10–20 mL/kg/d as tolerated for up to 5 days G2: Exclusive appropriately fortified HM | Feeding Practices (G1 = 29 vs. G2 = 24) | |
| Parenteral nutrition (d) (36 vs. 27, p = 0.04) | ↓ * | |||
| Morbidity (G1 = 29 vs. G2 = 24) | ||||
| NEC (n) (5 vs. 1, p = 0.08) | ↓◌ | |||
| Mortality (G1 = 29 vs. G2 = 24) | ||||
| Death (n) (2 vs. 0, NS) |  | |||
| Alan (2013) [27] | Preterm (VLBW) (GA: ≤32 wk, Bwt: ≤1500 g) Recruitment: NICU | Protein supplementation When mothers expressed first milk IG: HM with extra protein supplementation CG: HM with a standard fortification | Anthropometry (IG = 29 vs. CG = 29) | |
| During NICU stay (mean ± sd) | ||||
| Max. weight loss (9.5 ± 5.2 vs. 9.8 ± 4.1, p = 0.484) |  | |||
| Weight velocity (g/kg/d) (20.9 ± 4.7 vs. 18.9 ± 4.5, p = 0.053) | ↑◌ | |||
| Length velocity (mm/d) (1.60 ± 0.62 vs. 1.15 ± 0.53, p = 0.008) | ↑ ** | |||
| Arslanoglu (2006) [29] | Preterm (LBW, VLBW, ELBW) (GA: 26–34 wk, Bwt: 600–1750 g) Recruitment: Hospital | Protein supplementation When volume reached 150 mL/kg/d G1: HMF + additional protein G2: HM with HMF in the standard amount | Anthropometry (G1 = 16 vs. G2 = 16) | |
| Infants reached a weight of 2000 g (mean ± sd) | ||||
| Weight gain (g/d) (30.1 ± 5.8 vs. 24.8 ± 4.8, p < 0.01) | ↑ ** | |||
| Length gain (mm/d) (1.3 ± 0.5 vs. 1.1 ± 0.4, p > 0.05) |  | |||
| Florendo (2009) [36] | Preterm (VLBW) (GA: ≤32 wk, Bwt: 1200 g) Recruitment: New born medical centre | Protein supplementation IG: Partially hydrolysed whey protein CG: Non-hydrolysed whey casein preterm infant formula | Anthropometry (IG = 42 vs. CG = 38) | |
| Weight (g) 1,2, 3 wk (No differences between groups) |  | |||
| Length (cm) 1,2, 3 wk (No differences between groups) |  | |||
| Kim (2015) [40] | Preterm (VLBW) (GA: ≤33 wk, Bwt: 1174 g) Recruitment: NICU | Protein supplementation When volume reached 100 mL/kg/d IG: Conc. HMF containing liquid extensively hydrolysed protein CG: Powdered intake protein HMF | Anthropometry (IG = 66 vs. CG = 63) | |
| Until day 29 of study period or hospital discharge (mean ± sd) | ||||
| Weight gain (g/kg/d) (18.2 ± 0.3 vs. 17.5 ± 0.6, NS) |  | |||
| Length gain (cm/wk) (1.2 ± 0.06 vs. 1.2 ± 0.07, NS) |  | |||
| Erasmus (2002) [34] | Preterm (VLBW) (GA: 26–34 wk, Bwt: 1407 g) Recruitment: NICU | Lactase fortification From birth (day 1) to 36 wk or discharged IG: Fortified HM or preterm formula treated with lactaid drops (Lactase) CG: Untreated fortified HM or preterm formula | Anthropometry (IG = 52 vs. CG = 50) | |
| Weight gain (g/d) (mean ± sd) | ||||
| 7th d (17.4 ± 1.9 vs. 12.9 ± 1.9, NS) |  | |||
| 14th d (21.3 ± 1.6 vs. 18.6 ± 1.4, NS) |  | |||
| 24th d (25.2 ± 1.1 vs. 23.0 ± 1.2, NS) |  | |||
| Length gain (cm/wk) (1.0 ± 0.2 vs. 0.7 ± 0.1, NS) |  | |||
| Gathwala (2007) [37] | Term SGA (LBW) (GA: 40 wk, Bwt: 2000 g) Recruitment: Hospital | Lactase fortification When volume reached 100 mL/kg/d IG: HM fortified with Lactodex-HMF CG: Only BM | Anthropometry (IG = 25 vs. CG = 25) | |
| Follow-up at 28 d (mean ± sd) | ||||
| Weight gain (g/d) (38.77 ± 7.43 vs. 28.7 ± 3.18, p < 0.001) | ↑ *** | |||
| Length gain (cm/wk) (1.14 ± 0.33 vs. 0.87 ± 0.17, p < 0.01) | ↑ ** | |||
| Berseth (2004) [70] | Preterm (VLBW) (GA: ≤33 wk, Bwt: 1180 g) Recruitment: Hospital | Iron fortification When volume reached 100 mL/kg/d G1: HMF (iron fortified) G2: HMF (standard) | Anthropometry (G1 = 55 vs. G2 = 39) | |
| Day 1–28 (mean ± sd) | ||||
| Weight gain (g/kg/d) (17.5 ± 0.53 vs. 17.3 ± 0.59, p = 0.63) |  | |||
| Willeitner (2017) [55] | Preterm (VLBW, ELBW) (GA: 29 wk, Bwt: 500–1499 g) Recruitment: NICU | Iron fortification Birth day 3 IG: HM fortification (Conc. Preterm Formula 30 Similac Special Care 30 with iron) CG: Standard Powdered HMF (Similac HMF) | Anthropometry (IG = 35 vs. CG = 35) | |
| Weight gain, mean (g/kg/d) (18.3 vs. 16.9, p = 0.38) |  | |||
| Morbidity (IG = 35 vs. CG = 35) | ||||
| NEC (n) (3 vs. 4, p = 1) |  | |||
| Mortality (IG = 35 vs. CG = 35) | ||||
| Death (n) (0 vs. 2, p = 0.49) |  | |||
| Clarke (2007) [32] | Faltering growth (GA: 2–31 wk) <3rd centile for weight and height for age; weight gain <50% of expected Recruitment: Children’s Hospital | Nutrient fortification G1: Nutrient-dense formula G2: Energy-supplemented formula | Anthropometry (G1 = 26 vs. G2 = 23) | |
| During the study period for 6 wk (median) | ||||
| Change in weight (z-score) (0.29 vs. 0.49, p = 0.2) |  | |||
| Change in length (z-score) ( −0.18 vs. −0.28, p = 0.3) |  | |||
| Morlacchi (2016) [46] | Preterm (VLBW) (GA: <32 wk, Bwt: 1255 g) Recruitment: NICU | Nutrient fortification First day G1: Macronutrient fortification G2: Standardised fortification (Aptamil BMF, FM85) | Anthropometry (G1 = 10 vs. G2 = 10) | |
| Weekly (4–7 wk) (mean) | ||||
| Weight gain (g) (205.5 vs. 155, p = 0.025) | ↑ * | |||
| Length gain (cm) (1.6 vs. 1.1, p = 0.003) | ↑ ** | |||
| Worrell (2002) [56] | Preterm (VLBW) (GA: 27 ± 3 wk, Bwt: 925 g) Recruitment: NICU | Nutrient fortification G1: Transitional formula (TF-higher amounts of protein, Ca, p, and several trace minerals and vitamins) G2: Standard formula | Anthropometry (G1 = 66 vs. G2 = 114) | |
| Weight, mean ± sd (kg) | ||||
| 3 m (5.7 ± 0.9 vs. 5.4 ± 1.1, p = 0.12) |  | |||
| Length, mean ± sd (cm) | ||||
| 3 m (59.0 ± 2.9 vs. 58.2 ± 3.6, p = 0.10) |  | |||
| Hair (2014) [38] | Preterm (ELBW) (GA: 28 wk, Bwt: 970 g) Recruitment: NICU | Cream supplementation IG: HM derived cream supplement CG: Mothers own milk or donor’s HM derived fortifier | Anthropometry (IG = 39 vs. CG = 39) | |
| Initiation of enteral feeds until 36 wks PMA or weaned from fortifier (mean ± sd) | ||||
| Weight velocity (g/kg/d) (14.0 ± 2.5 vs. 12.4 ± 3.9, p = 0.03) | ↑ * | |||
| Length velocity (cm/wk) (1.03 ± 0.33 vs. 0.83 ± 0.41, p = 0.02) | ↑ * | |||
| Shah (2016) [71] | Preterm (VLBW) (GA: 27 wk, Bwt: <1500 g) Recruitment: NICU | Early and delayed fortification G1: Early fortification (20 mL/kg/d of HM feeds) G2: Delayed fortification (100 mL/kg/d of HM feeds) | Feeding Practices (G1 = 49 vs. G2 = 50) | |
| Days to full enteral feeds | ||||
| From birth (median) (20 vs. 20, p = 0.45) |  | |||
| From initiation (median) (19 vs. 18, p = 0.34) |  | |||
| Anthropometry (G1 = 49 vs. G2 = 50) | ||||
| 36 wk PMA (mean) | ||||
| Length change (z-score) (−1.58 vs. 1.59, p = 0.93) |  | |||
| Weight change (z-score) (−1.28 vs. 1.41, p = 0.22) |  | |||
| Weight velocity, median (g/k/d) (18.3 vs. 16.7, p = 0.30) |  | |||
| Taheri (2016) [53] | Preterm (VLBW) (GA: 28–34 wk, Bwt: 1294 g) Recruitment: NICU | Early and delayed fortification G1: Early fortification (1st feeding) G2: Late fortification (BF volume reached 75 mL/kg/d) | Feeding Practices (G1 vs. G2) | |
| Feeding intolerance (%) (13.9 vs. 8.6, p = 0.771) |  | |||
| Anthropometry (G1 vs. G2) | ||||
| 1 m after beginning of study (median) | ||||
| Weight after intervention (1725 vs. 760, p = 0.589) |  | |||
| Height after intervention (44 vs. 44, p = 0.387) |  | |||
| Morbidity (G1 vs. G2) | ||||
| End of the study | ||||
| NEC (%) (5.6 vs. 0, p = 0.223) |  | |||
| Sepsis (%) (5.6 vs. 2.9, p = 0.572) |  | |||
| Tillman (2012) [54] | Preterm (VLBW) (GA: <31 wk, Bwt: 1123 g) Recruitment: Neonatal database (NICU) | Early and delayed fortification Fortification with Enfamil, powdered HM fortifier G1: Early BM fortification (1st feed) G2: Delayed fortification (when volume reached 50–80 mL/kg/d) | Anthropometry (G1 = 36 vs. G2 = 36) | |
| At 34 wk (mean ± sd) | ||||
| Weight (g) (1867 ± 303 vs. 1895 ± 310, NS) |  | |||
| Bhat (2001) [31] | Preterm (VLBW) (GA: 26–34 wk, Bwt: 1242 g) Recruitment: Special care baby unit | Human milk fortification When clinical conditions permitted IG: Fortified HM CG: HM only Dose: 1 g of fortifier added to 100 mL of milk on day 1, and gradually increased to 4 g added to 100 mL on 3rd/4th day | Anthropometry (IG = 50 vs. CG = 50) | |
| 60 days | ||||
| Weight gain (g/d) | ||||
| <20 (n) (9 vs. 43), >20 (n) (41 vs. 7) | ↑ | |||
| Morlacchi (2018) [47] | Preterm (VLBW) (GA: <32 wk, Bwt: <1500 g) Recruitment: NICU | Human milk fortification and formula G1: Fortified HM G2: Preterm formula | Anthropometry (G1 = 17 vs. G2 = 15) | |
| At term CA (mean ± sd) | ||||
| Weight (g) (3080 ± 499 vs. 3264 ± 341, NS) |  | |||
| Length (cm) (47.5 ± 3.1 vs. 48.9 ± 1.4, NS) |  | |||
| Body FM (%) (14.9 ± 2.8 vs. 19.2 ± 3.2, p = 0.002) | ↑ ** | |||
| Body fat-free mass (%) (85.1 ± 2.8 vs. 80.8 ± 3.2, p = 0.002) | ↑ ** | |||
| Kim (2017) [41] | Preterm (ELBW) (GA: <32 wk, Bwt: 1087 g) Recruitment: NICU | Human milk and formula G1: Donor human milk G2: Preterm formula Infants fed G1 and G2 before achieving an enteral intake volume of 130 mL/kg/d | Anthropometry (G1 = 36 vs. G2 = 54) | |
| PMA 36 wk (mean ± sd) | ||||
| Weight (g) (2124 ± 345.1 vs. 2114.6 ± 415, p = 0.905) |  | |||
| Height (cm) (43.5 ± 1.8 vs. 43.9 ± 2.9, p = 0.399) |  | |||
| Lok (2017) [43] | Preterm (LBW, VLBW) (GA: <37 wk, Bwt: <2200 g, VLBW: <1500 g, LBW: ≥1500 g and <2200 g) Recruitment: NICU | Human milk and formula 1–4 d after birth and continued at 10–20 mL/kg/d as tolerated for up to 5 days Category 1: LBW, Category 2: VLBW; Both the groups further divided into G1: Any BM (human/donor) G2: No BM (infant formula) | Anthropometry | |
| (Category 1, G1 = 276 vs. G2 = 190) | ||||
| Birth to discharge (mean) | ||||
| Change in weight (z score) (−0.58 vs. −0.48, p = 0.070) | ↓◌ | |||
| (Category 2, G1 = 144 vs. G2 = 31) | ||||
| Change in weight (z score) (−0.68 vs. −0.55, p = 0.541) |  | |||
| Manea (2016) [45] | Preterm (ELBW) (GA: 25–33 wk, Bwt: <1000 g) Recruitment: Children hospital | Human milk and formula Once enteral nutrition (24–48 hrs of life) started until initiation of bottle feeding G1: Human BM G2: Formula | Anthropometry (G1 = 18 vs. G2 = 16) | |
| Within the first 5 wk of life (mean) | ||||
| Weight gain (g/wk) (120.83 vs. 97.27) | ↑ | |||
| Morbidity (G1 = 18 vs. G2 = 16) | ||||
| Infection rate (%) (66.7 vs. 100) | ↑ | |||
| NEC (n) (0 vs. 2) | ↑ | |||
| Morley (2000) [48] | Preterm (LBW) (GA: ≤31 wk, Bwt: <1850 g) Recruitment: Neonatal unit and breast milk bank centre/without BM banks | Human milk and formula Fed until they reach wt. of 2000 g or discharged from NICU Category 1: As sole diet Category 2: As supplement to HM G1: Banked donor milk G2: Preterm formula | Anthropometry (G1 vs. G2) | |
| At 9 m post-term (mean ± sd) | ||||
| Category 1 (G1 = 68 vs. G2 = 67) | ||||
| Weight (kg) (7.7 ± 1.2 vs. 7.9 ± 1.3, NS) |  | |||
| Length (cm) (68.8 ± 3.3 vs. 69.2 ± 3.7, NS) |  | |||
| Category 2 (G1 = 113 vs. G2 = 111) | ||||
| Weight (kg) (8 ± 1.1 vs. 7.9 ± 1.1, NS) |  | |||
| Length (cm) (69.5 ± 3.2 vs. 69.4 ± 3.2, NS) |  | |||
| O’Connor (2016) [50] | Preterm (ELBW) (GA: 27.5 wk, Bwt: 995 g) Recruitment: NICU | Human milk and formula Initiated after birth and advanced at a rate of 10–25 mL/kg/d up to 160 mL/kg/d G1: Donor milk G2: Preterm formula | Anthropometry (G1 = 164 vs. G2 = 162) | |
| At day 90 (mean) | ||||
| Weight-for-age change (z score) (−0.5 vs. −0.5, NS) |  | |||
| Length-for-age change (z score) (−1.0 vs. −0.9, NS) | ||||
| During hospital stay |  | |||
| Morbidity (G1 = 181 vs. G2 = 182) | ||||
| NEC (%) (3.9 vs. 11, p = 0.01) | ↑ * | |||
| Mortality (G1 = 181 vs. G2 = 182) | ||||
| Death (%) (9.4 vs. 11, p = 0.82) |  | |||
| Moya (2012) [49] | Preterm (ELBW) (GA: ≤30 wk, Bwt: 1000 g) Recruitment: NICU | Liquid and powdered fortification From birth to 28 days G1: Liquid HMF G2: Powdered HMF | Anthropometry (G1 = 51 vs. G2 = 58) | |
| At day 28 (mean ± se) | ||||
| Weight (g) (1770.0 ± 35.0 vs. 1670 ± 33.0, p = 0.038) | ↑ * | |||
| Length (cm) (41.8 ± 0.2 vs. 40.9 ± 0.2, p = 0.010) | ↑ * | |||
| Morbidity (G1 = 51 vs. G2 = 58) | ||||
| Sepsis (no differences between groups) |  | |||
| NEC (no differences between groups) |  | |||
| Kanmaz (2012) [39] | Preterm (ELBW) (GA: 28 wk, Bwt: 1092 g) Recruitment: NICU | Different levels of fortification When full feedings were achieved G1: Standard fortification (1.2 g HMF + 30 mL HM) G2: Moderate fortification (1.2 g HMF + 25 mL HM) G3: Aggressive fortification (1.2 g HMF + 20 mL HM) | Anthropometry (G1 = 26 vs. G2 = 29 vs. G3 = 29) | |
| Mean | ||||
| Daily weight gain (g/d) (19.7 vs. 20.6 vs. 21.4, p = 0.38) |  | |||
| Length at discharge (cm) (41.7 vs. 42.05 vs. 41.7, p = 0.85) |  | |||
| Porcelli (1999) [51] | Preterm (VLBW, ELBW) (GA: 25–32 wk, Bwt: 600–1500 g) Recruitment: NICU | Different fortifier G1: Test HMF (1 g of protein/100 mL of supplemented milk, 85% glucose polymers, 15% lactose, and calcium, phosphorus, sodium, copper) G2: Reference HMF (60% whey protein and 40% casein, 75% glucose polymers, 25% lactose and calcium, phosphorus, sodium, and copper) | Feeding Practices (G1 = 35 vs. G2 = 29) | |
| Mean Human milk intake (mL/d) (248.1 ± 7.1 vs. 228.9 ± 8.1, p < 0.05) | ↑ * | |||
| Anthropometry (G1 = 35 vs. G2 = 29) | ||||
| After fully weaned from assigned fortifier (2 wk), mean | ||||
| Weight gain (g/kg/d) (19.7 ± 0.98 vs. 16.8 ± 0.96, p = 0.04) | ↑ * | |||
| Length gain (cm/wk) (0.9 ± 0.1 vs. 0.8 ± 0.1, NS) |  | |||
| Kumar (2017) [42] | Preterm (ELBW) (GA: 27 wk, Bwt: 993 g) Recruitment: NICU | Different fortifier G1: Similac liquid human milk fortifier (Similac Human Milk Fortifier Hydrolysed Protein Conc. Liquid) G2: Enfamil liquid human milk fortifier (Enfamil human milk fortifier acidified liquid) | Anthropometry (G1 = 15 vs. G2 = 16) | |
| 0–40 days | ||||
| G1 had better weight gain (p = 0.008) than G2 | ↑ ** | |||
| Amesz (2010) [28] | Preterm (VLBW) (GA: ≤32 wk, Bwt: 1338 g) Recruitment: Neonatal unit | Different formulas Until term CA G1: Post discharge formula G2: Term formula G3: HM fortified formula | Anthropometry (G1 = 52 vs. G2 = 41 vs. G3 = 7) | |
| Between term and six months CA | ||||
| Change in weight (deviation score) (0.74 ± 1.12 vs. 0.7 ± 0.96 vs. 0.30 ± 0.62, NS) |  | |||
| Change in length (deviation score) (0.92 ± 1.03 vs. 0.84 ± 0.87 vs. 0.42 ± 0.56, NS) |  | |||
| Lucas (1992) [44] | Preterm (VLBW) (GA: 31 wk, Bwt: 1475 g) Recruitment: NICU | Different formula G1: Follow-on preterm formula (FPF Farley’s Premcare) G2: Standard term formula (STF-Farley’s Oster Milk) | Anthropometry (G1 = 16 vs. G2 = 15) | |
| Weight gain | ||||
| Start (37 wk Post Menstrual Age) vs. End (9 m) | ||||
| G1: 3–10 centile, 25 centile | ↑ | |||
| G2: 3–10 centile, 3–10 centile |  | |||
| Length gain | ||||
| Start (37 wk PMA) vs. End (9 m) | ||||
| G1: 25 centile vs. 50 centile | ↑ | |||
| G2: 25 centile vs. 25 centile |  | |||
| Flaherman (2013) [35] | Term infants (>37 wk who lost ≥5% Bwt before 36 h of age) Recruitment: Children hospital | Continued EBF and early limited formula Who lost ≥5% of birth weight before 36 h IG: Early limited formula (ELF 10 mL using feeding syringe) CG: Continued EBF | Feeding Practices (IG = 20 vs. CG = 20) | |
| EBF at 1 wk (%) (90 vs. 53, p = 0.01) | ↑ ** | |||
| EBF at 1 m (%) (70 vs. 42, p = 0.08) | ↑◌ | |||
| EBF at 2 m (%) (80 vs. 47, p = 0.04) | ↑ * | |||
| EBF at 3 m (%) (79 vs. 42, p = 0.02) | ↑ * | |||
| Anthropometry (IG = 20 vs. CG = 20) | ||||
| Weight loss at nadir, (mean ± sd) (% Bwt.) (6.8 ± 1.5 vs. 8.1 ± 2.3, p = 0.10) | ↑◌ | |||
| Enteral Feed Interventions (n = 8) | ||||
| Akintorin (1997) [57] | Preterm (VLBW, ELBW) (GA: 28 wk, Bwt: 700–1250 g) Category 1: 700–1000 g Category 2: 1001–1250 g Recruitment: NICU | CNG and IBG feeds Parenteral nutrition started on days 2 to 3 and continued until each infant tolerated full enteral feedings G1: CNG vs. IBG G2: CNG vs. IBG | Feeding Practices | |
| Full enteral feeds (d) | ||||
| Category 1 (G1 = 17 vs. G2 = 23) | ||||
| (19.7 ± 6.7 vs. 18 ± 5.4, NS) |  | |||
| Category 2 (G1 = 22 vs. G2 = 18) | ||||
| (13 ± 5.2 vs. 12.4 ± 3.9, NS) |  | |||
| Anthropometry | ||||
| 14 days (mean ± sd) | ||||
| Regain Bwt (g) | ||||
| Category 2 (G1 = 17 vs. G2 = 23) | ||||
| (12.8 ± 6.3 vs. 12.9 ± 3.9), NS |  | |||
| Category 2 (G1 = 22 vs. G2 = 18) | ||||
| (12.5 ± 4.0 vs. 12.0 ± 3.4, NS) |  | |||
| Mosqueda (2008) [61] | Preterm (ELBW) (GA: 26 wk, Bwt: 760 g) Recruitment: NICU | Intravenous and nasogastric feeds G1: Intravenous alimentation alone (NPO None per orem) G2: Small boluses of nasogastric feedings (Minimal enteral nutrition) | Anthropometry (G1 = 28 vs. G2 = 33) | |
| 32 wk CGA (mean ± sd) | ||||
| Weight gain (g/d) (13.27 ± 3.63 vs. 12.23 ± 3.06, p = 0.24) |  | |||
| Morbidity (G1 = 28 vs. G2 = 33) | ||||
| Sepsis (%) (32 vs. 39, p = 0.56) |  | |||
| NEC (%) (14 vs. 9, p = 0.53) |  | |||
| Kliethermes (1999) [60] | Preterm (LBW) (GA: ≤32 wk, Bwt: 1685 g) Recruitment: Regional perinatal centre | Nasogastric and bottle feeds G1: Nasogastric tube G2: Bottle feeding | Feeding practices (G1 = 38 vs. G2 = 46) | |
| BF (or) | ||||
| At discharge (4.5 times BF, 9.4 times fully BF, p < 0.05) | ↑ * | |||
| After 3 d (5 times BF, 6.4 times fully BF, p < 0.05) | ↑ * | |||
| After 3 m (4.3 times BF, 3.8 times fully BF, p < 0.05) | ↑ * | |||
| Bora (2017) [58] | Preterm (VLBW) (GA: 35 wk, Bwt: 1357 g) Recruitment: NICU | Complete and minimal feeds G1: Complete enteral feed (CEF) with EBM G2: Minimal enteral feed (MEF) with IVF (trophic feeds 20 mL/kg of EBM and 60 mL/kg 10% Dextrose by IV route) | Feeding practices (G1 = 51 vs. G2 = 52) | |
| Feed intolerance (%) (23.52 vs. 11.53, p = 0.12) |  | |||
| Anthropometry (G1 = 51 vs. G2 = 52) | ||||
| First 21 d of life or NICU discharge | ||||
| Regain Bwt (d) (10.6 ± 1.6 vs. 11.8 ± 1.6, p = 0.038) | ↑ * | |||
| Morbidity (G1 = 51 vs. G2 = 52) | ||||
| NEC (%) (7.8 vs. 1.9, p = 0.16) |  | |||
| Colaizy (2012) [59] | Preterm (ELBW) (GA: 27 wk, Bwt: 889 g) Recruitment: NICU | Different levels of feeds Total enteral intake as HM, donor milk, Mixed HM/DM G1: <25%, G2: 25–50%, G3: 50–75% G4: >75% | Anthropometry (G4 = 88 vs. G1 = 17, G2 = 30, G3 = 36) | |
| Birth to discharge | ||||
| Median change in weight z-score | ||||
| G4 vs. G1, G2, G3 (−0.6 vs. −0.1, −0.30, −0.32, p = 0.03) | ↑ * | |||
| Thomas (2012) [62] | Preterm (VLBW) (GA: 31.7 wk, Bwt: 1220 g) Recruitment: NICU | High and standard volume feeds G1: High volume feeds (300 mL/kg/d of EBM) G2: Standard volume feeds (200 mL/kg/d of EBM) | Feeding practices (G1 = 30 vs. G2 = 31) | |
| Feeding intolerance (n) (14 vs. 8, p = 0.076) | ↓◌ | |||
| Anthropometry (G1 = 30 vs. G2 = 31) | ||||
| When weight reached 1700 g | ||||
| Weight gain (g/kg/d) (24.9 vs. 18.7, p < 0.0001) | ↑ *** | |||
| Morbidity (G1 = 30 vs. G2 = 31) | ||||
| Sepsis (n) (1 vs. 0, p = 0.78) |  | |||
| NEC (n) (1 vs. 1, p = 0.98) |  | |||
| Salas (2018) [52] | Preterm (ELBW) (GA: 22–28 wk, Bwt: 833 g) Recruitment: NICU | Early and delayed feeding G1: Early progressive feeding without trophic feeding G2: Delayed progressive feeding after 4 d course of trophic feeding | Feeding Practices (G1 = 30 vs. G2 = 30) | |
| First 28 d after birth | ||||
| Full enteral feeding (d) (19 vs. 17, p = 0.02) | ↓ * | |||
| Anthropometry (G1 = 30 vs. G2 = 30) | ||||
| <10th percentile at 36 wk | ||||
| Weight (%) (50 vs. 62, p = 0.41) |  | |||
| Length (%) (54 vs. 69, p = 0.27) |  | |||
| Morbidity ((G1 = 30 vs. G2 = 30) | ||||
| NEC (%) (7 vs. 10, p = 1.00) |  | |||
| Mortality (G1 = 30 vs. G2 = 30) | ||||
| Death (%) (23 vs. 12, p = 0.37) |  | |||
| Zecca (2014) [63] | Preterm (LBW) (GA: 32–36 wk, Bwt: >1499 g) Recruitment: NICU | Proactive and standard feeds G1: Proactive feeding regimen (1st d of life 100 mL/kg/d of HM, day 2–130 mL/kg/d, day 3–165 mL/kg/d, day 4-discharge 200 mL/kg/d) G2: Standard Feeding Regimen (1st d of life 60 mL/kg/d of HM and gradually increased to 170 mL/kg/d by day 9) | Anthropometry (G1 = 36 vs. G2 = 36) | |
| At discharge (mean ± sd) | ||||
| Change in weight (z-score) (−0.29 ± 0.19 vs. −0.48 ± 0.29, p = 0.002) | ↑ ** | |||
| Change in length (z-score) (−0.19 ± 0.33 vs. −0.45 ± 0.50, p = 0.011) | ↑ ** | |||
| Other interventions (n = 6) | ||||
| Aly (2017) [64] | Preterm (VLBW) (GA: ≤34 wk, Bwt: 1300 g) Recruitment: NICU | Bee honey G1: 5 g, G2: 10 g, G3: 15 g G4: 0 g (control) | Anthropometry (G4 = 10 vs. G1 = 10, G2 = 10, G3 = 10) | |
| Compared with G4, other intervention groups (G1, G2, G3) demonstrated weight increase by 2 wk, p < 0.0001 | ↑ *** | |||
| Heon (2016) [65] | Mothers of EP infants Recruitment: NICU | Electric breast pump IG: Standard care + double electric breast pump + BM expression education and support intervention CG: Education and support | Feeding Practices (IG = 14 vs. CG = 19) | |
| wk 1–6 (mean) | ||||
| Volume of expressed BM (no difference between groups) |  | |||
| Slusher (2007) [69] | Mothers of preterm (GA: 31 wk) Recruitment: Teaching and mission hospital | Electric breast pump G1: Electric breast pump G2: Non-electric pedal Pump G3: Hand expression | Feeding practices (G1 = 21 vs. G2 = 24 vs. G3 = 18) | |
| Day 1–10 Maternal milk volume (mL) | ||||
| (578 ± 228 vs. 463 ± 302 vs. 323 ± 199) | ||||
| G1 vs. G3 (578 ± 228 vs. 463 ± 302, p < 0.01) | ↑ ** | |||
| G1 vs. G2 (NS), G2 vs. G3 (NS) |  | |||
| Kumar (2010) [66] | Preterm (VLBW) (GA: ≥32 wk, Bwt: >1250 ≤ 1600 g) Recruitment: Tertiary level Neonatal unit | Nasogastric and spoon feeds Trial 1 G1: NG feeding in hospital G2: Spoon feeding in hospital Trial 2 G1: Spoon feeding in hospital G2: Spoon feeding at home | Anthropometry (G1 vs. G2) | |
| Trial 1 (G1 = 36 vs. G2 = 36) | ||||
| Mean weight gain during transition to BF in hospital (1543.75 vs. 1578.47, p = 0.1793) |  | |||
| Trial 2 (G1 = 30 vs. G2 = 30) | ||||
| Mean weight gain till 4 wks of age | ||||
| (1827.88 vs. 1859.22, p = 0.5623) |  | |||
| Lau (2012) [67] | Preterm (VLBW) (GA: 28 wk, Bwt: 1103 g) Recruitment: NICU | Suckling and swallowing IG1: Non-nutritive sucking exercise (pacifier use) IG2: Swallowing exercise (placing a milk/formula bolus through syringe)CG: Standard care | Feeding Practices (IG1 = 25, IG2 = 22 vs. CG = 23) | |
| Start to independent oral feeding (mean ± SEM) | ||||
| Days of life at start of oral feeding | ||||
| IG1 vs. CG (44.4 ± 4.9 vs. 41.7 ± 3.6, p = 0.669) |  | |||
| IG2 vs. CG (43.5 ± 4.9 vs. 41.7 ± 3.6, p = 0.778) |  | |||
| Days of life at independent oral feeding | ||||
| IG1 vs. CG (62.3 ± 5.3 vs. 61.5 ± 4.6, p = 0.917) |  | |||
| IG2 vs. CG (57.1 ± 4.9 vs. 61.5 ± 4.6, p = 0.508) |  | |||
| Serrao (2018) [68] | Mothers of preterm (GA: 27–32 wk) Recruitment: Previously registered trial | Galactagogue From 3rd to 28th d after delivery G1: Silymarin-phosphatidylserine and galega (a daily single dose of 5 g of Piu`latte Plus MILTE) G2: Placebo (a daily single dose of 5 g of lactose) | Feeding Practices (G1 = 50 vs. G2 = 50) | |
| At 3 m | ||||
| Exclusive HM (n) (22 vs. 12, p < 0.05) | ↑ * | |||
| At 6 m | ||||
| Exclusive HM (n) (6 vs. 2, NS) |  | |||
 no effect, ◌ weak (0.05 < p < 0.1), * good (p < 0.05), ** strong (p < 0.01), *** very strong evidence (p < 0.001) Abbreviations BF = breastfeeding, BM = breastmilk, Bwt = birthweight, CA: corrected age, CEF = complete enteral feed, CG: control group, CM = cow milk, CNG: continuous nasogastric gavage, Conc. = concentrated, d: days, DM = donor milk, EBF: exclusive breastfeeding, EBM = expressed breastmilk, ELBW: extremely low birthweight, FM = fat mass, G = group, GA = gestational age, HM: human milk, HMF = human milk fortifier, IBG: intermittent bolus gavage, IG = intervention group, IV: intravenous, IVF = intravenous fluid, LBW: low birth weight, m = months, MEF: minimal enteral feed, MMV = maternal milk volume, nCPAP: nasal continuous positive airway pressure, NEC = necrotizing enterocolitis, NICU = neonatal intensive care unit, NNS: non nutritive sucking, NPO = none per orem, NS = non-significant, PMA: post menstrual age, SD = standard deviation, SE = standard error, SEM = standard error Mean, VLBW = very low birth weight, Wk = week
no effect, ◌ weak (0.05 < p < 0.1), * good (p < 0.05), ** strong (p < 0.01), *** very strong evidence (p < 0.001) Abbreviations BF = breastfeeding, BM = breastmilk, Bwt = birthweight, CA: corrected age, CEF = complete enteral feed, CG: control group, CM = cow milk, CNG: continuous nasogastric gavage, Conc. = concentrated, d: days, DM = donor milk, EBF: exclusive breastfeeding, EBM = expressed breastmilk, ELBW: extremely low birthweight, FM = fat mass, G = group, GA = gestational age, HM: human milk, HMF = human milk fortifier, IBG: intermittent bolus gavage, IG = intervention group, IV: intravenous, IVF = intravenous fluid, LBW: low birth weight, m = months, MEF: minimal enteral feed, MMV = maternal milk volume, nCPAP: nasal continuous positive airway pressure, NEC = necrotizing enterocolitis, NICU = neonatal intensive care unit, NNS: non nutritive sucking, NPO = none per orem, NS = non-significant, PMA: post menstrual age, SD = standard deviation, SE = standard error, SEM = standard error Mean, VLBW = very low birth weight, Wk = week References
- Victora, C.G.; de Onis, M.; Hallal, P.C.; Blössner, M.; Shrimpton, R. Worldwide timing of growth faltering: Revisiting implications for interventions. Pediatric 2010, 125, e473–e480. [Google Scholar] [CrossRef] [PubMed]
- Kerac, M.; Blencowe, H.; Grijalva-Eternod, C.S.; McGrath, M.; Shoham, J.; Cole, T.J.; Seal, A. Prevalence of wasting among under 6-month-old infants in developing countries and implications of new case definitions using WHO growth standards: A secondary data analysis. Arch. Dis. Child. 2011, 96, 1008–1013. [Google Scholar] [CrossRef]
- Kerac, M.; McGrath, M. Management of acute malnutrition in infants under 6 months of age. In The Biology of the First 1000 Days; CRC Press: Boca Raton, FL, USA, 2018; pp. 207–220. [Google Scholar]
- Management of Acute Malnutrition in Infants (MAMI) Project. 2010. Available online: https://reliefweb.int/sites/reliefweb.int/files/resources/8A7E77D26B35660F492576F70010D7DF-mami-report-complete.pdf (accessed on 16 August 2018).
- Global Strategy for Infant and Young Child Feeding. 2003. Available online: http://apps.who.int/iris/bitstream/handle/10665/42590/9241562218.pdf?sequence=1 (accessed on 16 August 2018).
- Victora, C.G.; Bahl, R.; Barros, A.J.D.; A França, G.V.; Horton, S.; Krasevec, J.; Murch, S.; Sankar, M.J.; Walker, N.; Rollins, N.C. Breastfeeding in the 21st century: Epidemiology, mechanisms, and lifelong effect. Lancet 2016, 387, 475–490. [Google Scholar] [CrossRef]
- Global Nutrition Targets 2025: Low Birth Weight Policy Brief. WHO, 2014. Available online: https://apps.who.int/iris/bitstream/handle/10665/149020/WHO_NMH_NHD_14.5_eng.pdf?ua=1 (accessed on 15 December 2018).
- Guidelines on Optimal Feeding of Low Birth-Weight Infants in Low—And Middle-Income Countries. World Health Organization, 2011. Available online: https://www.who.int/maternal_child_adolescent/documents/9789241548366.pdf?ua=1 (accessed on 10 December 2018).
- Balogun, O.O.; Dagvadorj, A.; Anigo, K.M.; Ota, E.; Sasaki, S. Factors influencing breastfeeding exclusivity during the first 6 months of life in developing countries: A quantitative and qualitative systematic review. Matern. Child. Nutr. 2015, 11, 433–451. [Google Scholar] [CrossRef] [PubMed]
- Kerac, M.; Frison, S.; Connell, N.; Page, B.; McGrath, M. Informing the management of acute malnutrition in infants aged under 6 months (MAMI): Risk factor analysis using nationally-representative demographic & health survey secondary data. PeerJ 2019, 6, e5848. [Google Scholar] [CrossRef]
- Flint, A.; New, K.; Davies, M.W. Cup feeding versus other forms of supplemental enteral feeding for newborn infants unable to fully breastfeed. Cochrane Database Syst. Rev. 2016, 2016, CD005092. [Google Scholar] [CrossRef]
- Henderson, G.; Anthony, M.Y.; McGuire, W. Formula milk versus maternal breast milk for feeding preterm or low birth weight infants. Cochrane Database Syst. Rev. 2007, 2007, CD002972. [Google Scholar] [CrossRef]
- Collins, C.T.; Gillis, J.; McPhee, A.J.; Suganuma, H.; Makrides, M. Avoidance of bottles during the establishment of breast feeds in preterm infants. Cochrane Database Syst. Rev. 2016, 10, CD005252. [Google Scholar] [CrossRef]
- Young, L.; Embleton, N.D.; McGuire, W. Nutrient-enriched formula versus standard formula for preterm infants following hospital discharge. Cochrane Database Syst. Rev. 2016, 12, CD004696. [Google Scholar] [CrossRef]
- Brown, J.V.; Embleton, N.D.; Harding, J.E.; McGuire, W. Multi-nutrient fortification of human milk for preterm infants. Cochrane Database Syst. Rev. 2016, 2016, CD000343. [Google Scholar] [CrossRef]
- Tan-Dy, C.R.; Ohlsson, A. Lactase treated feeds to promote growth and feeding tolerance in preterm infants. Cochrane Database Syst. Rev. 2013, 2013, CD004591. [Google Scholar] [CrossRef] [PubMed]
- Greene, Z.; O’Donnell, C.P.; Walshe, M. Oral stimulation for promoting oral feeding in preterm infants. Cochrane Database Syst. Rev. 2016, 9, CD009720. [Google Scholar] [CrossRef] [PubMed]
- Premji, S.S.; Chessell, L. Continuous nasogastric milk feeding versus intermittent bolus milk feeding for premature infants less than 1500 grams. Cochrane Database Syst. Rev. 2011, 2011, CD001819. [Google Scholar] [CrossRef] [PubMed]
- Basuki, F.; Hadiati, D.R.; Turner, T.; McDonald, S.; Hakimi, M. Dilute versus full strength formula in exclusively formula-fed preterm or low birth weight infants. Cochrane Database Syst. Rev. 2013, 2013, CD007263. [Google Scholar] [CrossRef] [PubMed]
- Amissah, E.A.; Brown, J.; Harding, J.E. Protein supplementation of human milk for promoting growth in preterm infants. Cochrane Database Syst. Rev. 2018, 6, CD000433. [Google Scholar] [CrossRef] [PubMed]
- Amissah, E.A.; Brown, J.; Harding, J.E. Fat supplementation of human milk for promoting growth in preterm infants. Cochrane Database Syst. Rev. 2018, 6, CD000341. [Google Scholar] [CrossRef]
- Guideline: Updates on the Management of Severe Acute Malnutrition in Infants and Children. World Health Organisation, 2013. Available online: http://apps.who.int/iris/bitstream/handle/10665/95584/9789241506328_eng.pdf?sequence=1 (accessed on 2 September 2018).
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses. PRISMA Statement 2009, 339, b2535. [Google Scholar] [CrossRef]
- Abouelfettoh, A.M.; Dowling, D.A.; Dabash, A.S.; Elguindy, S.R.; Seoud, I.A. Cup verus bottle feeding for hospitalized late preterm infants in Egypt: A quasi-experimental study. Int. Breastfeed. J. 2008, 3, 27. [Google Scholar] [CrossRef]
- Yilmaz, G.; Caylan, N.; Karacan Can, D.; Bodur, İ.; Gokcay, G. Effect of Cup Feeding and Bottle Feeding on Breastfeeding in Late Preterm Infants: A Randomized Controlled Study. J. Hum. Lact. 2014, 30, 174–179. [Google Scholar] [CrossRef]
- Abrams, S.A.; Schanler, R.J.; Lee, M.L.; Rechtman, D.J. Greater mortality and morbidity in extremely preterm infants fed a diet containing cow milk protein products. Breastfeed. Med. Off. J. Acad. Breastfeed. Med. 2014, 9, 281–295. [Google Scholar] [CrossRef]
- Alan, S.; Atasay, B.; Cakir, U.; Yildiz, D.; Kiliç, A.; Kahvecioglu, D.; Erdeve, O.; Arsan, S. An intention to achieve better postnatal in-hospital-growth for preterm infants: Adjustable protein fortification of human milk. Early Hum. Dev. 2013, 89, 1017–1023. [Google Scholar] [CrossRef] [PubMed]
- Amesz, E.M.; Schaafsma, A.; Cranendonk, A.; Lafeber, H.N. Optimal growth and lower fat mass in preterm infants fed a protein-enriched postdischarge formula. J. Pediatr. Gastroenterol. Nutr. 2010, 50, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Arslanoglu, S.; Moro, G.E.; Ziegler, E.E. Adjustable fortification of human milk fed to preterm infants: Does it make a difference? J. Perinatol. Off. J. Calif. Perinat. Assoc. 2006, 26, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Berseth, C.L.; Harris, C.L.; Wampler, J.L.; Hoffman, D.R.; Diersen-Schade, D.A. Liquid human milk fortifier significantly improves docosahexaenoic and arachidonic acid status in preterm infants. Prostaglandins Leukot. Essent. Fat. Acids 2014, 91, 97–103. [Google Scholar] [CrossRef]
- Bhat, B.A.; Gupta, B. Effects of human milk fortification on morbidity factors in very low birth weight infants. Ann. Saudi Med. 2001, 21, 292–305. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Clarke, S.E.; Evans, S.; Macdonald, A.; Davies, P.; Booth, I.W. Randomized comparison of a nutrient-dense formula with an energy-supplemented formula for infants with faltering growth. J. Hum. Nutr. Diet. 2007, 20, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Cristofalo, E.A.; Schanler, R.J.; Blanco, C.L.; Sullivan, S.; Trawoeger, R.; Kiechl-Kohlendorfer, U.; Dudell, G.; Rechtman, D.J.; Lee, M.L.; Lucas, A.; et al. Randomized trial of exclusive human milk versus preterm formula diets in extremely premature infants. J. Pediatr. 2013, 163, 1592–1595.e1. [Google Scholar] [CrossRef]
- Erasmus, H.D.; Ludwig-Auser, H.M.; Paterson, P.G.; Sun, D.; Sankaran, K. Enhanced weight gain in preterm infants receiving lactase-treated feeds: A randomized, double-blind, controlled trial. J. Pediatr. 2002, 141, 532–537. [Google Scholar] [CrossRef]
- Flaherman, V.J.; Aby, J.; Burgos, A.E.; Lee, K.A.; Cabana, M.D.; Newman, T.B. Effect of early limited formula on duration and exclusivity of breastfeeding in at-risk infants: An RCT. Pediatrics 2013, 131, 1059–1065. [Google Scholar] [CrossRef]
- Florendo, K.N.; Bellflower, B.; van, Z.; Cooke, R.J. Growth in preterm infants fed either a partially hydrolyzed whey or an intact casein/whey preterm infant formula. J. Perinatol. 2009, 29, 106–111. [Google Scholar] [CrossRef]
- Gathwala, G.; Chawla, M.; Gehlaut, V.S.; Gathwala, G.; Chawla, M.; Gehlaut Veena, S. Fortified human milk in the small for gestational age neonate. Indian J. Pediatr. 2007, 74, 815–818. [Google Scholar] [CrossRef] [PubMed]
- Hair, A.B.; Blanco, C.L.; Moreira, A.; Hawthorne, K.; Lee, M.L.; Rechtman, D.J.; Abrams, S.A. Randomized trial of human milk cream as a supplement to standard fortification of an exclusive human milk-based diet in infants 750-1250 g birth weight. J. Pediatr. 2014, 165, 915–920. [Google Scholar] [CrossRef] [PubMed]
- Kanmaz, H.G.; Mutlu, B. Human Milk Fortification with Differing Amounts of Fortifier and Its Association with Growth and Metabolic Responses in Preterm Infants. J. Hum. Lact. 2013, 29, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Chan, G.; Schanler, R.; Groh-Wargo, S.; Bloom, B.; Dimmit, R.; Williams, L.; Baggs, G.; Barrett-Reis, B. Growth and Tolerance of Preterm Infants Fed a New Extensively Hydrolyzed Liquid Human Milk Fortifier. J. Pediatr. Gastroenterol. Nutr. 2015, 61, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Kim Eun, J.; Lee Na, M.; Chung, S.-H. A retrospective study on the effects of exclusive donor human milk feeding in a short period after birth on morbidity and growth of preterm infants during hospitalization. Medicine 2017, 96, e7970. [Google Scholar] [CrossRef]
- Kumar, N.; Monga, R.; Sampath, V.; Ehrhart, B. Prospective Comparison of Enfamil and Similac Liquid Human Milk Fortifier on Clinical Outcomes in Premature Infants. Am. J. Perinatol. 2017, 34, 1411–1416. [Google Scholar] [CrossRef]
- Lok, K.Y.W.; Chau, P.H.; Fan, H.S.L.; Chan, K.M.; Chan, B.H.; Fung, G.P.C.; Tarrant, M. Increase in Weight in Low Birth Weight and Very Low Birth Weight Infants Fed Fortified Breast Milk versus Formula Milk: A Retrospective Cohort Study. Nutrients 2017, 9, 520. [Google Scholar] [CrossRef] [PubMed]
- Lucas, A.; Bishop, N.J.; King, F.J.; Cole, T.J. Randomised trial of nutrition for preterm infants after discharge. Arch. Dis. Child. 1992, 67, 324–327. [Google Scholar] [CrossRef]
- Manea, A.; Boia, M.; Iacob, D.; Dima, M.; Iacob Radu, E. Benefits of early enteral nutrition in extremely low birth weight infants. Singap. Med. J. 2016, 57, 616–618. [Google Scholar] [CrossRef]
- Morlacchi, L.; Mallardi, D.; Giannì, M.L.; Roggero, P.; Amato, O.; Pasqua, P.; Consonni, D.; Mosca, F. Is targeted fortification of human breast milk an optimal nutrition strategy for preterm infants? An interventional study. J. Transl. Med. 2016, 14, 195. [Google Scholar] [CrossRef]
- Morlacchi, L.; Roggero, P.; Giannì, M.L.; Bracco, B.; Porri, D.; Battiato, E.; Menis, C.; Liotto, N.; Mallardi, D.; Mosca, F. Protein use and weight-gain quality in very-low-birth-weight preterm infants fed human milk or formula. Am. J. Clin. Nutr. 2018, 107, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Morley, R.; Lucas, A. Randomized diet in the neonatal period and growth performance until 7.5-8 y of age in preterm children. Am. J. Clin. Nutr. 2000, 71, 822–828. [Google Scholar] [CrossRef] [PubMed]
- Moya, F.; Sisk, P.M.; Walsh, K.R.; Berseth, C.L. A new liquid human milk fortifier and linear growth in preterm infants. Pediatr 2012, 130, e928–e935. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, D.L.; Gibbins, S.; Kiss, A.; Bando, N.; Brennan-Donnan, J.; Ng, E.; Campbell, D.M.; Vaz, S.; Fusch, C.; Asztalos, E.; et al. Effect of Supplemental Donor Human Milk Compared With Preterm Formula on Neurodevelopment of Very Low-Birth-Weight Infants at 18 Months: A Randomized Clinical Trial. J. Am. Med. Assoc. 2016, 316, 1897–1905. [Google Scholar] [CrossRef] [PubMed]
- Porcelli, P.; Schanler, R.; Greer, F.; Chan, G.; Gross, S.; Mehta, N.; Spear, M.; Kerner, J.; Euler, A.R. Growth in human milk-Fed very low birth weight infants receiving a new human milk fortifier. Ann. Nutr. Metab. 2000, 44, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Salas, A.A.; Li, P.; Parks, K.; Lal, C.V.; Martin, C.R.; Carlo, W.A. Early progressive feeding in extremely preterm infants: A randomized trial. Am. J. Clin. Nutr. 2018, 107, 365–370. [Google Scholar] [CrossRef]
- Taheri Peymaneh, A.; Sajjadian, N.; Fargi Marzieh, A.; Shariat, M. Is early breast milk fortification more effective in preterm infants? A clinical trial: Early and late fortification. J. Perinat. Med. 2017, 45, 953–957. [Google Scholar] [CrossRef]
- Tillman, S.; Brandon, D.H.; Silva, S.G. Evaluation of human milk fortification from the time of the first feeding: Effects on infants of less than 31 weeks gestational age. J. Perinatol. Off. J. Calif. Perinat. Assoc. 2012, 32, 525–531. [Google Scholar] [CrossRef]
- Willeitner, A.; Anderson, M.; Lewis, J. Highly Concentrated Preterm Formula as an Alternative to Powdered Human Milk Fortifier: A Randomized Controlled Trial. J. Pediatr. Gastroenterol. Nutr. 2017, 65, 574–578. [Google Scholar] [CrossRef]
- Worrell, L.A.; Thorp, J.W.; Tucker, R.; McKinley, L.T.; Chen, J.; Chng, Y.-M.; Vohr, B.R. The effects of the introduction of a high-nutrient transitional formula on growth and development of very-low-birth-weight infants. J. Perinatol. Off. J. Calif. Perinat. Assoc. 2002, 22, 112–119. [Google Scholar] [CrossRef][Green Version]
- Akintorin, S.M.; Kamat, M.; Pildes, R.S.; Kling, P.; Andes, S.; Hill, J.; Pyati, S. A prospective randomized trial of feeding methods in very low birth weight infants. Pediatrics 1997, 100, E4. [Google Scholar] [CrossRef] [PubMed]
- Bora, R.; Murthy, N.B. In resource limited areas complete enteral feed in stable very low birth weight infants (1000–1500 g) started within 24 h of life can improve nutritional outcome. J. Matern. Fetal Neonatal Med. 2017, 30, 2572–2577. [Google Scholar] [CrossRef]
- Colaizy, T.T.; Carlson, S.; Saftlas, A.F.; Morriss, F.H., Jr. Growth in VLBW infants fed predominantly fortified maternal and donor human milk diets: A retrospective cohort study. BMC Pediatr. 2012, 12, 124. [Google Scholar] [CrossRef] [PubMed]
- Kliethermes, P.A.; Cross, M.L.; Lanese, M.G.; Johnson, K.M.; Simon, S.D. Transitioning preterm infants with nasogastric tube supplementation: Increased likelihood of breastfeeding. J. Obs. Gynecol. Neonatal Nurs. 1999, 28, 264–273. [Google Scholar] [CrossRef]
- Mosqueda, E.; Sapiegiene, L.; Glynn, L.; Wilson-Costello, D.; Weiss, M. The early use of minimal enteral nutrition in extremely low birth weight newborns. J. Perinatol. 2008, 28, 264–269. [Google Scholar] [CrossRef]
- Thomas, N.; Cherian, A.; Santhanam, S.; Jana, A.K. A randomized control trial comparing two enteral feeding volumes in very low birth weight babies. J. Trop. Pediatr. 2012, 58, 55–58. [Google Scholar] [CrossRef][Green Version]
- Zecca, E.; Costa, S.; Barone, G.; Giordano, L.; Zecca, C.; Maggio, L. Proactive enteral nutrition in moderately preterm small for gestational age infants: A randomized clinical trial. J. Pediatr. 2014, 165, 1135–1139.e1. [Google Scholar] [CrossRef]
- Aly, H.; Said, R.N.; Wali, I.E.; Elwakkad, A.; Soliman, Y.S.; Awad, A.R.; Shawky, M.A.; Abu Alam, M.S.; Mohamed, M.A. Medically Graded Honey Supplementation Formula to Preterm Infants as a Prebiotic: A Randomized Controlled Trial. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 966–970. [Google Scholar] [CrossRef]
- Héon, M.; Goulet, C.; Garofalo, C.; Nuyt Anne, M.; Levy, E. An Intervention to Promote Breast Milk Production in Mothers of Preterm Infants. West. J. Nurs. Res. 2016, 38, 529–552. [Google Scholar] [CrossRef]
- Kumar, A.; Dabas, P.; Singh, B. Spoon feeding results in early hospital discharge of low birth weight babies. J. Perinatol. Off. J. Calif. Perinat. Assoc. 2010, 30, 209–217. [Google Scholar] [CrossRef][Green Version]
- Lau, C.; Smith, E.O. Interventions to improve the oral feeding performance of preterm infants. Acta Paediatr. 2012, 101, e269–e274. [Google Scholar] [CrossRef] [PubMed]
- Serrao, F.; Corsello, M.; Romagnoli, C.; D’Andrea, V.; Zecca, E. The Long-Term Efficacy of a Galactagogue Containing Sylimarin-Phosphatidylserine and Galega on Milk Production of Mothers of Preterm Infants. Breastfeed. Med. 2018, 13, 67–69. [Google Scholar] [CrossRef] [PubMed]
- Slusher, T.; Slusher, I.L.; Biomdo, M.; Bode-Thomas, F.; Curtis, B.A.; Meier, P. Electric breast pump use increases maternal milk volume in African nurseries. J. Trop. Pediatr. 2007, 53, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Berseth, C.L.; Van Aerde, J.E.; Gross, S.; Stolz, S.I.; Harris, C.L.; Hansen, J.W. Growth, efficacy, and safety of feeding an iron-fortified human milk fortifier. Pediatrics 2004, 114, e699–e706. [Google Scholar] [CrossRef] [PubMed]
- Shah Sanket, D.; Dereddy, N.; Jones Tamekia, L.; Dhanireddy, R.; Talati Ajay, J. Early versus Delayed Human Milk Fortification in Very Low Birth Weight Infants-A Randomized Controlled Trial. J. Pediatr. 2016, 174, 126–131.e1. [Google Scholar] [CrossRef] [PubMed]
- Quigley, M.; Embleton, N.D.; McGuire, W. Formula versus donor breast milk for feeding preterm or low birth weight infants. Cochrane Database Syst. Rev. 2018, 6, CD002971. [Google Scholar] [CrossRef]
- Smith, H.A. Formula supplementation and the risk of cow’s milk allergy. Br. J. Midwifery 2012, 20, 345–350. [Google Scholar] [CrossRef]
- Bitton, A.; Ratcliffe, H.L.; Veillard, J.H.; Kress, D.H.; Barkley, S.; Kimball, M.; Secci, F.; Wong, E.; Basu, L.; Taylor, C.; et al. Primary health care as a foundation for strengthening health systems in low-and middle-income countries. J. Gen. Intern. Med. 2017, 32, 566–571. [Google Scholar] [CrossRef]
- Rowe, A.K.; Rowe, S.Y.; Peters, D.H.; Holloway, K.A.; Chalker, J.; Ross-Degnan, D. Effectiveness of strategies to improve health-care provider practices in low-income and middle-income countries: A systematic review. Lancet Glob. Health 2018, 6, e1163–e1175. [Google Scholar] [CrossRef]
- Special Section: Management of at Risk Mothers and Infants under 6 Months (MAMI). 2018. Available online: https://www.ennonline.net/attachments/2989/FEX_58_MAMI_special_section.pdf (accessed on 20 September 2018).
- Beck, K.; Kirk, C.M.; Bradford, J.; Mutaganzwa, C.; Nahimana, E.; Bigirumwami, O. The Paediatric Development Clinic: A model to improve medical, nutritional and developmental outcomes for high-risk children aged under-five in rural Rwanda. Field Exch. 2018, 58, 59. [Google Scholar]
- Kueter, A.M.; Burrell, A.; Butler, S.; Sarwar, M.; Rahaman, H. Piloting the C-MAMI approach in the Rohingya response in Bangladesh. Field Exch. 2018, 58, 51. [Google Scholar]
- Burrell, A.; Barthorp, H. GOAL’s experiences of management of at-risk mothers and infants (MAMI) programming in Ethiopia. Field Exch. 2020, 62, 30. [Google Scholar]
- Mandy, M.; Nyirenda, M. Developmental Origins of Health and Disease: The relevance to developing nations. Int. Health 2018, 10, 66–70. [Google Scholar] [CrossRef]
- Rowe, S.; Alexander, N.; Clydesdale, F.; Applebaum, R.; Atkinson, S.; Black, R.; Dwyer, J.; Hentges, E.; Higley, N.; Lefevre, M.; et al. Funding food science and nutrition research: Financial conflicts and scientific integrity. Nutr. Rev. 2009, 67, 264–272. [Google Scholar] [CrossRef] [PubMed]
| Author (Year) | Country | Study Design | Population | Sample Size * | Outcomes | |||
|---|---|---|---|---|---|---|---|---|
| Feeding | Anthropometry | Morbidity | Mortality | |||||
| Cup Feeding Interventions (2) | ||||||||
| Abouelfettoh (2008) [24] | Egypt | QE | Preterm (LBW) | 60 | ✓ | |||
| Yilmaz (2014) [25] | Turkey | RCT | Preterm (VLBW) | 607 | ✓ | ✓ | ||
| Formula Fortification/Supplementation Interventions (31) | ||||||||
| Abrams (2014) [26] | USA | RCT | Preterm (VLBW) | 260 | ✓ | ✓ | ✓ | |
| Alan (2013) [27] | Turkey | PO | Preterm (VLBW) | 58 | ✓ | |||
| Amesz (2010) [28] | Netherlands | RCT | Preterm (VLBW) | 102 | ✓ | |||
| Arslanoglu (2006) [29] | Italy | RCT | Preterm (LBW, VLBW, ELBW) | 34 | ✓ | |||
| Berseth (2004) [30] | USA | RCT | Preterm (VLBW) | 181 | ✓ | |||
| Bhat (2001) [31] | Oman | RCT | Preterm (VLBW) | 100 | ✓ | |||
| Clarke (2007) [32] | USA | RCT | Faltering growth | 60 | ✓ | |||
| Cristofalo (2013) [33] | USA | RCT | Preterm (ELBW) | 53 | ✓ | ✓ | ✓ | |
| Erasmus (2002) [34] | Canada | RCT | Preterm (VLBW) | 130 | ✓ | |||
| Flaherman (2013) [35] | USA | RCT | Term (weight loss) | 40 | ✓ | ✓ | ||
| Florendo (2009) [36] | USA | RCT | Preterm (VLBW) | 80 | ✓ | |||
| Gathwala (2007) [37] | India | RCT | Term SGA (LBW) | 65 | ✓ | |||
| Hair (2014) [38] | USA | RCT | Preterm (ELBW) | 78 | ✓ | |||
| Kanmaz (2012) [39] | Turkey | RCT | Preterm (ELBW) | 84 | ✓ | |||
| Kim (2015) [40] | USA | RCT | Preterm (VLBW) | 147 | ✓ | |||
| Kim (2017) [41] | South Korea | Cohort-R | Preterm (ELBW) | 132 | ✓ | |||
| Kumar (2017) [42] | USA | RCT | Preterm (ELBW) | 31 | ✓ | |||
| Lok (2017) [43] | Hong Kong | Cohort-R | Preterm (LBW, VLBW) | 642 | ✓ | |||
| Lucas (1992) [44] | UK | RCT | Preterm (VLBW) | 32 | ✓ | |||
| Manea (2016) [45] | Romania | QE | Preterm (ELBW) | 34 | ✓ | ✓ | ||
| Morlacchi (2016) [46] | Italy | QE | Preterm (VLBW) | 20 | ✓ | |||
| Morlacchi (2018) [47] | Italy | PO | Preterm (VLBW) | 32 | ✓ | |||
| Morley (2000) [48] | UK | RCT | Preterm (LBW) | 96 | ✓ | |||
| Moya (2012) [49] | USA | RCT | Preterm (ELBW) | 150 | ✓ | ✓ | ||
| O’Connor (2016) [50] | Canada | RCT | Preterm (ELBW) | 363 | ✓ | ✓ | ✓ | |
| Porcelli (1999) [51] | USA | RCT | Preterm (VLBW, ELBW) | 64 | ✓ | ✓ | ||
| Shah (2016) [52] | USA | RCT | Preterm (VLBW) | 100 | ✓ | ✓ | ||
| Taheri (2016) [53] | Iran | RCT | Preterm (VLBW) | 72 | ✓ | ✓ | ✓ | |
| Tillman (2012) [54] | USA | Pre-post | Preterm (VLBW) | 95 | ✓ | |||
| Willeitner (2017) [55] | USA | RCT | Preterm (VLBW, ELBW) | 70 | ✓ | ✓ | ✓ | |
| Worrell (2002) [56] | USA | Cohort-R | Preterm (VLBW) | 180 | ✓ | |||
| Enteral Feed Interventions (8) | ||||||||
| Akintorin (1997) [57] | USA | RCT | Preterm (VLBW, ELBW) | 80 | ✓ | ✓ | ||
| Bora (2017) [58] | India | RCT | Preterm (VLBW) | 107 | ✓ | ✓ | ✓ | |
| Colaizy (2012) [59] | USA | RCT | Preterm (ELBW) | 171 | ✓ | |||
| Kliethermes (1999) [60] | USA | RCT | Preterm (LBW) | 84 | ✓ | |||
| Mosqueda (2008) [61] | USA | RCT | Preterm (ELBW) | 84 | ✓ | ✓ | ||
| Salas (2018) [52] | USA | RCT | Preterm (ELBW) | 60 | ✓ | ✓ | ✓ | ✓ |
| Thomas (2012) [62] | India | RCT | Preterm (VLBW) | 61 | ✓ | ✓ | ✓ | |
| Zecca (2014) [63] | Italy | RCT | Preterm (LBW) | 72 | ✓ | |||
| Other Interventions (6) | ||||||||
| Aly (2017) [64] | Egypt | RCT | Preterm (VLBW) | 40 | ✓ | |||
| Heon (2016) [65] | Canada | RCT | Mothers of extremely preterm | 40 | ✓ | |||
| Kumar (2010) [66] | India | RCT | Preterm (VLBW) | 144 | ✓ | |||
| Lau (2012) [67] | USA | RCT | Preterm (VLBW) | 70 | ✓ | |||
| Serrao (2018) [68] | Italy | RCT | Mothers of preterm | 100 | ✓ | |||
| Slusher (2007) [69] | Nigeria and Kenya | RCT | Mothers of preterm | 65 | ✓ | |||
| Author (Year) | Population Characteristics | Intervention | Outcomes | |||
|---|---|---|---|---|---|---|
| Feeding Practices | Anthropometry | Morbidity | Mortality | |||
| Cup Feeding Interventions (n = 2) | ||||||
| Abouelfettoh (2008) [24] | Preterm (LBW) (GA: 35.13 wk, Bwt: 2150 g) | Cup feeding IG: Cup feeding vs. CG: Bottle feeding | Positive | |||
| Yilmaz (2014) [25] | Preterm (VLBW) (GA: 32–35 wk, Bwt: 1543 g) | Cup feeding G1: Cup feeding vs. G2: Bottle feeding | Positive | No effect | ||
| Formula Fortification/Supplementation Interventions (31) | ||||||
| Abrams (2014) [26] | Preterm (VLBW) (Bwt: <1250 g) | Bovine/cow milk G1: Cow milk (CM formula + CM based fortifier) G2: Human milk (HM (mother’s own/donor milk) + HM based fortifier) | No effect | Negative | Negative | |
| Cristofalo (2013) [33] | Preterm (ELBW) (GA: <27 wk, Bwt: 989 g) | Bovine/cow milk G1: Exclusive appropriately fortified HM G2: Bovine milk-based preterm formula | Negative | Negative | No effect | |
| Alan (2013) [27] | Preterm (VLBW) (GA: ≤32 wk, Bwt: ≤1500 g) | Protein supplementation IG: HM with extra protein supplementation CG: HM with a standard fortification | Mixed | |||
| Arslanoglu (2006) [29] | Preterm (LBW, VLBW, ELBW) (GA: 26–34 wk, Bwt: 600–1750 g) | Protein supplementation G1: HMF (with additional protein) G2: HM with HMF (standard amount) | Mixed | |||
| Florendo (2009) [36] | Preterm (VLBW) (GA: ≤32 wk, Bwt: 1200 g) | Protein supplementation IG: Partially hydrolysed whey protein CG: Non-hydrolysed whey casein preterm infant formula | No effect | |||
| Kim (2015) [40] | Preterm (VLBW) (GA: ≤33 wk, Bwt: 1174 g) | Protein supplementation IG: Conc. HMF containing liquid extensively hydrolysed protein CG: Powdered intake protein HMF | No effect | |||
| Erasmus (2002) [34] | Preterm (VLBW) (GA: 26–34 wk, Bwt: 1407 g) | Lactase fortification IG: Fortified HM or preterm formula treated with lactaid drops (Lactase) CG: Untreated fortified HM or preterm formula | No effect | |||
| Gathwala (2007) [37] | Term SGA (LBW) (GA: 40 wk, Bwt: 2000 g) | Lactase fortification IG: HM fortified with Lactodex-HMF vs. CG: Only BM | Positive | |||
| Berseth (2004) [70] | Preterm (VLBW) (GA: ≤33 wk, Bwt: 1180 g) | Iron fortification G1: HMF (iron fortified) vs. G2: HMF (standard) | No effect | |||
| Willeitner (2017) [55] | Preterm (VLBW, ELBW) (GA: 29 wk, Bwt: 500–1499 g) | Iron fortification IG: HM fortification (Concentrated preterm formula 30 Similac Special Care 30 with iron) CG: Standard Powdered HMF (Similac HMF) | No effect | No effect | No effect | |
| Clarke (2007) [32] | Faltering growth (GA: 2–31 wk) | Nutrient fortification G1: Nutrient-dense formula G2: Energy-supplemented formula | No effect | |||
| Morlacchi (2016) [46] | Preterm (VLBW) (GA: <32 wk, Bwt: 1255 g) | Nutrient fortification G1: Macronutrient fortification G2: Standardised fortification | Positive | |||
| Worrell (2002) [56] | Preterm (VLBW) (GA: 27 ± 3 wk, Bwt: 925 g) | Nutrient fortification G1: Transitional formula (higher amounts of protein, Ca, p, and several trace minerals and vitamins) G2: Standard formula | No effect | |||
| Hair (2014) [38] | Preterm (ELBW) (GA: 28 wk, Bwt: 970 g) | Cream supplementation IG: HM derived cream supplement CG: Mothers own milk or donor’s HM derived fortifier | Positive | |||
| Shah (2016) [71] | Preterm (VLBW) (GA: 27 wk, Bwt: <1500 g) | Early and delayed fortification G1: Early fortification (20 mL/kg/d of HM feeds) G2: Delayed fortification (100 mL/kg/d of HM feeds) | No effect | No effect | ||
| Taheri (2016) [53] | Preterm (VLBW) (GA: 28–34 wk, Bwt: 1294 g) | Early and delayed fortification G1: Early fortification (1st feeding) G2: Late fortification (BF volume reached 75 mL/kg/d) | No effect | No effect | No effect | |
| Tillman (2012) [54] | Preterm (VLBW) (GA: <31 wk, Bwt: 1123 g) | Early and delayed fortification Fortification with Enfamil, powdered HM fortifier G1: Early BM fortification (1st feed) G2: Delayed fortification (when volume reached 50–80 mL/kg/d) | No effect | |||
| Bhat (2001) [31] | Preterm (VLBW) (GA: 26–34 wk, Bwt: 1242 g) | Human milk fortification IG: Fortified HM vs. CG: HM only | Positive | |||
| Morlacchi (2018) [47] | Preterm (VLBW) (GA: <32 wk, Bwt: <1500 g) | Human milk fortification and formula G1: Fortified HM vs. G2: Preterm formula | Mixed | |||
| Kim (2017) [41] | Preterm (ELBW) (GA: <32 wk, Bwt: 1087 g) | Human milk and formula G1: Donor human milk vs. G2: Preterm formula | No effect | |||
| Lok (2017) [43] | Preterm (LBW, VLBW) (GA: <37 wk, Bwt: <2200 g, VLBW: <1500 g, LBW: ≥1500 g and <2200 g) | Human milk and formula Category 1: LBW, Category 2: VLBW; Both the groups further divided into G1: Any BM (human/donor) vs. G2: No BM (infant formula) | No effect | |||
| Manea (2016) [45] | Preterm (ELBW) (GA: 25–33 wk, Bwt: <1000 g) | Human milk and formula G1: Human BM vs. G2: Formula | Positive | Positive | ||
| Morley (2000) [48] | Preterm (LBW) (GA: ≤31 wk, Bwt: <1850 g) | Human milk and formula Category 1: As sole diet, Category 2: As supplement to HM G1: Banked donor milk vs. G2: Preterm formula | No effect | |||
| O’Connor (2016) [50] | Preterm (ELBW) (GA: 27.5 wk, Bwt: 995 g) | Human milk and formula G1: Donor milk vs. G2: Preterm formula | No effect | Positive | No effect | |
| Moya (2012) [49] | Preterm (ELBW) (GA: ≤30 wk, Bwt: 1000 g) | Liquid and powdered fortification G1: Liquid HMF vs. G2: Powdered HMF | Positive | No effect | ||
| Kanmaz (2012) [39] | Preterm (ELBW) (GA: 28 wk, Bwt: 1092 g) | Different levels of fortification G1: Standard fortification (1.2 g HMF + 30 mL HM) G2: Moderate fortification (1.2 g HMF + 25 mL HM) G3: Aggressive fortification (1.2 g HMF + 20 mL HM) | No effect | |||
| Porcelli (1999) [51] | Preterm (VLBW, ELBW) (GA: 25–32 wk, Bwt: 600–1500 g) | Different fortifier G1: Test HMF (1 g of protein/100 mL of supplemented milk, 85% glucose polymers, 15% lactose, and calcium, phosphorus, sodium, copper) G2: Reference HMF (60% whey protein and 40% casein, 75% glucose polymers, 25% lactose and calcium, phosphorus, sodium, and copper) | Positive | Mixed | ||
| Kumar (2017) [42] | Preterm (ELBW) (GA: 27 wk, Bwt: 993 g) | Different formula G1: Similac liquid HMF G2: Enfamil liquid HMF | Positive | |||
| Amesz (2010) [28] | Preterm (VLBW) (GA: ≤32 wk, Bwt: 1338 g) | Different formulas G1: Post discharge formula G2: Term formula G3: HM fortified formula | No effect | |||
| Lucas (1992) [44] | Preterm (VLBW) (GA: 31 wk, Bwt: 1475 g) | Different formula G1: Follow-on preterm formula G2: Standard term formula | Positive | |||
| Flaherman (2013) [35] | Term infants ( >37 wk who lost ≥5% Bwt before 36 h of age) | Continued EBF and early limited formula IG: Early limited formula (10 mL using feeding syringe) CG: Continued EBF | Positive | Positive | ||
| Enteral feed Interventions (8) | ||||||
| Akintorin (1997) [57] | Preterm (VLBW, ELBW) (GA: 28 wk, Bwt: 700–1250 g) Category 1: 700–1000 g Category 2: 1001–1250 g | Continuous nasogastric gavage(CNG) and intermittent bolus gavage (IBG) feeds G1: CNG vs. IBG G2: CNG vs. IBG | No effect | No effect | ||
| Mosqueda (2008) [61] | Preterm (ELBW) (GA: 26 wk, Bwt:760 g) | Intravenous and nasogastric feeds G1: Intravenous alimentation alone (NPO (none per orem)) G2: Small boluses of nasogastric feedings | No effect | No effect | ||
| Kliethermes (1999) [60] | Preterm (LBW) (GA: ≤32 wk, Bwt: 1685 g) | Nasogastric and bottle feeds G1: Nasogastric tube vs. G2: Bottle feeding | Positive | |||
| Bora (2017) [58] | Preterm (VLBW) (GA: 35 wk, Bwt: 1357 g) | Complete and minimal feeds G1: Complete enteral feed (CEF) with EBM G2: Minimal enteral feed (MEF) with IVF | No effect | Positive | No effect | |
| Colaizy (2012) [59] | Preterm (ELBW) (GA: 27 wk, Bwt: 889 g) | Different levels of feeds G1: <25%, G2: 25–50%, G3: 50–75% vs. G4: >75% | Positive | |||
| Thomas (2012) [62] | Preterm (VLBW) (GA: 31.7 wk, Bwt: 1220 g) | High and standard volume feeds G1: High volume feeds (300 mL/kg/d of EBM) G2: Standard volume feeds (200 mL/kg/d of EBM) | Negative | Positive | No effect | |
| Salas (2018) [52] | Preterm (ELBW) (GA: 22–28 wk, Bwt: 833 g) | Early and delayed feeding G1: Early progressive feeding without trophic feeding G2: Delayed progressive feeding after 4 d course of trophic feeding | Positive | No effect | No effect | No effect |
| Zecca (2014) [63] | Preterm (LBW) (GA: 32–36 wk, Bwt: >1499 g) | Proactive and standard feeds G1: Proactive Feeding Regimen G2: Standard Feeding Regimen | Positive | |||
| Other Interventions (n = 6) | ||||||
| Aly (2017) [64] | Preterm (VLBW) (GA: ≤34 wk, Bwt: 1300 g) | Bee honey G1: 5 g, G2: 10 g, G3: 15 g vs. G4: 0 g (control) | Positive | |||
| Heon (2016) [65] | Mothers of extremely preterm infants | Electric breast pump IG: Standard care + double electric breast pump + BM expression education and support intervention CG: Education and support | No effect | |||
| Slusher (2007) [69] | Mothers of preterm (GA: 31 wk) | Electric breast pump G1: Electric breast pump G2: Non-electric pedal Pump G3: Hand expression | Mixed | |||
| Kumar (2010) [66] | Preterm (VLBW) (GA: ≥32 wk, Bwt: >1250 ≤ 1600 g) | Nasogastric and spoon feeds Trial 1 G1: NG feeding in hospital vs. G2: Spoon feeding in hospital Trial 2 G1: Spoon feeding in hospital vs. G2: Spoon feeding at home | No effect | |||
| Lau (2012) [67] | Preterm (VLBW) (GA: 28 wk, Bwt: 1103 g) | Suckling and swallowing IG1: Non-nutritive sucking exercise (pacifier use) IG2: Swallowing exercise (placing a milk/formula bolus through syringe) CG: Standard care | No effect | |||
| Serrao (2018) [68] | Mothers of preterm (GA: 27–32 wk) | Galactagogue G1: Silymarin-phosphatidylserine and galega (a daily single dose of 5 g of Piu`latte Plus MILTE) G2: Placebo (a daily single dose of 5 g of lactose) | Mixed | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rana, R.; McGrath, M.; Gupta, P.; Thakur, E.; Kerac, M. Feeding Interventions for Infants with Growth Failure in the First Six Months of Life: A Systematic Review. Nutrients 2020, 12, 2044. https://doi.org/10.3390/nu12072044
Rana R, McGrath M, Gupta P, Thakur E, Kerac M. Feeding Interventions for Infants with Growth Failure in the First Six Months of Life: A Systematic Review. Nutrients. 2020; 12(7):2044. https://doi.org/10.3390/nu12072044
Chicago/Turabian StyleRana, Ritu, Marie McGrath, Paridhi Gupta, Ekta Thakur, and Marko Kerac. 2020. "Feeding Interventions for Infants with Growth Failure in the First Six Months of Life: A Systematic Review" Nutrients 12, no. 7: 2044. https://doi.org/10.3390/nu12072044
APA StyleRana, R., McGrath, M., Gupta, P., Thakur, E., & Kerac, M. (2020). Feeding Interventions for Infants with Growth Failure in the First Six Months of Life: A Systematic Review. Nutrients, 12(7), 2044. https://doi.org/10.3390/nu12072044








