Iron Supplementation Influence on the Gut Microbiota and Probiotic Intake Effect in Iron Deficiency—A Literature-Based Review
Abstract
1. Introduction
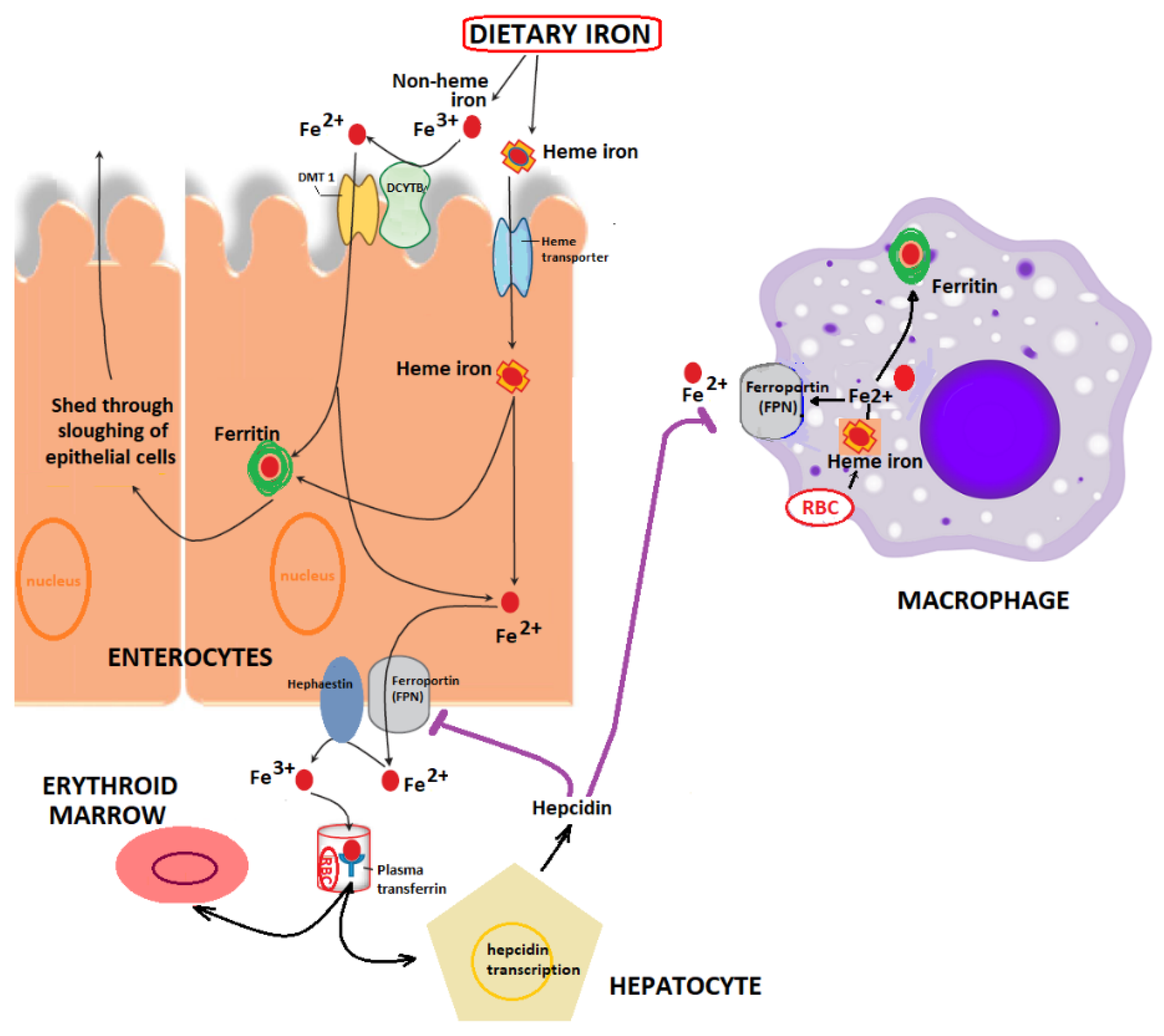
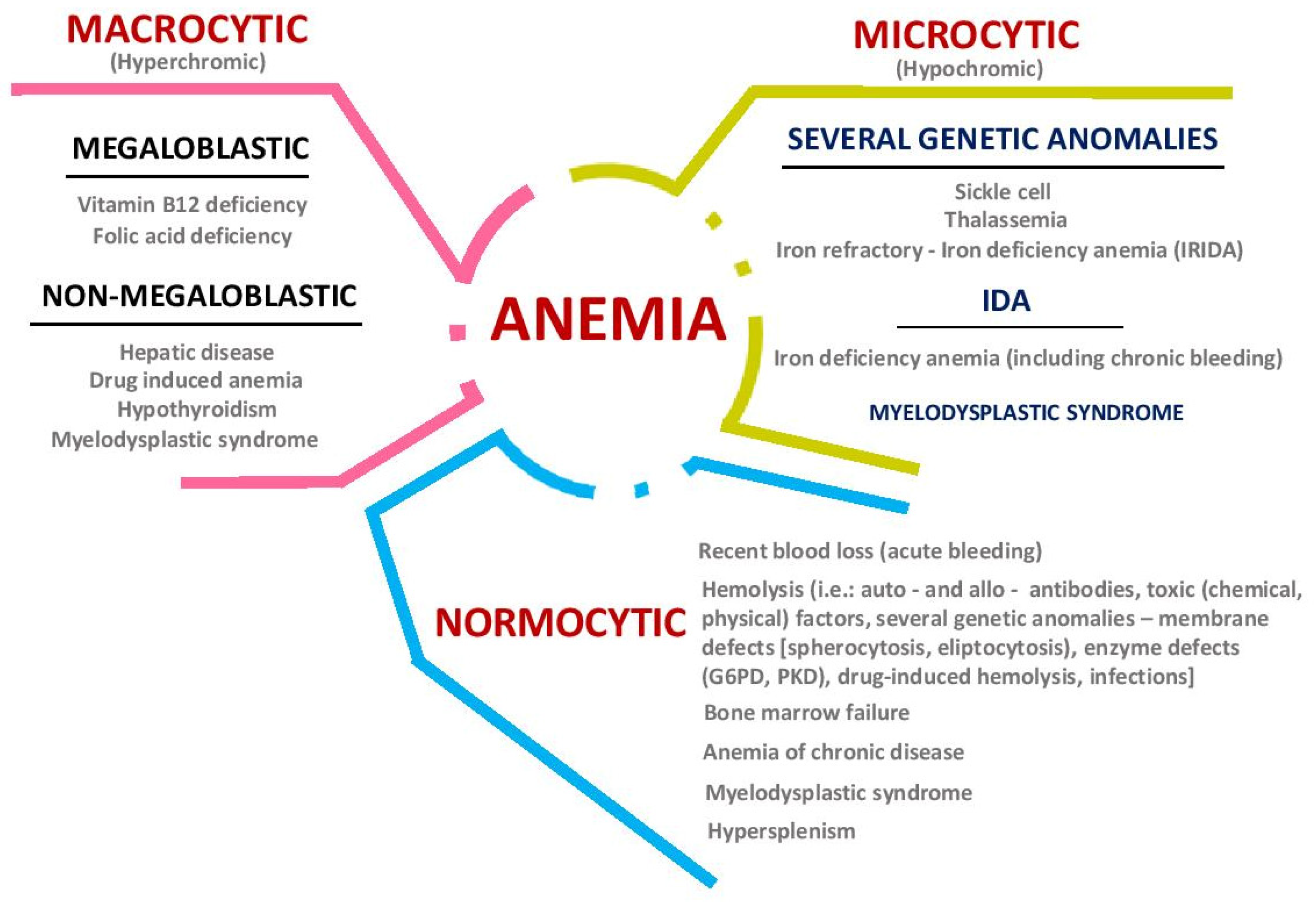
2. Iron Metabolism: Implications in Health and Disease
3. Gut Microbiota and Iron Deficiency
4. Iron Absorption from Supplements and from Foods
5. Probiotic, Prebiotic, and Synbiotic Approach in Iron Deficiency Treatment
5.1. Probiotics and Iron Deficiency Treatment
5.2. Prebiotics and Synbiotics in Iron Deficiency Treatment
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Boccio, J.R.; Iyengar, V. Iron deficiency: Causes, consequences, and strategies to overcome this nutritional problem. Biol. Trace Elem. Res. 2003, 94, 1–32. [Google Scholar] [CrossRef]
- Goddard, A.F.; James, M.W.; McIntyre, A.S.; Scott, B.B. Guidelines for the management of iron deficiency anaemia. Gut 2011, 60, 1309–1316. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.L. Iron deficiency anemia: A common and curable disease. Cold Spring Harb. Perspect Med. 2013, 3, a011866. [Google Scholar] [CrossRef] [PubMed]
- Chiplonkar, S.A.; Agte, V.V. Statistical model for predicting non-heme iron bioavailability from vegetarian meals. Int. J. Food Sci. Nutr. 2006, 57, 434–450. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.B. Algorithms to assess non-heme iron bioavailability. Int. J. Vitam. Nutr. Res. 2005, 75, 405–412. [Google Scholar] [CrossRef]
- Vonderheid, S.C.; Tussing-Humphreys, L.; Park, C.; Pauls, H.; Hemphill, N.O.; LaBomascus, B.; McLeod, A.; Koenig, M.D. A Systematic Review and Meta-Analysis on the Effects of Probiotic Species on Iron Absorption and Iron Status. Nutrients 2019, 11, 2938. [Google Scholar] [CrossRef]
- Hoppe, M.; Önning, G.; Berggren, A.; Hulthén, L. Probiotic strain Lactobacillus plantarum 299v increases iron absorption from an iron-supplemented fruit drink: A double-isotope cross-over single-blind study in women of reproductive age. Brit. J. Nutr. 2015, 114, 1195–1202. [Google Scholar] [CrossRef]
- Marciano, R.; Santamarina, A.B.; de Santana, A.A.; Silva, M.D.L.C.; Amancio, O.M.S.; do Nascimento, C.M.D.P.O.; Oyama, L.M.; de Morais, M.B. Effects of prebiotic supplementation on the expression of proteins regulating iron absorption in anaemic growing rats. Brit. J. Nutr. 2015, 113, 901–908. [Google Scholar] [CrossRef]
- Gupta, A. Iron Metabolism in Human Body. In Nutritional Anemia in Preschool Children; Springer: Singapore, 2017. [Google Scholar]
- Camaschella, C. Iron-deficiency anemia. N. Engl. J. Med. 2015, 372, 1832–1843. [Google Scholar] [CrossRef]
- Wilson, A.S.; Koller, K.R.; Ramaboli, M.C.; Nesengani, L.T.; Ocvirk, S.; Chen, C.X.; Flanagan, C.A.; Sapp, F.R.; Merritt, Z.T.; Bhatti, F.; et al. Diet and the Human Gut Microbiome: An International Review. Dig. Dis. Sci. 2020, 65, 723–740. [Google Scholar] [CrossRef]
- Andrews, S.C.; Robinson, A.K.; Rodríguez-Quiñones, F. Bacterial iron homeostasis. FEMS Microbiol. Rev. 2003, 27, 215–237. [Google Scholar] [CrossRef]
- Braun, V.; Hantke, K. Recent insights into iron import by bacteria. Curr. Opin. Chem. Biol. 2011, 15, 328–334. [Google Scholar] [CrossRef]
- Xi, R.; Wang, R.; Wang, Y.; Xiang, Z.; Su, Z.; Cao, Z.; Xu, X.; Zheng, X.; Li, J. Comparative analysis of the oral microbiota between iron-deficiency anaemia (IDA) patients and healthy individuals by high-throughput sequencing. BMC Oral Health 2019, 19, 255. [Google Scholar] [CrossRef] [PubMed]
- Beasley, F.C.; Marolda, C.L.; Cheung, J.; Buac, S.; Heinrichs, D.E. Staphylococcus aureus transporters Hts, Sir, and Sst capture iron liberated from human transferrin by Staphyloferrin A, Staphyloferrin B, and catecholamine stress hormones, respectively, and contribute to virulence. Infect. Immun. 2011, 79, 2345. [Google Scholar] [CrossRef] [PubMed]
- Cherayil, B.J.; Ellenbogen, S.; Shanmugam, N.N. Iron and intestinal immunity. Curr. Opin. Gastroenterol. 2011, 27, 523–528. [Google Scholar] [CrossRef] [PubMed]
- van de Lagemaat, M.; Amesz, E.M.; Schaafsma, A.; Lafeber, H.N. Iron deficiency and anemia in iron-fortified formula and human milk-fed preterm infants until 6 months post-term. Eur. J. Nutr. 2014, 53, 1263–1271. [Google Scholar] [CrossRef]
- Shubham, K.; Anukiruthika, T.; Dutta, S.; Kashyap, A.V.; Moses, J.A.; Anandharamakrishnan, C. Iron deficiency anemia: A comprehensive review on iron absorption, bioavailability and emerging food fortification approaches. Trends Food Sci. Technol. 2020, 99, 58–75. [Google Scholar] [CrossRef]
- Bierings, M.; Clayton, P.T.; Houwen, R.H. Disorders in the transport of copper, iron, magnesium, manganese, selenium and zinc. In Inborn Metabolic Diseases; Springer: Berlin, Germany, 2012; pp. 535–551. [Google Scholar]
- Jansen, V. Diagnosis of anemia—A synoptic overview and practical approach. Transfus. Apher. Sci. 2019, 58, 375–385. [Google Scholar] [CrossRef]
- Kowdley, K.V.; Brown, K.E.; Ahn, J.; Sundaram, V. ACG Clinical Guideline: Hereditary Hemochromatosis. Am. J. Gastroenterol. 2019, 114, 1202–1218. [Google Scholar] [CrossRef]
- Arredondo, M.; Núñez, M.T. Iron and copper metabolism. Mol. Asp. Med. 2005, 26, 313–327. [Google Scholar] [CrossRef]
- Fan, Y.; Dhaliwal, H.K.; Menon, A.V.; Chang, J.; Choi, J.E.; Amiji, M.M.; Kim, J. Site-specific intestinal DMT1 silencing to mitigate iron absorption using pH-sensitive multi-compartmental nanoparticulate oral delivery system. Nanomed. Nanotechnol. Biol. Med. 2019, 22, 102091. [Google Scholar] [CrossRef] [PubMed]
- Hentze, M.W.; Muckenthaler, M.U.; Galy, B.; Camaschella, C. Two to Tango: Regulation of Mammalian Iron Metabolism. Cell 2010, 142, 24–38. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.S.; Patnana, P.K.; Lomada, S.K.; Tomar, A.; Chatterjee, S. Co-regulation of Iron Metabolism and Virulence Associated Functions by Iron and XibR, a Novel Iron Binding Transcription Factor, in the Plant Pathogen Xanthomonas. PLoS Pathog. 2016, 12, e1006019. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Sheng, X.; Chang, Z.; Wu, Q.; Wang, S.; Xuan, Z.; Li, D.; Wu, Y.; Shang, Y.; Kong, X.; et al. Iron Metabolism Regulates p53 Signaling through Direct Heme-p53 Interaction and Modulation of p53 Localization, Stability, and Function. Cell Rep. 2014, 7, 180–193. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yu, L.; Ding, J.; Chen, Y. Iron Metabolism in Cancer. Int. J. Mol. Sci. 2019, 20, 95. [Google Scholar] [CrossRef]
- Yaskolka Meir, A.; Tsaban, G.; Zelicha, H.; Rinott, E.; Kaplan, A.; Youngster, I.; Rudich, A.; Shelef, I.; Tirosh, A.; Brikner, D. A green-Mediterranean diet, supplemented with Mankai duckweed, preserves iron-homeostasis in humans and is efficient in reversal of anemia in rats. J. Nutr. 2019, 149, 1004–1011. [Google Scholar] [CrossRef] [PubMed]
- Das, N.K.; Schwartz, A.J.; Barthel, G.; Inohara, N.; Liu, Q.; Sankar, A.; Hill, D.R.; Ma, X.; Lamberg, O.; Schnizlein, M.K.; et al. Microbial Metabolite Signaling Is Required for Systemic Iron Homeostasis. Cell Metab. 2020, 31, 115–130.e6. [Google Scholar] [CrossRef]
- Jia, H.X.; Han, J.H.; Li, H.Z.; Liang, D.; Deng, T.T.; Chang, S.Y. Mineral Intake in Urban Pregnant Women from Base Diet, Fortified Foods, and Food Supplements: Focus on Calcium, Iron, and Zinc. Biomed. Environ. Sci. 2016, 29, 898–901. [Google Scholar]
- World Health Organization. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Theml, H.; Diem, H.; Haferlach, T. Color Atlas of Hematology; Thieme: New York, NY, USA, 2004. [Google Scholar]
- Helmyati, S.; Rahayu, E.S.; Kandarina, B.J.I.; Juffrie, M. No Difference between Iron Supplementation Only and Iron Supplementation with Synbiotic Fermented Milk on Iron Status, Growth, and Gut Microbiota Profile in Elementary School Children with Iron Deficiency. Curr. Nutr. Food Sci. 2020, 16, 220–227. [Google Scholar] [CrossRef]
- Jaeggi, T.; Kortman, G.A.M.; Moretti, D.; Chassard, C.; Holding, P.; Dostal, A.; Boekhorst, J.; Timmerman, H.M.; Swinkels, D.W.; Tjalsma, H.; et al. Iron fortification adversely affects the gut microbiome, increases pathogen abundance and induces intestinal inflammation in Kenyan infants. Gut 2015, 64, 731. [Google Scholar] [CrossRef]
- Da Silva, W.R.; Silveira, L.; Fernandes, A.B. Diagnosing sickle cell disease and iron deficiency anemia in human blood by Raman spectroscopy. Lasers Med. Sci. 2019, 35, 1065–1074. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Nile, S.H.; Keum, Y.-S. Food science and technology for management of iron deficiency in humans: A review. Trends Food Sci. Technol. 2016, 53, 13–22. [Google Scholar] [CrossRef]
- Cammarota, G.; Ianiro, G.; Bibbò, S.; Gasbarrini, A. Gut microbiota modulation: Probiotics, antibiotics or fecal microbiota transplantation? Intern. Emerg. Med. 2014, 9, 365–373. [Google Scholar] [CrossRef]
- Gabbianelli, R.; Damiani, E. Epigenetics and neurodegeneration: Role of early-life nutrition. J. Nutr. Biochem. 2018, 57, 1–13. [Google Scholar] [CrossRef]
- Kim, Y.S.; Unno, T.; Kim, B.-Y.; Park, M.-S. Sex Differences in Gut Microbiota. World J. Mens Health 2020, 38, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Ursell, L.K.; Metcalf, J.L.; Parfrey, L.W.; Knight, R. Defining the human microbiome. Nutr. Rev. 2012, 70, S38–S44. [Google Scholar] [CrossRef]
- Tungland, B. Chapter 9—Dysbiosis of the Microbiota: Therapeutic Strategies Utilizing Dietary Modification, Pro- and Prebiotics and Fecal Transplant Therapies in Promoting Normal Balance and Local GI Functions. In Human Microbiota in Health and Disease; Tungland, B., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 381–419. [Google Scholar]
- Kim, S.R.; Kim, K.; Lee, S.A.; Kwon, S.O.; Lee, J.-K.; Keum, N.; Park, S.M. Effect of Red, Processed, and White Meat Consumption on the Risk of Gastric Cancer: An Overall and Dose–Response Meta-Analysis. Nutrients 2019, 11, 826. [Google Scholar] [CrossRef]
- Boran, P.; Baris, H.E.; Kepenekli, E.; Erzik, C.; Soysal, A.; Dinh, D.M. The impact of vitamin B12 deficiency on infant gut microbiota. Eur. J. Pediatr. 2020, 179, 385–393. [Google Scholar] [CrossRef]
- Deschemin, J.C.; Noordine, M.L.; Remot, A.; Willemetz, A.; Afif, C.; Canonne-Hergaux, F.; Langella, P.; Karim, Z.; Vaulont, S.; Thomas, M.; et al. The microbiota shifts the iron sensing of intestinal cells. FASEB J. 2016, 30, 252–261. [Google Scholar] [CrossRef]
- Forth, W.; Rummel, W. Iron absorption. Physiol. Rev. 1973, 53, 724–792. [Google Scholar] [CrossRef]
- Wollenberg, P.; Rummel, W. Dependence of intestinal iron absorption on the valency state of iron. N-S Arch. Pharmacol. 1987, 336, 578–582. [Google Scholar] [CrossRef] [PubMed]
- Dietzfelbinger, H. Bioavailability of bi-and trivalent oral iron preparations. Investigations of iron absorption by postabsorption serum iron concentrations curves. Arzneimittel-Forschung 1987, 37, 107–112. [Google Scholar]
- Bezkorovainy, A. Biochemistry of nonheme iron in man. I. Iron proteins and cellular iron metabolism. Clin. Physiol. Biochem. 1989, 7, 1–17. [Google Scholar] [PubMed]
- Cremonesi, P.; Acebron, A.; Raja, K.B.; Simpson, R.J. Iron absorption: Biochemical and molecular insights into the importance of iron species for intestinal uptake. Pharmacol. Toxicol. 2002, 91, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Simonyté Sjödin, K.; Domellöf, M.; Lagerqvist, C.; Hernell, O.; Lönnerdal, B.; Szymlek-Gay, E.A.; Sjödin, A.; West, C.E.; Lind, T. Administration of ferrous sulfate drops has significant effects on the gut microbiota of iron-sufficient infants: A randomised controlled study. Gut 2019, 68, 2095–2097. [Google Scholar] [CrossRef]
- Balamurugan, R.; Mary, R.R.; Chittaranjan, S.; Jancy, H.; Shobana Devi, R.; Ramakrishna, B.S. Low levels of faecal lactobacilli in women with iron-deficiency anaemia in south India. Brit. J. Nutr. 2010, 104, 931–934. [Google Scholar] [CrossRef] [PubMed]
- Jahani-Sherafat, S.; Alebouyeh, M.; Moghim, S.; Ahmadi Amoli, H.; Ghasemian-Safaei, H. Role of gut microbiota in the pathogenesis of colorectal cancer; a review article. Gastroenterol. Hepatol. Bed. Bench. 2018, 11, 101–109. [Google Scholar]
- Louis, P.; Hold, G.L.; Flint, H.J. The gut microbiota, bacterial metabolites and colorectal cancer. Nat. Rev. Microbiol. 2014, 12, 661–672. [Google Scholar] [CrossRef]
- Gagniere, J.; Raisch, J.; Veziant, J.; Barnich, N.; Bonnet, R.; Buc, E.; Bringer, M.A.; Pezet, D.; Bonnet, M. Gut microbiota imbalance and colorectal cancer. World J. Gastroenterol. 2016, 22, 501–518. [Google Scholar] [CrossRef]
- Gao, Z.; Guo, B.; Gao, R.; Zhu, Q.; Qin, H. Microbiota disbiosis is associated with colorectal cancer. Front. Microbiol. 2015, 6, 20. [Google Scholar] [CrossRef] [PubMed]
- Ng, O. Iron, microbiota and colorectal cancer. Wien. Med. Wochenschr. 2016, 166, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Dostal, A.; Baumgartner, J.; Riesen, N.; Chassard, C.; Smuts, C.M.; Zimmermann, M.B.; Lacroix, C. Effects of iron supplementation on dominant bacterial groups in the gut, faecal SCFA and gut inflammation: A randomised, placebo-controlled intervention trial in South African children. Brit. J. Nutr. 2014, 112, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Dostal, A.; Chassard, C.; Hilty, F.M.; Zimmermann, M.B.; Jaeggi, T.; Rossi, S.; Lacroix, C. Iron depletion and repletion with ferrous sulfate or electrolytic iron modifies the composition and metabolic activity of the gut microbiota in rats. J. Nutr. 2012, 142, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Buhnik-Rosenblau, K.; Moshe-Belizowski, S.; Danin-Poleg, Y.; Meyron-Holtz, E.G. Genetic modification of iron metabolism in mice affects the gut microbiota. BioMetals 2012, 25, 883–892. [Google Scholar] [CrossRef]
- Perez-Conesa, D.; Lopez, G.; Ros, G. Effect of probiotic, prebiotic and synbiotic follow-up infant formulas on iron bioavailability in rats. Food Sci. Technol. Int. 2007, 13, 69–77. [Google Scholar] [CrossRef]
- Collins, J.F.; Flores, S.R.; Wang, X.; Anderson, G.J. Mechanisms and Regulation of Intestinal Iron Transport. In Physiology of the Gastrointestinal Tract; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1451–1483. [Google Scholar]
- González, A.; Gálvez, N.; Martín, J.; Reyes, F.; Pérez-Victoria, I.; Dominguez-Vera, J.M. Identification of the key excreted molecule by Lactobacillus fermentum related to host iron absorption. Food Chem. 2017, 228, 374–380. [Google Scholar] [CrossRef]
- Gan, E.K.; Powell, L.W.; Olynyk, J.K. Natural History and Management of HFE-Hemochromatosis. Semin. Liver Dis. 2011, 31, 293–301. [Google Scholar] [CrossRef]
- Oh, C.-K.; Moon, Y. Dietary and Sentinel Factors Leading to Hemochromatosis. Nutrients 2019, 11, 1047. [Google Scholar] [CrossRef]
- Camaschella, C.; Poggiali, E. Inherited disorders of iron metabolism. Curr. Opin. Pediatrics 2011, 23, 14–20. [Google Scholar] [CrossRef]
- Pantopoulos, K. Inherited Disorders of Iron Overload. Front. Nutr. 2018, 5, 5. [Google Scholar] [CrossRef]
- Gulec, S.; Anderson, G.J.; Collins, J.F. Mechanistic and regulatory aspects of intestinal iron absorption. Am. J. Physiol. Gastrointest. Liver Physiol. 2014, 307, G397–G409. [Google Scholar] [CrossRef]
- Aslam, M.F.; Frazer, D.M.; Faria, N.; Bruggraber, S.F.A.; Wilkins, S.J.; Mirciov, C.; Powell, J.J.; Anderson, G.J.; Pereira, D.I.A. Ferroportin mediates the intestinal absorption of iron from a nanoparticulate ferritin core mimetic in mice. FASEB J. 2014, 28, 3671–3678. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, L.; Brait, D.; Vaz, M.; Lollo, P.; Morato, P.; Oesterreich, S.; Raposo, J.; Freitas, K. Partially Hydrolyzed Guar Gum Increases Ferroportin Expression in the Colon of Anemic Growing Rats. Nutrients 2017, 9, 228. [Google Scholar] [CrossRef]
- Liu, B.D.; Pan, X.H.; Liu, Z.H.; Han, M.L.; Xu, G.H.; Dai, X.S.; Wang, W.; Zhang, H.B.; Xie, L.W. Fecal microbiota as a noninvasive biomarker to predict the tissue iron accumulation in intestine epithelial cells and liver. FASEB J. 2020, 34, 3006–3020. [Google Scholar] [CrossRef] [PubMed]
- Kalipatnapu, S.; Kuppuswamy, S.; Venugopal, G.; Kaliaperumal, V.; Ramadass, B. Fecal total iron concentration is inversely associated with fecal Lactobacillus in preschool children. J. Gastroenterol. Hepatol. 2017, 32, 1475–1479. [Google Scholar] [CrossRef]
- Buret, A.G.; Motta, J.-P.; Allain, T.; Ferraz, J.; Wallace, J.L. Pathobiont release from dysbiotic gut microbiota biofilms in intestinal inflammatory diseases: A role for iron? J. Biomed. Sci. 2019, 26, 1. [Google Scholar] [CrossRef] [PubMed]
- Martınez-Navarrete, N.; Camacho, M.M.; Martınez-Lahuerta, J.; Martınez-Monzó, J.; Fito, P. Iron deficiency and iron fortified foods—A review. Food Res. Int. 2002, 35, 225–231. [Google Scholar] [CrossRef]
- Brazaca, S.G.C.; da Silva, F.C. Enhancers and inhibitors of iron availability in legumes. Plant Foods Hum. Nutr. 2003, 58, 1–8. [Google Scholar] [CrossRef]
- Record, I.R.; McInerney, J.K.; Dreosti, I.E. Black tea, green tea, and tea polyphenols. Biol. Trace Elem. Res. 1996, 53, 27–43. [Google Scholar] [CrossRef]
- Milne, D.B.; Canfield, W.K.; Mahalko, J.R.; Sandstead, H.H. Effect of oral folic acid supplements on zinc, copper, and iron absorption and excretion. Am. J. Clin. Nutr. 1984, 39, 535–539. [Google Scholar] [CrossRef]
- Shu, E.; Ogbodo, S. Role of Ascorbic Acid in the Prevention of Iron-Deficiency Anaemia in Pregnancy. Biomed. Res. 2005, 16, 40–44. [Google Scholar]
- Martínez-Torres, C.; Romano, E.; Layrisse, M. Effect of cysteine on iron absorption in man. Am. J. Clin. Nutr. 1981, 34, 322–327. [Google Scholar] [CrossRef]
- García-Casal, M.N.; Layrisse, M.; Solano, L.; Barón, M.A.; Arguello, F.; Llovera, D.; Ramírez, J.; Leets, I.; Tropper, E. Vitamin A and β-Carotene Can Improve Nonheme Iron Absorption from Rice, Wheat and Corn by Humans. J. Nutr. 1998, 128, 646–650. [Google Scholar] [CrossRef] [PubMed]
- Amos, A.; Alvan, A.; Florence, A. The Anti-nutritional Effect of Phytate on Zinc, Iron and Calcium Bioavailabilities of Some Cereals Staple Foods in Zaria, Nigeria. Eur. J. Nutr. Food Saf. 2020, 1–6. [Google Scholar] [CrossRef]
- Rosen, G.M.; Morrissette, S.; Larson, A.; Stading, P.; Griffin, K.H.; Barnes, T.L. Use of a Probiotic to Enhance Iron Absorption in a Randomized Trial of Pediatric Patients Presenting with Iron Deficiency. J. Pediatr. 2019, 207, 192–197.e1. [Google Scholar] [CrossRef] [PubMed]
- Skrypnik, K.; Bogdanski, P.; Schmidt, M.; Suliburska, J. The Effect of Multispecies Probiotic Supplementation on Iron Status in Rats. Biol. Trace Elem. Res. 2019, 192, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Helmyati, S.; Sudargo, T.; Kandarina, I.; Yuliati, E.; Wisnusanti, S.U.; Puspitaningrum, V.A.D.; Juffrie, M. Tempeh extract fortified with iron and synbiotic as a strategy against anemia. Int. Food Res. J. 2016, 23, 2296–2299. [Google Scholar]
- Deriu, E.; Liu, J.Z.; Pezeshki, M.; Edwards, R.A.; Ochoa, R.J.; Contreras, H.; Libby, S.J.; Fang, F.C.; Raffatellu, M. Probiotic bacteria reduce salmonella typhimurium intestinal colonization by competing for iron. Cell Host Microbe 2013, 14, 26–37. [Google Scholar] [CrossRef]
- Adeyanju, A.A.; Kruger, J.; Taylor, J.R.; Duodu, K.G. Effects of different souring methods on the protein quality and iron and zinc bioaccessibilities of non-alcoholic beverages from sorghum and amaranth. Int. J. Food Sci. Technol. 2019, 54, 798–809. [Google Scholar] [CrossRef]
- Adiki, S.K.; Perla, C.K.; Saha, G.; Katakam, P.; Theendra, V. Enhancement in Iron Absorption on Intake of Chemometrically Optimized Ratio of Probiotic Strain Lactobacillus plantarum 299v with Iron Supplement Pearl Millet. Biol. Trace Elem. Res. 2019, 190, 150–156. [Google Scholar] [CrossRef]
- El-Azeem, A.S.A.; Hegazy, A.M.; Badawy, I.; Ibrahim, G.A.; El-Shafei, K.; El-Sayed, H.S.; Sharaf, O.M. Effectiveness of Functional Wheat-Fermented Milk Beverage against Tannic Acid Induced Anemia. Res. J. Pharm. Biol. Chem. Sci. 2016, 7, 2622. [Google Scholar]
- Garcés, V.; Rodríguez-Nogales, A.; González, A.; Gálvez, N.; Rodríguez-Cabezas, M.E.; García-Martin, M.L.; Gutiérrez, L.; Rondón, D.; Olivares, M.; Gálvez, J. Bacteria-carried iron oxide nanoparticles for treatment of anemia. Bioconj. Chem. 2018, 29, 1785–1791. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, M.; Önning, G.; Hulthén, L. Freeze-dried Lactobacillus plantarum 299v increases iron absorption in young females—Double isotope sequential single-blind studies in menstruating women. PLoS ONE 2017, 12, e0189141. [Google Scholar] [CrossRef] [PubMed]
- Khodaii, Z.; Zadeh, M.N.; Kamali, J.; Natanzi, M.M. Enhanced iron absorption from lactic acid fermented bread (an in vivo/ex vivo study). Gene Rep. 2019, 15, 100389. [Google Scholar] [CrossRef]
- Korčok, D.J.; Tršić-Milanoviće, N.; Ivanović, N.; Đorđević, B. Development of Probiotic Formulation for the Treatment of Iron Deficiency Anemia. Chem. Pharm. Bull. 2018, 66, 347–352. [Google Scholar] [CrossRef]
- Scheers, N.; Rossander-Hulthen, L.; Torsdottir, I.; Sandberg, A.-S. Increased iron bioavailability from lactic-fermented vegetables is likely an effect of promoting the formation of ferric iron (Fe 3+). Eur. J. Nutr. 2016, 55, 373–382. [Google Scholar] [CrossRef]
- Skrypnik, K.; Bogdański, P.; Sobieska, M.; Suliburska, J. The effect of multistrain probiotic supplementation in two doses on iron metabolism in obese postmenopausal women: A randomized trial. Food Funct. 2019, 10, 5228–5238. [Google Scholar] [CrossRef]
- Tungland, B. Chapter 5—Direct Physiological Effects on Local Gi and Indirect Systemic Effects of Prebiotic Fructan Treatment, and its Role in Disease Prevention and Therapy. In Human Microbiota in Health and Disease; Tungland, B., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 155–196. [Google Scholar]
- Rizwan Ahmad, A.M.; Ahmed, W.; Iqbal, S.; Mushtaq, M.H.; Anis, R.A. Iron and prebiotic fortified flour improves the immune function of iron deficient women of childbearing age. Pak. J. Pharm. Sci. 2020, 33, 253–261. [Google Scholar]
- Castro, L.C.V.; Costa, N.M.B.; Sant’Anna, H.M.P.; Ferreira, C.L.d.L.F.; Franceschini, S.d.C.d.C. Improvement the nutritional status of pre-school children following intervention with a supplement containing iron, zinc, copper, vitamin A, vitamin C and prebiotic. Ciên. Saú. Colet. 2017, 22, 359–368. [Google Scholar] [CrossRef]
- Christides, T.; Ganis, J.C.; Sharp, P.A. In vitro assessment of iron availability from commercial Young Child Formulae supplemented with prebiotics. Eur. J. Nutr. 2018, 57, 669–678. [Google Scholar] [CrossRef]
- Dabour, N.; Dyab, N.; Kheadr, E. Iron fortification of reduced-fat bioyoghurt containing either short-or long-chain inulin. Int. J. Dairy Technol. 2019, 72, 229–239. [Google Scholar] [CrossRef]
- Sazawal, S.; Dhingra, U.; Hiremath, G.; Sarkar, A.; Dhingra, P.; Dutta, A.; Menon, V.P.; Black, R.E. Effects of Bifidobacterium lactis HN019 and prebiotic oligosaccharide added to milk on iron status, anemia, and growth among children 1 to 4 years old. J. Ped. Gastroenterol. Nutr. 2010, 51, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Feruś, K.; Drabińska, N.; Krupa-Kozak, U.; Jarocka-Cyrta, E. A randomized, placebo-controlled, pilot clinical trial to evaluate the effect of supplementation with prebiotic Synergy 1 on iron homeostasis in children and adolescents with celiac disease treated with a gluten-free diet. Nutrients 2018, 10, 1818. [Google Scholar] [CrossRef] [PubMed]
- Ghibaudo, F.; Gerbino, E.; Copello, G.J.; Campo Dall’ Orto, V.; Gómez-Zavaglia, A. Pectin-decorated magnetite nanoparticles as both iron delivery systems and protective matrices for probiotic bacteria. Colloids Surf. B Biointerfaces 2019, 180, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Jeroense, F.M.; Michel, L.; Zeder, C.; Herter-Aeberli, I.; Zimmermann, M.B. Consumption of galacto-oligosaccharides increases iron absorption from ferrous fumarate: A stable iron isotope study in iron-depleted young women. J. Nutr. 2019, 149, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Laparra, J.M.; Díez-Municio, M.; Herrero, M.; Moreno, F.J. Structural differences of prebiotic oligosaccharides influence their capability to enhance iron absorption in deficient rats. Food Funct. 2014, 5, 2430–2437. [Google Scholar] [CrossRef]
- Paganini, D.; Uyoga, M.A.; Cercamondi, C.I.; Moretti, D.; Mwasi, E.; Schwab, C.; Bechtler, S.; Mutuku, F.M.; Galetti, V.; Lacroix, C. Consumption of galacto-oligosaccharides increases iron absorption from a micronutrient powder containing ferrous fumarate and sodium iron EDTA: A stable-isotope study in Kenyan infants. Am. J. Clin. Nutr. 2017, 106, 1020–1031. [Google Scholar] [CrossRef]
- Paganini, D.; Uyoga, M.A.; Kortman, G.A.; Boekhorst, J.; Schneeberger, S.; Karanja, S.; Hennet, T.; Zimmermann, M.B. Maternal Human Milk Oligosaccharide Profile Modulates the Impact of an Intervention with Iron and Galacto-Oligosaccharides in Kenyan Infants. Nutrients 2019, 11, 2596. [Google Scholar] [CrossRef]
- Petry, N.; Egli, I.; Chassard, C.; Lacroix, C.; Hurrell, R. Inulin modifies the bifidobacteria population, fecal lactate concentration, and fecal pH but does not influence iron absorption in women with low iron status. Am. J. Clin. Nutr. 2012, 96, 325–331. [Google Scholar] [CrossRef]
- Weinborn, V.; Valenzuela, C.; Olivares, M.; Arredondo, M.; Weill, R.; Pizarro, F. Prebiotics increase heme iron bioavailability and do not affect non-heme iron bioavailability in humans. Food Funct. 2017, 8, 1994–1999. [Google Scholar] [CrossRef]
- Maawia, K.; Iqbal, S.; Qamar, T.R.; Rafiq, P.; ullah, A.; Ahmad, M.-U.-D. Production of impure prebiotic galacto-oligosaccharides and their effect on calcium, magnesium, iron and zinc absorption in Sprague-Dawley rats. Pharma Nutr. 2016, 4, 154–160. [Google Scholar] [CrossRef]
- Pitarresi, G.; Tripodo, G.; Cavallaro, G.; Palumbo, F.S.; Giammona, G. Inulin–iron complexes: A potential treatment of iron deficiency anaemia. Eur. J. Pharm. Biopharm. 2008, 68, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Laparra, J.M.; Tako, E.; Glahn, R.P.; Miller, D.D. Supplemental inulin does not enhance iron bioavailability to Caco-2 cells from milk- or soy-based, probiotic-containing, yogurts but incubation at 37 °C does. Food Chem. 2008, 109, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Asemi, Z.; Aarabi, M.H.; Hajijafari, M.; Alizadeh, S.A.; Razzaghi, R.; Mazoochi, M.; Esmaillzadeh, A. Effects of synbiotic food consumption on serum minerals, liver enzymes, and blood pressure in patients with type 2 diabetes: A double-blind randomized cross-over controlled clinical trial. Int. J. Prev. Med. 2017, 8, 43. [Google Scholar] [CrossRef] [PubMed]
| Type of Iron Deficiency | Probiotic Strain | Type of Administration | Results | References |
|---|---|---|---|---|
| Low iron absorption | Lactobacillus. plantarum (FS2) | Orally; non-alcoholic sorghum-based beverages | ↑ iron bioavailability by 128–372% in the fermented beverages | [85] |
| Anemia (IDA) | L. plantarum 299v | Orally; pearl millet seeds | ↑ iron absorption | [86] |
| Anemia (IDA) | Streptococcus thermophilus | Orally; fermented milk beverage | ↑ iron absorption and utilization (amelioration of blood hemoglobin, serum iron, total iron binding capacity, ferritin) | [87] |
| Anemia (IDA) | L. fermentum | Orally; nanoparticles | probiotic internalize into the enterocyte delivering the nanoparticles and providing an adequate iron level | [88] |
| Anemia (IDA) | L. fermentum | In vitro | ↑ iron absorption | [62] |
| In menstruation | L. plantarum 299v | Orally; capsules, with a meal with a high iron bioavailability | ↑ iron absorption when administered together | [89] |
| Anemia (IDA) | L. plantarum 299v | Orally; fruit drink | ↑iron absorption | [7] |
| Anemia (IDA) | L. acidophilus | Orally; fermented bread | ↑ ferritin formation significantly in the intestinal cells (in vitro) and animal serum (in vivo) ↑ iron absorption | [90] |
| Anemia (IDA) | L. plantarum 299v | Orally; capsules together with iron and vitamin C | ↑ iron level in the blood | [91] |
| Low iron bioavailability | Bifidobacterium bifidum and B. longum | Orally; powder follow-up infant formulas | ↑ apparent iron absorption or retention (p < 0.05) | [60] |
| Iron deficiency | L. plantarum 299v | Orally; capsules | The treatments were well-tolerated, with mild side effects No significant difference in the increase in serum ferritin in children | [81] |
| Healthy | L. plantarum | Orally; mix of raw vegetables | ↑ bioavailability of iron | [92] |
| Abnormalities of iron metabolism related to obesity | Probiotic mixture (B. bifidum W23, B. lactis W51, B. lactis W52, L. acidophilus W37, L. brevis W63, L. casei W56, L. salivarius W24, Lactococcus lactis W19, and Lc. lactis W58) | Orally; powder | Multistrain probiotic supplementation may influence iron metabolism in obese postmenopausal female patients; further studies are needed | [93] |
| Anemia (IDA) | Lactobacillus plantarum Dad 13 | Orally; fermented milk | No difference on the iron status, height, weight, and gut microbiota profile | [33] |
| Type of Iron Deficiency | Prebiotic/Synbiotics | Type of Administration | Results | References |
|---|---|---|---|---|
| Anemia (IDA) | Galacto oligosaccharides and inulin | Orally; wheat flour | Improved immune function of iron-deficient women | [95] |
| Healthy | Inulin | Orally; supplement | Improved the iron and anthropometric status | [96] |
| Iron deficiency (ID) and IDA | Fructo- and galacto oligosaccharides | Milk-derived products | Improved iron bioavailability | [97] |
| Anemia (IDA) | Inulin | Bioyoghurt—inulin and iron salts | ↑ ferric sulphate bioavailability ↓ calcium bioavailability inulin addition significant ↓ iron bioavailability | [98] |
| Anemia (IDA) | Bifidobacterium lactis HN019 and oligosaccharides | Orally; milk | ↓ risk of anemia and iron deficiency and helped to gain weight | [99] |
| Anemia in celiac disease | Oligofructose-enriched inulin | Orally; supplement | Decreased serum hepcidin conc. - ↑ iron absorption | [100] |
| Vitro | Lactobacillus plantarum CIDCA * 83114 and pectin | Capsules | ↑ iron absorption | [101] |
| Iron-depleted | Galacto-oligosaccharides | Orally | ↑ iron absorption | [102] |
| Anemia (IDA) | Galacto-oligosaccharides | Injection | ↑ iron absorption | [103] |
| Anemia (IDA) | Inulin and oligofructose | Orally; dietary fibre | ↑ the expression of the divalent metal transporter protein in the caecum and oligofructose decreased the expression of the protein ferroportin in the duodenum Helps regulate of intestinal iron absorption | [8] |
| Anemia (IDA) | Galacto-oligosaccharides | Orally; maize porridge fortified with a micronutrient powder (ferrous fumarate + sodium iron + galacto-oligosaccharide) | ↑ fractional iron absorption (62%) Improved the relative iron bioavailability | [104] |
| Healthy (Kenyan mothers) | Galacto-oligosaccharides | Orally; micronutrient powder (ferrous fumarate + sodium iron EDTA ** + galacto-oligosaccharides) | Modulate the infant gut microbiota response to fortificant iron | [105] |
| Anemia (IDA) | B. bifidum, B. longum galactooligosaccharides | Orally; powder follow-up infant formula | ↑ the apparent iron absorption or retention | [60] |
| Low iron status | Inulin | Orally; cooked rice and a pureed, boiled vegetable sauce | ↑ iron absorption | [106] |
| Anemia (IDA) | inulin, polidextrose, arabic gum, and guar gum | Orally in yoghurt, 2 g per day | ↑ heme iron bioavailability not influence non-heme iron bioavailability | [107] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rusu, I.G.; Suharoschi, R.; Vodnar, D.C.; Pop, C.R.; Socaci, S.A.; Vulturar, R.; Istrati, M.; Moroșan, I.; Fărcaș, A.C.; Kerezsi, A.D.; et al. Iron Supplementation Influence on the Gut Microbiota and Probiotic Intake Effect in Iron Deficiency—A Literature-Based Review. Nutrients 2020, 12, 1993. https://doi.org/10.3390/nu12071993
Rusu IG, Suharoschi R, Vodnar DC, Pop CR, Socaci SA, Vulturar R, Istrati M, Moroșan I, Fărcaș AC, Kerezsi AD, et al. Iron Supplementation Influence on the Gut Microbiota and Probiotic Intake Effect in Iron Deficiency—A Literature-Based Review. Nutrients. 2020; 12(7):1993. https://doi.org/10.3390/nu12071993
Chicago/Turabian StyleRusu, Ioana Gabriela, Ramona Suharoschi, Dan Cristian Vodnar, Carmen Rodica Pop, Sonia Ancuța Socaci, Romana Vulturar, Magdalena Istrati, Ioana Moroșan, Anca Corina Fărcaș, Andreea Diana Kerezsi, and et al. 2020. "Iron Supplementation Influence on the Gut Microbiota and Probiotic Intake Effect in Iron Deficiency—A Literature-Based Review" Nutrients 12, no. 7: 1993. https://doi.org/10.3390/nu12071993
APA StyleRusu, I. G., Suharoschi, R., Vodnar, D. C., Pop, C. R., Socaci, S. A., Vulturar, R., Istrati, M., Moroșan, I., Fărcaș, A. C., Kerezsi, A. D., Mureșan, C. I., & Pop, O. L. (2020). Iron Supplementation Influence on the Gut Microbiota and Probiotic Intake Effect in Iron Deficiency—A Literature-Based Review. Nutrients, 12(7), 1993. https://doi.org/10.3390/nu12071993











