L-Theanine Reduced the Development of Knee Osteoarthritis in Rats via Its Anti-Inflammation and Anti-Matrix Degradation Actions: In Vivo and In Vitro Study
Abstract
1. Introduction
2. Materials and Methods
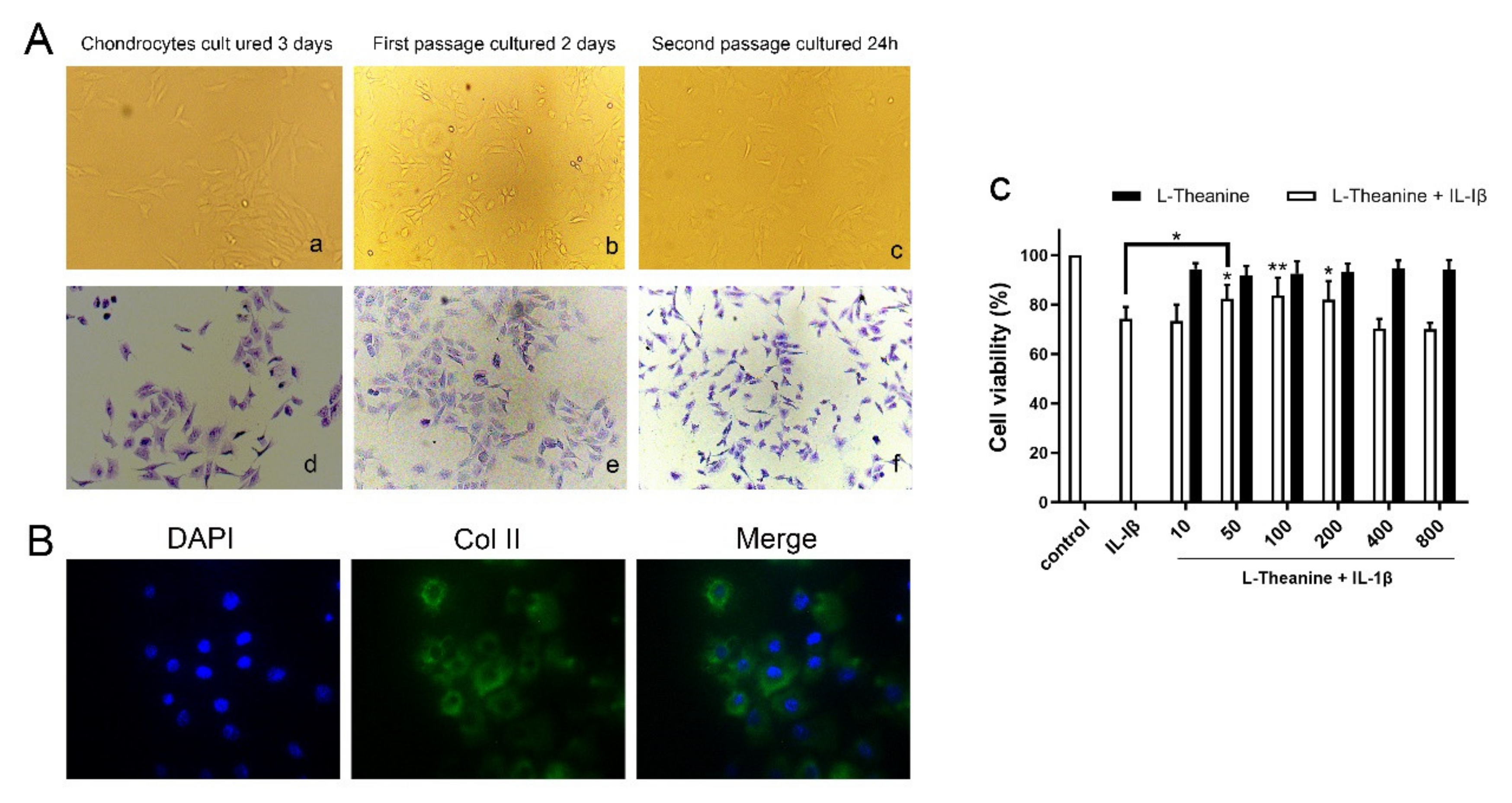
2.1. Preparation of Rat Primary Chondrocytes
2.2. Cell Proliferation Assay
2.3. In Vitro Immunofluorescence Assay
2.4. In Vitro Western Blot Analysis
2.5. In Vitro Real-Time Polymerase Chain Reaction (PCR) Analysis
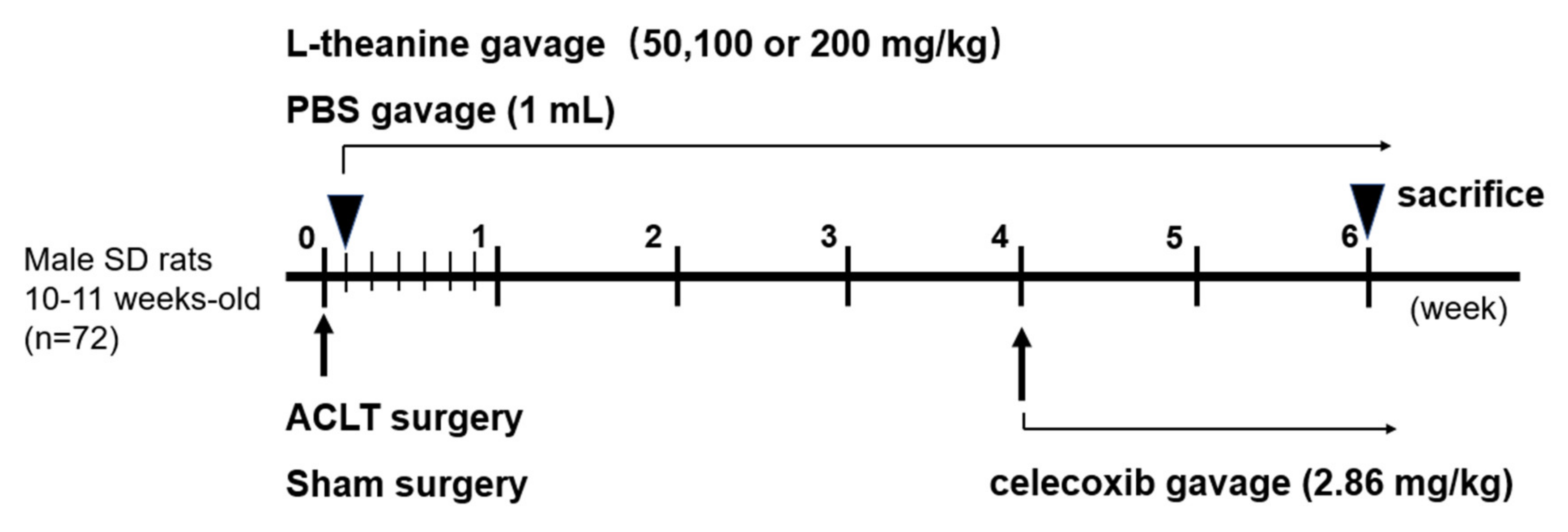
2.6. In Vivo Rat Anterior Cruciate Ligament (ACL) Transection-Induced Osteoarthritis (OA) Model
2.7. Histological Assessment and Osteoarthritis Research Society International (OARSI) Grading System
2.8. In Vivo Enzyme-Linked Immunosorbent Assay (ELISA) Kits
2.9. Statistical Analysis
3. Results
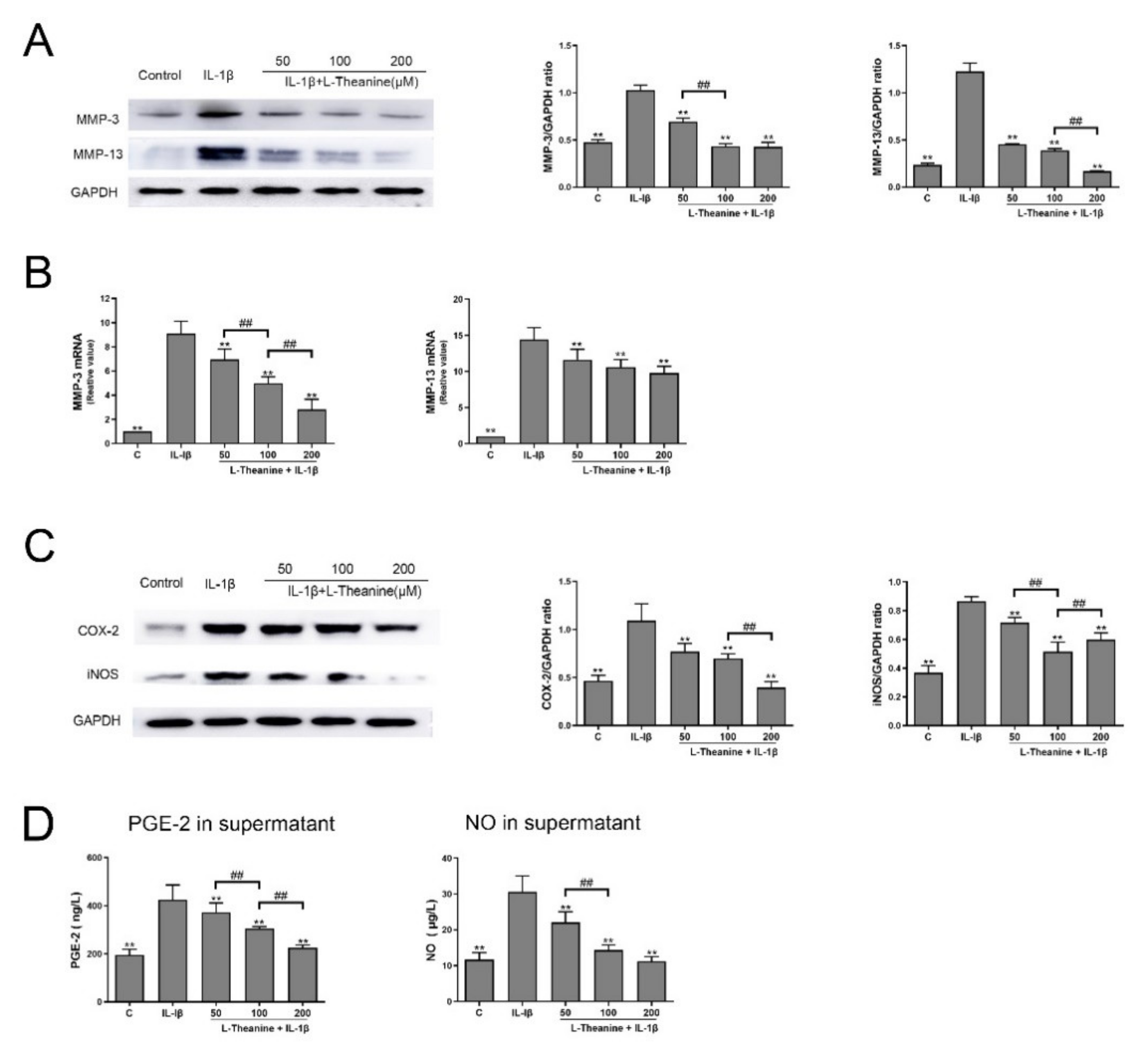
3.1. L-Theanine Reduces the Release of Catabolic Enzymes and Inflammatory Mediates from IL-1-Induced Chondrocytes In Vitro
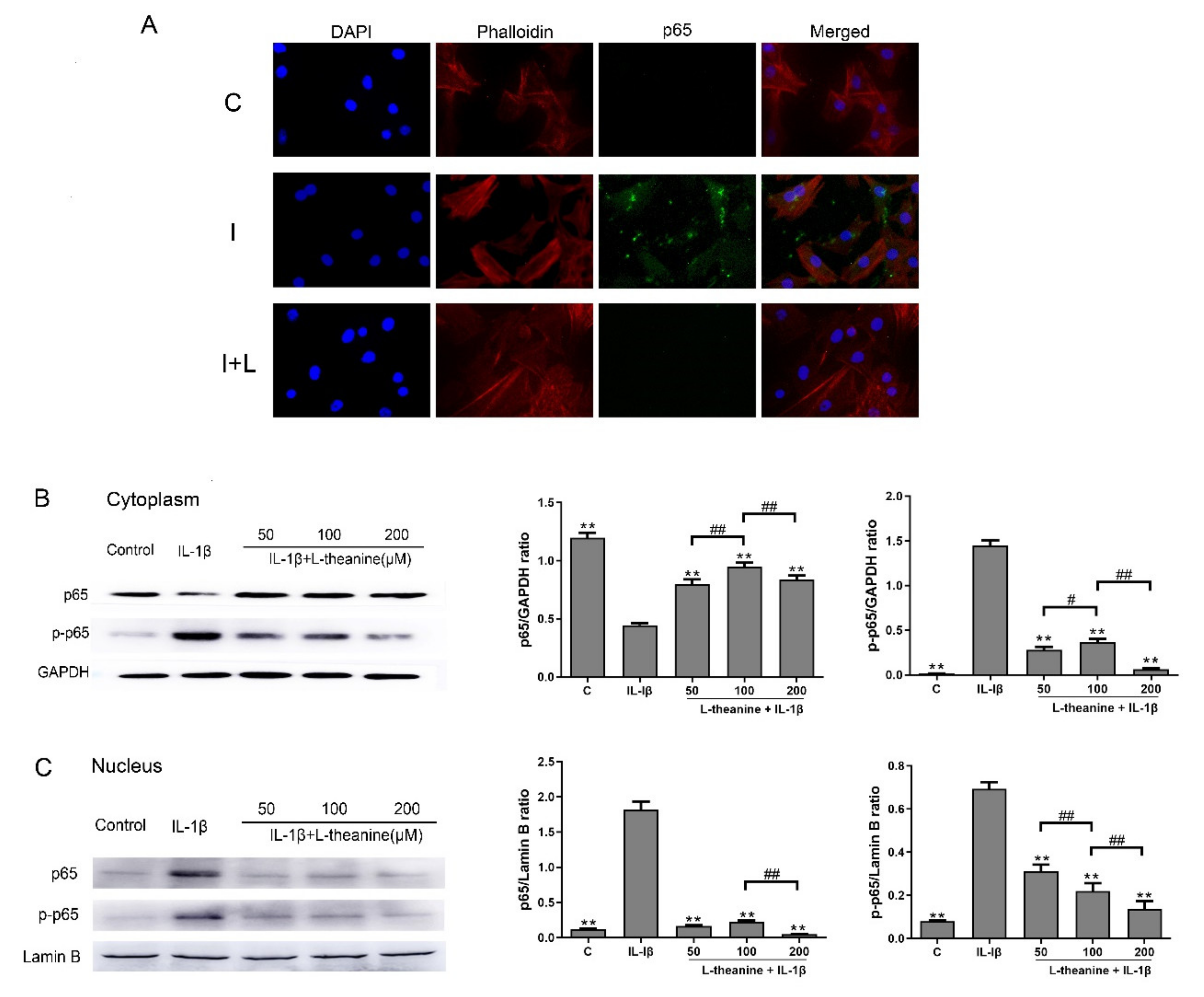
3.2. L-Theanine Inhibits Nuclear Factor Kappa B (NF-κB) p65 Phosphorylation and Expression In Vitro
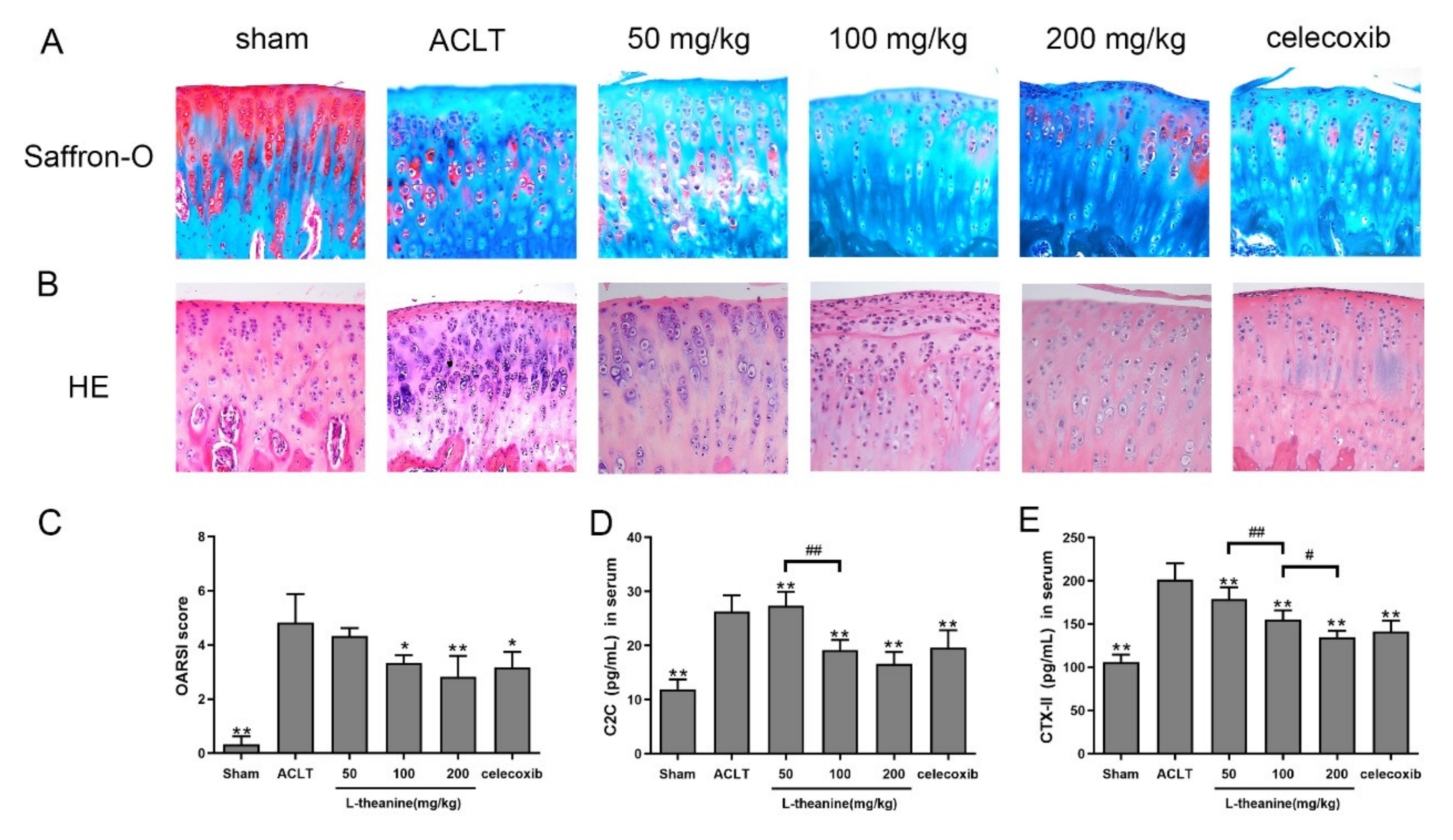
3.3. L-Theanine Ameliorates Knee Joint Histopathology and Reduces Extracellular Matrix (ECM) Degradation in the Rat Anterior Cruciate Ligament Transection (ACLT) Model
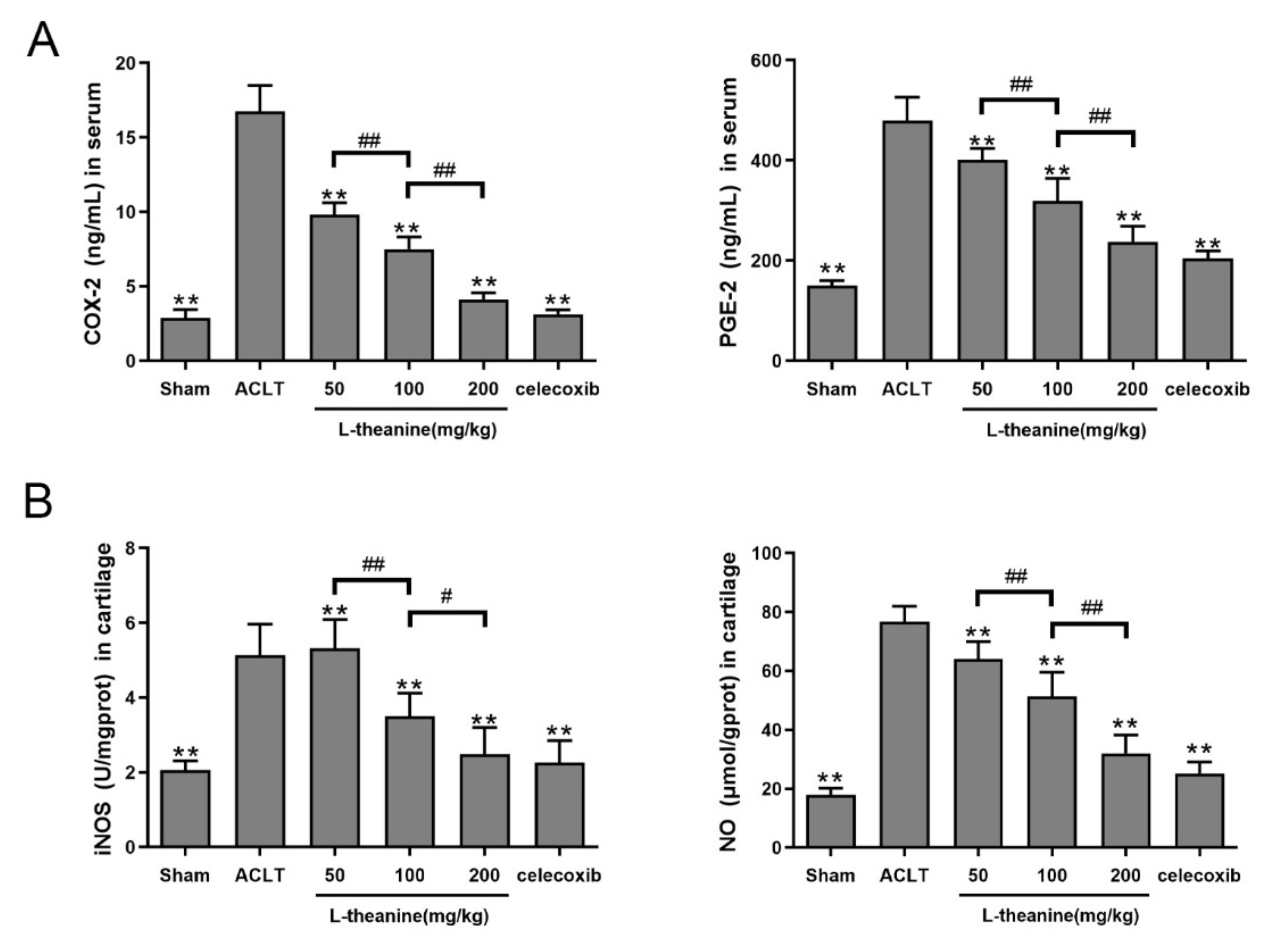
3.4. Systemic L-Theanine Treatment Exerts Anti-Inflammatory Activity In Vivo
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Declarations
Availability of Data and Material
Abbreviations
References
- Robinson, W.H.; Lepus, C.M.; Wang, Q.; Raghu, H.; Mao, R.; Lindstrom, T.M.; Sokolove, J. Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2016, 12, 580–592. [Google Scholar] [CrossRef]
- Lieberthal, J.; Sambamurthy, N.; Scanzello, C.R. Inflammation in joint injury and post-traumatic osteoarthritis. Osteoarthr. Cartil. 2015, 23, 1825–1834. [Google Scholar] [CrossRef]
- Nakata, K.; Hanai, T.; Take, Y.; Osada, T.; Tsuchiya, T.; Shima, D.; Fujimoto, Y. Disease-modifying effects of COX-2 selective inhibitors and non-selective NSAIDs in osteoarthritis: A systematic review. Osteoarthr. Cartil. 2018, 26, 1263–1273. [Google Scholar] [CrossRef]
- Kapoor, M.; Martel-Pelletier, J.; Lajeunesse, D.; Pelletier, J.P.; Fahmi, H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat. Rev. Rheumatol. 2011, 7, 33–42. [Google Scholar] [CrossRef]
- Blom, A.B.; van Lent, P.L.E.; Holthuysen, A.E.M.; van der Kraan, P.M.; van Rooijen, N.; van den Berg, W.B. Stromelysin (MMP-3) plays a pivotal role in spontaneous osteoarthritis: Involvement of synovial macrophages. Arthritis Rheum. 2003, 48, S431. [Google Scholar]
- Burrage, P.S.; Mix, K.S.; Brinckerhoff, C.E. Matrix metalloproteinases: Role in arthritis. Front. Biosci. 2006, 11, 529–543. [Google Scholar] [CrossRef]
- Martel-Pelletier, J.; Welsch, D.J.; Pelletier, J.P. Metalloproteases and inhibitors in arthritic diseases. Best Pract. Res. Clin. Rheumatol. 2001, 15, 805–829. [Google Scholar] [CrossRef]
- Pearle, A.D.; Warren, R.F.; Rodeo, S.A. Basic science of articular cartilage and osteoarthritis. Clin. Sports Med. 2005, 24, 1–12. [Google Scholar] [CrossRef]
- Tak, P.P.; Firestein, G.S. NF-kappaB: A key role in inflammatory diseases. J. Clin. Investig. 2001, 107, 7–11. [Google Scholar] [CrossRef]
- Saito, T.; Tanaka, S. Molecular mechanisms underlying osteoarthritis development: Notch and NF-kB. Arthritis Res. Ther. 2017, 19, 1–7. [Google Scholar] [CrossRef]
- Frank, S.; Peters, M.A.; Wehmeyer, C.; Strietholt, S.; Koerswunrau, C.; Bertrand, J.; Heitzmann, M.; Hillmann, A.; Sherwood, J.; Seyfert, C. Regulation of matrixmetalloproteinase-3 and matrixmetalloproteinase-13 by SUMO-2/3 through the transcription factor NF-κB. Ann. Rheum. Dis. 2013, 72, 1874–1881. [Google Scholar] [CrossRef] [PubMed]
- Agents, A.I.; Effects, N.S.A. Recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update. Arthritis Rheumatol. 2000, 43, 1905–1915. [Google Scholar]
- Henrotin, Y.; Lambert, C.; Couchourel, D.; Ripoll, C.; Chiotelli, E. Nutraceuticals: Do they represent a new era in the management of osteoarthritis?—A narrative review from the lessons taken with five products. Osteoarthr. Cartil. 2011, 19, 1. [Google Scholar] [CrossRef] [PubMed]
- Henrotin, Y.; Kurz, B.; Aigner, T. Oxygen and reactive oxygen species in cartilage degradation: Friends or foes? Osteoarthr. Cartil. 2005, 13, 643–654. [Google Scholar] [CrossRef]
- Henrotin, Y.; Kurz, B. Antioxidant to treat osteoarthritis: Dream or reality? Curr. Drug Targets 2007, 8, 347–357. [Google Scholar] [CrossRef]
- Juneja, L.R.; Chu, D.C.; Okubo, T.; Nagato, Y.; Yokogoshi, H. L-theanine--a unique amino acid of green tea and its relaxation effect in humans. Trends Food Sci. Technol. 2012, 10, 199–204. [Google Scholar] [CrossRef]
- Vuong, Q.V.; Bowyer, M.C.; Roach, P.D. L-Theanine: Properties, synthesis and isolation from tea. J. Sci. Food Agric. 2011, 91, 1931–1939. [Google Scholar] [CrossRef]
- Rasheed, Z.; Rasheed, N.; Al-Shaya, O. Epigallocatechin-3-O-gallate modulates global microRNA expression in interleukin-1β-stimulated human osteoarthritis chondrocytes: Potential role of EGCG on negative co-regulation of microRNA-140-3p and ADAMTS5. Eur. J. Nutr. 2018, 57, 917–928. [Google Scholar] [CrossRef]
- Leong, J.D.; Choudhury, M.; Hanstein, R.; Hirsh, M.D.; Kim, J.S.; Majeska, R.J.; Schaffler, M.B.; Hardin, J.A.; Spray, D.C.; Goldring, M.B.; et al. Green tea polyphenol treatment is chondroprotective, anti-inflammatory and palliative in a mouse post-traumatic osteoarthritis model. Arthritis Res. Ther. 2014, 6, 1–11. [Google Scholar]
- Nathan, P.J. The acute effects of L-theanine in comparison with alprazolam on anticipatory anxiety in humans. Hum. Psychopharmacol. 2004, 19, 457–465. [Google Scholar]
- Haskell, C.F.; Kennedy, D.O.; Milne, A.L.; Wesnes, K.A.; Scholey, A.B. The effects of L-theanine, caffeine and their combination on cognition and mood. Biol. Psychol. 2008, 77, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Duan, H.Y.; Luan, J.L.; Yagasaki, K.; Zhang, G.Y. Effects of theanine on growth of human lung cancer and leukemia cells as well as migration and invasion of human lung cancer cells. Cytotechnology 2009, 59, 211–217. [Google Scholar] [CrossRef]
- Liu, J.N.; Sun, Y.P.; Zhang, H.R.; Ji, D.X.; Wu, F.; Tian, H.H.; Liu, K.; Zhang, Y.; Wu, B.H.; Zhang, G.Y. Theanine from tea and its semi-synthetic derivative TBrC suppress human cervical cancer growth and migration by inhibiting EGFR/Met-Aktil\IF-kappa B signaling. Eur. J. Pharmacol. 2016, 791, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, J.P.; Dean, D.D.; Schwartz, Z.; Cochran, D.L.; Grant, G.M.; Klebe, R.J.; Nakaya, H.; Boyan, B.D. Chondrocyte cultures express matrix metalloproteinase mRNA and immunoreactive protein; stromelysin-1 and 72 kDa gelatinase are localized in extracellular matrix vesicles. J. Cell. Biochem. 1996, 61, 375–391. [Google Scholar] [CrossRef]
- Sumathi, T.; Asha, D.; Nagarajan, G.; Sreenivas, A.; Nivedha, R. L-Theanine alleviates the neuropathological changes induced by PCB (Aroclor 1254) via inhibiting upregulation of inflammatory cytokines and oxidative stress in rat brain. Environ. Toxicol. Pharmacol. 2016, 41, 99–117. [Google Scholar] [CrossRef] [PubMed]
- Yong, P.H.; Sun, W.J.; Choi, J.H.; Choi, C.Y.; Kim, H.G.; Kim, S.J.; Kim, Y.; Lee, K.J.; Chung, Y.C.; Jeong, H.G. Inhibitory effects of L-theanine on airway inflammation in ovalbumin-induced allergic asthma. Food Chem. Toxicol. 2017, 99, 162–169. [Google Scholar]
- Akhtar, N.; Haqqi, T.M. MicroRNA-199a* regulates the expression of cyclooxygenase-2 in human chondrocytes. Ann. Rheum. Dis. 2012, 71, 1073–1080. [Google Scholar] [CrossRef]
- Panahifar, A.; Jaremko, J.L.; Tessier, A.G.; Lambert, R.G.; Maksymowych, W.P.; Fallone, B.G.; Doschak, M.R. Development and reliability of a multi-modality scoring system for evaluation of disease progression in pre-clinical models of osteoarthritis: Celecoxib may possess disease-modifying properties. Osteoarthr. Cartil. 2014, 22, 1639–1650. [Google Scholar] [CrossRef]
- Hanstein, R.; Zhao, J.B.; Basak, R.; Smith, D.N.; Zuckerman, Y.Y.; Hanani, M.; Spray, D.C.; Gulinello, M. Focal Inflammation Causes Carbenoxolone-Sensitive Tactile Hypersensitivity in Mice. Open Pain J. 2010, 3, 123. [Google Scholar] [CrossRef]
- Scotece, M.; Conde, J.; Abella, V.; López, V.; Francisco, V.; Ruiz, C.; Campos, V.; Lago, F.; Gomez, R.; Pino, J.; et al. Oleocanthal Inhibits Catabolic and Inflammatory Mediators in LPS-Activated Human Primary Osteoarthritis (OA) Chondrocytes Through MAPKs/NF-κB Pathways. Cell. Physiol. Biochem. 2018, 49, 2414–2426. [Google Scholar] [CrossRef]
- Liacini, A.; Sylvester, J.; Li, W.Q.; Zafarullah, M. Inhibition of interleukin-1-stimulated MAP kinases, activating protein-1 (AP-1) and nuclear factor kappa B (NF-κB) transcription factors down-regulates matrix metalloproteinase gene expression in articular chondrocytes. Matrix Biol. 2002, 21, 251–262. [Google Scholar] [CrossRef]
- Feng, Z.; Li, X.; Lin, J.; Zheng, W.; Hu, Z.; Xuan, J.; Ni, W.; Pan, X. Oleuropein inhibits IL-1β-induced expression of inflammatory mediators by suppressing the activation of NF-κB and MAPK in human Osteoarthritis chondrocytes. Food Funct. 2017, 8, 3737–3744. [Google Scholar] [CrossRef]
- Li, G.; Ye, Y.; Kang, J.; Yao, X.; Zhang, Y.; Jiang, W.; Gao, M.; Dai, Y.; Xin, Y.; Wang, Q. l-Theanine prevents alcoholic liver injury through enhancing the antioxidant capability of hepatocytes. Food Chem. Toxicol. 2012, 50, 363. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Ye, X.; Ji, D.; Zhang, H.; Sun, F.; Shang, C.; Zhang, Y.; Wu, E.; Wang, F.; Wu, F. Inhibition of lung tumor growth by targeting EGFR/VEGFR-Akt/NF-κB pathways with novel theanine derivatives. Oncotarget 2014, 5, 8528–8543. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Gao, M.; Sun, S.; Bi, A.; Xin, Y.; Han, X.; Wang, L.; Yin, Z.; Luo, L. Protective effect of L-theanine on carbon tetrachloride-induced acute liver injury in mice. Biochem. Biophys Res. Commun. 2012, 422, 344–350. [Google Scholar]
- Little, C.B.; Hunter, D.J. Post-traumatic osteoarthritis: From mouse models to clinical trials. Nat. Rev. Rheumatol. 2013, 9, 485. [Google Scholar] [CrossRef] [PubMed]
- Piskin, A.; Gulbahar, M.Y.; Tomak, Y.; Gulman, B.; Kabak, Y.B. Osteoarthritis models after anterior cruciate ligament resection and medial meniscectomy in rats: A histological and immunohistochemical study. Saudi Med. J. 2008, 28, 1796–1802. [Google Scholar]
- Jamwal, S.; Kumar, P. L-theanine, a Component of Green Tea Prevents 3-Nitropropionic Acid (3-NP)-Induced Striatal Toxicity by Modulating Nitric Oxide Pathway. Mol. Neurobiol. 2017, 54, 2327–2337. [Google Scholar] [CrossRef]
- Elsaid, K.A.; Fleming, B.C.; Oksendahl, H.L.; Machan, J.T.; Fadale, P.D.; Hulstyn, M.J.; Shalvoy, R.; Jay, G.D. Decreased lubricin concentrations and markers of joint inflammation in the synovial fluid of patients with anterior cruciate ligament injury. Arthritis Rheum. 2008, 58, 1707–1715. [Google Scholar] [CrossRef]
- Scanzello, C.R. Role of low-grade inflammation in osteoarthritis. Curr. Opin. Rheumatol. 2017, 29, 79–85. [Google Scholar] [CrossRef]
- Ge, Z.; Hu, Y.; Heng, B.C.; Yang, Z.; Cao, T. Osteoarthritis and therapy. Arthritis Care Res. 2006, 55, 493–500. [Google Scholar] [CrossRef] [PubMed]
- JR, V. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat. New Biol. 1971, 231, 232–235. [Google Scholar] [CrossRef]
- Aikawa, J.; Uchida, K.; Takano, S.; Inoue, G.; Iwase, D.; Miyagi, M.; Mukai, M.; Shoji, S.; Sekiguchi, H.; Takaso, M. Regulation of calcitonin gene-related peptide expression through the COX-2/mPGES-1/PGE2 pathway in the infrapatellar fat pad in knee osteoarthritis. Lipids Health Dis. 2018, 17, 1–6. [Google Scholar] [CrossRef]
- Rasheed, Z.; Rasheed, N.; Al-Shobaili, H.A. Epigallocatechin-3-O-gallate up-regulates microRNA-199a-3p expression by down-regulating the expression of cyclooxygenase-2 in stimulated human osteoarthritis chondrocytes. J. Cell. Mol. Med. 2016, 20, 2241–2248. [Google Scholar] [CrossRef] [PubMed]
- Zweers, M.C.; de Boer, T.N.; van Roon, J.; Bijlsma, J.W.; Lafeber, F.P.; Mastbergen, S.C. Celecoxib: Considerations regarding its potential disease-modifying properties in osteoarthritis. Arthritis Res. Ther. 2011, 13, 239. [Google Scholar] [CrossRef] [PubMed]
- Martel-Pelletier, J.; Pelletier, J.P.; Fahmi, H. Cyclooxygenase-2 and Prostaglandins in Articular Tissues. Semin. Arthritis Rheum. 2004, 33, 155–167. [Google Scholar] [CrossRef]
- Abramson, S.B. Nitric oxide in inflammation and pain associated with osteoarthritis. Arthritis Res. Ther. 2008, 10, S2. [Google Scholar] [CrossRef] [PubMed]
- Abramson, S.B. Osteoarthritis and nitric oxide. Osteoarthr. Cartil. 2008, 16, S15–S20. [Google Scholar] [CrossRef]
- Vuolteenaho, K.; Moilanen, T.; Knowles, R.G.; Moilanen, E. The role of nitric oxide in osteoarthritis. Scand. J. Rheumatol. 2007, 36, 247–258. [Google Scholar] [CrossRef]
- Li, H.; Wang, D.; Yuan, Y.J.; Min, J.K. New insights on the MMP-13 regulatory network in the pathogenesis of early osteoarthritis. Arthritis Res. Ther. 2017, 19, 248. [Google Scholar] [CrossRef]
- Li, H.; Poulos, T.L. Structure–function studies on nitric oxide synthases. J. Inorg. Biochem. 2005, 99, 293–305. [Google Scholar] [CrossRef]
- Kerwin, J.F.; Lancaster, J.R.; Feldman, P.L. Nitric oxide: A new paradigm for second messengers. J. Med. Chem. 1995, 38, 4343. [Google Scholar] [CrossRef]
- Berenbaum, F. Signaling transduction: Target in osteoarthritis. Curr. Opin. Rheumatol. 2004, 16, 616–622. [Google Scholar] [CrossRef]
- Leonidou, A.; Lepetsos, P.; Mintzas, M.; Kenanidis, E.; Macheras, G.; Tzetis, M.; Potoupnis, M.; Tsiridis, E. Inducible nitric oxide synthase as a target for osteoarthritis treatment. Expert Opin. Ther. Targets 2018, 22, 299–318. [Google Scholar] [CrossRef]
- Agarwal, S.; Long, P.; Seyedain, A.; Piesco, N.; Gassner, R. A central role for nuclear factor- B pathway in the anti-inflammatory and proinflammatory actions of mechanical strain. Faseb J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2003, 17, 899–901. [Google Scholar]
- Bonizzi, G.; Karin, M. The two NF-kappa B activation pathways and their role in innate and adaptive immunity. Trends Immunol. 2004, 25, 280–288. [Google Scholar] [CrossRef]
- Tilstra, J.S.; Clauson, C.L.; Niedernhofer, L.J.; Robbins, P.D. NF-kappa B in Aging and Disease. Aging Dis. 2011, 2, 449–465. [Google Scholar]
- Marcu, K.B.; Otero, M.; Olivotto, E.; Borzi, R.M.; Goldring, M.B. NF-kappa B Signaling: Multiple Angles to Target OA. Curr. Drug Targets 2010, 11, 599–613. [Google Scholar] [CrossRef]
- Goldring, M.B.; Goldring, S.R. Osteoarthritis. J. Cell. Physiol. 2007, 213, 626–634. [Google Scholar] [CrossRef]
- Ceuninck, F.D.; Sabatini, M.; Pastoureau, P. Recent progress toward biomarker identification in osteoarthritis. Drug Discov. Today 2011, 16, 443–449. [Google Scholar] [CrossRef]
- Jordan, K.M.; Syddall, H.E.; Garnero, P.; Gineyts, E.; Dennison, E.M.; Sayer, A.A.; Delmas, P.D.; Cooper, C.; Arden, N.K. Urinary CTX-II and glucosyl-galactosyl-pyridinoline are associated with the presence and severity of radiographic knee osteoarthritis in men. Ann. Rheum. Dis. 2006, 65, 871–877. [Google Scholar] [CrossRef] [PubMed]
| Gene | Primer Sequence | Product Length (bp) |
|---|---|---|
| MMP-3 | F: TTTGGCCGTCTCTTCCATCC | 175 |
| R: GCATCGATCTTCTGGACGGT | ||
| MMP13 | F: TTCTGGTCTTCTGGCACACG | 92 |
| R: TGGAGCTGCTTGTCCAGGT | ||
| GAPDH | F: GATGCCCCCATGTTTGTGAT | 150 |
| R: GGCATGGACTGTGGTCATGAG |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, H.; Zhang, Z.; Li, Y.; Song, X.; Ma, T.; Liu, C.; Liu, L.; Yuan, R.; Wang, X.; Gao, L. L-Theanine Reduced the Development of Knee Osteoarthritis in Rats via Its Anti-Inflammation and Anti-Matrix Degradation Actions: In Vivo and In Vitro Study. Nutrients 2020, 12, 1988. https://doi.org/10.3390/nu12071988
Bai H, Zhang Z, Li Y, Song X, Ma T, Liu C, Liu L, Yuan R, Wang X, Gao L. L-Theanine Reduced the Development of Knee Osteoarthritis in Rats via Its Anti-Inflammation and Anti-Matrix Degradation Actions: In Vivo and In Vitro Study. Nutrients. 2020; 12(7):1988. https://doi.org/10.3390/nu12071988
Chicago/Turabian StyleBai, Hui, Zhiheng Zhang, Yue Li, Xiaopeng Song, Tianwen Ma, Chunpeng Liu, Lin Liu, Rui Yuan, Xinyu Wang, and Li Gao. 2020. "L-Theanine Reduced the Development of Knee Osteoarthritis in Rats via Its Anti-Inflammation and Anti-Matrix Degradation Actions: In Vivo and In Vitro Study" Nutrients 12, no. 7: 1988. https://doi.org/10.3390/nu12071988
APA StyleBai, H., Zhang, Z., Li, Y., Song, X., Ma, T., Liu, C., Liu, L., Yuan, R., Wang, X., & Gao, L. (2020). L-Theanine Reduced the Development of Knee Osteoarthritis in Rats via Its Anti-Inflammation and Anti-Matrix Degradation Actions: In Vivo and In Vitro Study. Nutrients, 12(7), 1988. https://doi.org/10.3390/nu12071988




