A Double-Blind, Randomized, Placebo-Controlled Trial of Heat-Killed Pediococcus acidilactici K15 for Prevention of Respiratory Tract Infections among Preschool Children
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Clinical Test Foods
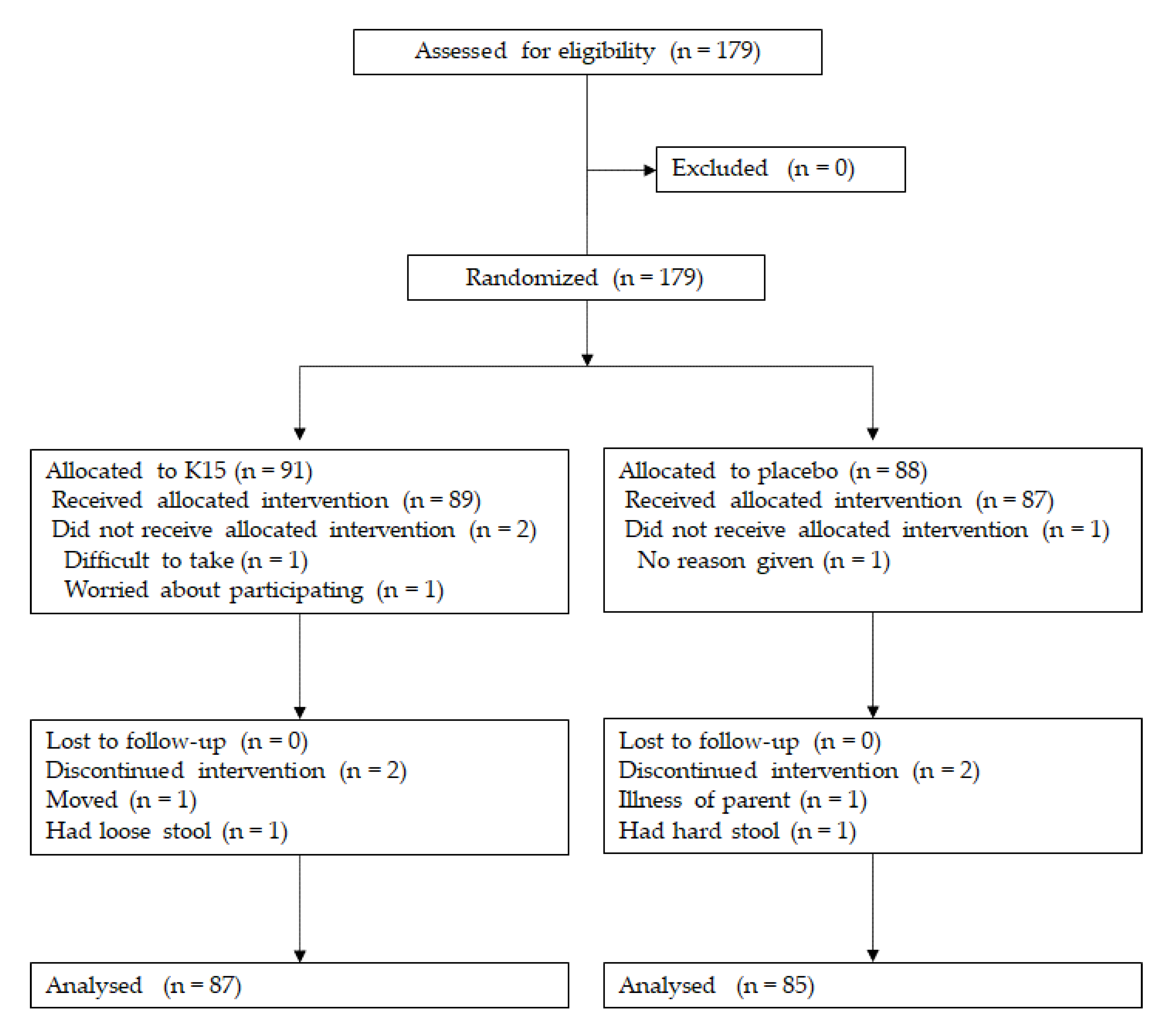
2.2. Clinical Study Design
2.3. Outcomes
2.4. Subjects for the Clinical Trial
2.5. Determination of Salivary sIgA Secretion Rate and Concentration
2.6. Statistical Analysis
3. Results
3.1. Participants’ Baseline Characteristics
3.2. Primary Outcome (Number of Febrile Days during the Exam Period)
3.3. Secondary Outcomes
3.4. Salivary sIgA
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Suzuki, K.; Kondo, K.; Washio, M.; Nakashima, K.; Kan, S.; Imai, S.; Yoshimura, K.; Ota, C.; Ohfuji, S.; Fukushima, W.; et al. Preventive effects of pneumococcal and influenza vaccines on community-acquired pneumonia in older individuals in Japan: A case-control study. Hum. Vaccines Immunother. 2019, 15, 2171–2177. [Google Scholar] [CrossRef] [PubMed]
- Leidner, A.J.; Murthy, N.; Chesson, H.W.; Biggerstaff, M.; Stoecker, C.; Harris, A.M.; Acosta, A.; Dooling, K.; Bridges, C.B. Cost-effectiveness of adult vaccinations: A systematic review. Vaccine 2019, 37, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Cetinoglu, E.D.; Uzaslan, E.; Sayıner, A.; Cilli, A.; Kılınc, O.; Coskun, A.S.; Hazar, A.; Kokturk, N.; Filiz, A.; Polatli, M. Pneumococcal and influenza vaccination status of hospitalized adults with community acquired pneumonia and the effects of vaccination on clinical presentation. Hum. Vaccines Immunother. 2017, 13, 2072–2077. [Google Scholar] [CrossRef] [PubMed]
- Siedler, S.; Balti, R.; Neves, A.R. Bioprotective mechanisms of lactic acid bacteria against fungal spoilage of food. Curr. Opin. Biotechnol. 2019, 56, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Chikindas, M.L.; Weeks, R.; Drider, D.; Chistyakov, V.A.; Dicks, L.M. Functions and emerging applications of bacteriocins. Curr. Opin. Biotechnol. 2017, 49, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Ruggirello, M.; Giordano, M.; Bertolino, M.; Ferrocino, I.; Cocolin, L.; Dolci, P. Study of Lactococcus lactis during advanced ripening stages of model cheeses characterized by GC-MS. Food Microbiol. 2018, 74, 132–142. [Google Scholar] [CrossRef]
- Parvez, S.; Malik, K.; Kang, S.A.; Kim, H.-Y. Probiotics and their fermented food products are beneficial for health. J. Appl. Microbiol. 2006, 100, 1171–1185. [Google Scholar] [CrossRef]
- Kawashima, T.; Hayashi, K.; Kosaka, A.; Kawashima, M.; Igarashi, T.; Tsutsui, H.; Tsuji, N.M.; Nishimura, I.; Hayashi, T.; Obata, A. Lactobacillus platarum strain YU from fermented foods activates Th1 and protective immune responses. Int. Immunopharmacol. 2011, 11, 2017–2024. [Google Scholar] [CrossRef]
- Masuda, S.; Yamaguchi, H.; Kurokawa, T.; Shirakami, T.; Tsuji, R.; Nishimura, I. Immunomodulatory effect of halophilic lactic acid bacterium Tetragenococcus halophilus Th221 from soy sauce moromi grown in high-salt medium. Int. J. Food Microbiol. 2008, 121, 245–252. [Google Scholar] [CrossRef]
- Maeda, N.; Nakamura, R.; Hirose, Y.; Murosaki, S.; Yamamoto, Y.; Kase, T.; Yoshikai, Y. Oral administration of heat-killed Lactobacillus platarum L-137 enhances protection against influenza virus infection by stimulation of type I interferon production in mice. Int. Immunopharmacol. 2009, 9, 1122–1125. [Google Scholar] [CrossRef]
- Asama, T.; Uematsu, T.; Kobayashi, N.; Tatefuji, T.; Hashimoto, K. Oral administration of heat-killed Lactobacillus kunkeei YB38 improves murine influenza pneumonia by enhancing IgA production. Biosci. Microbiota Food Health 2017, 36, 1–9. [Google Scholar] [CrossRef]
- Kalliomäki, M.; Salminen, S.; Arvilommi, H.; Kero, P.; Koskinen, P.; Isolauri, E. Probiotics in primary prevention of atopic disease: A randomised placebo-controlled trial. Lancet 2001, 357, 1076–1079. [Google Scholar] [CrossRef]
- Kosaka, A.; Yan, H.; Ohashi, S.; Gotoh, Y.; Sato, A.; Tsutsui, H.; Kaisho, T.; Toda, T.; Tsuji, N.M. Lactococcus lactis subsp. cremoris FC triggers IFN-γ production from NK and T cells via IL-12 and IL-18. Int. Immunopharmacol. 2012, 14, 729–733. [Google Scholar] [CrossRef] [PubMed]
- Karrich, J.J.; Jachimowski, L.C.M.; Uittenbogaart, C.H.; Blom, B. The plasmacytoid dendritic cell as the Swiss army knife of the immune system: Molecular regulation of its multifaceted functions. J. Immunol. 2014, 193, 5772–5778. [Google Scholar] [CrossRef] [PubMed]
- Siegal, F.P.; Kadowaki, N.; Shodell, M.; Fitzgerald-Bocarsly, P.A.; Shah, K.; Ho, S.; Antonenko, S.; Liu, Y.-J. The Nature of the Principal Type 1 Interferon-Producing Cells in Human Blood. Sci. 1999, 284, 1835–1837. [Google Scholar] [CrossRef] [PubMed]
- Kotani, Y.; Kunisawa, J.; Suzuki, Y.; Sato, I.; Saito, T.; Toba, M.; Kohda, N.; Kiyono, H. Role of Lactobacillus pentosus Strain b240 and the Toll-Like Receptor 2 Axis in Peyer’s Patch Dendritic Cell-Mediated Immunoglobulin A Enhancement. PLOS ONE 2014, 9, e91857. [Google Scholar] [CrossRef] [PubMed]
- Kawase, M.; He, F.; Kubota, A.; Harata, G.; Hiramatsu, M. Oral administration of lactobacilli from human intestinal tract protects mice against influenza virus infection. Lett. Appl. Microbiol. 2010, 51, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Mestecky, J.; Russell, M. Specific antibody activity, glycan heterogeneity and polyreactivity contribute to the protective activity of S-IgA at mucosal surfaces. Immunol. Lett. 2009, 124, 57–62. [Google Scholar] [CrossRef]
- Kawashima, T.; Ikari, N.; Kouchi, T.; Kowatari, Y.; Kubota, Y.; Shimojo, N.; Tsuji, N.M. The molecular mechanism for activating IgA production by Pediococcus acidilactici K15 and the clinical impact in a randomized trial. Sci. Rep. 2018, 8, 5065. [Google Scholar] [CrossRef]
- Kawashima, T.; Kosaka, A.; Yan, H.; Guo, Z.; Uchiyama, R.; Fukui, R.; Kaneko, D.; Kumagai, Y.; You, N.-J.; Carreras, J.; et al. Double-Stranded RNA of Intestinal Commensal but Not Pathogenic Bacteria Triggers Production of Protective Interferon-β. Immunity 2013, 38, 1187–1197. [Google Scholar] [CrossRef]
- Asong, J.; Wolfert, M.A.; Maiti, K.K.; Miller, D.; Boons, G.-J. Binding and Cellular Activation Studies Reveal That Toll-like Receptor 2 Can Differentially Recognize Peptidoglycan from Gram-positive and Gram-negative Bacteria. J. Boil. Chem. 2009, 284, 8643–8653. [Google Scholar] [CrossRef] [PubMed]
- Grangette, C.; Nutten, S.; Palumbo, E.; Morath, S.; Hermann, C.; Dewulf, J.; Pot, B.; Hartung, T.; Hols, P.; Mercenier, A. Enhanced anti-inflammatory capacity of a Lactobacillus plantarum mutant synthesizing modified teichoic acids. Proc. Natl. Acad. Sci. USA 2005, 102, 10321–10326. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, I.; Igarashi, T.; Enomoto, T.; Dake, Y.; Okuno, Y.; Obata, A. Clinical Efficacy of Halophilic Lactic Acid Bacterium Tetragenococcus halophilus Th221 from Soy Sauce Moromi for Perennial Allergic Rhinitis. Allergol. Int. 2009, 58, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Piqué, N.; Berlanga, M.; Miñana-Galbis, D. Health Benefits of Heat-Killed (Tyndallized) Probiotics: An Overview. Int. J. Mol. Sci. 2019, 20, 2534. [Google Scholar] [CrossRef]
- Kawashima, T.; Ikari, N.; Watanabe, Y.; Kubota, Y.; Yoshio, S.; Kanto, T.; Motohashi, S.; Shimojo, N.; Tsuji, N.M. Double-Stranded RNA Derived from Lactic Acid Bacteria Augments Th1 Immunity via Interferon-β from Human Dendritic Cells. Front. Immunol. 2018, 9, 27. [Google Scholar] [CrossRef]
- Mizuno, D.; Ide-Kurihara, M.; Ichinomiya, T.; Kubo, I.; Kido, H. Modified pulmonary surfactant is a potent adjuvant that stimulates the mucosal IgA production in response to the influenza virus antigen. J. Immunol. 2006, 176, 1122–1130. [Google Scholar] [CrossRef]
- Sawabuchi, T.; Suzuki, S.; Iwase, K.; Ito, C.; Mizuno, D.; Togari, H.; Watanabe, I.; Talukder, S.R.; Chida, J.; Kido, H. Boost of mucosal secretory immunoglobulin A response by clarithromycin in paediatric influenza. Respirology 2009, 14, 1173–1179. [Google Scholar] [CrossRef]
- Kumpu, M.; Lehtoranta, L.; Roivainen, M.; Rönkkö, E.; Ziegler, T.; Söderlund-Venermo, M.; Kautiainen, H.; Järvenpää, S.; Kekkonen, R.; Hatakka, K.; et al. The use of the probioticLactobacillus rhamnosusGG and viral findings in the nasopharynx of children attending day care. J. Med. Virol. 2013, 85, 1632–1638. [Google Scholar] [CrossRef]
- Leyer, G.J.; Li, S.; Mubasher, M.E.; Reifer, C.; Ouwehand, A.C. Probiotic Effects on Cold and Influenza-Like Symptom Incidence and Duration in Children. Pediatrics 2009, 124, 172–179. [Google Scholar] [CrossRef]
- Rerksuppaphol, S.; Rerksuppaphol, L. Randomized controlled trial of probiotics to reduce common cold in schoolchildren. Pediatr. Int. 2012, 54, 682–687. [Google Scholar] [CrossRef]
- Laursen, R.P.; Larnkjær, A.; Hauger, H.; Michaelsen, K.F.; Mølgaard, C.; Ritz, C. Probiotics and Child Care Absence Due to Infections: A Randomized Controlled Trial. Pediatrics 2017, 140, e20170735. [Google Scholar] [CrossRef] [PubMed]
- Laursen, R.P.; Hojsak, I. Probiotics for respiratory tract infections in children attending day care centers—A systematic review. Eur. J. Nucl. Med. Mol. Imaging 2018, 177, 979–994. [Google Scholar] [CrossRef] [PubMed]
- Arimori, Y.; Nakamura, R.; Hirose, Y.; Murosaki, S.; Yamamoto, Y.; Shidara, O.; Ichikawa, H.; Yoshikai, Y. Daily intake of heat-killed Lactobacillus plantarum L-137 enhances type I interferon production in healthy humans and pigs. Immunopharmacol. Immunotoxicol. 2012, 34, 937–943. [Google Scholar] [CrossRef] [PubMed]
- Shinkai, S.; Toba, M.; Saito, T.; Sato, I.; Tsubouchi, M.; Taira, K.; Kakumoto, K.; Inamatsu, T.; Yoshida, H.; Fujiwara, Y.; et al. Immunoprotective effects of oral intake of heat-killed Lactobacillus pentosus strain b240 in elderly adults: A randomised, double-blind, placebo-controlled trial. Br. J. Nutr. 2012, 109, 1856–1865. [Google Scholar] [CrossRef]
- Szajewska, H.; Skorka, A.; Ruszczyński, M.; Gieruszczak-Białek, D. Meta-analysis:LactobacillusGG for treating acute gastroenteritis in children - updated analysis of randomised controlled trials. Aliment. Pharmacol. Ther. 2013, 38, 467–476. [Google Scholar] [CrossRef]
- Banchereau, J.; Steinman, R.M. Dendritic cells and the control of immunity. Nature 1998, 392, 245–252. [Google Scholar] [CrossRef]
- Paineau, D.; Carcano, D.; Leyer, G.; Darquy, S.; Alyanakian, M.-A.; Simoneau, G.; Bergmann, J.-F.; Brassart, D.; Bornet, F.; Ouwehand, A.C. Effects of seven potential probiotic strains on specific immune responses in healthy adults: A double-blind, randomized, controlled trial. FEMS Immunol. Med. Microbiol. 2008, 53, 107–113. [Google Scholar] [CrossRef]
- De Vrese, M.; Rautenberg, P.; Laue, C.; Koopmans, M.; Herremans, T.; Schrezenmeir, J. Probiotic bacteria stimulate virus–specific neutralizing antibodies following a booster polio vaccination. Eur. J. Nutr. 2004, 44, 406–413. [Google Scholar] [CrossRef]
- Kobayashi, N.; Saito, T.; Uematsu, T.; Kishi, K.; Toba, M.; Kohda, N.; Suzuki, T. Oral administration of heat-killed Lactobacillus pentosus strain b240 augments protection against influenza virus infection in mice. Int. Immunopharmacol. 2011, 11, 199–203. [Google Scholar] [CrossRef]
- Trinchieri, G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat. Rev. Immunol. 2003, 3, 133–146. [Google Scholar] [CrossRef]
| K15 (n = 87) | Placebo (n = 85) | p Value | |
|---|---|---|---|
| Male/female | 0.88 | ||
| Male, n (%) | 41 (49.4) | 42 (50.6) | |
| Female, n (%) | 46 (51.7) | 43 (48.3) | |
| Preschool year | 0.92 | ||
| The 3rd year, n (%) | 28 (49.1) | 29 (50.9) | |
| The 2nd year, n (%) | 30 (50.0) | 30 (50.0) | |
| The 1st year, n (%) | 29 (52.7) | 26 (47.3) | |
| Family history of allergy | |||
| Yes, n (%) | 63 (49.2) | 65 (50.7) | 0.48 |
| Consumption of test foods | |||
| Average days (mean ± SD) | 105.13 ± 10.08 | 105.28 ± 10.77 | 0.50 |
| Intake of foods including lactic acid bacteria | |||
| Average days (mean ± SD) | 37.29 ± 32.36 | 40.92 ± 38.21 | 0.50 |
| Number of vaccination | 0.44 | ||
| None, n (%) | 34 (50.5) | 33 (49.3) | |
| 1, n (%) | 11 (64.7) | 6 (35.3) | |
| 2, n (%) | 42 (47.7) | 46 (52.3) | |
| Frequency of common cold symptoms in family | |||
| Average days (mean ± SD) | 11.93 ± 19.09 | 13.01 ± 17.23 | 0.70 |
| K15 (n = 87) | Placebo (n = 85) | p Value | |
|---|---|---|---|
| Average days (mean ± SD) | 2.24 ± 2.54 | 2.67 ± 3.43 | 0.35 |
| K15 (n = 87) | Placebo (n = 85) | p Value | |
|---|---|---|---|
| Absence from preschool | |||
| Average days (mean ± SD) | 2.14 ± 3.65 | 2.31 ± 2.96 | 0.74 |
| Incidence of influenza virus infections | |||
| n (%) | 14 (16.1) | 19 (22.4) | 0.34 |
| Febrile days by influenza virus infection | |||
| Average days (mean ± SD) | 0.37 ± 0.92 | 0.52 ± 1.07 | 0.32 |
| Adverse events, n (%) | |||
| Respiratory tract | 83 (51.6) | 82 (49.4) | 0.74 |
| Gastrointestinal tract | 46 (28.6) | 47 (28.3) | 1.00 |
| Others | 30 (18.7) | 32 (28.3) | 0.89 |
| K15 (n = 36) | Placebo (n = 41) | p Value | |
|---|---|---|---|
| Average days (mean ± SD) | 1.69 ± 2.08 | 3.17 ± 3.98 | 0.042 |
| K15 | Placebo | p Value | |
|---|---|---|---|
| Total sIgA concentrations, mg/dL (mean ± SD) | |||
| Before (n) | 53.63 ± 42.26 (83) | 54.63 ± 50.80 (83) | 0.892 |
| After (n) | 53.31 ± 42.22 (82) | 42.82 ± 28.20 (83) | 0.063 |
| Change (n) | 3.20 ± 47.21 (81) | −12.49 ± 51.24 (81) | 0.044 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hishiki, H.; Kawashima, T.; Tsuji, N.M.; Ikari, N.; Takemura, R.; Kido, H.; Shimojo, N. A Double-Blind, Randomized, Placebo-Controlled Trial of Heat-Killed Pediococcus acidilactici K15 for Prevention of Respiratory Tract Infections among Preschool Children. Nutrients 2020, 12, 1989. https://doi.org/10.3390/nu12071989
Hishiki H, Kawashima T, Tsuji NM, Ikari N, Takemura R, Kido H, Shimojo N. A Double-Blind, Randomized, Placebo-Controlled Trial of Heat-Killed Pediococcus acidilactici K15 for Prevention of Respiratory Tract Infections among Preschool Children. Nutrients. 2020; 12(7):1989. https://doi.org/10.3390/nu12071989
Chicago/Turabian StyleHishiki, Haruka, Tadaomi Kawashima, Noriko M. Tsuji, Naho Ikari, Ryo Takemura, Hiroshi Kido, and Naoki Shimojo. 2020. "A Double-Blind, Randomized, Placebo-Controlled Trial of Heat-Killed Pediococcus acidilactici K15 for Prevention of Respiratory Tract Infections among Preschool Children" Nutrients 12, no. 7: 1989. https://doi.org/10.3390/nu12071989
APA StyleHishiki, H., Kawashima, T., Tsuji, N. M., Ikari, N., Takemura, R., Kido, H., & Shimojo, N. (2020). A Double-Blind, Randomized, Placebo-Controlled Trial of Heat-Killed Pediococcus acidilactici K15 for Prevention of Respiratory Tract Infections among Preschool Children. Nutrients, 12(7), 1989. https://doi.org/10.3390/nu12071989




