Recommendations Based on Evidence by the Andalusian Group for Nutrition Reflection and Investigation (GARIN) for the Pre- and Postoperative Management of Patients Undergoing Obesity Surgery
Abstract
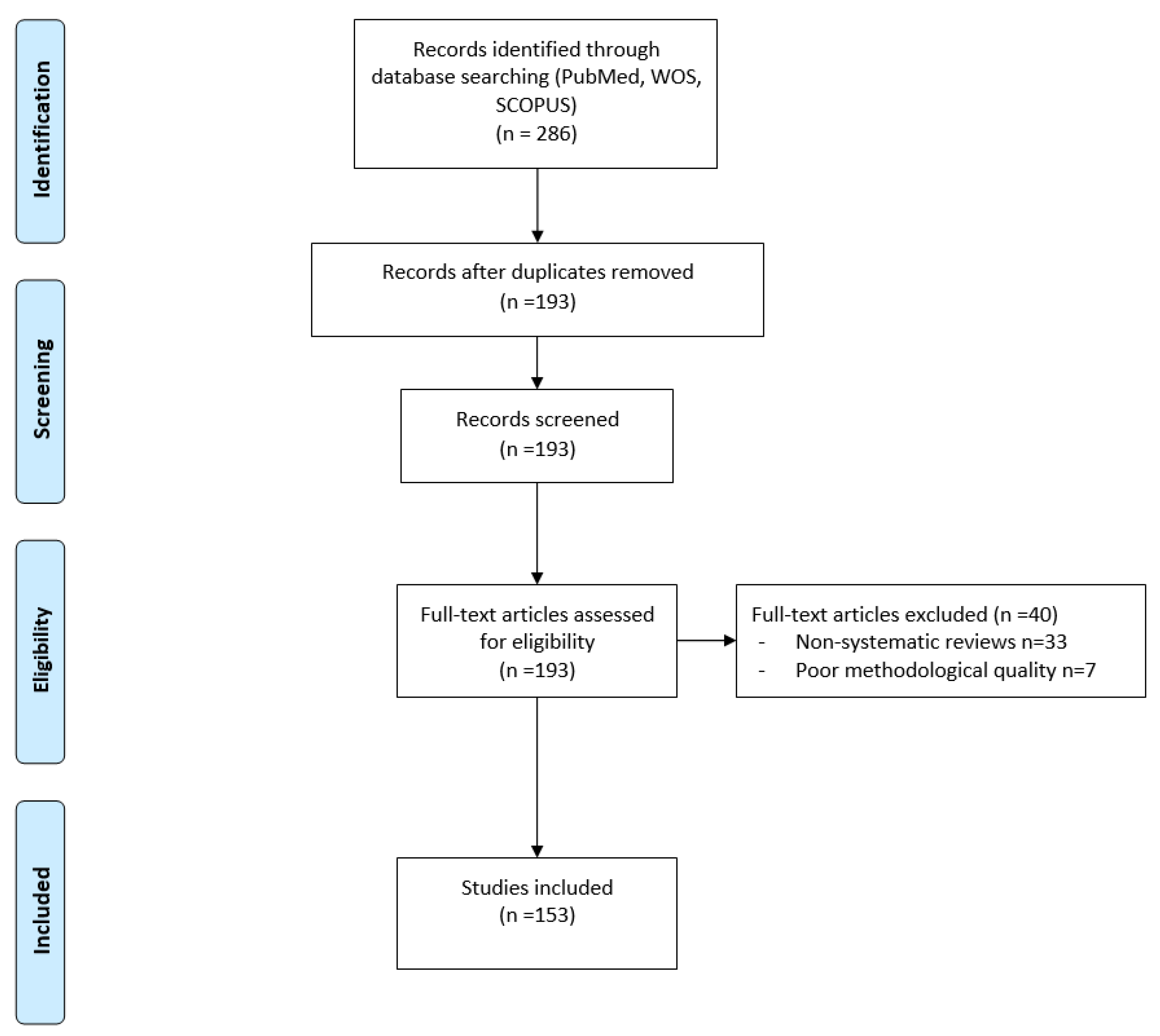
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
4.1. Preoperative Management
4.1.1. Should Micronutrient and Vitamin Levels Be Measured Preoperatively in Patients Who Are Candidates for Obesity Surgery?
4.1.2. Should Deficits Be Supplemented? Are There Any Deficits that Have Been Shown to Be Critical?
4.1.3. Is the VLCD (Very Low-Calorie Diet) Recommended Preoperatively in All Patients? Is Liquid VLCD Better Than Solid VLCD?
4.2. Postoperative Management
4.2.1. What Protein Provisions Should the Diet Contain?
4.2.2. What Contributions of Calcium and Vitamin D Should Be Recommended Postoperatively?
4.2.3. Should Iron Supplementation Be a Routine Postoperative Necessity?
4.2.4. Should Vitamin B12 Supplementation Be a Routine Postoperative Necessity?
4.2.5. Should an Increase of the Dietary Intake of Other Micronutrients Be a Routine Postoperative Necessity?
4.2.6. What Iron and Calcium Preparations Should Be Used?
4.2.7. What Analytical Determinations Should Be Made in the Follow-Up of Patients Who Have Undergone Obesity Surgery? How Often?
4.2.8. Is the Systematic Use of Proton Pump Inhibitors (PPIs) Necessary?
4.2.9. Is Systematic Supplementation with Multivitamin Complexes and Micronutrients Necessary?
4.2.10. Does the Composition of Currently Available Multivitamin Supplements Fit the Recommendations?
4.2.11. Is It Necessary to Keep an Eye Out for the Appearance of Kidney Stones? What Measures Should Be Used to Prevent Their Appearance?
4.2.12. Is Long-Term Follow-Up of Obesity Patients Who Have Undergone Restrictive Techniques Necessary? For How Long? Is Long-Term Follow-Up of Obesity Patients Who Have Undergone Malabsorptive Techniques Necessary? For How Long? Is Long-Term Follow-Up of Obesity Patients Who Have Undergone Mixed Techniques Necessary? For How Long?
4.3. Obesity Surgery in Special Situations
4.3.1. What Technique Should We Recommend in Patients with Type 2 Diabetes Mellitus (T2DM) and Other Metabolic Comorbidities?
4.3.2. Pregnancy after Obesity Surgery
- (a)
- Pregnancy should be avoided in the first year after Obesity Surgery, and contraceptive measures should be routinely recommended.
- (b)
- All women of childbearing age undergoing Obesity Surgery should program their gestation at an experienced centre.
- (c)
- Possible micronutrient deficits (Vitamin A, D, E, K, B12, folic, iron) should be detected (AND treated) at least every six months prior to gestation, in each trimester of pregnancy (or sooner if any type of deficit is detected) and at 6-8 weeks post-partum, especially in case of breastfeeding.
- (d)
- Breastfeeding is advocated.
4.3.3. Obesity Surgery in the Two Non-Working Age Groups: Adolescents and over 65 Years
4.3.4. Obesity Surgery Re-operation. To Whom and with What Technique?
5. Conclusions
Funding
Conflicts of Interest
References
- Buchwald, H. The evolution of metabolic/bariatric surgery. Obes. Surg. 2014, 24, 1126–1135. [Google Scholar] [CrossRef] [PubMed]
- Colquitt, J.L.; Pickett, K.; Loveman, E.; Frampton, G.K. Surgery for weight loss in adults. In Cochrane Database of Systematic Reviews; John Wiley & Sons, Ltd.: Chichester, UK, 2014. [Google Scholar]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339. [Google Scholar] [CrossRef] [PubMed]
- SIGN 50: A Guideline Developer’s Handbook, 3rd ed.; Scottish Intercollegiate Guidelines Network: Edinburg, UK, 2015; ISBN 978-1-909103-30-6.
- Carifio, J.; Perla, R. Resolving the 50-year debate around using and misusing Likert scales. Med. Educ. 2008, 42, 1150–1152. [Google Scholar] [CrossRef] [PubMed]
- Hartley, J. Some thoughts on Likert-type scales. Int. J. Clin. Health Psychol. 2014, 14, 83–86. [Google Scholar] [CrossRef]
- Aaseth, E.; Fagerland, M.W.; Aas, A.-M.; Hewitt, S.; Risstad, H.; Kristinsson, J.; Bøhmer, T.; Mala, T.; Aasheim, E.T. Vitamin concentrations 5 years after gastric bypass. Eur. J. Clin. Nutr. 2015, 69, 1249–1255. [Google Scholar] [CrossRef]
- Abdemur, A.; Han, S.-M.; Lo Menzo, E.; Szomstein, S.; Rosenthal, R. Reasons and outcomes of conversion of laparoscopic sleeve gastrectomy to Roux-en-Y gastric bypass for nonresponders. Surg. Obes. Relat. Dis. 2016, 12, 113–118. [Google Scholar] [CrossRef]
- Adams, T.D.; Hammoud, A.O.; Davidson, L.E.; Laferrère, B.; Fraser, A.; Stanford, J.B.; Hashibe, M.; Greenwood, J.L.J.; Kim, J.; Taylor, D.; et al. Maternal and neonatal outcomes for pregnancies before and after gastric bypass surgery. Int. J. Obes. 2015, 39, 686–694. [Google Scholar] [CrossRef]
- Alexandrou, A.; Armeni, E.; Kouskouni, E.; Tsoka, E.; Diamantis, T.; Lambrinoudaki, I. Cross-sectional long-term micronutrient deficiencies after sleeve gastrectomy versus Roux-en-Y gastric bypass: A pilot study. Surg. Obes. Relat. Dis. 2014, 10, 262–268. [Google Scholar] [CrossRef]
- Aron-Wisnewsky, J.; Verger, E.O.; Bounaix, C.; Dao, M.C.; Oppert, J.-M.; Bouillot, J.-L.; Chevallier, J.-M.; Clément, K. Nutritional and protein deficiencies in the short term following both gastric bypass and gastric banding. PLoS ONE 2016, 11, e0149588. [Google Scholar] [CrossRef]
- Bailly, L.; Schiavo, L.; Sebastianelli, L.; Fabre, R.; Pradier, C.; Iannelli, A. Anemia and bariatric surgery: Results of a national french survey on administrative data of 306,298 consecutive patients between 2008 and 2016. Obes. Surg. 2018. [Google Scholar] [CrossRef]
- Basfi-Fer, K.; Rojas, P.; Carrasco, F.; Valencia, A.; Inostroza, J.; Codoceo, J.; Pizarro, F.; Olivares, M.; Papapietro, K.; Csendes, A.; et al. Evolución de la ingesta y del estado nutricional de zinc, hierro y cobre. Nutr. Hosp. 2012, 27, 1527–1535. [Google Scholar] [CrossRef] [PubMed]
- Ben-Porat, T.; Elazary, R.; Goldenshluger, A.; Sherf Dagan, S.; Mintz, Y.; Weiss, R. Nutritional deficiencies four years after laparoscopic sleeve gastrectomy—Are supplements required for a lifetime? Surg. Obes. Relat. Dis. 2017, 13, 1138–1144. [Google Scholar] [CrossRef] [PubMed]
- Bennasar Remolar, M.Á.; Martínez Ramos, D.; Ortega Serrano, J.; Salvador Sanchís, J.L. Nutritional alterations after very low-calorie diet before bariatric surgery. Cir. Esp. Engl. Ed. 2016, 94, 159–164. [Google Scholar] [CrossRef]
- Botella-Carretero, J.I.; Lafuente, C.; Montes-Nieto, R.; Balsa, J.; Vega-Piñero, B.; Garcia-Moreno, F.; Peromingo, R.; Galindo, J.; San-Millan, J.L.; Escobar-Morreale, H. Serum bioavailable vitamin D concentrations and bone mineral density in women after obesity surgery. Obes. Surg. 2016, 26, 2732–2737. [Google Scholar] [CrossRef]
- Botella Romero, F.; Milla Tobarra, M.; Alfaro Martínez, J.J.; García Arce, L.; García Gómez, A.; Ángeles Salas Sáiz, M.; Soler Marín, A. Bariatric surgery in duodenal switch procedure: Weight changes and associated nutritional deficiencies. Endocrinol. Nutr. Engl. Ed. 2011, 58, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Boyce, S.G.; Goriparthi, R.; Clark, J.; Cameron, K.; Roslin, M.S. Can composite nutritional supplement based on the current guidelines prevent vitamin and mineral deficiency after weight loss surgery? Obes. Surg. 2016, 26, 966–971. [Google Scholar] [CrossRef] [PubMed]
- Brethauer, S.A.; Kothari, S.; Sudan, R.; Williams, B.; English, W.J.; Brengman, M.; Kurian, M.; Hutter, M.; Stegemann, L.; Kallies, K.; et al. Systematic review on reoperative bariatric surgery. Surg. Obes. Relat. Dis. 2014, 10, 952–972. [Google Scholar] [CrossRef]
- Busetto, L.; Dicker, D.; Azran, C.; Batterham, R.L.; Farpour-Lambert, N.; Fried, M.; Hjelmesæth, J.; Kinzl, J.; Leitner, D.R.; Makaronidis, J.M.; et al. Practical recommendations of the obesity management task force of the European association for the study of obesity for the post-bariatric surgery medical management. Obes. Facts 2017, 10, 597–632. [Google Scholar] [CrossRef]
- Cabral, J.A.V.; de Souza, G.P.; Nascimento, J.d.A.; Simoneti, L.F.; Marchese, C.; Sales-Peres, S.H.d.C. Impact of vitamin D and calcium deficiency in the bones of patients undergoing bariatric surgery: A systematic review. ABCD Arq. Bras. Cir. Dig. São Paulo 2016, 29, 120–123. [Google Scholar] [CrossRef]
- Caron, M.; Hould, F.S.; Lescelleur, O.; Marceau, S.; Lebel, S.; Julien, F.; Simard, S.; Biertho, L. Long-term nutritional impact of sleeve gastrectomy. Surg. Obes. Relat. Dis. 2017, 13, 1664–1673. [Google Scholar] [CrossRef]
- Casillas, R.A.; Um, S.S.; Zelada Getty, J.L.; Sachs, S.; Kim, B.B. Revision of primary sleeve gastrectomy to Roux-en-Y gastric bypass: Indications and outcomes from a high-volume center. Surg. Obes. Relat. Dis. 2016, 12, 1817–1825. [Google Scholar] [CrossRef] [PubMed]
- Chagas, C.; Saunders, C.; Pereira, S.; Silva, J.; Saboya, C.; Ramalho, A. Vitamin A status and its relationship with serum zinc concentrations among pregnant women who have previously undergone Roux-en-Y gastric bypass. Int. J. Gynecol. Obstet. 2016, 133, 94–97. [Google Scholar] [CrossRef] [PubMed]
- Chakhtoura, M.T.; Nakhoul, N.; Akl, E.A.; Mantzoros, C.S.; El Hajj Fuleihan, G.A. Guidelines on vitamin D replacement in bariatric surgery: Identification and systematic appraisal. Metabolism 2016, 65, 586–597. [Google Scholar] [CrossRef] [PubMed]
- Chakhtoura, M.T.; Nakhoul, N.N.; Shawwa, K.; Mantzoros, C.; El Hajj Fuleihan, G.A. Hypovitaminosis D in bariatric surgery: A systematic review of observational studies. Metabolism 2016, 65, 574–585. [Google Scholar] [CrossRef]
- Chakhtoura, M.; Rahme, M.; El-Hajj Fuleihan, G. Vitamin D metabolism in bariatric surgery. Endocrinol. Metab. Clin. North Am. 2017, 46, 947–982. [Google Scholar] [CrossRef]
- Coblijn, U.K.; Goucham, A.B.; Lagarde, S.M.; Kuiken, S.D.; van Wagensveld, B.A. Development of ulcer disease after roux-en-y gastric bypass, incidence, risk factors, and patient presentation: A systematic review. Obes. Surg. 2014, 24, 299–309. [Google Scholar] [CrossRef]
- Coblijn, U.K.; Verveld, C.J.; van Wagensveld, B.A.; Lagarde, S.M. Laparoscopic roux-en-y gastric bypass or laparoscopic sleeve gastrectomy as revisional procedure after adjustable gastric band—A systematic review. Obes. Surg. 2013, 23, 1899–1914. [Google Scholar] [CrossRef]
- Menegati, G.C.; de Oliveira, L.C.; Santos, A.L.A.; Cohen, L.; Mattos, F.; Mendonça, L.M.C.; Carneiro, J.R.I.; Farias, M.L.F.; Rosado, E.L. Nutritional status, body composition, and bone health in women after bariatric surgery at a university hospital in Rio de Janeiro. Obes. Surg. 2016, 26, 1517–1524. [Google Scholar] [CrossRef]
- Costa, T.L.; Paganotto, M.; Radominski, R.B.; Kulak, C.M.; Borba, V.C. Calcium metabolism, vitamin D and bone mineral density after bariatric surgery. Osteoporos. Int. 2015, 26, 757–764. [Google Scholar] [CrossRef]
- Sherf Dagan, S.; Tovim, T.B.; Keidar, A.; Raziel, A.; Shibolet, O.; Zelber-Sagi, S. Inadequate protein intake after laparoscopic sleeve gastrectomy surgery is associated with a greater fat free mass loss. Surg. Obes. Relat. Dis. 2017, 13, 101–109. [Google Scholar] [CrossRef]
- Daigle, C.R.; Andalib, A.; Corcelles, R.; Cetin, D.; Schauer, P.R.; Brethauer, S.A. Bariatric and metabolic outcomes in the super-obese elderly. Surg. Obes. Relat. Dis. 2016, 12, 132–137. [Google Scholar] [CrossRef] [PubMed]
- de Luis, D.A.; Pacheco, D.; Izaola, O.; Terroba, M.C.; Cuellar, L.; Cabezas, G. Micronutrient status in morbidly obese women before bariatric surgery. Surg. Obes. Relat. Dis. 2013, 9, 323–327. [Google Scholar] [CrossRef] [PubMed]
- del Villar Madrigal, E.; Neme-Yunes, Y.; Clavellina-Gaytan, D.; Sanchez, H.A.; Mosti, M.; Herrera, M.F. Anemia after Roux-en-Y gastric bypass. How feasible to eliminate the risk by proper supplementation? Obes. Surg. 2015, 25, 80–84. [Google Scholar] [CrossRef]
- Dogan, K.; Homan, J.; Aarts, E.O.; de Boer, H.; van Laarhoven, C.J.H.M.; Berends, F.J. Long-term nutritional status in patients following Roux-en-Y gastric bypass surgery. Clin. Nutr. 2018, 37, 612–617. [Google Scholar] [CrossRef]
- Dunstan, M.J.D.; Molena, E.J.; Ratnasingham, K.; Kamocka, A.; Smith, N.C.; Humadi, S.; Irukulla, S. Variations in oral vitamin and mineral supplementation following bariatric gastric bypass surgery: A national survey. Obes. Surg. 2015, 25, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Edholm, D. Early intake of solid food after Roux-en-Y gastric bypass and complications. A cohort study from the Scandinavian obesity surgery registry. Surg. Obes. Relat. Dis. 2018. [Google Scholar] [CrossRef]
- Edholm, D.; Svensson, F.; Näslund, I.; Karlsson, F.A.; Rask, E.; Sundbom, M. Long-term results 11 years after primary gastric bypass in 384 patients. Surg. Obes. Relat. Dis. 2013, 9, 708–713. [Google Scholar] [CrossRef] [PubMed]
- Elbahrawy, A.; Bougie, A.; Loiselle, S.-E.; Demyttenaere, S.; Court, O.; Andalib, A. Medium to long-term outcomes of bariatric surgery in older adults with super obesity. Surg. Obes. Relat. Dis. 2018, 14, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Elhag, W.; El Ansari, W.; Abdulrazzaq, S.; Abdullah, A.; Elsherif, M.; Elgenaied, I. Evolution of 29 anthropometric, nutritional, and cardiometabolic parameters among morbidly obese adolescents 2 years post sleeve gastrectomy. Obes. Surg. 2018, 28, 474–482. [Google Scholar] [CrossRef]
- Fashandi, A.Z.; Mehaffey, J.H.; Hawkins, R.B.; Schirmer, B.; Hallowell, P.T. Bariatric surgery increases risk of bone fracture. Surg. Endosc. 2018, 32, 2650–2655. [Google Scholar] [CrossRef] [PubMed]
- Ferreira Nicoletti, C.; Morandi Junqueira-Franco, M.V.; dos Santos, J.E.; Sergio Marchini, J.; Junior, W.S.; Nonino, C.B. Protein and amino acid status before and after bariatric surgery: A 12-month follow-up study. Surg. Obes. Relat. Dis. 2013, 9, 1008–1012. [Google Scholar] [CrossRef] [PubMed]
- Flores, L.; Moizé, V.; Ortega, E.; Rodríguez, L.; Andreu, A.; Filella, X.; Vidal, J. Prospective study of individualized or high fixed doses of vitamin D supplementation after bariatric surgery. Obes. Surg. 2015, 25, 470–476. [Google Scholar] [CrossRef]
- Flores, L.; Martínez Osaba, M.J.; Andreu, A.; Moizé, V.; Rodríguez, L.; Vidal, J. Calcium and vitamin D supplementation after gastric bypass should be individualized to improve or avoid hyperparathyroidism. Obes. Surg. 2010, 20, 738–743. [Google Scholar] [CrossRef]
- Froylich, D.; Sadeh, O.; Mizrahi, H.; Kafri, N.; Pascal, G.; Daigle, C.R.; Geron, N.; Hazzan, D. Midterm outcomes of sleeve gastrectomy in the elderly. Surg. Obes. Relat. Dis. 2018, 14, 1495–1500. [Google Scholar] [CrossRef] [PubMed]
- Fulton, C.; Sheppard, C.; Birch, D.; Karmali, S.; de Gara, C. A comparison of revisional and primary bariatric surgery. Can. J. Surg. 2017, 60, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Gadgil, M.D.; Chang, H.-Y.; Richards, T.M.; Gudzune, K.A.; Huizinga, M.M.; Clark, J.M.; Bennett, W.L. Laboratory testing for and diagnosis of nutritional deficiencies in pregnancy before and after bariatric surgery. J. Womens Health 2014, 23, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Gebhart, A.; Young, M.T.; Nguyen, N.T. Bariatric surgery in the elderly: 2009–2013. Surg. Obes. Relat. Dis. 2015, 11, 393–398. [Google Scholar] [CrossRef]
- Gesquiere, I.; Lannoo, M.; Augustijns, P.; Matthys, C.; Van der Schueren, B.; Foulon, V. Iron deficiency after Roux-en-Y gastric bypass: Insufficient iron absorption from oral iron supplements. Obes. Surg. 2014, 24, 56–61. [Google Scholar] [CrossRef]
- Gesquiere, I.; Foulon, V.; Augustijns, P.; Gils, A.; Lannoo, M.; Van der Schueren, B.; Matthys, C. Micronutrient intake, from diet and supplements, and association with status markers in pre- and post-RYGB patients. Clin. Nutr. 2017, 36, 1175–1181. [Google Scholar] [CrossRef]
- Gillon, S.; Jeanes, Y.M.; Andersen, J.R.; Våge, V. Micronutrient status in morbidly obese patients prior to laparoscopic sleeve gastrectomy and micronutrient changes 5 years post-surgery. Obes. Surg. 2017, 27, 606–612. [Google Scholar] [CrossRef]
- Gimenes, J.C.; Nicoletti, C.F.; de Souza Pinhel, M.A.; Cortes-Oliveira, C.; Salgado Júnior, W.; Nonino, C.B. Nutritional status of children from women with previously bariatric surgery. Obes. Surg. 2018, 28, 990–995. [Google Scholar] [CrossRef] [PubMed]
- Gimenes, J.C.; Nicoletti, C.F.; de Souza Pinhel, M.A.; de Oliveira, B.A.P.; Salgado Júnior, W.; Marchini, J.S.; Nonino, C.B. Pregnancy after Roux en Y gastric bypass: Nutritional and biochemical aspects. Obes. Surg. 2017, 27, 1815–1821. [Google Scholar] [CrossRef] [PubMed]
- Giordano, S.; Victorzon, M. Laparoscopic Roux-en-Y gastric bypass in elderly patients (60 years or older): A meta-analysis of comparative studies. Scand. J. Surg. 2018, 107, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Victorzon, M.; Giordano, S. Bariatric surgery in elderly patients: A systematic review. Clin. Interv. Aging 2015, 1627. [Google Scholar] [CrossRef]
- Gobato, R.C.; Seixas Chaves, D.F.; Chaim, E.A. Micronutrient and physiologic parameters before and 6 months after RYGB. Surg. Obes. Relat. Dis. 2014, 10, 944–951. [Google Scholar] [CrossRef]
- Goldberg, H.R.; Chin, V.L.; Zitsman, J.L.; Zhang, C.; Williams, K.M.; Oberfield, S.; Fennoy, I. Bariatric surgery in adolescents: Is routine nutrient supplementation sufficient to avoid anemia following bariatric surgery? Nutr. Clin. Pract. 2017, 32, 502–507. [Google Scholar] [CrossRef]
- Lima, K.V.G.; Lima, R.P.A.; Gonçalves, M.C.R.; Faintuch, J.; Morais, L.C.S.L.; Asciutti, L.S.R.; Costa, M.J.C. High frequency of serum chromium deficiency and association of chromium with triglyceride and cholesterol concentrations in patients awaiting bariatric surgery. Obes. Surg. 2014, 24, 771–776. [Google Scholar] [CrossRef]
- González Navarro, I.; Pereira Cunill, J.L.; Serrano Aguayo, P.; Morales Conde, S.; Martos Martínez, J.M.; García Luna, P.P. Resultados materno-fetales de la gestación tras cirugía bariátrica. Nutr. Hosp. 2011, 376–383. [Google Scholar] [CrossRef]
- Grace, C.; Vincent, R.; Aylwin, S.J. High prevalence of vitamin D insufficiency in a United Kingdom urban morbidly obese population: Implications for testing and treatment. Surg. Obes. Relat. Dis. 2014, 10, 355–360. [Google Scholar] [CrossRef]
- Gregory, D.M.; Twells, L.K.; Lester, K.K.; Midodzi, W.K.; Pedersen, M.R.; Pace, D.; Smith, C.; Boone, D.; Randell, E.W.; Kovacs, C.S. Preoperative and postoperative assessments of biochemical parameters in patients with severe obesity undergoing laparoscopic sleeve gastrectomy. Obes. Surg. 2017. [Google Scholar] [CrossRef]
- Haywood, C.; Sumithran, P. Treatment of obesity in older persons-A systematic review. Obes. Rev. 2019. [Google Scholar] [CrossRef] [PubMed]
- Homan, J.; Betzel, B.; Aarts, E.O.; van Laarhoven, K.J.H.M.; Janssen, I.M.C.; Berends, F.J. Secondary surgery after sleeve gastrectomy: Roux-en-Y gastric bypass or biliopancreatic diversion with duodenal switch. Surg. Obes. Relat. Dis. 2015, 11, 771–777. [Google Scholar] [CrossRef] [PubMed]
- Hsin, M.-C.; Huang, C.-K.; Tai, C.-M.; Yeh, L.-R.; Kuo, H.-C.; Garg, A. A case-matched study of the differences in bone mineral density 1 year after 3 different bariatric procedures. Surg. Obes. Relat. Dis. 2015, 11, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Iannelli, A.; Debs, T.; Martini, F.; Benichou, B.; Ben Amor, I.; Gugenheim, J. Laparoscopic conversion of sleeve gastrectomy to Roux-en-Y gastric bypass: Indications and preliminary results. Surg. Obes. Relat. Dis. 2016, 12, 1533–1538. [Google Scholar] [CrossRef] [PubMed]
- James, H.; Lorentz, P.; Collazo-Clavell, M.L. Patient-reported adherence to empiric vitamin/mineral supplementation and related nutrient deficiencies after Roux-en-Y gastric bypass. Obes. Surg. 2016, 26, 2661–2666. [Google Scholar] [CrossRef]
- Jans, G.; Matthys, C.; Bogaerts, A.; Lannoo, M.; Verhaeghe, J.; Van der Schueren, B.; Devlieger, R. Maternal micronutrient deficiencies and related adverse neonatal outcomes after bariatric surgery: A systematic review. Adv. Nutr. 2015, 6, 420–429. [Google Scholar] [CrossRef]
- Jans, G.; Guelinckx, I.; Voets, W.; Galjaard, S.; Van Haard, P.M.M.; Vansant, G.M.; Devlieger, R. Vitamin K1 monitoring in pregnancies after bariatric surgery: A prospective cohort study. Surg. Obes. Relat. Dis. 2014, 10, 885–890. [Google Scholar] [CrossRef]
- Jáuregui-Lobera, I. Iron deficiency and bariatric surgery. Nutrients 2013, 5, 1595–1608. [Google Scholar] [CrossRef]
- Kalani, A.; Bami, H.; Tiboni, M.; Jaeschke, R.; Adachi, J.D.; Lau, A.N. The effect of bariatric surgery on serum 25-OH vitamin D levels: A systematic review and meta-analysis: Impact on bone health in bariatric surgery. Obes. Sci. Pract. 2017, 3, 319–332. [Google Scholar] [CrossRef]
- Kim, M.K.; Kim, W.; Kwon, H.-S.; Baek, K.-H.; Kim, E.K.; Song, K.-H. Effects of bariatric surgery on metabolic and nutritional parameters in severely obese Korean patients with type 2 diabetes: A prospective 2-year follow up. J. Diabetes Investig. 2014, 5, 221–227. [Google Scholar] [CrossRef]
- Ito, M.K.; Gonçalves, V.S.S.; Faria, S.L.C.M.; Moizé, V.; Porporatti, A.L.; Guerra, E.N.S.; De Luca Canto, G.; de Carvalho, K.M.B. Effect of protein intake on the protein status and lean mass of post-bariatric surgery patients: A systematic review. Obes. Surg. 2017, 27, 502–512. [Google Scholar] [CrossRef] [PubMed]
- Kornerup, L.S.; Hvas, C.L.; Abild, C.B.; Richelsen, B.; Nexo, E. Early changes in vitamin B12 uptake and biomarker status following Roux-en-Y gastric bypass and sleeve gastrectomy. Clin. Nutr. 2018. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.; Kim, H.J.; Lo Menzo, E.; Park, S.; Szomstein, S.; Rosenthal, R.J. Anemia, iron and vitamin B12 deficiencies after sleeve gastrectomy compared to Roux-en-Y gastric bypass: A meta-analysis. Surg. Obes. Relat. Dis. 2014, 10, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Krzizek, E.-C.; Brix, J.M.; Herz, C.T.; Kopp, H.P.; Schernthaner, G.-H.; Schernthaner, G.; Ludvik, B. Prevalence of micronutrient deficiency in patients with morbid obesity before bariatric surgery. Obes. Surg. 2018, 28, 643–648. [Google Scholar] [CrossRef] [PubMed]
- Lecube, A.; Zafon, C.; Gromaz, A.; Fort, J.M.; Caubet, E.; Baena, J.A.; Tortosa, F. Iodine deficiency is higher in morbid obesity in comparison with late after bariatric surgery and non-obese women. Obes. Surg. 2015, 25, 85–89. [Google Scholar] [CrossRef]
- Lefebvre, P.; Letois, F.; Sultan, A.; Nocca, D.; Mura, T.; Galtier, F. Nutrient deficiencies in patients with obesity considering bariatric surgery: A cross-sectional study. Surg. Obes. Relat. Dis. 2014, 10, 540–546. [Google Scholar] [CrossRef]
- Faria, S.L.; Faria, O.P.; Cardeal, M.d.A.; Ito, M.K. Effects of a very low calorie diet in the preoperative stage of bariatric surgery: A randomized trial. Surg. Obes. Relat. Dis. 2015, 11, 230–237. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, X.; Fu, W. Vitamin D supplementation for the prevention of vitamin D deficiency after bariatric surgery: A systematic review and meta-analysis. Eur. J. Clin. Nutr. 2017. [Google Scholar] [CrossRef]
- Liu, C.; Wu, D.; Zhang, J.-F.; Xu, D.; Xu, W.-F.; Chen, Y.; Liu, B.-Y.; Li, P.; Li, L. Changes in bone metabolism in morbidly obese patients after bariatric surgery: A meta-analysis. Obes. Surg. 2016, 26, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Soares, F.L.; Bissoni de Sousa, L.; Corradi-Perini, C.; Ramos da Cruz, M.R.; Nunes, M.G.J.; Branco-Filho, A.J. Food quality in the late postoperative period of bariatric surgery: An evaluation using the bariatric food pyramid. Obes. Surg. 2014, 24, 1481–1486. [Google Scholar] [CrossRef] [PubMed]
- Luger, M.; Kruschitz, R.; Kienbacher, C.; Traussnigg, S.; Langer, F.B.; Prager, G.; Schindler, K.; Kallay, E.; Hoppichler, F.; Trauner, M.; et al. Vitamin D3 loading is superior to conventional supplementation after weight loss surgery in vitamin D-deficient morbidly obese patients: A double-blind randomized placebo-controlled trial. Obes. Surg. 2017, 27, 1196–1207. [Google Scholar] [CrossRef]
- Majumder, S.; Soriano, J.; Louie Cruz, A.; Dasanu, C.A. Vitamin B12 deficiency in patients undergoing bariatric surgery: Preventive strategies and key recommendations. Surg. Obes. Relat. Dis. 2013, 9, 1013–1019. [Google Scholar] [CrossRef] [PubMed]
- Malone, M.; Alger-Mayer, S.; Lindstrom, J.; Bailie, G.R. Management of iron deficiency and anemia after Roux-en-Y gastric bypass surgery: An observational study. Surg. Obes. Relat. Dis. 2013, 9, 969–974. [Google Scholar] [CrossRef] [PubMed]
- Mann, J.P.; Jakes, A.D.; Hayden, J.D.; Barth, J.H. Systematic review of definitions of failure in revisional bariatric surgery. Obes. Surg. 2015, 25, 571–574. [Google Scholar] [CrossRef]
- Manousou, S.; Carlsson, L.M.S.; Eggertsen, R.; Hulthén, L.; Jacobson, P.; Landin-Wilhelmsen, K.; Trimpou, P.; Svensson, P.-A.; Nyström, H.F. Iodine status after bariatric surgery—A prospective 10-year report from the swedish obese subjects (SOS) study. Obes. Surg. 2018, 28, 349–357. [Google Scholar] [CrossRef]
- Marczuk, P.; Kubisa, M.J.; Święch, M.; Walędziak, M.; Kowalewski, P.; Major, P.; Pędziwiatr, M.; Paśnik, K.; Janik, M.R. Effectiveness and safety of Roux-en-Y gastric bypass in elderly patients—Systematic review and meta-analysis. Obes. Surg. 2018. [Google Scholar] [CrossRef]
- Martín García-Almenta, E.; Ruiz-Tovar, J.; Sánchez Santos, R.; Garcia-Moreno, F. (Eds.) Vía Clínica de Cirugía Bariátrica, 1st ed.; Im3diA Comunicación S.L: Albacete, Spain, 2017; ISBN 978-84-697-7104-4. [Google Scholar]
- McCracken, E.; Wood, G.C.; Prichard, W.; Bistrian, B.; Still, C.; Gerhard, G.; Rolston, D.; Benotti, P. Severe anemia after Roux-en-Y gastric bypass: A cause for concern. Surg. Obes. Relat. Dis. 2018. [Google Scholar] [CrossRef] [PubMed]
- McGlone, E.R.; Bond, A.; Reddy, M.; Khan, O.A.; Wan, A.C. Super-obesity in the elderly: Is bariatric surgery justified? Obes. Surg. 2015, 25, 1750–1755. [Google Scholar] [CrossRef]
- Mead, N.C.; Sakkatos, P.; Sakellaropoulos, G.C.; Adonakis, G.L.; Alexandrides, T.K.; Kalfarentzos, F. Pregnancy outcomes and nutritional indices after 3 types of bariatric surgery performed at a single institution. Surg. Obes. Relat. Dis. 2014, 10, 1166–1173. [Google Scholar] [CrossRef]
- Mechanick, J.I.; Youdim, A.; Jones, D.B.; Timothy Garvey, W.; Hurley, D.L.; Molly McMahon, M.; Heinberg, L.J.; Kushner, R.; Adams, T.D.; Shikora, S.; et al. Clinical practice guidelines for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient—2013 update: Cosponsored by American Association of Clinical Endocrinologists, The Obesity Society, and American Society for Metabolic & Bariatric Surgery. Surg. Obes. Relat. Dis. 2013, 9, 159–191. [Google Scholar] [CrossRef]
- Mendes-Filho, A.M.; Godoy, E.S.N.; Alhinho, H.C.A.W.; Galvão-Neto, M.D.P.; Ramos, A.C.; Ferraz, Á.A.B.; Campos, J.M. Fundoplication conversion in Roux-en-Y gastric bypass for control of obesity and gastroesophageal reflux: Systematic review. ABCD Arq. Bras. Cir. Dig. São Paulo 2017, 30, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Mingrone, G.; Bornstein, S.; Le Roux, C.W. Optimisation of follow-up after metabolic surgery. Lancet Diabetes Endocrinol. 2018, 6, 487–499. [Google Scholar] [CrossRef]
- Mischler, R.A.; Armah, S.M.; Craig, B.A.; Rosen, A.D.; Banerjee, A.; Selzer, D.J.; Choi, J.N.; Gletsu-Miller, N. Comparison of oral iron supplement formulations for normalization of iron status following Roux-en-Y gastric bypass surgery: A randomized trial. Obes. Surg. 2018, 28, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Mischler, R.A.; Armah, S.M.; Wright, B.N.; Mattar, S.G.; Rosen, A.D.; Gletsu-Miller, N. Influence of diet and supplements on iron status after gastric bypass surgery. Surg. Obes. Relat. Dis. 2016, 12, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Moizé, V.; Andreu, A.; Flores, L.; Torres, F.; Ibarzabal, A.; Delgado, S.; Lacy, A.; Rodriguez, L.; Vidal, J. Long-term dietary intake and nutritional deficiencies following sleeve gastrectomy or Roux-en-Y gastric bypass in a mediterranean population. J. Acad. Nutr. Diet. 2013, 113, 400–410. [Google Scholar] [CrossRef]
- Moore, C.E.; Sherman, V. Effectiveness of B vitamin supplementation following bariatric surgery: Rapid increases of serum vitamin B12. Obes. Surg. 2015, 25, 694–699. [Google Scholar] [CrossRef]
- Morales, M.P.; Wheeler, A.A.; Ramaswamy, A.; Scott, J.S.; de la Torre, R.A. Laparoscopic revisional surgery after Roux-en-Y gastric bypass and sleeve gastrectomy. Surg. Obes. Relat. Dis. 2010, 6, 485–490. [Google Scholar] [CrossRef]
- Nicoletti, C.F.; Lima, T.P.; Donadelli, S.P.; Salgado, W.; Marchini, J.S.; Nonino, C.B. New look at nutritional care for obese patient candidates for bariatric surgery. Surg. Obes. Relat. Dis. 2013, 9, 520–525. [Google Scholar] [CrossRef]
- O’Kane, M.; Parretti, H.M.; Hughes, C.A.; Sharma, M.; Woodcock, S.; Puplampu, T.; Blakemore, A.I.; Clare, K.; MacMillan, I.; Joyce, J.; et al. Guidelines for the follow-up of patients undergoing bariatric surgery: Follow-up of bariatric surgery patients. Clin. Obes. 2016, 6, 210–224. [Google Scholar] [CrossRef]
- Obeid, N.R.; Malick, W.; Concors, S.J.; Fielding, G.A.; Kurian, M.S.; Ren-Fielding, C.J. Long-term outcomes after Roux-en-Y gastric bypass: 10- to 13-year data. Surg. Obes. Relat. Dis. 2016, 12, 11–20. [Google Scholar] [CrossRef]
- Obinwanne, K.M.; Riess, K.P.; Kallies, K.J.; Mathiason, M.A.; Manske, B.R.; Kothari, S.N. Effects of laparoscopic Roux-en-Y gastric bypass on bone mineral density and markers of bone turnover. Surg. Obes. Relat. Dis. 2014, 10, 1056–1062. [Google Scholar] [CrossRef] [PubMed]
- Obinwanne, K.M.; Fredrickson, K.A.; Mathiason, M.A.; Kallies, K.J.; Farnen, J.P.; Kothari, S.N. Incidence, treatment, and outcomes of iron deficiency after laparoscopic Roux-en-Y gastric bypass: A 10-year analysis. J. Am. Coll. Surg. 2014, 218, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Olbers, T.; Beamish, A.J.; Gronowitz, E.; Flodmark, C.-E.; Dahlgren, J.; Bruze, G.; Ekbom, K.; Friberg, P.; Göthberg, G.; Järvholm, K.; et al. Laparoscopic Roux-en-Y gastric bypass in adolescents with severe obesity (AMOS): A prospective, 5-year, Swedish nationwide study. Lancet Diabetes Endocrinol. 2017, 5, 174–183. [Google Scholar] [CrossRef]
- Parmar, C.; Mahawar, K.K.; Carr, W.R.J.; Schroeder, N.; Balupuri, S.; Small, P.K. Bariatric surgery in septuagenarians: A comparison with <60 year olds. Obes. Surg. 2017, 27, 3165–3169. [Google Scholar] [CrossRef]
- Parrott, J.; Frank, L.; Rabena, R.; Craggs-Dino, L.; Isom, K.A.; Greiman, L. American Society for Metabolic and Bariatric Surgery integrated health nutritional guidelines for the surgical weight loss patient 2016 update: Micronutrients. Surg. Obes. Relat. Dis. 2017, 13, 727–741. [Google Scholar] [CrossRef] [PubMed]
- Pędziwiatr, M.; Małczak, P.; Wierdak, M.; Rubinkiewicz, M.; Pisarska, M.; Major, P.; Wysocki, M.; Karcz, W.K.; Budzyński, A. Revisional gastric bypass is inferior to primary gastric bypass in terms of short- and long-term outcomes—Systematic review and meta-analysis. Obes. Surg. 2018, 28, 2083–2091. [Google Scholar] [CrossRef]
- Pellitero, S.; Martínez, E.; Puig, R.; Leis, A.; Zavala, R.; Granada, M.L.; Pastor, C.; Moreno, P.; Tarascó, J.; Puig-Domingo, M. Evaluation of vitamin and trace element requirements after sleeve gastrectomy at long term. Obes. Surg. 2017, 27, 1674–1682. [Google Scholar] [CrossRef]
- Pereira, S.; Saboya, C.; Ramalho, A. Impact of different protocols of nutritional supplements on the status of vitamin A in class III obese patients after Roux-en-Y gastric bypass. Obes. Surg. 2013, 23, 1244–1251. [Google Scholar] [CrossRef]
- Da Cruz, S.P.; Matos, A.; Pereira, S.; Saboya, C.; da Cruz, S.P.; Ramalho, A. Roux-en-Y gastric bypass aggravates vitamin A deficiency in the mother-child group. Obes. Surg. 2018, 28, 114–121. [Google Scholar] [CrossRef]
- Pereira-Santos, M.; Costa, P.R.F.; Assis, A.M.O.; Santos, C.A.S.T.; Santos, D.B. Obesity and vitamin D deficiency: A systematic review and meta-analysis: Obesity and vitamin D. Obes. Rev. 2015, 16, 341–349. [Google Scholar] [CrossRef]
- Quirante, F.P.; Montorfano, L.; Rammohan, R.; Dhanabalsamy, N.; Lee, A.; Szomstein, S.; Lo Menzo, E.; Rosenthal, R.J. Is bariatric surgery safe in the elderly population? Surg. Endosc. 2017, 31, 1538–1543. [Google Scholar] [CrossRef] [PubMed]
- Peterson, L.A.; Zeng, X.; Caufield-Noll, C.P.; Schweitzer, M.A.; Magnuson, T.H.; Steele, K.E. Vitamin D status and supplementation before and after bariatric surgery: A comprehensive literature review. Surg. Obes. Relat. Dis. 2016, 12, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Peterson, L.A.; Cheskin, L.J.; Furtado, M.; Papas, K.; Schweitzer, M.A.; Magnuson, T.H.; Steele, K.E. Malnutrition in bariatric surgery candidates: Multiple micronutrient deficiencies prior to surgery. Obes. Surg. 2016, 26, 833–838. [Google Scholar] [CrossRef] [PubMed]
- Pinto-Bastos, A.; Conceição, E.M.; Machado, P.P.P. Reoperative bariatric surgery: A systematic review of the reasons for surgery, medical and weight loss outcomes, relevant behavioral factors. Obes. Surg. 2017, 27, 2707–2715. [Google Scholar] [CrossRef] [PubMed]
- Pratt, J.S.A.; Browne, A.; Browne, N.T.; Bruzoni, M.; Cohen, M.; Desai, A.; Inge, T.; Linden, B.C.; Mattar, S.G.; Michalsky, M.; et al. ASMBS pediatric metabolic and bariatric surgery guidelines, 2018. Surg. Obes. Relat. Dis. 2018. [Google Scholar] [CrossRef]
- Quezada, N.; Hernández, J.; Pérez, G.; Gabrielli, M.; Raddatz, A.; Crovari, F. Laparoscopic sleeve gastrectomy conversion to Roux-en-Y gastric bypass: Experience in 50 patients after 1 to 3 years of follow-up. Surg. Obes. Relat. Dis. 2016, 12, 1611–1615. [Google Scholar] [CrossRef]
- Rodríguez-Carmona, Y.; López-Alavez, F.J.; González-Garay, A.G.; Solís-Galicia, C.; Meléndez, G.; Serralde-Zúñiga, A.E. Bone mineral density after bariatric surgery. A systematic review. Int. J. Surg. 2014, 12, 976–982. [Google Scholar] [CrossRef]
- Rottenstreich, A.; Barkai, A.; Arad, A.; Raccah, B.H.; Kalish, Y. The effect of bariatric surgery on direct-acting oral anticoagulant drug levels. Thromb. Res. 2018, 163, 190–195. [Google Scholar] [CrossRef]
- Rousseau, C.; Jean, S.; Gamache, P.; Lebel, S.; Mac-Way, F.; Biertho, L.; Michou, L.; Gagnon, C. Change in fracture risk and fracture pattern after bariatric surgery: Nested case-control study. BMJ 2016, i3794. [Google Scholar] [CrossRef]
- Ruiz-Tovar, J.; Oller, I.; Priego, P.; Arroyo, A.; Calero, A.; Diez, M.; Zubiaga, L.; Calpena, R. Short- and Mid-term Changes in Bone Mineral Density After Laparoscopic Sleeve Gastrectomy. Obes. Surg. 2013, 23, 861–866. [Google Scholar] [CrossRef]
- Sakhaee, K.; Pak, C. Superior calcium bioavailability of effervescent potassium calcium citrate over tablet formulation of calcium citrate after Roux-en-Y gastric bypass. Surg. Obes. Relat. Dis. 2013, 9, 743–748. [Google Scholar] [CrossRef] [PubMed]
- Sakhaee, K.; Griffith, C.; Pak, C.Y.C. Biochemical control of bone loss and stone-forming propensity by potassium-calcium citrate after bariatric surgery. Surg. Obes. Relat. Dis. 2012, 8, 67–72. [Google Scholar] [CrossRef]
- Salgado, W.; Modotti, C.; Nonino, C.B.; Ceneviva, R. Anemia and iron deficiency before and after bariatric surgery. Surg. Obes. Relat. Dis. 2014, 10, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Sallé, A.; Demarsy, D.; Poirier, A.L.; Lelièvre, B.; Topart, P.; Guilloteau, G.; Bécouarn, G.; Rohmer, V. Zinc deficiency: A frequent and underestimated complication after bariatric surgery. Obes. Surg. 2010, 20, 1660–1670. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, A.; Rojas, P.; Basfi-fer, K.; Carrasco, F.; Inostroza, J.; Codoceo, J.; Valencia, A.; Papapietro, K.; Csendes, A.; Ruz, M. Micronutrient deficiencies in morbidly obese women prior to bariatric surgery. Obes. Surg. 2016, 26, 361–368. [Google Scholar] [CrossRef]
- Santarpia, L.; Grandone, I.; Alfonsi, L.; Sodo, M.; Contaldo, F.; Pasanisi, F. Long-term medical complications after malabsorptive procedures: Effects of a late clinical nutritional intervention. Nutrition 2014, 30, 1301–1305. [Google Scholar] [CrossRef]
- Schiavo, L.; Scalera, G.; Pilone, V.; De Sena, G.; Quagliariello, V.; Iannelli, A.; Barbarisi, A. A Comparative study examining the impact of a protein-enriched vs. normal protein postoperative diet on body composition and resting metabolic rate in obese patients after sleeve gastrectomy. Obes. Surg. 2017, 27, 881–888. [Google Scholar] [CrossRef]
- Schijns, W.; Schuurman, L.T.; Melse-Boonstra, A.; van Laarhoven, C.J.H.M.; Berends, F.J.; Aarts, E.O. Do specialized bariatric multivitamins lower deficiencies after RYGB? Surg. Obes. Relat. Dis. 2018. [Google Scholar] [CrossRef]
- Schneider, J.; Peterli, R.; Gass, M.; Slawik, M.; Peters, T.; Wölnerhanssen, B.K. Laparoscopic sleeve gastrectomy and Roux-en-Y gastric bypass lead to equal changes in body composition and energy metabolism 17 months postoperatively: A prospective randomized trial. Surg. Obes. Relat. Dis. 2016, 12, 563–570. [Google Scholar] [CrossRef]
- Schollenberger, A.E.; Karschin, J.; Meile, T.; Küper, M.A.; Königsrainer, A.; Bischoff, S.C. Impact of protein supplementation after bariatric surgery: A randomized controlled double-blind pilot study. Nutrition 2016, 32, 186–192. [Google Scholar] [CrossRef]
- Shah, M.; Sharma, A.; Wermers, R.A.; Kennel, K.A.; Kellogg, T.A.; Mundi, M.S. Hypocalcemia after bariatric surgery: Prevalence and associated risk factors. Obes. Surg. 2017, 27, 2905–2911. [Google Scholar] [CrossRef] [PubMed]
- Sheng, B.; Truong, K.; Spitler, H.; Zhang, L.; Tong, X.; Chen, L. The long-term effects of bariatric surgery on type 2 diabetes remission, microvascular and macrovascular complications, and mortality: A systematic review and meta-analysis. Obes. Surg. 2017, 27, 2724–2732. [Google Scholar] [CrossRef] [PubMed]
- Sherf-Dagan, S.; Hod, K.; Buch, A.; Mardy-Tilbor, L.; Regev, Z.; Ben-Porat, T.; Sakran, N.; Goitein, D.; Raziel, A. Health and nutritional status of vegetarian candidates for bariatric surgery and practical recommendations. Obes. Surg. 2018, 28, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Sjöström, L.; Peltonen, M.; Jacobson, P.; Ahlin, S.; Andersson-Assarsson, J.; Anveden, Å.; Bouchard, C.; Carlsson, B.; Karason, K.; Lönroth, H.; et al. Association of bariatric surgery with long-term remission of type 2 diabetes and with microvascular and macrovascular complications. JAMA 2014, 311, 2297. [Google Scholar] [CrossRef]
- Silva, J.S.; Chaves, G.V.; Stenzel, A.P.; Pereira, S.E.; Saboya, C.J.; Ramalho, A. Improvement of anthropometric and biochemical, but not of vitamin A, status in adolescents who undergo Roux-en-Y gastric bypass: A 1-year follow up study. Surg. Obes. Relat. Dis. 2017, 13, 227–233. [Google Scholar] [CrossRef]
- Susmallian, S.; Barnea, R.; Weiss, Y.; Raziel, A. Outcome of bariatric surgery in older patients. Surg. Obes. Relat. Dis. 2018, 14, 1705–1713. [Google Scholar] [CrossRef]
- Susmallian, S.; Raziel, A.; Barnea, R.; Paran, H. Bariatric surgery in older adults: Should there be an age limit? Medicine (Baltimore) 2019, 98, e13824. [Google Scholar] [CrossRef]
- Tang, L.; Alsulaim, H.A.; Canner, J.K.; Prokopowicz, G.P.; Steele, K.E. Prevalence and predictors of postoperative thiamine deficiency after vertical sleeve gastrectomy. Surg. Obes. Relat. Dis. 2018. [Google Scholar] [CrossRef] [PubMed]
- Tondapu, P.; Provost, D.; Adams-Huet, B.; Sims, T.; Chang, C.; Sakhaee, K. Comparison of the absorption of calcium carbonate and calcium citrate after Roux-en-Y gastric bypass. Obes. Surg. 2009, 19, 1256–1261. [Google Scholar] [CrossRef]
- Topart, P.; Becouarn, G.; Sallé, A.; Ritz, P. Biliopancreatic diversion requires multiple vitamin and micronutrient adjustments within 2 years of surgery. Surg. Obes. Relat. Dis. 2014, 10, 936–941. [Google Scholar] [CrossRef]
- Tran, D.D.; Nwokeabia, I.D.; Purnell, S.; Zafar, S.N.; Ortega, G.; Hughes, K.; Fullum, T.M. Revision of Roux-en-Y gastric bypass for weight regain: A systematic review of techniques and outcomes. Obes. Surg. 2016, 26, 1627–1634. [Google Scholar] [CrossRef] [PubMed]
- Upala, S.; Jaruvongvanich, V.; Sanguankeo, A. Risk of nephrolithiasis, hyperoxaluria, and calcium oxalate supersaturation increased after Roux-en-Y gastric bypass surgery: A systematic review and meta-analysis. Surg. Obes. Relat. Dis. 2016, 12, 1513–1521. [Google Scholar] [CrossRef] [PubMed]
- Van Nieuwenhove, Y.; Dambrauskas, Z.; Campillo-Soto, A.; van Dielen, F.; Wiezer, R.; Janssen, I.; Kramer, M.; Thorell, A. Preoperative very low-calorie diet and operative outcome after laparoscopic gastric bypass: A randomized multicenter study. Arch. Surg. Chic. Ill. 1960 2011, 146, 1300–1305. [Google Scholar] [CrossRef]
- Van Rutte, P.W.J.; Aarts, E.O.; Smulders, J.F.; Nienhuijs, S.W. Nutrient deficiencies before and after sleeve gastrectomy. Obes. Surg. 2014, 24, 1639–1646. [Google Scholar] [CrossRef] [PubMed]
- Verger, E.O.; Aron-Wisnewsky, J.; Dao, M.C.; Kayser, B.D.; Oppert, J.-M.; Bouillot, J.-L.; Torcivia, A.; Clément, K. Micronutrient and protein deficiencies after gastric bypass and sleeve gastrectomy: A 1-year follow-up. Obes. Surg. 2016, 26, 785–796. [Google Scholar] [CrossRef] [PubMed]
- Vinan-Vega, M.; Diaz Vico, T.; Elli, E.F. Bariatric surgery in the elderly patient: Safety and short-time outcome. A case match analysis. Obes. Surg. 2018. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Guan, B.; Yang, W.; Yang, J.; Cao, G.; Lee, S. Prevalence of electrolyte and nutritional deficiencies in Chinese bariatric surgery candidates. Surg. Obes. Relat. Dis. 2016, 12, 629–634. [Google Scholar] [CrossRef]
- Wang, F.-G.; Yu, Z.-P.; Yan, W.-M.; Yan, M.; Song, M.-M. Comparison of safety and effectiveness between laparoscopic mini-gastric bypass and laparoscopic sleeve gastrectomy: A meta-analysis and systematic review. Medicine (Baltimore) 2017, 96, e8924. [Google Scholar] [CrossRef]
- Ward, E.K.; Jensen-Otsu, E.; Schoen, J.A.; Rothchild, K.; Mitchell, B.; Austin, G.L. Acid suppression medications are associated with suboptimal weight loss after laparoscopic Roux-en-Y gastric bypass in patients older than 40 years. Surg. Obes. Relat. Dis. 2015, 11, 585–590. [Google Scholar] [CrossRef]
- Wolf, E.; Utech, M.; Stehle, P.; Büsing, M.; Stoffel-Wagner, B.; Ellinger, S. Preoperative micronutrient status in morbidly obese patients before undergoing bariatric surgery: Results of a cross-sectional study. Surg. Obes. Relat. Dis. 2015, 11, 1157–1163. [Google Scholar] [CrossRef]
- Wei, J.-H.; Lee, W.-J.; Chong, K.; Lee, Y.-C.; Chen, S.-C.; Huang, P.-H.; Lin, S.-J. High incidence of secondary hyperparathyroidism in bariatric patients: Comparing different procedures. Obes. Surg. 2018, 28, 798–804. [Google Scholar] [CrossRef] [PubMed]
- Weng, T.-C.; Chang, C.-H.; Dong, Y.-H.; Chang, Y.-C.; Chuang, L.-M. Anaemia and related nutrient deficiencies after Roux-en-Y gastric bypass surgery: A systematic review and meta-analysis. BMJ Open 2015, 5, e006964. [Google Scholar] [CrossRef] [PubMed]
- White, M.G.; Ward, M.A.; Applewhite, M.K.; Wong, H.; Prachand, V.; Angelos, P.; Kaplan, E.L.; Grogan, R.H. Rates of secondary hyperparathyroidism after bypass operation for super-morbid obesity: An overlooked phenomenon. Surgery 2017, 161, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Ying, V.W.C.; Kim, S.H.H.; Khan, K.J.; Farrokhyar, F.; D’Souza, J.; Gmora, S.; Anvari, M.; Hong, D. Prophylactic PPI help reduce marginal ulcers after gastric bypass surgery: A systematic review and meta-analysis of cohort studies. Surg. Endosc. 2015, 29, 1018–1023. [Google Scholar] [CrossRef] [PubMed]
- Yorke, E.; Sheppard, C.; Switzer, N.J.; Kim, D.; de Gara, C.; Karmali, S.; Kanji, A.; Birch, D. Revision of sleeve gastrectomy to Roux-en-Y gastric bypass: A Canadian experience. Am. J. Surg. 2017, 213, 970–974. [Google Scholar] [CrossRef]
- Yska, J.P.; van Roon, E.N.; de Boer, A.; Leufkens, H.G.M.; Wilffert, B.; de Heide, L.J.M.; de Vries, F.; Lalmohamed, A. Remission of type 2 diabetes mellitus in patients after different types of bariatric surgery: A population-based cohort study in the United Kingdom. JAMA Surg. 2015, 150, 1126. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, Y.; Li, J.; Chen, D.; Cheng, Z.; Xu, S.; Huang, Y.; Wang, Q. A meta-analysis of the effects of bariatric surgery on fracture risk: Bariatric surgery and fracture. Obes. Rev. 2018, 19, 728–736. [Google Scholar] [CrossRef]
- Mechanick, J.I.; Apovian, C.; Brethauer, S.; Timothy Garvey, W.; Joffe, A.M.; Kim, J.; Kushner, R.F.; Lindquist, R.; Pessah-Pollack, R.; Seger, J.; et al. Clinical practice guidelines for the perioperative nutrition, metabolic, and nonsurgical support of patients undergoing bariatric procedures—2019 update: Cosponsored by American Association of Clinical Endocrinologists/American College of Endocrinology, The Obesity Society, American Society for Metabolic and Bariatric Surgery, Obesity Medicine Association, and American Society of Anesthesiologists. Obes. Silver Spring Md. 2020, 28, O1–O58. [Google Scholar] [CrossRef]
- Lima, K.V.G.D.; Costa, M.J.D.C.; Gonçalves, M.D.C.R.; Sousa, B.S.D. Deficiências de micronutrientes no pré-operatório de cirurgia bariátrica. ABCD Arq. Bras. Cir. Dig. São Paulo 2013, 26, 63–66. [Google Scholar] [CrossRef][Green Version]
- Amaya García, M.J.; Vílchez López, F.J.; Campos Martín, C.; Sánchez Vera, P.; Pereira Cunill, J.L. Micronutrientes en Cirugía Bariátrica. Nutr. Hosp. 2012, 349–361. [Google Scholar] [CrossRef]
- Vilchez López, F.J.; Campos Martín, C.; Amaya García, M.J.; Sánchez Vera, P.; Pereira Cunill, J.L. Very low calorie diets in clinical management of morbid obesity. Nutr. Hosp. 2013, 275–285. [Google Scholar] [CrossRef]
- Gargallo Fernández, M.; Basulto Marset, J.; Breton Lesmes, I.; Quiles Izquierdo, J.; Formiguera Sala, X.; Salas-Salvadó, J. Recomendaciones nutricionales basadas en la evidencia para la prevención y el tratamiento del sobrepeso y la obesidad en adultos (consenso FESNAD-SEEDO): Metodología y resumen ejecutivo (I/III). Nutr. Hosp. 2012, 27, 800–832. [Google Scholar] [CrossRef]
- Alami, R.S.; Morton, J.M.; Schuster, R.; Lie, J.; Sanchez, B.R.; Peters, A.; Curet, M.J. Is there a benefit to preoperative weight loss in gastric bypass patients? A prospective randomized trial. Surg. Obes. Relat. Dis. Off. J. Am. Soc. Bariatr. Surg. 2007, 3, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Van Wissen, J.; Bakker, N.; Doodeman, H.J.; Jansma, E.P.; Bonjer, H.J.; Houdijk, A.P.J. Preoperative methods to reduce liver volume in bariatric surgery: A systematic review. Obes. Surg. 2016, 26, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Gerber, P.; Anderin, C.; Gustafsson, U.O.; Thorell, A. Weight loss before gastric bypass and postoperative weight change: Data from the Scandinavian Obesity Registry (SOReg). Surg. Obes. Relat. Dis. Off. J. Am. Soc. Bariatr. Surg. 2016, 12, 556–562. [Google Scholar] [CrossRef]
- Martínez-Ramos, D.; Salvador-Sanchis, J.L.; Escrig-Sos, J. Pérdida de peso preoperatoria en pacientes candidatos a cirugía bariátrica. Recomendaciones basadas en la evidencia. Cir. Esp. 2012, 90, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Sakhaee, K.; Poindexter, J.; Aguirre, C. The effects of bariatric surgery on bone and nephrolithiasis. Bone 2016, 84, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kominiarek, M.A. Preparing for and managing a pregnancy after bariatric surgery. Semin. Perinatol. 2011, 35, 356–361. [Google Scholar] [CrossRef]
- Mathus-Vliegen, E.M.H.; Basdevant, A.; Finer, N.; Hainer, V.; Hauner, H.; Micic, D.; Maislos, M.; Roman, G.; Schutz, Y.; Tsigos, C.; et al. Prevalence, pathophysiology, health consequences and treatment options of obesity in the elderly: A guideline. Obes. Facts 2012, 5, 460–483. [Google Scholar] [CrossRef]
- Mathus-Vliegen, E.M.H. Obesity and the elderly. J. Clin. Gastroenterol. 2012, 46, 533–544. [Google Scholar] [CrossRef] [PubMed]
| LoE | Interpretation |
|---|---|
| 1++ | High quality meta-analyses, systematic reviews of CTs, or high quality CTs with a very low risk of bias |
| 1+ | Well conducted meta-analyses, systematic reviews of CTs, or well conducted CTs with a low risk of bias |
| 1− | Meta-analyses, systematic reviews of CTs, or CTs with a high risk of bias |
| 2++ | High quality systematic reviews of case control or cohort studies. Case control or cohort studies with a low risk of bias and a high probability that the relationship is causal |
| 2+ | Well conducted case control or cohort studies with a low risk of bias and a moderate probability that the relationship is causal |
| 2− | Case control or cohort studies with a high risk of bias and a significant risk that the relationship is not causal |
| 3 | Non-analytic studies, e.g., case reports and case series |
| 4 | Expert opinion |
| Grade of Recommendation | Interpretation |
|---|---|
| A | At least one meta-analysis, systematic review, or CT rated as 1++, and directly applicable to the guidelines target population; or A body of scientific evidence consisting of studies rated as 1+ and demonstrating overall consistency of results. |
| B | A body of scientific evidence including studies rated as 2++, directly applicable to the guidelines target population, and demonstrating overall consistency of results; or Extrapolated scientific evidence from studies rated as 1++ or 1+ |
| C | A body of scientific evidence including studies rated as 2+, directly applicable to the guidelines target population and demonstrating overall consistency of results; or Extrapolated scientific evidence from studies rated as 2++ |
| D | Scientific evidence level 3 or 4; or Extrapolated scientific evidence from studies rated as 2+ |
| Articles Used to Answer Each Question | ||||
|---|---|---|---|---|
| First Author | Type of Study | SIGN/AGREE II Score | LoE | Reference |
| Aaseth E | Case series | No checklist required | 3 | [7] |
| Abdemur A | Case series | No checklist required | 3 | [8] |
| Adams TD | Case-control study | High quality (++) | 2++ | [9] |
| Alexandrou A | Transversal study | No checklist required | 3 | [10] |
| Aron-Wisnewsky J | Case series | No checklist required | 3 | [11] |
| Bailly L | Case series | No checklist required | 3 | [12] |
| Basfi-Fer K | Case series | No checklist required | 3 | [13] |
| Ben-Porat T | Case series | No checklist required | 3 | [14] |
| Benassar Remolar MA | Case series | No checklist required | 3 | [15] |
| Botella-Carretero JI | Case series | No checklist required | 3 | [16] |
| Botella Romero F | Case series | No checklist required | 3 | [17] |
| Boyce SG | Case series | No checklist required | 3 | [18] |
| Brethauer SA | Systematic review | Low quality (−) | 1− | [19] |
| Busetto L | Practice guideline | 74.53% Good quality | NA | [20] |
| Cabral J | Systematic review | Low quality (−) | 1− | [21] |
| Caron M | Case series | No checklist required | 3 | [22] |
| Casillas RA | Case series | No checklist required | 3 | [23] |
| Chagas C | Case series | No checklist required | 3 | [24] |
| Chakhtoura MT | Systematic review | Acceptable (+) | 1+ | [25] |
| Chakhtoura MT | Systematic review | High quality (++) | 1++ | [26] |
| Chakhtoura MT | Systematic review | High quality (++) | 1++ | [27] |
| Coblijn UK | Systematic review | Acceptable (+) | 1+ | [28] |
| Coblijn UK | Systematic review | Low quality (−) | 1− | [29] |
| Cosendey Menegati G | Case-control study | Acceptable (+) | 2+ | [30] |
| Costa TL | Case-control study | Acceptable (+) | 2+ | [31] |
| Dagan SS | Cohort study | Acceptable (+) | 2+ | [32] |
| Daigle CR | Case series | No checklist required | 3 | [33] |
| De Luis DA | Case series | No checklist required | 3 | [34] |
| Del Villar Madrigal E | Case series | No checklist required | 3 | [35] |
| Dogan K | Case series | No checklist required | 3 | [36] |
| Dunstan M | Case series | No checklist required | 3 | [37] |
| Edholm D | Case series | No checklist required | 3 | [38] |
| Edholm D | Case series | No checklist required | 3 | [39] |
| Elbahrawy A | Case series | No checklist required | 3 | [40] |
| Elhag W | Case series | No checklist required | 3 | [41] |
| Fashandi AZ | Case series | No checklist required | 3 | [42] |
| Ferreira-Nicoletti C | Pre-post study | No checklist required | 3 | [43] |
| Flores L | NRCT | Acceptable (+) | 1+ | [44] |
| Flores L | Case series | No checklist required | 3 | [45] |
| Froylich D | Case series | No checklist required | 3 | [46] |
| Fulton C | Case series | No checklist required | 3 | [47] |
| Gadgil MD | Case-control study | High quality (++) | 2++ | [48] |
| Gebhart A | Case series | No checklist required | 3 | [49] |
| Gesquiere I | Case series | No checklist required | 3 | [50] |
| Gesquiere I | Case series | No checklist required | 3 | [51] |
| Gillon S | Case series | No checklist required | 3 | [52] |
| Gimenes JC | Transversal study | No checklist required | 3 | [53] |
| Gimenes JC | Case series | No checklist required | 3 | [54] |
| Giordano S | Systematic review | High quality (++) | 1++ | [55] |
| Giordano S | Systematic review | Acceptable (+) | 1+ | [56] |
| Gobato RC | Case series | No checklist required | 3 | [57] |
| Goldberg HR | Case series | No checklist required | 3 | [58] |
| Gomes de Lima KV | Case series | No checklist required | 3 | [59] |
| González-Navarro I | Case series | No checklist required | 3 | [60] |
| Grace, C | Case series | No checklist required | 3 | [61] |
| Gregory DM | Pre-post study | No checklist required | 3 | [62] |
| Haywood C | Systematic review | Low quality (−) | 1− | [63] |
| Homan J | Case series | No checklist required | 3 | [64] |
| Hsin MC | Cohort study | Acceptable (+) | 2+ | [65] |
| Iannelli A | Case series | No checklist required | 3 | [66] |
| James H | Case series | No checklist required | 3 | [67] |
| Jans G | Systematic review | Low quality (−) | 2++ | [68] |
| Jans G | Cohort study | Acceptable (+) | 2+ | [69] |
| Jáuregui-Lobera I | Systematic review | Acceptable (+) | 1+ | [70] |
| Kalani A | Systematic review | High quality (++) | 1++ | [71] |
| Kim MK | Case series | No checklist required | 3 | [72] |
| Kiyomi-Ito M | Systematic review | High quality (++) | 1++ | [73] |
| Kornerup LS | Case series | No checklist required | 3 | [74] |
| Kwon Y | Systematic review | High quality (++) | 1++ | [75] |
| Krzizek EC | Case series | No checklist required | 3 | [76] |
| Lecube A | Transversal study | No checklist required | 3 | [77] |
| Lefebvre P | Case series | No checklist required | 3 | [78] |
| Leite Faria, S | Open RCT | Acceptable (+) | 1− | [79] |
| Li Z | Systematic review | High quality (++) | 1++ | [80] |
| Liu C | Systematic review | High quality (++) | 1++ | [81] |
| Lucas Soares F | Case series | No checklist required | 3 | [82] |
| Luger M | RCT | High quality (++) | 1+ | [83] |
| Majumder S | Systematic review | Low quality (−) | 1− | [84] |
| Malone M | Case series | No checklist required | 3 | [85] |
| Mann JP | Systematic review | Low quality (−) | 1− | [86] |
| Manousou S | Case-control study | High quality (++) | 2++ | [87] |
| Marczuk P | Systematic review | High quality (++) | 1++ | [88] |
| Martín García-Almenta E | Practice guideline | 54.03% Low quality | NA | [89] |
| McCracken E | Case-control study | High quality (++) | 2++ | [90] |
| McGlone ES | Case series | No checklist required | 3 | [91] |
| Mead NC | Transversal study | No checklist required | 3 | [92] |
| Mechanick JL | Practice guideline | 90.06% Excellent quality | NA | [93] |
| Mendes-Filho AM | Systematic review | Low quality (−) | 1− | [94] |
| Mingrone G | Systematic review | Low quality (−) | 1− | [95] |
| Mischler R | RCT | Low quality (−) | 1− | [96] |
| Mischler R | Transversal study | No checklist required | 3 | [97] |
| Moizé V | Case series | No checklist required | 3 | [98] |
| Moore CE | Case series | No checklist required | 3 | [99] |
| Morales MP | Case series | No checklist required | 3 | [100] |
| Nicoletti CF | Case series | No checklist required | 3 | [101] |
| O’Kane M | Practice guideline | 77.63% Good quality | NA | [102] |
| Obeid NR | Case series | No checklist required | 3 | [103] |
| Obinwanne K | Case series | No checklist required | 3 | [104] |
| Obinwanne K | Case series | No checklist required | 3 | [105] |
| Olbers T | Cohort study | High quality (++) | 2+ | [106] |
| Parmar C | Transversal study | No checklist required | 3 | [107] |
| Parrot J | Practice guideline | 63.35% Acceptable quality | NA | [108] |
| Pędziwiatr M | Systematic review | High quality (++) | 1++ | [109] |
| Pellitero S | Case series | No checklist required | 3 | [110] |
| Pereira S | NRCT | Acceptable (+) | 1− | [111] |
| Pereira Da Cruz S | Cohort study | High quality (++) | 2+ | [112] |
| Pereira-Santos M | Systematic review | High quality (++) | 1++ | [113] |
| Pérez Quirante F | Transversal study | No checklist required | 3 | [114] |
| Peterson LA | Systematic review | Low quality (−) | 1− | [115] |
| Peterson LA | Transversal study | No checklist required | 3 | [116] |
| Pinto-Bastos A | Systematic review | Low quality (−) | 1− | [117] |
| Pratt JSA | Practice guideline | 70.8% Good quality | NA | [118] |
| Quezada N | Case series | No checklist required | 3 | [119] |
| Rodríguez-Carmona Y | Systematic review | High quality (++) | 1++ | [120] |
| Rottenstreich A | Case series | No checklist required | 3 | [121] |
| Rousseau C | Case-control study | High quality (++) | 2++ | [122] |
| Ruíz-Tovar J | Case series | No checklist required | 3 | [123] |
| Sakhaee K | RCT | Acceptable (+) | 1+ | [124] |
| Sakhaee K | RCT | Acceptable (+) | 1+ | [125] |
| Salgado W | Case series | No checklist required | 3 | [126] |
| Sallé A | Case series | No checklist required | 3 | [127] |
| Sánchez A | Transversal study | No checklist required | 3 | [128] |
| Santarpia, L | Case series | No checklist required | 3 | [129] |
| Schiavo L | Pre-post study | No checklist required | 3 | [130] |
| Schijns W | Cohort study | Acceptable (+) | 2+ | [131] |
| Schneider J | RCT | Acceptable (+) | 1+ | [132] |
| Schollenberger AE | RCT | Acceptable (+) | 1+ | [133] |
| Shah M | Case series | No checklist required | 3 | [134] |
| Sheng B | Systematic review | High quality (++) | 1+ | [135] |
| Sherf-Dagan S | Transversal study | No checklist required | 3 | [136] |
| Sjöström L | Cohort study | Acceptable (+) | 2+ | [137] |
| Souza Silva J | Pre-post study | No checklist required | 3 | [138] |
| Susmallian S | Case series | No checklist required | 3 | [139] |
| Susmallian S | Case series | No checklist required | 3 | [140] |
| Tang L | Case series | No checklist required | 3 | [141] |
| Tondapu P | RCT | Acceptable (+) | 1+ | [142] |
| Topart P | Case series | No checklist required | 3 | [143] |
| Tran DD | Systematic review | Low quality (−) | 1− | [144] |
| Upala S | Systematic review | High quality (++) | 1+ | [145] |
| Van Nieuwenhove Y | RCT | Acceptable (+) | 1+ | [146] |
| Van Rutte PWJ | Pre-post study | No checklist required | 3 | [147] |
| Verger EO | Case series | No checklist required | 3 | [148] |
| Vinan-Vega M | Case-control study | Acceptable (+) | 2+ | [149] |
| Wang C | Case series | No checklist required | 3 | [150] |
| Wang FG | Systematic review | High quality (++) | 1++ | [151] |
| Ward EK | Retrospective cohort study | Acceptable (+) | 2+ | [152] |
| Wolf E | Transversal study | No checklist required | 3 | [153] |
| Wei JH | Pre-post study | No checklist required | 3 | [154] |
| Wang TC | Systematic review | High quality (++) | 1++ | [155] |
| White MG | Case series | No checklist required | 3 | [156] |
| Wu Chao Ying V | Systematic review | High quality (++) | 1++ | [157] |
| Yorke E | Case series | No checklist required | 3 | [158] |
| Yska JP | Case series | No checklist required | 3 | [159] |
| Zhang Q | Systematic review | High quality (++) | 1++ | [160] |
| Total Caloric Value (Kcal/day) | 450–800 |
|---|---|
| Carbohydrate content | At least 55 gr/day |
| Protein content | 50 gr/day (high biological value) |
| Lipids | 7 gr |
| Linoleic acid | 3 gr |
| Alpha-linolenic acid | 0.5 gr |
| Fibre | 10 gr |
| Vitamins, micronutrients and trace elements | 100% of daily requirements |
| Pre-Surgery | 1 Month | 3 Months | 6 Months | 12 Months | Annual | |
|---|---|---|---|---|---|---|
| CBC/Biochemistry | X | X | X | X | X | X |
| Albumin | X | X | X | X | X | X |
| Prealbumin | X | Optional | Optional | Optional | Optional | Optional |
| CRP | X | X | X | X | X | X |
| Iron/Ferritin | X | X | X | X | X | |
| Ca/P/Mg | X | X | X | X | X | |
| iPTH | X | X | X | |||
| B12/Folic acid | X | X * | XA | XA | XA | |
| Vitamin D | X | X | X | X | ||
| Zn/Cu | Optional | Optional | Optional | |||
| B1 | Optional | Optional | Optional | |||
| Vitamin A and E | X | X A | X A |
| Recommendations of the American Society for Metabolic and Bariatric Surgery (ASMBS) | Multicentrum (Per tablet) | Multi-Tenex (Per tablet) | Supradyn (Per tablet) | Micebrina Complex | |
|---|---|---|---|---|---|
| Vit A, μg | 1500–3000 | 800 | 800 | 800 | 450 |
| Vit B1, mg | 1.2 | 1.4 | 1.4 | 1.1 | 10 |
| Vit B12, μg | 350–500 | 2.5 | 1 | 2.5 | 12 |
| Vit D3, μg | 75 | 5 | 5 | 5 | 10 |
| Vit E, mg | 15 | 15 | 10 | 12 | 30 |
| Vit K, μg | 90–120 | 30 | - | 25 | - |
| Copper, mg | 1–2 | 0.5 | 2 | 1 | 2 |
| Iron, mg | 45–60 | 5 | 14 | 14 | 18 |
| Zinc, mg | 8–22 | 5 | 14 | 10 | 15 |
| Calcium, mg | 1200–1500 | 162 | 100 | 120 | 0.15 (Calcium iodate) |
| Recommendation | Grade of Recommendation/Consensus Level |
|---|---|
| It is convenient to determine the levels of certain micronutrients and vitamins preoperatively, at least vitamin D and iron metabolism. Folic acid and B12 vitamin should be included in certain populations | D/93% |
| Specific micronutrient supplements should be used if there is any evidence of any preoperative deficit following the current treatment recommendations | D/100% |
| We recommend the use of a liquid VLCD diet preoperatively, for at least 4-8 weeks minimum prior to surgery and ideally for a longer length of time in selected patients | B/91% |
| After surgery, a liquid diet should be maintained for about 4 weeks, and then a semi-solid diet for another 4 weeks | D/84% |
| The GARIN group advises against calculating the protein provision based on a percentage of the diet’s total caloric value, since this method often results in insufficient intake. Instead, it is advisable to use a direct calculation based on the adjusted weight, at least 1 to 1.5 gr of high biological value protein per Kg of weight and day | D/96% |
| The use of protein supplements could be beneficial in the 6-12 months after surgery | B/96% |
| The postoperative use of calcium (1000 mg of calcium element at least) and vitamin D (880 IU of cholecalciferol) supplements are recommended | A/91% |
| In biliopancreatic diversion/Scopinaro surgeries the GARIN group recommends a higher intake of calcium (2000 mg/d) and especially a higher intake of vitamin D (2000 IU/d) | D/91% |
| Periodic monitoring of iron levels after surgery should be performed, and in the case of deficit, treated accordingly | D/85% |
| Use of parenteral treatment for vitamin B12 deficiency only if the deficit is evident | D/85% |
| Although there is no scientific evidence, a consideration of the pathophysiological mechanism of Obesity Surgery, especially malabsorptive surgery, would make an increase of the dietary intake of other micronutrients, including supplements recommendable | D/89% |
| Calcium citrate preparations should be recommended above other calcium compounds, especially in RYGBP | B/93% |
| The GARIN group suggest periodic and customised analytical follow-up after surgery. Vitamin A, E, B12 and folic acid are mandatory in malabsorptive surgery (See text for details) | D/89% |
| The GARIN group recommends individualising the use and duration of PPI therapy | D/96% |
| We recommend the systematic use of multivitamin and mineral complexes | C/98% |
| The GARIN group recommends, whenever available, the use of supplements that are specifically designed for patients undergoing Obesity Surgery | D/85% |
| We recommend periodical kidney function monitoring using serum creatinine and specific formulas to estimate glomerular filtration, occasional 24-hourour urine calcium, and, in selected cases, imaging tests, at least in patients who underwent RYGBP to rule out kidney stones. | D/84% |
| After one year post-surgery, the GARIN group recommends annual check-ups in Specialised Care for at least five years of all patients who underwent Obesity Surgery. After this time, it is advisable to maintain annual check-ups in patients who underwent malabsorptive techniques, while those patients without complications who underwent purely restrictive techniques do not require specialised follow-up, except in selected cases | D/87% |
| The GARIN group recommends that in patients with T2DM, RYGBP should be considered before LSG; independently of the technique used, it should be performed by a Surgical Team with experience in that technique | D/91% |
| Pregnancy should be avoided in the first year after Obesity Surgery, and contraceptive measures should be routinely recommended | D/87% |
| All women of childbearing age undergoing Obesity Surgery should programme their gestation at an experienced centre | D/87% |
| Possible micronutrient deficits (Vitamin A, D, E, K, B12, folic, iron) should be detected (AND treated) at least every six months prior to gestation, in each trimester of pregnancy (or sooner if any type of deficit is detected ) and at 6-8 weeks post-partum, especially in case of breastfeeding. | C/87% |
| Breastfeeding is encouraged | D/87% |
| In selected adolescent population between 13 and 18 years LSG can be considered for weight loss | D/87% |
| In selected adolescent patients between 13 and 18 years, RYGBP can be considered for weight loss, especially in patients with prediabetes/T2DM, hypertension and/or dyslipidaemia | C/87% |
| BGYR should be discouraged in people over 65, especially in cases of high cardiovascular risk | A/87% |
| In selected patients over 65 years LSG could be considered as an option | B/87% |
| If re-intervention is needed due to poor results in weight loss, RYGBP after LSG is an effective and safe option, allowing additional weight loss and improvement of comorbidities. If the first surgery was RYGBP, biliopancreatic diversion seems to be the most favourable technique | B/75% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Ortega, A.J.; Olveira, G.; Pereira-Cunill, J.L.; Arraiza-Irigoyen, C.; García-Almeida, J.M.; Irles Rocamora, J.A.; Molina-Puerta, M.J.; Molina Soria, J.B.; Rabat-Restrepo, J.M.; Rebollo-Pérez, M.I.; et al. Recommendations Based on Evidence by the Andalusian Group for Nutrition Reflection and Investigation (GARIN) for the Pre- and Postoperative Management of Patients Undergoing Obesity Surgery. Nutrients 2020, 12, 2002. https://doi.org/10.3390/nu12072002
Martínez-Ortega AJ, Olveira G, Pereira-Cunill JL, Arraiza-Irigoyen C, García-Almeida JM, Irles Rocamora JA, Molina-Puerta MJ, Molina Soria JB, Rabat-Restrepo JM, Rebollo-Pérez MI, et al. Recommendations Based on Evidence by the Andalusian Group for Nutrition Reflection and Investigation (GARIN) for the Pre- and Postoperative Management of Patients Undergoing Obesity Surgery. Nutrients. 2020; 12(7):2002. https://doi.org/10.3390/nu12072002
Chicago/Turabian StyleMartínez-Ortega, Antonio J., Gabriel Olveira, José L. Pereira-Cunill, Carmen Arraiza-Irigoyen, José M. García-Almeida, José A. Irles Rocamora, María J. Molina-Puerta, Juan B. Molina Soria, Juana M. Rabat-Restrepo, María I. Rebollo-Pérez, and et al. 2020. "Recommendations Based on Evidence by the Andalusian Group for Nutrition Reflection and Investigation (GARIN) for the Pre- and Postoperative Management of Patients Undergoing Obesity Surgery" Nutrients 12, no. 7: 2002. https://doi.org/10.3390/nu12072002
APA StyleMartínez-Ortega, A. J., Olveira, G., Pereira-Cunill, J. L., Arraiza-Irigoyen, C., García-Almeida, J. M., Irles Rocamora, J. A., Molina-Puerta, M. J., Molina Soria, J. B., Rabat-Restrepo, J. M., Rebollo-Pérez, M. I., Serrano-Aguayo, M. P., Tenorio-Jiménez, C., Vílches-López, F. J., & García-Luna, P. P. (2020). Recommendations Based on Evidence by the Andalusian Group for Nutrition Reflection and Investigation (GARIN) for the Pre- and Postoperative Management of Patients Undergoing Obesity Surgery. Nutrients, 12(7), 2002. https://doi.org/10.3390/nu12072002





