Influence of Dietary Supplementation for Hyperhomocysteinemia Treatments
Abstract
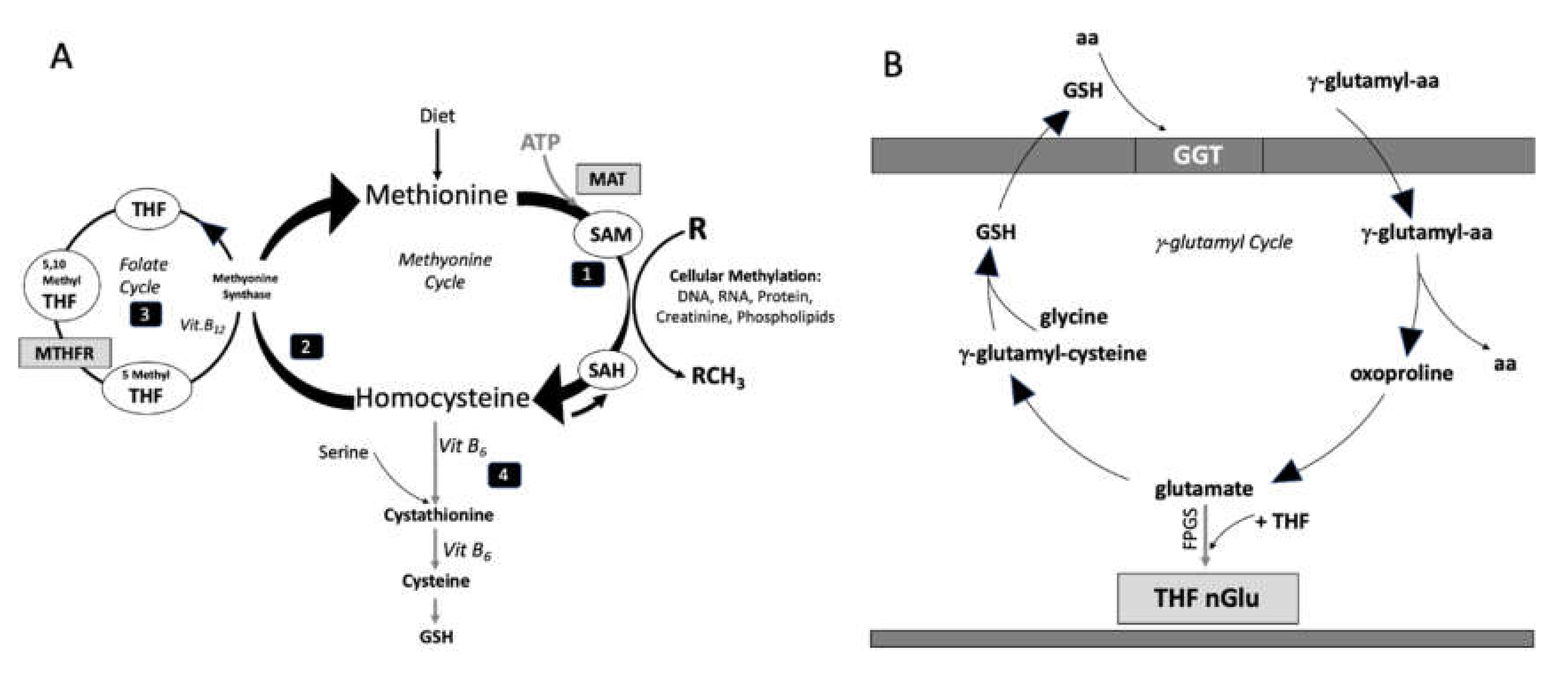
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Homocysteine and Aminothiols Assay
2.3. Sample Size and Statistical Analysis
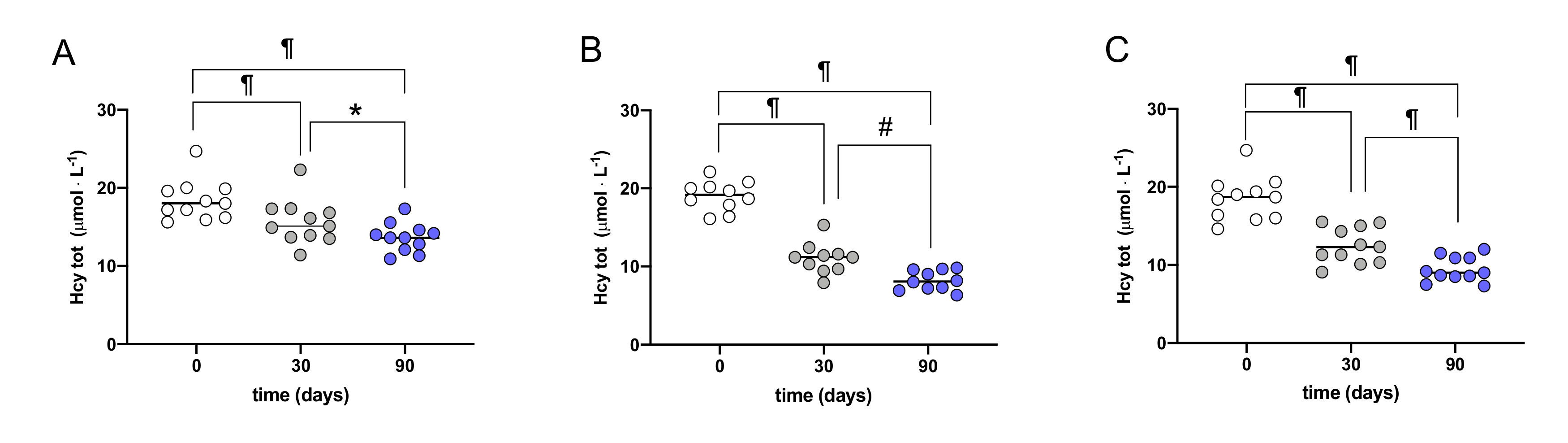
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Baggott, J.E.; Tamura, T. Homocysteine, iron and cardiovascular disease: A hypothesis. Nurients 2015, 7, 1108–1118. [Google Scholar] [CrossRef]
- Weiss, N.; Keller, C.; Hoffmann, U.; Loscalzo, J. Endothelial dysfunction and atherothrombosis in mild hyperhomocysteinemia. Vasc. Med. 2002, 7, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Harker, L.A.; Slichter, S.J.; Scott, C.R.; Ross, R. Homocystinemia: Vascular injury and arterial thrombosis. N. Engl. J. Med. 1974, 291, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Harker, L.A.; Ross, R.; Slichter, S.J.; Scott, C.R. Homocystine-induced arteriosclerosis: The role of endothelial cell injury and platelet response in its genesis. J. Clin. Investig. 1976, 58, 731–741. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Ghosh, A.K.; Eren, M.; Miyata, T.; Vaughan, D.E. PAI-1 contributes to homocysteine-induced cellular senescence. Cell. Signal. 2019, 64, 109394. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Sun, X.; Liu, J.; Xie, X.; Cui, W.; Zhu, Y. Homocysteine Accelerates Senescence of Endothelial Cells via DNA Hypomethylation of Human Telomerase Reverse Transcriptase. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 71–78. [Google Scholar] [CrossRef]
- Rane, G.; Koh, W.P.; Kanchi, M.M.; Wang, R.; Yuan, J.M.; Wang, X. Association Between Leukocyte Telomere Length and Plasma Homocysteine in a Singapore Chinese Population. Rejuvenation Res. 2015, 18, 203–210. [Google Scholar] [CrossRef]
- Selhub, J. The many facets of hyperhomocysteinemia: Studies from the Framingham cohorts. J. Nutr. 2006, 136, 1726S–1730S. [Google Scholar] [CrossRef]
- Sanderson, P.; McNulty, H.; Mastroiacovo, P.; McDowell, I.F.; Melse-Boonstra, A.; Finglas, P.M.; Gregory, J.F., 3rd; UK Food Standards Agency. Folate bioavailability: UK Food Standards Agency workshop report. Br. J. Nutr. 2003, 90, 473–479. [Google Scholar] [CrossRef]
- Winkels, R.; Brouwer, I.; Siebelink, E.; Katan, M.; Verhoef, P. Bioavailability of food folates is 80% of that of folic acid. Am. J. Clin. Nutr. 2007, 85, 465–473. [Google Scholar] [CrossRef]
- Jenkins, D.J.A.; Spence, J.D.; Giovannucci, E.L.; Kim, Y.I.; Josse, R.; Vieth, R.; Blanco Mejia, S.; Viguiliouk, E.; Nishi, S.; Sahye-Pudaruth, S.; et al. Supplemental vitamins and minerals for CVD prevention and treatment. J. Am. Coll. Cardiol. 2018, 71, 2570–2584. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jin, Y.; Wang, Y.; Li, L.; Liao, Y.; Zhang, Y.; Yu, D. The effect of folic acid in patients with cardiovascular didease. A systematic review and meta-analysis. Medicine 2019. [Google Scholar] [CrossRef]
- National Institute of Health Office of Dietary Supplements. 2016. Available online: https://ods.od.nih.gov/factsheets/Folate-HealthProfessional/ (accessed on 30 June 2020).
- Crider, K.S.; Bailey, L.B.; Berry, R.J. Folic acid fortification—Its history, effect, concerns, and future directions. Nutrients 2011, 3, 370–384. [Google Scholar] [CrossRef] [PubMed]
- Christensen, K.E.; Mikael, L.G.; Leung, K.Y.; Lévesque, N.; Deng, L.; Wu, Q.; Malysheva, O.V.; Best, A.; Caudill, M.A.; Greene, N.D.; et al. High folic acid consumption leads to pseudo-MTHFR deficiency, altered lipid metabolism, and liver injury in mice. Am. J. Clin. Nutr. 2015, 101, 646–658. [Google Scholar] [CrossRef]
- Ware, W.R. Raising concerns about unmetabolized folic acid. J. Orthomol. Med. 2008, 23, 43–51. [Google Scholar]
- Cawley, S.; Mullaney, L.; McKeating, A.; Farren, M.; McCartney, D.; Turner, M.J. A review of European guidelines on periconceptional folic acid supplementation. Eur. J. Clin. Nutr. 2016, 70, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Shane, B. Folate chemistry and metabolism. In Folate in Health and Disease; Bailey, L.B., Ed.; Marcel Dekker: New York, NY, USA, 1995; pp. 1–22. [Google Scholar]
- Raz, S.; Stark, M.; Assaraf, Y.G. Folypoly-γ-glutamate synthetase: A key determinant of folate homeostasis and antifolate resistance in cancer. Drug Res. Updates 2016, 28, 43–64. [Google Scholar] [CrossRef]
- Ueland, P.M.; Mansoor, M.A.; Guttormsen, A.B. Reduced, oxidised and protein-bound forms of homocysteine and other aminothiols in plasma comprise the redox thiols status a possible element of the extracellular antioxidant defence system. J. Nutr. 1996, 126, 1281S–1284S. [Google Scholar] [CrossRef]
- Ravaglia, G.; Forti, P.; Maioli, F.; Chiappelli, M.; Montesi, F.; Bianchin, M.; Licastro, F.; Patterson, C. Apolipoprotein E e4 allele affects risk of hyperhomocysteinemia in the elderly. Am. J. Clin. Nutr. 2006, 84, 1473–1480. [Google Scholar] [CrossRef]
- Accinni, R.; Campolo, J.; Bartesaghi, S.; De Leo, G.; Lucarelli, C.; Cursano, C.F.; Parodi, O. High-performance liquid chromatographic determination of total plasma homocysteine with or without internal standards. J. Chromat. 1998, 828, 397–400. [Google Scholar] [CrossRef]
- Accinni, R.; Parodi, O. Determination of plasma homocysteine. In Atherosclerosis: Experimental Methods and Protocols; Drew, A.F., Ed.; Humana Press: Totowa, NJ, USA, 2001; pp. 77–103. [Google Scholar]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods. 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Basu, P.; Qipshidze, N.; Tyagi, S.C.; Sen, U. Remodeling in vein expresses arterial phenotype in hyperhomocysteinemia. Int. J. Physiol. Pathophysiol. Pharmacol. 2011, 3, 266–279. [Google Scholar] [PubMed]
- Basu, P.; Qipshidze, N.; Sen, U.; Givvimani, S.; Munjal, C.; Mishra, P.K.; Tyagi, S.C. Chronic hyperhomocysteinemia causes vascular remodelling by instigating vein phenotype in artery. Arch. Physiol. Biochem. 2011, 117, 270–282. [Google Scholar] [CrossRef] [PubMed]
- Fan, R.; Zhang, A.; Zhong, F. Association between Homocysteine Levels and All-cause Mortality: A Dose-Response Meta-Analysis of Prospective Studies. Sci. Rep. 2017, 7, 4769. [Google Scholar] [CrossRef]
- Eto, K.; Asada, T.; Arima, K.; Makifuchi, T.; Kimura, H. Brain hydrogen sulfide is severely decreased in Alzheimer’s disease. Biochem. Biophys. Res. Commun. 2002, 293, 1485–1488. [Google Scholar] [CrossRef]
- Seshadri, S.; Beiser, A.; Selhub, J.; Jacques, P.F.; Rosenberg, I.H.; D’Agostino, R.B.; Wilson, P.W.; Wolf, P.A. Plasma homocysteine as a risk factor for dementia and Alzheimer’s disease. N. Engl. J. Med. 2002, 346, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Sachdev, P. Homocysteine, cerebrovascular disease and brain atrophy. J. Neurol. Sci. 2004, 226, 25–29. [Google Scholar] [CrossRef]
- Van Dam, F.; Van Gool, W.A. Hyperhomocysteinemia and Alzheimer’s disease: A systematic review. Arch. Gerontol. Geriatr. 2009, 48, 425–430. [Google Scholar] [CrossRef]
- Henry, O.R.; Benghuzzi, H.; Taylor, H.A., Jr.; Tucci, M.; Butler, K.; Jones, L. Suppression of homocysteine levels by vitamin B12 and folates: Age and gender dependency in the Jackson Heart Study. Am. J. Med. Sci. 2012, 344, 110–115. [Google Scholar] [CrossRef]
- Ostrakhovitch, E.A.; Tabibzade, S. Homocysteine and age-associated disorders. Ageing Res. Rev. 2019, 49, 144–164. [Google Scholar] [CrossRef]
- Nijhout, H.F.; Reed, M.C.; Budu, P.; Ulrich, C.M. A mathematical model of the folate cycle: New insights into folate homeostasis. J. Biol. Chem. 2004, 279, 55008–55016. [Google Scholar] [CrossRef] [PubMed]
- Ward, M.; McNulty, H.; McPartlin, J.; Strain, J.J.; Weir, D.G.; Scott, J.M. Plasma homocysteine, a risk factor for cardiovascular disease, is lowered by physiological doses of folic acid. Quart. J. Med. 1997, 90, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Venn, B.J.; Mann, J.L.; Williams, S.M.; Riddell, L.J.; Chisholm, A.; Harper, M.J.; Aitken, W.; Rossaak, J.L. Assessment of three levels of folic acid on serum folate and plasma homocysteine: A randomised placebo-controlled double-blind dietary intervention trial. Eur. J. Clin. Nutr. 2002, 56, 748–754. [Google Scholar] [CrossRef] [PubMed]
- Zappacosta, B.; Mastroiacovo, P.; Persichilli, S.; Pounis, G.; Ruggeri, S.; Minucci, A.; Carnovale, E.; Andria, G.; Ricci, R.; Scala, I.; et al. Homocysteine lowering by folate-rich diet or pharmacological supplementations in subjects with moderate hyperhomocysteinemia. Nutrients 2013, 5, 1531–1543. [Google Scholar] [CrossRef]
- Homocysteine Lowering Trialists’ Collaboration. Dose-dependent effects of folic acid on blood concentrations of homocysteine: A meta-analysis of the randomized trials. Am. J. Clin. Nutr. 2005, 82, 806–812. [Google Scholar] [CrossRef]
- Dudman, N.P.B.; Wilcken, D.E.L.; Stocker, R. Circulating lipidhydroperoxide levels in human hyperhomocysteinemia: Relevance to development of atherosclerosis. Arterioscl. Thromb. 1993, 13, 512–516. [Google Scholar] [CrossRef]
- Saez, G.; Thornalley, P.J.; Hill, H.A.; Herms, R.; Bannister, J.V. The production of free radicals during the auto-oxidation of cysteine and their effect on isolated rat hepatocytes. Biochim. Biophys. Acta 1982, 719, 24–31. [Google Scholar] [CrossRef]
| Magnesio+ | Oxifolic | |
|---|---|---|
| Magnesium pidolate | 588 mg | 652 mg |
| Magnesium | 57 mg | |
| Oxoproline | 538 mg | 595 mg |
| Folic acid | – | 300 μg |
| Therapy | Sex (Male) | Age (Years) | Hight (cm) | Weight (kg) | BMI (kg·m−2) | Hcy (μM) |
|---|---|---|---|---|---|---|
| Magnesio+ | 5 (46%) | 50 ± 19 | 168 ± 8 | 73.4 ± 9.7 | 26.2 ± 3.6 | 18.4 ± 2.6 |
| Oxifolic | 5 (50%) | 48 ± 9 | 176 ± 5 | 81.4 ± 9.6 | 26.5 ± 4.1 | 19.0 ± 1.9 |
| Traditional | 6 (55%) | 46 ± 13 | 171 ± 10 | 73.1 ± 12.7 | 24.9 ± 4.1 | 18.5 ± 2.8 |
| Thiols | Magnesio+ | Oxifolic | Traditional | |||
|---|---|---|---|---|---|---|
| μmol·L−1 | Pre | Post | Pre | Post | Pre | Post |
| Cys | 267.8± 48.0 | 216.8 ± 40.8 * | 259.7± 61.9 | 203.1 ± 37.8 * | 266.2 ± 54.3 | 215.9 ± 55.7 # |
| Hcy | 18.4 ± 2.6 | 13.6 ± 1.8 ¶ | 19.0 ± 1.9 | 8.2 ± 1.2 ¶ | 18.5 ± 2.8 | 9.5 ± 1.6 ¶ |
| CysGly | 26.9 ± 5.0 | 27.9 ± 5.0 | 25.3 ± 4.5 | 25.8 ± 4.7 | 28.1 ± 4.5 | 29.4 ± 4.1 |
| GSH | 4.99 ± 1.77 | 6.08 ± 1.29 | 5.04 ± 0.88 | 6.85 ± 2.13 # | 4.71 ± 0.61 | 6.30 ± 2.18 * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vezzoli, A.; Dellanoce, C.; Maria Caimi, T.; Vietti, D.; Montorsi, M.; Mrakic-Sposta, S.; Accinni, R. Influence of Dietary Supplementation for Hyperhomocysteinemia Treatments. Nutrients 2020, 12, 1957. https://doi.org/10.3390/nu12071957
Vezzoli A, Dellanoce C, Maria Caimi T, Vietti D, Montorsi M, Mrakic-Sposta S, Accinni R. Influence of Dietary Supplementation for Hyperhomocysteinemia Treatments. Nutrients. 2020; 12(7):1957. https://doi.org/10.3390/nu12071957
Chicago/Turabian StyleVezzoli, Alessandra, Cinzia Dellanoce, Teresa Maria Caimi, Daniele Vietti, Michela Montorsi, Simona Mrakic-Sposta, and Roberto Accinni. 2020. "Influence of Dietary Supplementation for Hyperhomocysteinemia Treatments" Nutrients 12, no. 7: 1957. https://doi.org/10.3390/nu12071957
APA StyleVezzoli, A., Dellanoce, C., Maria Caimi, T., Vietti, D., Montorsi, M., Mrakic-Sposta, S., & Accinni, R. (2020). Influence of Dietary Supplementation for Hyperhomocysteinemia Treatments. Nutrients, 12(7), 1957. https://doi.org/10.3390/nu12071957







