Inhibition of UVB-Induced Inflammation by Laminaria japonica Extract via Regulation of nc886-PKR Pathway
Abstract
:1. Introduction
2. Material and Methods
2.1. Extraction of Laminaria japonica
2.2. Cell Culture, Cell Treatment with LJE and UVB Irradiation, qRT-PCR, and Methylation-Specific PCR
2.3. DNA and siRNA Transfection for nc886 Overexpression and Knockdown
2.4. Western Blotting
2.5. Analysis of MMP-9, PGE2, IL-8, and TNF-α by ELISA
2.6. Data Availability
2.7. Statistical Analysis
3. Results
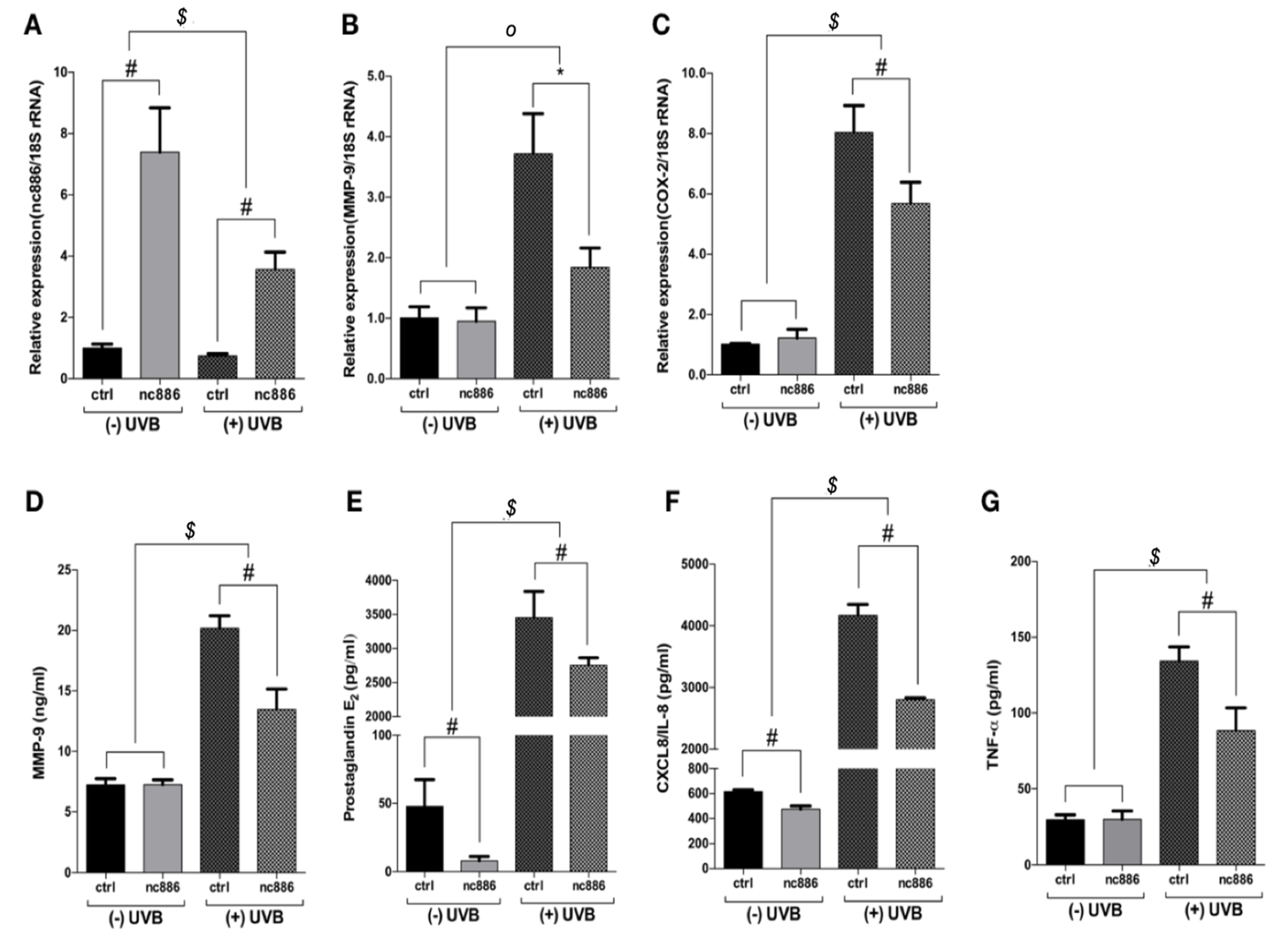
3.1. Overexpression of nc886 Reduces the Production of MMP-9 and Inflammatory Cytokines
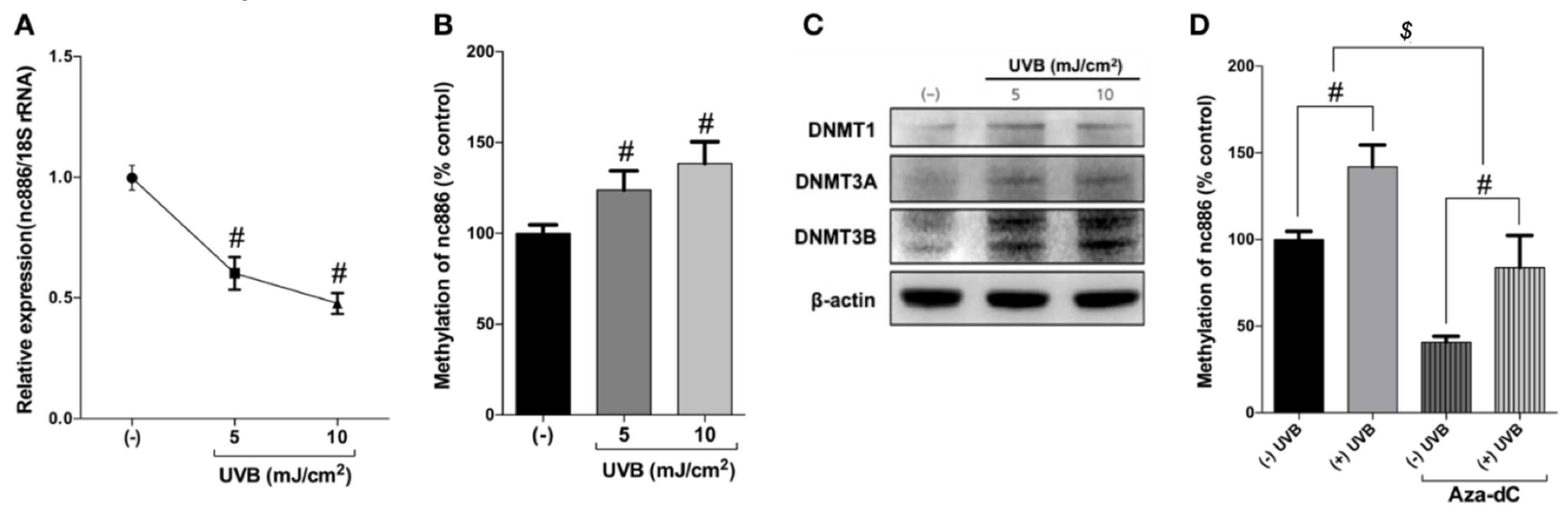
3.2. UVB Inhibits nc886 Expression by Increasing nc886 Methylation
3.3. Laminaria japonica Extract Reduces the Production of MMP-9 and Inflammatory Cytokines
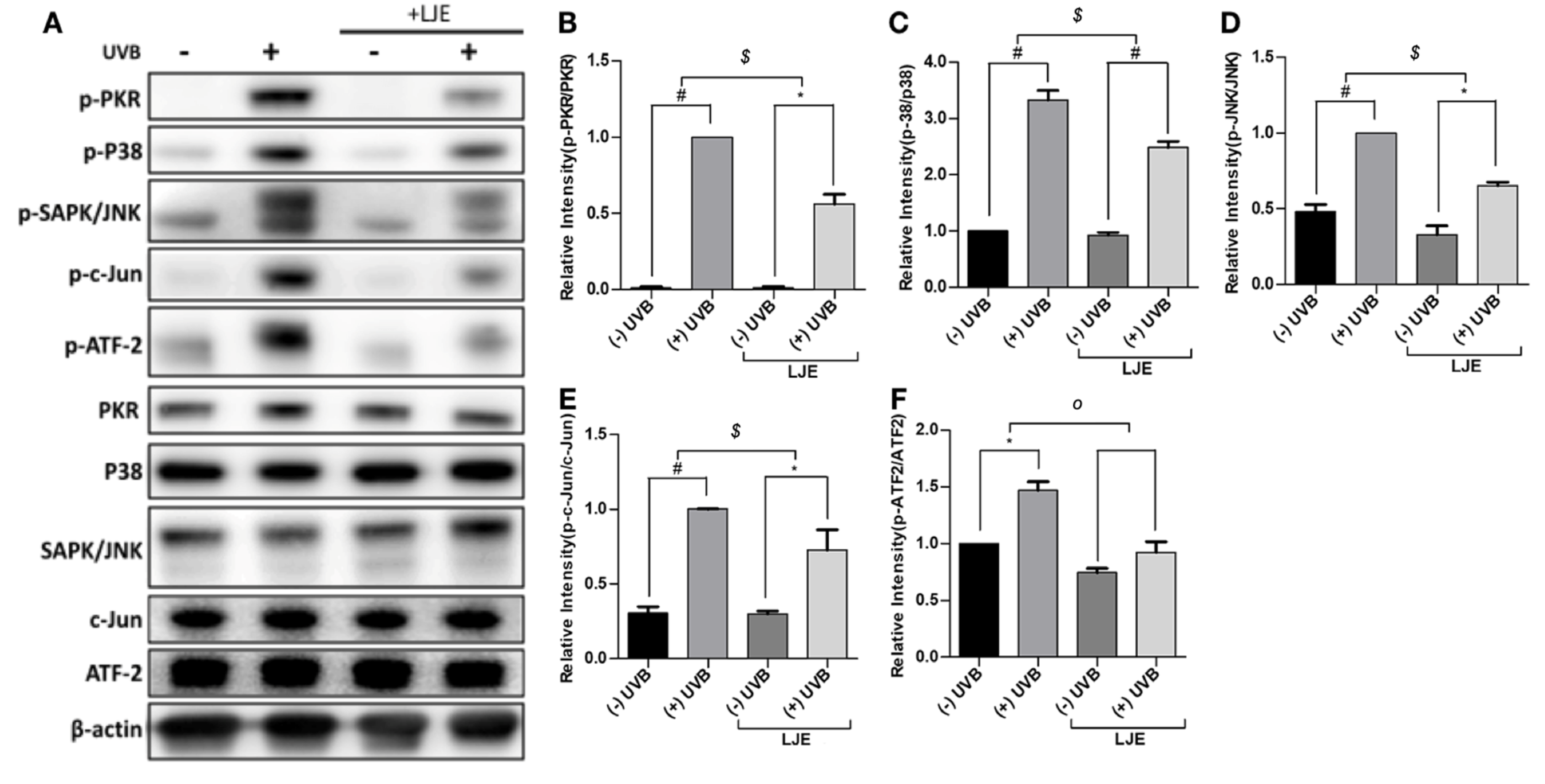
3.4. Laminaria japonica Extract Attenuates the PKR-MAPKs Pathway Activated by UVB
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Choi, J.-S.; Moon, W.S.; Choi, J.N.; Do, K.H.; Moon, S.H.; Cho, K.K.; Han, C.-J.; Choi, I.S.; Center, R. Effects of seaweed Laminaria japonica extracts on skin moisturizing activity in vivo. J. Cosmet. Sci. 2013, 64, 193–205. [Google Scholar] [PubMed]
- Kyung, J.; Kim, D.; Park, D.; Yang, Y.-H.; Choi, E.-K.; Lee, S.-P.; Kim, T.-S.; Lee, Y.-B.; Kim, Y.-B. Synergistic anti-inflammatory effects of Laminaria japonica fucoidan and Cistanche tubulosa extract. Lab. Anim. Res. 2012, 28, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuda, T.; Nakamura, S.; An, C.; Takahashi, H.; Kimura, B.; Nishizawa, M. Effects of Holdfast of Laminaria japonica on Listeria Invasion on Enterocyte-Like Caco-2 Cells and NO Production of Macrophage RAW 264.7 Cells. Appl. Biochem. Biotechnol. 2012, 168, 928–935. [Google Scholar] [CrossRef] [PubMed]
- Jang, E.J.; Kim, S.C.; Lee, J.-H.; Lee, J.R.; Kim, I.K.; Baek, S.Y.; Kim, Y.W. Fucoxanthin, the constituent of Laminaria japonica, triggers AMPK-mediated cytoprotection and autophagy in hepatocytes under oxidative stress. BMC Complement. Altern. Med. 2018, 18. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.-S.; Haj, F.G.; Lee, M.; Kang, I.; Zhang, G.; Lee, Y. Laminaria japonica Extract Enhances Intestinal Barrier Function by Altering Inflammatory Response and Tight Junction-Related Protein in Lipopolysaccharide-Stimulated Caco-2 Cells. Nutrients 2019, 11, 1001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, L.; Tan, J.; Yang, X.; Tan, H.; Xu, X.; You, M.; Qin, W.; Huang, L.; Li, S.; Mo, M.; et al. Polysaccharide Extracted from Laminaria japonica Delays Intrinsic Skin Aging in Mice. Evid. Based Complement. Altern. Med. 2016, 2016, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.M.; Im, A.-R.; Park, S.K.; Shin, H.S.; Chae, S. Protective effects of Timosaponin AIII against UVB- radiation induced inflammation and DNA injury in human epidermal keratinocytes. Biol. Pharm. Bull. 2019. [Google Scholar] [CrossRef] [Green Version]
- Li, X.-Q.; Cai, L.-M.; Liu, J.; Ma, Y.-L.; Kong, Y.-H.; Li, H.; Jiang, M. Liquiritin suppresses UVB-induced skin injury through prevention of inflammation, oxidative stress and apoptosis through the TLR4/MyD88/NF-κB and MAPK/caspase signaling pathways. Int. J. Mol. Med. 2018, 42, 1445–1459. [Google Scholar] [CrossRef] [Green Version]
- Buckman, S.Y.; Gresham, A.; Hale, P.; Hruza, G.; Anast, J.; Masferrer, J.; Pentland, A.P. COX-2 expression is induced by UVB exposure in human skin: Implications for the development of skin cancer. Carcinogenesis 1998, 19, 723–729. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.; Cho, A.; Kwon, S.; Kim, M.; Song, M.; Han, H.; Shin, E.; Park, E.; Lee, S. Potential Photoprotective Effect of Dietary Corn Silk Extract on Ultraviolet B-Induced Skin Damage. Molecules 2019, 24, 2587. [Google Scholar] [CrossRef] [Green Version]
- Cha, H.J.; Lee, G.T.; Lee, K.S.; Lee, K.K.; Hong, J.T.; Lee, N.K.; Kim, S.-Y.; Lee, B.M.; An, I.-S.; Hahn, H.J.; et al. Photoprotective effect of arctiin against ultraviolet B-induced damage in HaCaT keratinocytes is mediated by microRNA expression changes. Mol. Med. Rep. 2014, 10, 1363–1370. [Google Scholar] [CrossRef]
- Chen, F.; Tang, Y.; Sun, Y.; Veeraraghavan, V.P.; Mohan, S.K.; Cui, C. 6-shogaol, a active constiuents of ginger prevents UVB radiation mediated inflammation and oxidative stress through modulating NrF2 signaling in human epidermal keratinocytes (HaCaT cells). J. Photochem. Photobiol. B Biol. 2019, 197, 111518. [Google Scholar] [CrossRef]
- Syed, D.N.; Khan, M.I.; Shabbir, M.; Mukhtar, H. MicroRNAs in skin response to UV radiation. Curr. Drug Targets 2013, 14, 1128–1134. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Li, F.; Yang, B.; Liang, J.; Qin, H.; Xu, J. Effects of ultraviolet B exposure on DNA methylation in patients with systemic lupus erythematosus. Exp. Ther. Med. 2013, 5, 1219–1225. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.-S.; Park, J.-L.; Lee, K.; Richardson, L.E.; Johnson, B.H.; Lee, H.-S.; Lee, J.-S.; Kim, S.-B.; Kwon, O.-H.; Song, K.S.; et al. nc886, a non-coding RNA of anti-proliferative role, is suppressed by CpG DNA methylation in human gastric cancer. Oncotarget 2014, 5, 3944–3955. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.-S.; Lee, K.; Jang, H.-J.; Lee, G.K.; Park, J.-L.; Kim, S.-Y.; Kim, S.-B.; Johnson, B.H.; Zo, J.I.; Lee, J.-S.; et al. Epigenetic silencing of the non-coding RNA nc886 provokes oncogenes during human esophageal tumorigenesis. Oncotarget 2014, 5, 3472–3481. [Google Scholar] [CrossRef] [Green Version]
- Katiyar, S.K.; Singh, T.; Prasad, R.; Sun, Q.; Vaid, M. Epigenetic alterations in ultraviolet radiation-induced skin carcinogenesis: Interaction of bioactive dietary components on epigenetic targets. Photochem. Photobiol. 2012, 88, 1066–1074. [Google Scholar] [CrossRef]
- Jeon, S.H.; Lee, K.; Lee, K.S.; Kunkeaw, N.; Johnson, B.H.; Holthauzen, L.M.F.; Gong, B.; Leelayuwat, C.; Lee, Y.S. Characterization of the direct physical interaction of nc886, a cellular non-coding RNA, and PKR. FEBS Lett. 2012, 586, 3477–3484. [Google Scholar] [CrossRef] [Green Version]
- Golec, E.; Lind, L.; Qayyum, M.; Blom, A.M.; King, B.C. The Noncoding RNA nc886 Regulates PKR Signaling and Cytokine Production in Human Cells. J. Immunol. 2019, 202, 131–141. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.S.; Kunkeaw, N.; Lee, Y.-S. Protein kinase R and its cellular regulators in cancer: An active player or a surveillant? Wires RNA 2019, e1558. [Google Scholar] [CrossRef]
- Jeon, S.; Johnson, B.; Lee, Y. A Tumor Surveillance Model: A Non-Coding RNA Senses Neoplastic Cells and Its Protein Partner Signals Cell Death. Int. J. Mol. Sci. 2012, 13, 13134–13139. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.S. A Novel Type of Non-coding RNA, nc886, Implicated in Tumor Sensing and Suppression. Genom. Inf. 2015, 13, 26–30. [Google Scholar] [CrossRef] [Green Version]
- Kunkeaw, N.; Jeon, S.H.; Lee, K.; Johnson, B.H.; Tanasanvimon, S.; Javle, M.; Pairojkul, C.; Chamgramol, Y.; Wongfieng, W.; Gong, B.; et al. Cell death/proliferation roles for nc886, a non-coding RNA, in the protein kinase R pathway in cholangiocarcinoma. Oncogene 2013, 32, 3722–3731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.; Kunkeaw, N.; Jeon, S.H.; Lee, I.; Johnson, B.H.; Kang, G.-Y.; Bang, J.Y.; Park, H.S.; Leelayuwat, C.; Lee, Y.S. Precursor miR-886, a novel noncoding RNA repressed in cancer, associates with PKR and modulates its activity. RNA 2011, 17, 1076–1089. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.-S.; Shin, S.; Cho, E.; Im, W.K.; Lee, Y.S.; Jeon, S.H.; Kim, Y.; Park, D.; Fréchet, M.; Chajra, H.; et al. nc886, a non-coding RNA, inhibits UVB-induced MMP-9 and COX-2 expression via the PKR pathway in human keratinocytes. Biochem. Biophys. Res. Commun. 2019. [Google Scholar] [CrossRef]
- Goh, K.C. The protein kinase PKR is required for p38 MAPK activation and the innate immune response to bacterial endotoxin. EMBO J. 2000, 19, 4292–4297. [Google Scholar] [CrossRef] [Green Version]
- Zhong, J.L.; Yang, L.; Lü, F.; Xiao, H.; Xu, R.; Wang, L.; Zhu, F.; Zhang, Y. UVA, UVB and UVC induce differential response signaling pathways converged on the eIF2α phosphorylation. Photochem. Photobiol. 2011, 87, 1092–1104. [Google Scholar] [CrossRef]
- Pittayapruek, P.; Meephansan, J.; Prapapan, O.; Komine, M.; Ohtsuki, M. Role of Matrix Metalloproteinases in Photoaging and Photocarcinogenesis. Int. J. Mol. Sci. 2016, 17, 868. [Google Scholar] [CrossRef] [Green Version]
- Bahar-Shany, K.; Ravid, A.; Koren, R. Upregulation of MMP-9 production by TNFalpha in keratinocytes and its attenuation by vitamin D. J. Cell. Physiol. 2010, 222, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Koźák, I.; Klisenbauer, D.; Juhás, T. UV-B induced production of MMP-2 and MMP-9 in human corneal cells. Physiol. Res. 2003, 52, 229–234. [Google Scholar]
- Onoue, S.; Kobayashi, T.; Takemoto, Y.; Sasaki, I.; Shinkai, H. Induction of matrix metalloproteinase-9 secretion from human keratinocytes in culture by ultraviolet B irradiation. J. Dermatol. Sci. 2003, 33, 105–111. [Google Scholar] [CrossRef]
- Silva, A.M.; Whitmore, M.; Xu, Z.; Jiang, Z.; Li, X.; Williams, B.R.G. Protein kinase R (PKR) interacts with and activates mitogen-activated protein kinase kinase 6 (MKK6) in response to double-stranded RNA stimulation. J. Biol. Chem. 2004, 279, 37670–37676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, B.; Hwang, E.; Seo, S.A.; Zhang, M.; Park, S.-Y.; Yi, T.-H. Dietary Rosa damascena protects against UVB-induced skin aging by improving collagen synthesis via MMPs reduction through alterations of c-Jun and c-Fos and TGF-β1 stimulation mediated smad2/3 and smad7. J. Funct. Foods 2017, 36, 480–489. [Google Scholar] [CrossRef]
- Bonneville, M.; Saint-Mezard, P.; Benetiere, J.; Hennino, A.; Pernet, I.; Denis, A.; Nicolas, J. Laminaria ochroleuca extract reduces skin inflammation. J. Eur. Acad. Dermatol. Venereol. 2007, 21, 1124–1125. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, K.-S.; Cho, E.; Weon, J.B.; Park, D.; Fréchet, M.; Chajra, H.; Jung, E. Inhibition of UVB-Induced Inflammation by Laminaria japonica Extract via Regulation of nc886-PKR Pathway. Nutrients 2020, 12, 1958. https://doi.org/10.3390/nu12071958
Lee K-S, Cho E, Weon JB, Park D, Fréchet M, Chajra H, Jung E. Inhibition of UVB-Induced Inflammation by Laminaria japonica Extract via Regulation of nc886-PKR Pathway. Nutrients. 2020; 12(7):1958. https://doi.org/10.3390/nu12071958
Chicago/Turabian StyleLee, Kwang-Soo, Eunae Cho, Jin Bae Weon, Deokhoon Park, Mathilde Fréchet, Hanane Chajra, and Eunsun Jung. 2020. "Inhibition of UVB-Induced Inflammation by Laminaria japonica Extract via Regulation of nc886-PKR Pathway" Nutrients 12, no. 7: 1958. https://doi.org/10.3390/nu12071958
APA StyleLee, K.-S., Cho, E., Weon, J. B., Park, D., Fréchet, M., Chajra, H., & Jung, E. (2020). Inhibition of UVB-Induced Inflammation by Laminaria japonica Extract via Regulation of nc886-PKR Pathway. Nutrients, 12(7), 1958. https://doi.org/10.3390/nu12071958



